Abstract
Cancer stem cells (CSCs) represent a small yet pivotal subset of tumor cells endowed with self-renewal capabilities. These cells are intricately linked to tumor progression and are central to drug resistance, metastasis, and recurrence. The tumor microenvironment (TME) encompasses the cancer cells and their surrounding milieu, including immune and inflammatory cells, cancer-associated fibroblasts, adjacent stromal tissues, tumor vasculature, and a variety of cytokines and chemokines. Within the TME, cells such as immune and inflammatory cells, endothelial cells, adipocytes, and fibroblasts release growth factors, cytokines, chemokines, and exosomes, which can either sustain or disrupt CSCs, thereby influencing tumor progression. Conversely, CSCs can also secrete cytokines, chemokines, and exosomes, affecting various components of the TME. Exosomes, a subset of extracellular vesicles (EVs), carry a complex cargo of nucleic acids, proteins, and lipids, playing a crucial role in the communication between CSCs and the TME. This review primarily focuses on the impact of exosomes secreted by CSCs (CSC-exo) on tumor progression, including their roles in maintaining stemness, promoting angiogenesis, facilitating metastasis, inducing immune suppression, and contributing to drug resistance. Additionally, we discuss how exosomes secreted by different cells within the TME affect CSCs. Finally, we explore the potential of utilizing exosomes to mitigate the detrimental effects of CSCs or to target and eliminate them. A thorough understanding of the exosome-mediated crosstalk between CSCs and the TME could provide valuable insights for developing targeted therapies against CSCs.
Keywords: Cancer stem cells, Exosomes, Extracellular vesicles, Tumor microenvironment, Cancer treatment
Introduction
Cancer remains the leading cause of death worldwide and is a major impediment to increasing human lifespan across all nations globally [1]. A recent statistics indicate approximately 20 million new cancer cases and about 9.7 million deaths attributed to the disease in 2020 [2]. At present, there are several therapeutic options available for cancer, such as surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy. However, issues like tumor recurrence, metastasis, and resistance to drugs continue to pose significant challenges in effective cancer management [3]. A key reason for these challenges is that most treatments predominantly target bulk tumor cells and do not effectively address cancer stem cells (CSCs) [4]. CSCs were first discovered and isolated in leukemia in 1994 [5]. These cells share many characteristics with stem cells, including self-renewal and the ability to proliferate indefinitely. CSCs are distinguished by unique stem cell-like properties and express specific markers that differ from those of other tumor cells. These markers vary across different types of cancer, allowing for the relatively specific isolation of CSCs. Since their discovery in leukemia, CSCs have been identified and isolated from various other tumors, including those affecting the esophagus, pancreas, ovaries, colon, liver, breast, prostate, and skin, among others. Many scientists widely recognize the pivotal role of CSCs in tumor progression due to their capacity for self-renewal and unlimited proliferation. CSCs also possess robust migratory and invasive capabilities, enabling them to travel to distant sites through the bloodstream or lymphatic system and establish metastases. Furthermore, their resistance to conventional therapies means that any surviving CSCs can regenerate the tumor, leading to treatment failures and recurrence. CSCs also demonstrate “plasticity,” which allows them to differentiate into various cell states and adapt to environmental changes, thereby enhancing tumor progression and contributing to increased drug resistance [6, 7]. Currently, there are two models explaining tumor cell heterogeneity. The first is the hierarchical organization theory, which suggests that a structured hierarchy exists within tumors, with cancer stem cells at the apex. These cells are characterized by their unique ability to self-renew and differentiate into various cell types found within the tumor. The CSC model posits that certain cancer cell populations possess self-renewal capabilities that can initiate tumor growth, and these cells generally exhibit lower levels of differentiation compared to most cancer cells [8]. The second model related to CSCs is the concept of clonal evolution. This model proposes that tumors evolve within the host’s biological environment through mutations and natural selection. Within this framework, CSCs are considered clonal populations with specific mutations that confer stem-like properties, such as the ability to self-renew and resist treatments. This concept emphasizes the genetic heterogeneity and adaptability of tumors, highlighting the complexities of targeting CSCs for cancer therapy [7, 9]. As a result, CSCs are often seen as the primary drivers behind tumor growth and pose significant obstacles in cancer therapy.
The tumor microenvironment (TME) encompasses not only the cancer cells but also the surrounding environment, which is made up of a variety of elements such as immune and inflammatory cells, carcinoma-associated fibroblasts, nearby stromal tissue, tumor blood vessels, and a range of cytokines and chemokines [10, 11]. CSCs are intricately connected to their environment, engaging in continuous interactions. They can alter the TME by releasing signaling molecules, enhancing angiogenesis, promoting immune tolerance, and aiding metastasis. Conversely, cells within the TME, along with their secreted factors, can bolster the self-renewal and stemness of CSCs, stimulate angiogenesis, attract immune and stromal cells, and facilitate tumor invasion and metastasis. Extracellular vesicles (EVs) play a pivotal role in facilitating communication between CSCs and TME cells.
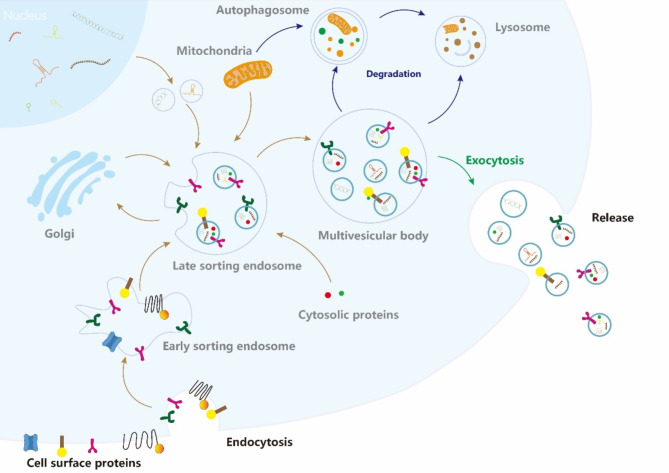
EVs are lipid bilayer-enclosed particles released by all cell types. They contain cargo from their donor cells but lack functional nuclei and cannot replicate. Found in various biological fluids such as blood, urine, semen, saliva, breast milk, cerebrospinal fluid, and bile. EVs are considered a mechanism for cells to eliminate harmful or redundant intracellular components. They transport a variety of substances, including DNA, RNA, lipids, and proteins, enabling them to transmit signals and effect physiological changes in recipient cells, thereby serving as crucial conduits for intercellular communication. The powerful cargo-carrying capacity of EVs makes them crucial in the interaction between CSCs and cells within TME. Exosomes, a type of EVs, are produced through a two-step invagination process of the cytoplasmic membrane, resulting in the formation of multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). These ILVs are eventually released as exosomes, which are approximately 40 to 160 nm in diameter. MVBs fuse with the cytoplasmic membrane and release exosomes into the extracellular space via exocytosis [12, 13](Fig. 1). These exosomes carry host cell-specific cargo from their host cell and are internalized by recipient cells via endocytosis, releasing their contents and promoting intercellular communication. This mechanism is crucial for interactions between CSCs and TME cells. Exosomes transport bioactive molecules like proteins, lipids, and RNAs, which regulate the behavior of both CSCs and the surrounding stromal cells. This bidirectional communication not only supports tumor growth, angiogenesis, and immune evasion but also plays a role in tumor progression. For instance, exosomes secreted by CSCs (CSC-exo) can transform normal stromal cells into a state conducive to tumorigenesis, thereby supporting the survival and proliferation of CSCs. Moreover, exosomes within the TME can affect the plasticity and resistance to therapy of CSCs. Given the focus of current research on EV-mediated communication in the TME mainly focuses on exosomes, this review will specifically highlight the role of exosomes.
Fig. 1.
Exosomes biogenesis and release. Exosome formation and release encompass several steps, starting with endocytosis, followed by the formation of multivesicular bodies (MVBs), and culminating in the release of exosomes into the extracellular space via fusion with the plasma membrane
The role of CSC-exo in the TME
CSC-exo promotes stemness in Non-CSCs
Currently, substantial evidence suggests that CSCs can impart stemness traits from parental cells to non-CSCs via exosomes, thus increasing tumor stemness and accelerating tumor progression. Researches are finding that CSC-exo contain proteins that trigger stemness signaling pathways in non-CSCs, like Wnt, Notch, and Hedgehog, to regulate tumor stemness. For instance, exosomes produced by glioblastoma stem cells (GSCs) function as carriers, transporting Notch1 protein to non-CSCs. This transfer activates the Notch1 signaling pathway, inducing the differentiation of glioma cells into GSCs and thus enhancing tumor stemness and tumorigenic potential. When Notch1 RNA interference is applied, it decreases Notch1 protein levels in GSCs and their exosomes, subsequently reducing the levels of stemness-related proteins in the targeted non-GSCs [14]. Huang et al. discovered that the release of liver CSC-exo was Rab27A dependent, while Rab27A expression was closely related to Nanog in hepatocellular carcinoma (HCC) tissues. CSC-exo can elevate Nanog expression in non-CSCs, indicating that Rab27A contributes to the enhancement of non-CSC stemness in HCC by regulating the release of CSC-exo [15].
Additionally, numerous studies have found that RNA molecules encapsulated in CSC-exo can activate stemness-related pathways, conferring stemness to non-CSCs. Li and colleagues demonstrated that FMR1-AS1, a long non-coding RNA (lncRNA) found in exosomes from esophageal CSCs, binds to the endosomal toll-like receptor 7 (TLR7) and activates NFκB signaling. This activation promotes the expression of the stemness-related gene c-Myc in recipient non-CSCs, enhancing their malignant potential [16]. Similarly, colorectal CSC-exo and thyroid CSC-exo can carry miRNAs and lncRNAs that target cancer cells to activate the stemness-related Wnt-β-catenin signaling pathway, thereby conferring stemness to non-CSCs [17, 18]. Han et al. discovered that that circular RNAs (circRNAs), specifically circ-ZEB1 and circ-AFAP1, in liver CSC-exo significantly elevate the expression of the stemness marker CD133, while simultaneously decreasing levels of E-cadherin and epithelial cell adhesion molecule (EpCAM), markers associated with the epithelial-mesenchymal transition (EMT) process. This activity not only boosts the stemness and EMT process in cancer cells but also advances the progression of HCC. Additionally, the levels of circ-ZEB1 and circ-AFAP1 are markedly elevated in HCC tissues compared to peritumoral tissue, and Their expression shows a positive correlation with the stemness marker CD133. Therefore, these two indicators can also serve as prognostic markers for HCC [19].
CSC-exo promotes tumor angiogenesis
Tumor angiogenesis is a critical mechanism in tumor development, as it supplies the necessary oxygen and nutrients to tumor cells through blood vessels [20]. CSCs primarily promote angiogenesis by secreting pro-angiogenic factors and exosomes [21]. Some evidence suggests that CSC-exo contains numerous pro-angiogenic components and facilitates tumor angiogenesis by interacting with endothelial and stromal cells within the TME, activating key signaling pathways. For example, some researches have shown that CSCs can influence the differentiation of endothelial progenitor cells through the exosomal delivery of miRNAs [22]. Additionally, exosomal miR-26a secreted by GSCs enhances the angiogenic capability of human brain microvascular endothelial cells (ECs) by triggering the PI3K/Akt signaling pathway and reducing PTEN expression [23]. Interestingly, other study found that exosomes derived from GSCs (GSC-exo) are also rich in miR-21 and VEGF, which enhance the angiogenic capability of ECs by activating the VEGF/VEGFR2 signaling pathway [24, 25]. Moreover, Lindoso and his colleagues discovered that renal CSC-exo can modify the secretion profiles of mesenchymal stem cells (MSCs), inducing them to release interleukin-8 (IL-8), osteopontin (OPN), and myeloperoxidase (MPO). This alteration enhances tumor vascularization and growth, significantly increasing tumor migration and stroma remodeling [26]. IL-8 can induce ECs to produce VEGF-A and raise VEGFR2 expression, as well as enhancing MMP secretion which breaks down the extracellular matrix, facilitating the proliferation of ECs to form new blood vessels [27]. Additionally, OPN is abundantly expressed in the tumor stroma and participates in the regulatory signaling processes associated with angiogenesis, metastasis and tumor growth across various cancers [28]. MPO plays a role in ECs activities linked to angiogenesis, promoting the expression of angiogenic signaling pathways and their associated genes [29].
CSC-exo promotes tumor invasion, migration, and metastasis
CSCs are one of the root causes of metastatic spread [30], which may be mainly dependent on CSC-exo, and transfer CSC characteristics to non-CSCs. EMT plays a vital role in this process, marking a key transition in tumor invasion and metastasis. During EMT, epithelial cells lose their characteristic features and gain mesenchymal traits, including enhanced mobility and invasiveness [31]. This transformation involves the loss of intercellular adhesion and epithelial markers such as cytokeratins and E-cadherin, coupled with an increase in mesenchymal markers like N-cadherin, vimentin, and fibronectin [32]. Numerous factors termed EMT-inducing transcription factors (EMT-TFs), including ZEB1/2, SNAIL, SLUG, and TWIST, can stimulate EMT in cancer cells, thereby promoting tumor invasion [33]. CSC-exo can act as a signal carrier, transferring EMT signals to tumor cells, hence promoting tumor migration, invasion, and metastasis. In thyroid cancer, CSC-exo significantly upregulates lncRNAs MALAT1 and linc-ROR, along with the EMT marker SLUG and the stem cell transcription factor SOX2. These molecules are transferred to non-CSCs, triggering EMT and thus enhancing tumor metastasis [34]. Multiple studies indicate that CSC-exo cannot only directly transfer EMT-TFs but also convey EMT signals via non-coding ncRNAs to promote EMT. Alzahrani et al. found significant levels of miR21, lncTuc339, lncHEIH, and HCC lncHOTAIR in liver cancer CSC-exo. These non-coding RNAs affect the mRNA levels of Bcl2, TGFβ1, NFκB, VEGF, and matrix metalloproteinase 9 (MMP9), thereby activating corresponding signaling pathways that significantly increase cancer cell metastasis and induce EMT [35]. Furthermore, Wu et al. discovered that lncRNA CDKN2B-AS1 in CSC-exo can enhance tumor cell viability, invasion, and EMT was achieved by decreasing the levels of E-cadherin and increasing the levels of P4HA1, which is an enzyme responsible for collagen production and deposition, as well as N-cadherin and vimentin expression [36]. Wang and colleagues discovered that miR-210-3p in lung CSC-exo elevates the expression of N-cadherin, vimentin, MMP-9, and matrix metalloproteinase 1 (MMP-1) in lung cancer cells, while simultaneously decreasing E-cadherin expression to promote cell migration and invasion [37]. Additionally, miR-19b-3p in clear cell renal cell carcinoma CSC-exo augments the expression of EMT-related genes by targeting the PTEN signaling pathway. Research has also shown that integrin CD103, carried by CSC-exo, enables organ-specific metastasis to the lungs, highlighting the role of CSC-exo in directing tumor metastasis [38].
The non-coding RNAs transported by CSC-exo can directly trigger gene expression related to the EMT in cancer cells to promote EMT, and they also indirectly influence tumor metastasis through alternative pathways. Some studies have found that the suppression of the autophagy pathway by CSC-exo can promote the metastasis and colonization of tumors. For instance, miR-4535 and miR-1268a in melanoma CSC-exo have been found to enhance the metastatic colonization of melanoma cells by inhibiting the autophagy pathway [39, 40]. Zhu et al. identified that the SOX2-β-catenin/Beclin1/autophagy signaling axis in colorectal CSCs plays a role in regulating chemoresistance, CSC traits, and EMT in colorectal cancer (CRC) [41]. SOX2, which is typically a marker of CSCs, is commonly released via exosomes [42]. Additional studies indicate that CSC-exo can indirectly boost tumor metastasis by increasing glycolysis. In lung CSC-exo, the highly expressed lncRNA Mir100hg targets miR-15a-5p and miR-31-5p in tumor cells, elevating glycolytic activity and thereby enhancing metastatic potential [43]. Ji et al. demonstrated that in liver cancer, exosomal ZFPM2-AS1 modulates glycolysis in HCC via a pyruvate pathway dependent on HIF-1α, affecting tumor metastasis and growth, while also promoting macrophage recruitment and M2 polarization [44]. Additionally, CSC-exo can influence EMT by regulating immune cells. Hwang et al. found that colorectal CSC-exo enhances the activity of the neutrophils from bone marrow and promotes their pro-tumor characteristics. Moreover, patients with CRC displaying active CSC signaling (characterized by Snail + and IL8 + markers) showed elevated levels of MPO + tumor-infiltrating neutrophils, suggesting neutrophils are involved in mediating EMT and maintaining the CSC niche [45].
CSC-exo promotes immunosuppression
The immune system performs three fundamental functions: immune defense, which combats pathogenic microorganisms and eradicates invading pathogens and harmful biomolecules; immune surveillance, which monitors and swiftly eliminates mutated cells; and immune stability, which ensures self-regulation by recognizing self, distinguishing between “foreign” or “harmful” entities, and maintaining overall homeostasis. Typically, immune cells actively execute these functions by recognizing, monitoring, and clearing malignant cells. However, these malignant cells often deploy sophisticated strategies to escape immune detection and destruction, with CSCs playing a pivotal role in this process. The mechanisms by which CSCs influence infiltrating immune cells are currently understood to be highly complex. Research indicates that CSC-exo can specifically target certain subsets of immune cells, such as tumor-associated macrophages (TAMs) and T cells [46].
CSC-exo can polarize TAMs into a pro-tumor MHC-II phenotype, thereby impacting tumor immunity [46]. In recent years, some research found MHC-II-macrophages play multiple roles in the TME, including immune suppression [47], angiogenesis [48], ECM deposition [49], and promoting metastasis [50, 51]. Gabrusiewicz and colleagues found that GSC-exo drives the differentiation of monocytes into M2 macrophages via the EIF2, mTOR, ephrinB signaling pathways, and STAT3 phosphorylation. This process enhances the expression of programmed death-ligand 1 (PD-L1), a key immune checkpoint extensively studied in clinical trials. PD-L1 binds to the PD-1 receptor on T cells, creating an immunosuppressive environment that facilitates tumor immune evasion [52, 53]. Furthermore, research has revealed that small EVs secreted by oral squamous cell carcinoma (OSCC) CSCs promote M2 macrophage polarization through the transfer of lncRNA UCA1. This lncRNA targets the LAMC2-mediated PI3K/AKT signaling axis and also inhibits the proliferation of CD4+ T cells and the production of interferon-gamma (IFN-γ) [54].
Additionally, some research has found that exosomes can influence tumor immunity by modulating the cytotoxic function of natural killer (NK) cells and T cells through TAMs [55]. Apart from these indirect effects through TAMs, CSC-exo directly influences T cells as well. For example, esophageal cancer CSC-exo can deliver O-GlcNAc transferase (OGT) to adjacent CD8+ T cells, increasing their PD-1 expression. This activation adversely affects the proliferation and functionality of CD8+ T cells, facilitating cancer immune evasion [56]. In CRC, CSC-exo increases TNF-α expression in neutrophils, and this upregulation, stimulated by IL-1β, may lead to activation-induced cell death of T cells, thus intensifying tumor immune suppression [45]. Another study found that colorectal CSC-exo containing miRNA-146a-5p promotes the stem-like properties and tumor-forming ability of CRC cells by targeting Numb [57]. Patients with elevated serum levels of exosomal miRNA-146a-5p show increased CSC characteristics, fewer tumor-infiltrating CD8+ T cells, and more tumor-infiltrating CD66b+ neutrophils, leading to tumor immune suppression. Further research indicates that brain CSC-exo contains the extracellular matrix protein tenascin-C (TNC), which interacts with integrin receptors α5β1 and αvβ6. This interaction suppresses T cell mTOR pathway signaling, reducing T cell activity in co-culture and allowing tumor cells to evade destruction by immune cells [58]. Moreover, Naseri et al. found that CSC-exo can increase the ratio of IL-12 to IL-10 in dendritic cells (DCs). They also discovered that CSC-exo contains immunogenic antigens that promote anti-tumor responses. DCs loaded with these CSC-exo can observably promote the growth of autologous T cells and activate cytotoxic T cells targeted at spheroids, enhancing in vivo anti-tumor effects [59]. While the focus has largely been on these specific immune cells, further studies may gradually uncover the direct effects of CSC-exo on other immune cells like mast cells and NK cells, which play significant roles in the body’s defense against tumors. Future studies may gradually uncover the connections between CSC-exo and these cells, potentially revealing more mechanisms of tumor immune suppression.
CSC-exo promotes tumor drug resistance
Since cancer is often diagnosed at advanced stages, targeted therapies and chemotherapy are typically the most viable treatment options [60]. However, tumors frequently develop resistance to these treatments, posing a significant hurdle in cancer therapy. Thus, understanding the mechanisms underlying tumor drug resistance is crucial for improving treatment outcomes.
Drug resistance is also a notable trait of CSCs. Studies have shown that CSC-exo can transport RNAs to adjacent non-CSCs, activating specific signaling pathways and imparting drug resistance to tumor cells. For instance, Yang and colleagues found that pancreatic CSC-exo resistant to gemcitabine can transfer miR-210 to tumor cells, enhancing their drug resistance by upregulating resistance-associated proteins such as MDR1, YB-1, and BCRP. They noted that miR-210 predominantly facilitates the horizontal transfer of resistance traits via the mTOR signaling pathway [61]. Santos and colleagues discovered that CSCs secrete exosomal miRNA-155, which can lead to the downregulation of C/EBP-β, thereby inhibiting the expression of TGF-β, C/EBP-β, and FOXO3a, resulting in the development of EMT and chemoresistance in cancer cells [62]. Yang and colleagues have found that GSC-derived EVs contain the lncRNA MALAT1, which influences immune effector cells in the central nervous system. This occurs through the miR-129-5p/HMGB1 axis, modulating lipopolysaccharide (LPS) response and promoting microglial polarization to an M2 phenotype. This polarization results in increased secretion of IL-6, IL-8, and TNF-α, impacting tumor resistance, growth, angiogenesis, and other facets of tumor progression [63]. Exosomes from breast CSCs also contribute to regulating tumor resistance by modulating autophagy. These exosomes deliver annexin-A6 (ANXA6), which influences the YAP1/Hippo pathway, enhancing autophagy and stemness in BC cells, thereby augmenting their resistance to paclitaxel (PTX) [64]. Similarly, Lu and Yao et al. confirmed that the YAP1 pathway influences autophagy, enhancing stemness and drug resistance in tumor cells [65, 66](Fig. 2) Table 1.
Fig. 2.
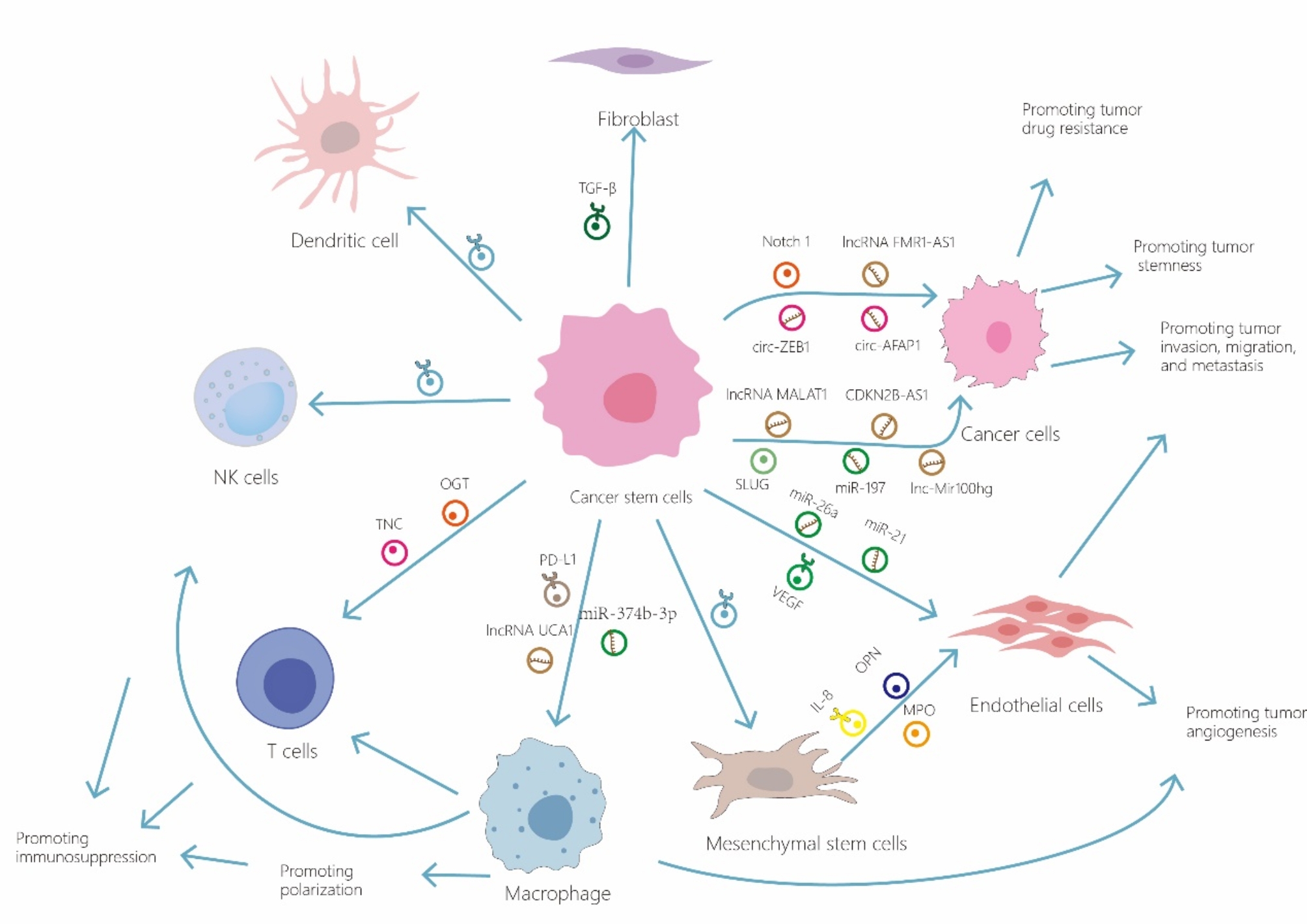
Cancer stem cell-derived exosomes are released into the cells within the tumor microenvironment, where they play a role in influencing tumor progression
Table 1.
Examples of exosomes derived from cancer stem cells discussed in this review
| Exosomal cargo |
Source of exosomes | Target cells | Effect of tumor progression | Mechanisms affecting tumors | Reference |
|---|---|---|---|---|---|
| Notch1 protein | Glioblastoma CSC | Glioma cell | Promoting tumor stemness | Activating the Notch1 signaling | [14] |
| lncRNA FMR1-AS1 | Esophageal CSC | esophageal cancer cell | Promoting tumor stemness | Activating TLR7-NFκB pathway | [16] |
| miR-146a-5p | Colorectal CSC | Colorectal cancer cell | Promoting tumor stemness | Targeting Numb to regulate the Wnt-β-catenin pathway | [17] |
| lncRNA DOCK9-AS2 | Thyroid CSC | Thyroid carcinoma cells | Promoting tumor stemness | Activating Wnt/β-catenin pathway | [18] |
| circ-ZEB1 | Liver CSC | Liver cancer cell | Promoting tumor stemness and metastasis | Increasing the expression of stemness marker CD133 and downregulating E-cadherin and EpCAM | [19] |
| circ-AFAP1 | Liver CSC | Liver cancer cell | Promoting tumor stemness and metastasis | Increasing the expression of stemness marker CD133 and downregulating E-cadherin and EpCAM | [19] |
| miR-26a | Glioblastoma CSC | Microvessel endothelial cell | Promoting tumor angiogenesis | Targeting PTEN and activating the PI3K/Akt signaling pathway | [23] |
| miR-21 | Glioblastoma CSC | Endothelial cell | Promoting tumor angiogenesis | Upregulating VEGF expression | [24] |
| VEGF-A | Glioblastoma CSC | Endothelial cell | Promoting tumor angiogenesis | Promoting angiogenesis of endothelial cells | [25] |
| lncRNA MALAT1 | Thyroid CSC | Thyroid cell | Promoting tumor invasion | Inducing EMT | [34] |
| linc-ROR | Thyroid CSC | Thyroid cell | Promoting tumor invasion | Inducing EMT | [34] |
| SLUG | Thyroid CSC | Thyroid cell | Promoting tumor invasion | Inducing EMT | [34] |
| lncRNA CDKN2B-AS1 | Thyroid CSC | Thyroid cancer cell | Affected tumor growth and metastasis | Decreasing the expression of E-cadherin and increasing the expression of P4HA1 | [36] |
| miR-210-3p | Lung CSC | Lung cancer cell | Promoting migration and invasion | Upregulating the expression of N-cadherin, vimentin, MMP-9, and MMP-1 in lung cancer cells, and downregulating E-cadherin expression | [37] |
| miR-197 | Breast CSC | Breast cancer cell | Promoting tumor growth and metastasis | Facilitating EMT and inhibiting PPARG expression | [127] |
| miR-19b-3p | CSC of clear cell renal cell carcinoma | Clear cell renal cell carcinoma cell | Promoting tumor proliferation and metastasis | Inducing EMT via repressing the expression of PTEN. | [38] |
| miR-4535 | Melanoma CSC | Melanoma cell | Promoting tumor metastasis | Inhibiting autophagy pathway | [39] |
| miR-1268a | Melanoma CSC | Melanoma cell | Promoting tumor metastasis | Inhibiting autophagy pathway | [40] |
| lncRNA Mir100hg | Lung CSC | Lung cancer cell | Promoting tumor metastasis | Targeting miR-15a-5p and miR-31-5p and increasing the glycolytic activity | [43] |
| lncRNA UCA1 | Oral CSC | Oral squamous cell carcinoma and Macrophage | Facilitating tumor progression and immunosuppression | Promoting M2 macrophage polarization and Suppressing CD4+T cell activity by Targeting LAMC2+ | [54] |
| O-GlcNAc transferase | Esophageal CSC | CD8+T cell | Promoting cancer immunosuppression | Protecting esophageal carcinoma stem cells from CD8+T cells through up-regulation of PD-1 | [56] |
| miRNA-146a-5p | Colorectal CSC | Colorectal cancer cell | Promoting tumor stemness and immunosuppression | Targeting Numb | [57] |
| tenascin-C | Brain CSC | T cell | Promoting tumor immunosuppression | Targeting the integrin receptors α5β1 and αvβ6 and attenuating T cell mTOR pathway signaling | [58] |
| miR-210 | Pancreatic CSC | Pancreatic cancer cell | Promoting tumor drug resistance | Activating mTOR signaling pathway | [61] |
| miR-155 | Breast CSC | Breast cancer cell | Promoting tumor drug resistance | Downregulating of C/EBP-β and inhibiting the expression of TGF-β, C/EBP-β, and FOXO3a | [62] |
| MALAT1 | Glioma CSC | Microglia | Promoting tumor immunosuppression, resistance, growth, and angiogenesis | Regulating the miR-129-5p/HMGB1 axis and modulating LPS and polarizing microglia to an M2 phenotype and influencing the secretion of IL-6, IL-8, and TNF-α. | [63] |
| ANXA6 | Breast CSC | Breast cancer cell | Promoting tumor drug resistance | Regulating the YAP1/Hippo pathway | [64] |
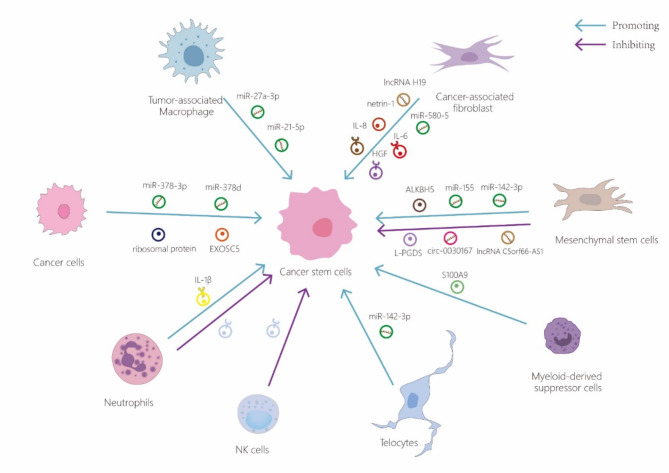
Effects of exosomes derived from various sources within the TME on CSCs
Effects of exosomes derived from cancer cells on CSCs
Non-CSC-derived EVs commonly transport specific cargo molecules that elevate the expression of stemness markers and activate various stemness-related signaling pathways in recipient CSCs. These pathways help maintain CSC characteristics, thereby driving tumor progression. Common stemness signaling pathways cover WNT/β-catenin [67], Notch [68], Hedgehog [69], and STAT pathways [70]. Studies have demonstrated that chemotherapy triggers cancer cells to release exosomes enriched with miRNAs associated with drug resistance. These miRNAs operate through stem cell pathways to augment CSC stemness and drug resistance. For example, chemotherapy triggers the EZH2/STAT3 pathway, leading to the secretion of exosomes from BC cells that harbor miR-378a-3p and miR-378d. These miRNAs target NUMB and DKK3, modulating the WNT/β-catenin and Notch pathways, improving CSC traits and drug resistance [71]. Additionally, exosomes released by endometrial cancer cells, containing Exosome component 5 (EXOSC5), can target netrin4 (NTN4) and bind to integrin β1, activating the FAK/SRC/β-catenin signaling axis and enhancing c-MYC activity, which in turn strengthens CSC properties and promotes tumor progression [72]. Additional studies suggest that non-CSC-derived EVs in bladder cancer carry ribosome-rich cargo that CSCs can absorb and use to rapidly initiate protein synthesis, boosting the expression of stemness factors such as ALDH1α1, OCT-4, TWIST, and CD44. Intriguingly, these studies have observed that while chemotherapy eliminates most cancer cells, these cells, prior to their demise, release EVs that support CSC survival in a chemotherapeutic environment, leading to drug resistance and disease progression [73]. In summary, exosomes derived from cancer cells play a pivotal role in the TME, profoundly affecting CSC behavior. They bolster CSC stemness, foster drug resistance, facilitate metastasis, and aid immune evasion, all of which highlight their significance in cancer progression and treatment challenges. A deeper understanding of these mechanisms could reveal new therapeutic targets.
Effect of EVs released by cancer-associated fibroblasts on CSCs
Cancer-associated fibroblasts (CAFs) are key components of the TME in solid tumors, displaying significant heterogeneity in their phenotype, origin, and function. CAFs can originate from various sources including native fibroblasts reprogrammed by tumor-derived factors, mesenchymal cells recruited from the bone marrow, or cells derived from adipocyte precursors, endothelial cells, mesothelial cells, or pericytes [74]. As primary producers of the extracellular matrix in the TME, CAFs play critical roles that extend to promoting cancer cell proliferation, angiogenesis, and remodeling of the extracellular matrix (ECM). Furthermore, CAFs orchestrate tumor-promoting inflammation and manipulate the immune microenvironment to foster immune suppression. These functions are executed through the release of factors, exosomes, and metabolites that participate in complex signaling interactions with cancer cells, stromal components, and infiltrating immune cells [75].
For instance, exosomes from CAFs secreting netrin-1 can indirectly induce the secretion of cytokines like IL6 and IL8, which in turn promote cancer stemness. It has been observed that CAFs can directly secrete IL-6, and IL-8 stimulates STAT3/Notch signaling pathways to sustain the stemness of tumor cells [75]. In addition, miR-146a-5p exosomes secreted by CAF in urothelial bladder cancer can also enhance CSC characteristics and drug resistance by activating STAT3 and mTOR signaling pathways [76]. CAF-derived exosomes (CAF-exo) can also enhance the characteristics of CSCs through the regulation of the TGF-β signaling pathway [77, 78], and the Wnt/β-catenin pathway [79], leading to cancer progression and increased chemotherapy resistance. Factors within the TME, like hepatocyte growth factor (HGF), which binds to the c-MET tyrosine kinase receptor activating β-catenin-dependent transcription, support CSC phenotypes and contribute to tumor heterogeneity by providing suitable niches for CSCs [80]. Hu and colleagues discovered that miR-92a-3p in CAF-secreted exosomes targets FBXW7 and MOAP1 in tumor cells, enhancing β-catenin expression, tumor stemness, and EMT, while inhibiting apoptosis and leading to metastasis and chemoresistance in CRC [81]. Additionally, studies have shown that upregulation of PD-L1 in chemotherapy-treated CAFs leads to increased HGF secretion, stimulating cancer progression, enhancing cell invasion and stem cell properties, and inhibiting apoptosis [82]. Moreover, Li et al. found that CAF-secreted HGF could also promote CSC properties through phosphorylation of STAT3 [83]. Moreover, CAF-derived exosomes can also deliver non-coding RNAs to target HIF-1α to regulate stem cell properties and glycolysis, thereby regulating tumor progression [84, 85].
In summary, CAF-exo serve an essential function in sustaining CSC traits and their resistance to drugs. However, there are not many studies on CAF-exo for the management of tumors, possibly due to the complex origins of CAFs and the challenges in identifying the specific cells they target.
Effects of exosomes released by mesenchymal stem cells on CSCs
MSCs are versatile cells capable of self-renewal and differentiation into various lineages. Numerous studies have shown that MSCs can migrate to tumor sites and influence tumor matrix formation. Moreover, within the TME, interactions between MSCs and CSCs can promote tumor growth and metastasis through various mechanisms [86]. MSCs, known for their high plasticity, can modify their behavior and role depending on the cytokines produced by nearby immune cells, displaying either tumor-promoting or tumor-inhibiting characteristics depending on the tumor environment [87]. The most typical is bone marrow-derived mesenchymal stem cells (BM-MSCs), which can have an impact on stem cell traits in many tumors, such as acute myeloid leukemia [88], triple-negative breast cancer (TNBC) [89], HCC [90], myeloma [91], CRC [92], and pancreatic cancer [93]. BM-MSC-derived exosomes can modulate CSC traits by carrying RNAs or cytokines that impact downstream pathways. For example, BM-MSC-derived exosomal miR-142-3p promotes CSC traits by repressing Numb expression and promoting Notch target gene expression [92]. Additionally, BM-MSCs secrete AlkB homolog 5, an exosomal m6A regulator that increases TNBC stem cell traits by upregulating m6A modification of UBE2C and downregulating p53, thereby fostering TNBC growth and metastasis [89]. MSC-derived exosomes (MSC-exo) can both promote and inhibit malignant behaviors in CSCs. For example, exosomes from BM-MSCs containing circ0030167 suppress CSC traits in pancreatic cancer by targeting the miR-338-5p/Wif1/Wnt8/β-catenin axis [93]. In HCC, MSC-derived exosomal lncRNAs C5orf66-AS1 inhibit malignant behaviors like self-renewal, stemness, invasion, and migration by targeting the miR-127-3p/DUSP1/ERK axis [90]. Another study also showed that MSC-derived EV, prostaglandin D2 synthase (L-PGDS) reduced the levels of stem cell markers (such as Oct4, Nanog, and Sox2) and suppressed the phosphorylation of STAT3, thereby affecting CSC properties and tumor progression [94]. Thus, MSC-exo not only regulates CSCs but also regulates tumor immunity and drug resistance. MSC-exo has been extensively studied for its roles in regulating tumor immunity, resistance, and stemness, making it a focal point in cancer treatment research due to its significant potential and advantages [95].
Effects of exosomes released by other cells within the TME on CSCs
In the TME, not only tumor cells, CAFs and MSCs can act on CSCs, but exosomes secreted by other cells like TAMs, myeloid-derived suppressor cells (MDSCs), and adipose-derived stem cells can also regulate CSCs. Among them, M2 macrophages in TAMs primarily facilitate immune evasion, angiogenesis, tissue remodeling, and resistance to chemoradiotherapy, which in turn support tumor proliferation, growth, and metastasis. For example, exosomal miR-27a-3p secreted by M2 macrophages boosts liver CSC traits by reducing the expression of thioredoxin-interacting protein (TXNIP) in liver cancer [96]. Chang et al. found that miR-21-5p levels in exosomes derived from TAMs were down-regulated in pancreatic cancer, inhibiting Nanog/Oct4 expression, and targeting Krüppel-like factor 3 (KLF3) decreased, thereby inhibiting CSC activity in pancreatic cancer [97].
MDSCs are heterogeneous populations of immature myeloid cells that mainly shape immune responses to support tumor niches, with their mechanisms being complex and encompassing both immunosuppressive and non-immune roles, such as enhancing stemness and promoting angiogenesis [98]. MDSC-derived exosomes can stimulate and maintain CSC properties through IL-6/STAT3 and NO/NOTCH signaling pathways [99, 100]. Furthermore, bone marrow-derived telocytes and their mitochondria have been shown to influence CSC traits via miR-146a-5p, which mainly affects the expression of EMT markers (N-cadherin, vimentin, E-cadherin) and tumorigenic markers (BRCA1, P53, SOX2) in CSCs [101]. Numerous studies have demonstrated that miR-146a-5p communicates information through exosomes, such as CAF [76] and melanoma cells [102], potentially including communication between telocytes and CSCs in this context.
Exosomes secreted by NK cells (NK-exo) are enriched with various bioactive molecules, including cytotoxic proteins like perforin and Fas/FasL, which can stimulate apoptosis in cancer cells. Additionally, the microRNAs contained within these exosomes can suppress gene expression, leading to reduced cell proliferation and enhanced apoptosis in cancer cells [103]. At present, the majority of research on NK-exo focuses on their impact on tumor and immune cells, with limited studies exploring their effects on the relatively small population of CSCs within tumors. Future investigations are expected to further elucidate their interactions. Recent findings have demonstrated that canine NK-exo can inhibit tumor growth by downregulating CSC-associated markers and augmenting the tumor suppressor activity of p53 [104]. Therefore, under existing targeted immunotherapy with NK-exo against tumor cells, NK-exo immunotherapy with more precise targeting of CSC may be just around the corner (Fig. 3) Table 2.
Fig. 3.
Other cells in the tumor microenvironment also secrete exosomes that impact cancer stem cells
Table 2.
Examples of exosomes from different sources within the tumor microenvironment that target cancer stem cells discussed in this review
| Exosomal cargo | Source of exosomes | Mechanisms affecting tumors | Effect of tumor progression | Reference |
|---|---|---|---|---|
| miR-378a-3p /miR-378d | Breast cancer cell | Targeting of DKK3 and NUMB and activating the WNT and NOTCH stem cell pathways | Promoting tumor drug resistance | [71] |
| EXOSC5 | Endometrial carcinoma cell | Increasing NTN4 expression and activating c-MYC via the integrin β1/FAK/SRC pathway | Promoting tumor stemness | [72] |
| ribosomal protein | Non-stem bladder cancer cell | Promoting the expression of stemness factors | Promoting tumor stemness | [73] |
| netrin-1 | Colon and lung CAF | trigger the secretion of IL6 | Promoting tumor stemness | [75] |
| miR-146a-5p | Cancer-associated fibroblast of urothelial bladder cancer | Activating STAT3 and mTOR signaling pathways | Promoting tumor stemness and drug resistance | [76] |
| lncRNA H19 | CAF of colorectal cancer | Activating the β-catenin pathway | Promoting tumor stemness and drug resistance | [79] |
| miR-92a-3p | CAF of colorectal cancer | Targeting FBXW7 and MOAP1, and promoting the β-catenin expression | Promoting tumor metastasis and chemoresistance | [81] |
| miR-7641 | CAF of breast cancer | Targeting the HIF-1α pathway suppressing glycolysis | Inhibiting tumor stemness | [84] |
| circHIF1A | CAF of breast cancer | Sponging miR-580-5p and targeting CD44 expression | Promoting tumor stemness | [85] |
| miR-142-3p | BM-MSC /stromal cell | Promoting the Notch signaling pathway by downregulating Numb. | Promoting tumor stemness | [92] |
| ALKBH5 | BM-MSC in triple-negative breast cancer | Upregulating UBE2C and downregulating p53 | Promoting tumor stemness, growth, and metastasis | [89] |
| circ_0030167 | BM-MSC in pancreatic cancer | Sponging miR-338-5p and targeting the Wif1/Wnt8/β-catenin axis | Inhibiting tumor invasion, migration, proliferation, and stemness | [93] |
| lncRNAs C5orf66-AS1 | MSC in hepatocellular carcinoma | Targeting C5orf66-AS1/miR-127-3p/DUSP1/ERK axis | Inhibiting tumor proliferation, migration, invasion, angiogenesis-stimulating | [90] |
| L-PGDS | MSC in gastric cancer | reducing the levels of stem cell markers and suppressing the phosphorylation of STAT3 | Inhibiting tumor stemness | [94] |
| miR-27a-3p | M2 macrophage in hepatocellular carcinoma | downregulating TXNIP | Promoting tumor stemness, proliferation, drug resistance, migration, invasion | [96] |
| miR-21-5p | M2 macrophage in pancreatic cancer | Mediating KLF3 | Promoting tumor stemness | [97] |
| S100A9 | Myeloid-derived suppressor cell in colorectal cancer | Enhancing the phosphorylation of STAT3 and NF-κB | Promoting tumor stemness | [99] |
Significance of CSCs and exosomes for clinical treatment
Targeting CSC-exo for tumor therapy
The interaction between CSC-exo and other elements in the TME is pivotal for tumor progression, making their integration into precision therapy a crucial clinical objective. By targeting CSCs and disrupting the communication between CSC-exo and the TME, we could potentially impede tumor progression, including the acquisition of stemness, invasiveness, and drug resistance by cancer cells, thus enhancing therapeutic outcomes. Research focused on the direct effects of modulating exosome development is expanding and shows promise as a treatment modality. Studies have indicated that IFN-α can decrease EV secretion in subsets of malignant melanoma CSCs, significantly reducing their tumorigenicity and stemness, as well as downregulating the expression of several oncogenic miRNAs in exosomes, including miR98-5p, miR-191, miR-744-3p, and let-7e-3p [105]. Pharmacological interventions targeting specific pathways to reduce CSC-exo secretion are also being explored. For instance, aspirin has been shown to inhibit exosome release under hypoxic conditions via the HIF-1α/COX-2 pathway, consequently reducing CSC traits and tumor proliferation, migration, and angiogenesis [106]. Furthermore, Chen et al. discovered that targeting CSC-EVs with Ovatodiolide treatment sensitized CSCs to cisplatin, primarily by inhibiting oral squamous cell carcinoma tumorigenesis through a reduction of CSC-EV cargo, including mTOR, PI3K, STAT3, β-catenin, and miR-21-5p [107]. While numerous studies are directed towards reducing CSC-exo release to mitigate CSC-related harm, currently, there appears to be no research employing CSC-exo as a treatment modality for tumors Table 3.
Table 3.
Therapeutic modalities affecting exosomes secreted by the cancer stem cells discussed in this review
| Substances related to exosomes | Targeting cell | Effects on exosomes | Effects on tumor progression | Function model | Reference |
|---|---|---|---|---|---|
| IFN-α | Malignant melanoma CSC | Affecting exosome production and cargo | Reducing CSC formation and stemness properties | Down-regulating the expression of miR98-5p, miR-191, miR-744-3p, and let-7e-3p | [105] |
| Aspirin | non-small cell lung cancer cell or CSC | Affecting exosome production and cargo | Suppressing of stemness through inhibiting of exosome secretion by CSC | Targeting HIF-1α/COX-2 pathway | [106] |
| Ovatodiolide | OSCC CSC | Affecting exosome cargo | Suppressing CSC self-renewal, reducing drug resistance | Inhibiting the expressions of miR-21-5p, STAT3, and mTOR in CSC-exo. | [107] |
The biology of exosome biogenesis and release is inherently intricate, involving numerous pathways such as the endosomal sorting complexes required for transport (ESCRT) machinery, tetraspanins, and lipid raft-associated mechanisms. Specifically targeting these pathways in CSCs without impacting normal cells presents significant challenges due to the commonality and overlap of exosome production mechanisms across various cell types. Systemic inhibition of these pathways could unintentionally disrupt normal physiological processes that rely on exosome communication. Additionally, many of the pathways integral to exosome release are also vital for the function of normal stem cells and other healthy tissues. Non-specific suppression of exosome release might lead to off-target effects and toxicity, potentially impairing normal cellular communication, immune functions, and tissue homeostasis. This raises substantial safety concerns, complicating the translation of these strategies into clinical settings. Furthermore, developing treatments that can specifically target exosome release from CSCs without affecting other cells remains a considerable technical hurdle. Current methods lack the precision necessary to selectively inhibit exosome release from CSCs, which is crucial to prevent damage to normal cells. Advances in targeting technologies are needed to achieve the specificity required for effective and safe clinical use. Additionally, CSCs display significant heterogeneity within and between different tumor types. This diversity complicates the task of identifying universal targets for inhibiting exosome release, as different CSC subpopulations may depend on distinct exosomal pathways. Personalized approaches would be essential, necessitating comprehensive characterization of CSCs in each patient, which is resource-intensive and currently not feasible for broad clinical application. Present therapeutic strategies that focus on CSCs typically aim at direct targeting and elimination of these cells through cytotoxic agents, immunotherapy, or differentiation therapy. These approaches have demonstrated more immediate and observable benefits in preclinical and clinical trials. Therefore, the development and refinement of these strategies are prioritized over the emerging field of targeting CSC-exo release. Hence, overcoming these challenges will require significant advancements in our understanding of exosome biology, improved targeting techniques, and thorough evaluation of the efficacy and safety of these methods to make the modulation of CSC-exo release viable for widespread clinical application.
Targeting exosomes secreted by other cells in the TME for tumor therapy
In tumor therapy, targeting cell-secreted exosomes within the TME presents a promising avenue, particularly the application of MSC-exo in combating CSCs. MSC-exo possess unique properties that allow them to effectively target CSCs, potentially enhancing cancer treatment outcomes. These exosomes can be engineered to carry therapeutic agents directly to CSCs, including chemotherapeutic drugs, small interfering RNAs (siRNAs), or miRNAs that target critical pathways for CSC survival and proliferation. This targeted strategy helps minimize the systemic toxicity typically associated with traditional chemotherapy, thus reducing adverse side effects. For instance, exosomes derived from GRP7-siRNA modified BMSCs can enhance the responsiveness of HCC cells to sorafenib. The combination of exosomes from GRP78-siRNA modified MSCs and sorafenib effectively inhibits the proliferation and invasion of HCC cells in vitro [108]. MSC-exo loaded with miRNAs that downregulate drug resistance genes can restore the sensitivity of CSCs to existing treatments, improving their efficacy. For example, MSC-derived exosomes overexpressing miR-199a can suppress glioma growth, invasion, and migration while boosting sensitivity to temozolomide both in vitro and in vivo [109]. Another example involves MSC-exo recombinant miR-193a, which can inhibit tumor colony formation, invasion, migration, and proliferation, and induce apoptosis in resistant cells by downregulating LRRC1 [110]. Additionally, modifying MSCs can change the signaling properties of their exosomes, reducing their pro-tumorigenic signals. For example, reducing TGF-β1 expression in MSCs can diminish their exosomes’ ability to mediate EMT while enhancing their capability to inhibit cell proliferation and promote apoptosis [111]. Interestingly, past studies have shown that MSC-exo can actively modulate immune responses within the TME. These exosomes contain immunomodulatory molecules that bolster anti-tumor immunity, which can be leveraged to modulate the tumor immune microenvironment for more effective immunotherapy. For instance, BMSC-exo loaded with galectin-9 siRNA and the oxaliplatin (OXA) prodrug can modulate macrophage polarization, attract cytotoxic T lymphocytes, and decrease Treg levels, thereby triggering a robust anti-tumor immune response and improving immunotherapy outcomes in vitro and in vivo [112].
Therapeutic approaches utilizing CAF-derived exosomes predominantly aim to convert CAFs from promoting tumors to inhibiting them. This transformation can be achieved through drugs, cytokines, or genetic modifications that modify CAF behavior. For instance, in pancreatic ductal adenocarcinoma, using the vitamin D receptor agonist calcipotriol to reprogram stromal cells has shown to curtail tumor growth and improve the efficacy of pancreatic cancer treatments [113]. Such reprogrammed CAFs might release exosomes that possess anti-tumor characteristics. CAFs have the capability to directly influence immune cell activity and indirectly lead to immune effector cell dysfunction by elevating immune checkpoint molecules on their surfaces and restructuring the ECM within the TME. Additionally, CAFs can suppress immune cell-driven anti-tumor responses through the release of various chemokines, cytokines, and other effector molecules [114]. As with other exosomes, modifying the contents of CAF-derived exosomes to include anti-tumor molecules is another promising approach.
Exosomes from DCs, T cells, NK cells, and other immune cells are packed with proteins, lipids, and nucleic acids that can activate and modulate the immune system. These immune cells can target CSCs or tumor cells directly and indirectly affect them by altering the immune microenvironment, thereby playing a role in tumor therapy. Exosomes from DCs are particularly effective as they are powerful antigen-presenting vesicles that can induce robust anti-tumor immune responses. They transport tumor antigens, MHC molecules, and co-stimulatory signals, efficiently priming T cells to initiate strong cytotoxic attacks on tumor cells. Clinical trials have shown that vaccines based on DC-derived exosomes can trigger tumor-specific immune responses, marking an innovative and promising direction in cancer immunotherapy. These exosomes’ ability to present antigens and activate T cells positions them as excellent candidates for creating personalized cancer vaccines [115]. Li et al. developed a nanovaccine platform utilizing DC-derived exosomes loaded with neoantigens, which considerably curbed tumor growth, extended survival, delayed tumor onset, and fostered long-term memory, thus preventing tumor recurrence and metastasis while enhancing survival rates [116].
T cell-derived exosomes (T-exo) contain proteins and RNA, reflecting the activation state and cytotoxic potential of T cells. These exosomes can transfer functional molecules such as granzyme, perforin, and cytokines to target cells, inducing tumor cell apoptosis. Additionally, they can regulate the TME by promoting immune cell infiltration and enhancing the antitumor activity of other immune cells. Leveraging T-exo can amplify the antitumor immune response, thereby increasing the efficacy of T cell therapies and checkpoint inhibitors [117] Zhu et al. developed hybrid nanovesicles by merging exosomes from bispecific CAR-T cells, which target mesothelin and PD-L1, with liposomes to administer paclitaxel (PTX) for treating metastatic lung cancer. These nanovesicles blocked PD-L1 on tumors, reducing T cell exhaustion and enhancing the anticancer effects by facilitating PTX-induced immunogenic cell death [118].
The potential of NK-exo in cancer therapy is notable due to their capacity to specifically target and eradicate cancer cells, including CSCs. NK-exo possess inherent cytotoxic properties attributed to the inclusion of perforin and granzyme B, which can trigger apoptosis in cancer cells. This capability enables them to target CSCs, which are frequently resistant to standard treatments, thus overcoming a significant hurdle in cancer therapy. Additionally, NK-exo can boost the effectiveness of existing treatments by making tumor cells more susceptible to chemotherapy and radiotherapy. They also play a role in altering the TME, creating conditions that are unfavorable for CSCs and cancer cells. Moreover, NK-exo can activate other immune cells such as T cells and macrophages by transferring proteins and RNA, which helps to initiate an immune response. This immunomodulatory function can amplify the overall anti-tumor response and complement other immunotherapeutic strategies. The typically immunosuppressive TME can impair the success of immunotherapies, but remodeling the TME with NK-exo can significantly improve immunotherapy outcomes. NK-exo can also be integrated with other therapeutic approaches to enhance their impact, for example, they can be combined with checkpoint inhibitors to counteract tumor immune evasion tactics. Their inherent ability to target and destroy cancer cells positions them as promising agents for developing exosome-based immunotherapies. Additionally, these exosomes can be engineered to transport chemotherapeutic drugs, RNA interference molecules, or CRISPR/Cas9 components, targeting specific genetic mutations in cancer cells. This multifaceted approach could lead to more thorough and effective cancer treatment strategies [119].
A therapeutic strategy involving TAM-derived exosomes in cancer treatment focuses on converting TAMs from a tumor-promoting M2 phenotype to an anti-tumor M1 phenotype. This transformation can be accomplished using drugs, cytokines, or genetic interventions that influence macrophage polarization. For example, Chen et al. developed engineered exosomes containing siPDL, specifically targeting PDL1 on M2 TAMs, which reprogrammed them into anti-tumor M1 macrophages and reversed the immunosuppressive TME [120]. Exosomes from these reprogrammed M1 macrophages convey anti-tumor signals within the TME and to CSCs, thereby inhibiting tumor growth and improving the effectiveness of existing cancer treatments. Additionally, Kim et al. utilized exosomes derived from M1 macrophages to trigger a phenotypic switch in M2-like TAMs, shifting them from an anti-inflammatory to a pro-inflammatory M1 macrophage state. These reprogrammed M1 macrophages exhibited protein expression profiles characteristic of classical M1 macrophages, enhanced their phagocytic function and cross-presentation capabilities, and significantly bolstered anti-tumor immunity near the tumor site [121].
In certain instances, exosomes released by neutrophils (N-exo) can promote tumor growth by facilitating angiogenesis and dampening immune responses. Therapeutic approaches could involve blocking the release or uptake of these pro-tumorigenic exosomes. By targeting pathways critical to N-exo mediated communication, it may be feasible to interrupt the supportive interactions between neutrophils and tumor cells, thus impeding tumor progression. Additionally, N-exo can be utilized to bolster anti-tumor immune responses. By transmitting pro-inflammatory signals and activating other immune cells, these exosomes can contribute to creating a more inhospitable environment for tumor cells. This strategy could complement other immunotherapies, such as checkpoint inhibitors, to amplify the overall immune response against tumors. Another therapeutic avenue involves using N-exo to directly target CSCs by incorporating miRNAs or other molecules that suppress CSC characteristics into N-exo. This method targets the fundamental factors behind tumor recurrence and metastasis, aiming to enhance long-term cancer treatment outcomes. For instance, Zhang et al. harvested N-exo with enhanced tumor-targeting capabilities and combined them with DOX to significantly enhancing the efficacy of tumor treatment [122].
In conclusion, the rationale behind utilizing exosomes to target non-CSCs and non-CSCs-derived exosomes for tumor therapy lies in the complex and dynamic nature of TME, where interactions between CSCs and non-CSCs play a vital role in sustaining the CSC niche and enhancing tumor diversity. Non-CSCs, while not initiating tumors themselves, support CSC activities through mechanisms like paracrine signaling, extracellular matrix modification, and immunomodulation. Disrupting these supportive interactions via exosome-mediated delivery of therapeutic agents or siRNAs that target specific signaling pathways in non-CSCs can destabilize the CSC niche, potentially leading to CSC depletion or re-differentiation. By focusing on non-CSCs, which make up the majority of the tumor, exosome-based treatments could decrease the overall tumor mass and indirectly hinder CSC functionality. This approach may result in more sustained and effective cancer treatment outcomes, possibly preventing tumor recurrence and metastasis, often driven by CSC persistence. Moreover, this method could be integrated with other CSC-specific therapies to improve therapeutic efficacy. Ultimately, targeting non-CSCs with exosomes presents a novel and supplementary approach in CSC-centric cancer therapy, aiming to undermine the supportive role of non-CSCs within the TME, reduce tumor diversity, and enhance the effectiveness of cancer treatments Table 4.
Table 4.
Therapeutic modalities using exosomes discussed in this review
| Therapeutic exosome | Parental cell | Function material | Targeting cell | Effect | Reference |
|---|---|---|---|---|---|
| GRP7-siRNA load exosome | Mesenchymal stem cell | GRP7-siRNA | HCC cell | Inhibiting the growth and invasion of the cancer cells in vitro and inhibiting the growth and metastasis of the cancer cells in vivo. | [108] |
| Exosomes overexpressing miR-199a | Mesenchymal stem cell | miR-199a | Glioma cell | Suppressing the proliferation, invasion and migration of glioma cell | [109] |
| miR-193a load exosome | Mesenchymal stem cell | miR-193a | Non-small cell lung cancer cell | Suppressing the colony formation, invasion, migration, and proliferation and promoting apoptosis of resistant cell | [110] |
| Electroporation-loaded galectin-9 siRNA with oxaliplatin | Mesenchymal stem cell | galectin-9 siRNA, oxaliplatin | Macrophage and cytotoxic T lymphocyte | Eliciting anti-tumor immunity | [112] |
| Neoantigens load exosome | Dendritic cell | Neoantigens | Melanoma cell | Inhibiting tumor growth, prolonging survival, delaying tumor onset, inducing long-term memory, and preventing recurrence and metastasis | [116] |
| Chimeric antigen receptor-T (CAR-T) cell-derived exosomes with paclitaxel | T cell | Mesothelin and PD-L1 | Lung cancer cell | Promoting immunogenic cell death | [118] |
| Exosomes erived from M1-type macrophage | M1-type macrophage | - | M2-type macrophage | Leading to the transformation of human patient-derived macrophage into M1-like macrophages | [121] |
| Doxorubicin load exosome | Neutrophil | Doxorubicin | Gastric cancer cell | Restraining tumor growth | [122] |
Targeting CSCs with exosomal drug delivery
As our research on exosomes and EVs has become more and more intensive, exosomes have now emerged as carriers and engineered EVs to explore cancer therapy. Exosomes offer numerous advantages over traditional delivery systems like liposomes, viral vectors, and polymers, including high biocompatibility, stability, specific homing capabilities, low toxicity, and the ability to cross the blood-brain barrier [123]. These properties enable more precise drug delivery to target cells and substantially boost the effectiveness of cancer treatment. At present, more and more studies on engineered EVs are becoming more and more mature, and many studies have explored engineered EVs to target CSC for cancer therapy. For example, some researchers have engineered EVs to perform a dual knockdown of IQGAP1 and FOXM1, coupled with sorafenib treatment. This combination has shown to disrupt key signaling pathways such as PI3K/Akt and MAPK/ERK, improving sorafenib’s efficacy in tumor treatment by preventing β-catenin’s nuclear translocation, essential for CSC maintenance. These engineered EVs demonstrate enhanced targeting and homing capabilities compared to sorafenib alone, reducing CSC populations and reversing tumor resistance, thereby enhancing the efficacy of cancer therapy [124]. Moreover, Ishiguro et al. have developed engineered EVs targeting liver CSCs expressing EpCAM, facilitating the delivery of siRNA to β-catenin. This approach depletes β-catenin expression, curtails CSC proliferation, and achieves therapeutic effects. EpCAM is not only a CSC marker in the liver and other epithelial tissues but also a transcriptional target of the canonical Wnt/β-catenin signaling pathway that regulates liver CSC proliferation [125]. In recent years, nanomaterials with tumor therapeutic potential have emerged as effective therapeutic tools, with exosomes providing more precise tumor targeting than other carriers. Yong and colleagues have developed biocompatible tumor-cell-exocytosed exosome-biomimetic porous silicon nanoparticles (PSiNPs) for encapsulating doxorubicin (DOX). These PSiNPs, first loaded with DOX through endocytosis by tumor cells (termed DOX@-PSiNPs), are then exocytosed, resulting in exosome-sheathed doxorubicin-loaded PSiNPs (DOX@E-PSiNPs). Treatment with DOX@E-PSiNPs shows superior intracellular abundance and more effectively inhibits CSC spheroid number and size compared to other forms. Moreover, DOX@E-PSiNPs exhibit strong cytotoxicity targeted specifically at tumor cells and CSCs, with minimal toxicity to normal cells, and do not trigger immune responses in various mouse models, further confirming the high biocompatibility and safety of exosomes [126].
Targeted drug delivery to CSCs via exosomes marks a groundbreaking advancement in contemporary cancer treatment, tackling many limitations of conventional therapies. This method holds considerable clinical promise, notably in boosting the effectiveness of cancer therapies while lessening side effects, thereby improving patient outcomes. A primary benefit of this exosome-mediated approach is its ability to circumvent therapeutic resistance. CSCs notoriously resist standard chemotherapies and radiation due to their dormant nature, proficient DNA repair capabilities, and drug efflux transporter expression. These traits allow CSCs to withstand usual treatments, leading to tumor resurgence and spread. Exosome-based delivery systems, however, can be tailored to encapsulate potent anticancer drugs and specifically target CSCs, directly tackling the fundamental causes of treatment resistance. Successfully eliminating CSCs greatly diminishes the likelihood of tumor recurrence and metastasis, promoting more enduring and long-term therapeutic results. Additionally, exosome-mediated drug delivery offers enhanced precision and specificity compared to conventional treatment methods. By bioengineering, exosomes can be customized to recognize and bind to specific CSC markers like CD133, CD44, and ALDH. This targeting ability ensures that drugs are delivered directly to CSCs within a diverse tumor environment, minimizing unintended impacts and reducing toxicity to normal stem cells and surrounding healthy tissues. This targeted approach is particularly vital for decreasing the harmful side effects commonly associated with traditional chemotherapy, which indiscriminately impacts both cancerous and non-cancerous cells. The biocompatibility and low immunogenicity of exosomes also make them ideal candidates for clinical applications. Unlike synthetic drug delivery systems, exosomes are natural vesicles derived from cells, reducing the risk of eliciting immune responses. This characteristic enhances their safety profile, making them suitable for repeated administration, which may be necessary for effectively eradicating CSCs. Using patient-derived exosomes further minimizes the risk of adverse immune reactions, promoting a personalized medicine approach in cancer treatment. The potential for combination therapies is another crucial feature. Exosomes can carry a mix of therapeutic agents, including chemotherapeutics, RNA-based treatments, and proteins, facilitating a comprehensive assault on CSCs. This multi-drug strategy can enhance therapeutic efficacy synergistically, as different agents target multiple survival pathways in CSCs, countering these cells’ adaptability and redundancy. Moreover, exosomes can be engineered to alter the TME, making it less supportive of CSC survival and proliferation. The clinical translation of exosome-mediated targeted drug delivery systems to CSCs also represents a step forward in personalized medicine. By isolating and engineering exosomes from a patient’s own cells, treatment regimens can be tailored to the specific biological characteristics of the patient’s tumor, enhancing therapeutic effectiveness and minimizing adverse effects. This personalized approach not only betters patient outcomes but also shifts the paradigm in the development and application of cancer therapies.
In conclusion, utilizing exosomes as drug delivery vehicles targeting CSCs holds significant potential for revolutionizing cancer treatment. These exosome-mediated systems tackle challenges like therapeutic resistance, improve targeting accuracy, guarantee biocompatibility, facilitate combination therapies, and accommodate personalized treatment approaches. This innovative method offers a promising avenue for effectively combating cancer and enhancing patient outcomes.
Conclusion and prospects
CSCs are pivotal in driving tumor progression and are often resistant to standard treatments, which can lead to tumor recurrence and metastasis. Recent advances in the study of CSCs and exosomes highlight the critical role of CSC-exo in tumor dynamics. Consequently, there is a focused effort to counter the detrimental impacts of CSCs by targeting CSC-exo. Moreover, the natural carrier capabilities of exosomes are being exploited to deliver therapeutic agents specifically to CSCs, aiming for precise and effective treatment strategies that could potentially eliminate CSCs.
Despite these promising theoretical approaches, several challenges persist in the research and practical application of strategies targeting CSCs and CSC-exo. Current methods for identifying and isolating CSCs rely primarily on surface markers, which may lack precision. Moreover, standardization is needed in the extraction methods for CSC exosomes to reduce the potential interference from exosomes of other origins. Additionally, as CSCs and normal stem cells share certain signaling pathways, developing therapeutic strategies that specifically target CSCs without affecting normal stem cells remains a significant scientific challenge.
Non-CSCs, while not directly initiating tumors, are crucial in maintaining the CSC niche and facilitating cancer progression. Hence, utilizing exosomes to target non-CSCs could indirectly affect CSC dynamics, potentially disrupting the supportive TME and diminishing tumor recurrence. By inhibiting critical signaling pathways used by non-CSCs to support CSCs, the CSC niche may become destabilized, which could decrease CSC survival, proliferation, and differentiation capabilities. This might enhance the effectiveness of therapies aimed at CSCs, which typically face challenges due to the intrinsic adaptability and resilience of CSCs. However, significant hurdles are associated with this approach, particularly concerning the role of exosomes secreted by non-CSCs. These exosomes can transport various bioactive molecules that might unintentionally promote CSC survival, stemness, and metastatic capabilities. For instance, non-CSC-derived exosomes might carry growth factors, cytokines, or pro-inflammatory signals that sustain the CSC phenotype or protect CSCs from apoptosis. Hence, the dual function of exosomes from non-CSCs as both potential therapeutic vectors and contributors to CSC resilience adds complexity to this therapeutic strategy. The risk of exosome-based therapies inadvertently supporting CSC survival highlights the need for careful design and rigorous testing of these approaches. Potential strategies to address these challenges may involve engineering exosomes with highly specific targeting ligands, developing combination treatments that target both CSCs and non-CSCs, and utilizing advanced drug delivery systems to control the release and localization of therapeutic agents. Ultimately, achieving precise targeting, leveraging exosomes effectively, and accurately focusing treatments on CSCs are believed to be promising directions for improving clinical cancer treatment.
Acknowledgements
The authors declare that they have not use AI-generated work in this manuscript.
Abbreviations
- ANXA6
Annexin-a6
- BC
Breast cancer
- BCRP
Breast cancer resistance protein
- BM-MSCs
Marrow-derived mesenchymal stem cells
- CAF-exo
Cancer-associated fibroblast-derived exosome
- CAFs
Cancer-associated fibroblasts
- CCRCC
Clear cell renal cell carcinoma
- C/EBP-β
Ccaat enhancer binding protein β
- CRC
Colorectal cancer
- CSC-exo
Exosomes secreted by cancer stem cells
- CSCs
Cancer stem cells
- DCs
Dendritic cells
- ECM
Extracellular matrix
- ECs
Endothelial cells
- EMT
Epithelial-mesenchymal transition
- EMT-TFs
Epithelial-mesenchymal transition-inducing transcription factors
- EpCAM
Epithelial cell adhesion molecule
- ESCRT
endosomal sorting complexes required for transport
- ER
Endoplasmic reticulum
- ESEs
Early sorting endosomes
- EVs
Extracellular vesicles
- FOXO3a
Forkhead box o3
- GSCs
Glioblastoma stem cells
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HMGB
High mobility group box 1 protein
- IFN-γ
Interferon gamma
- ILVs
Intraluminal vesicles
- KLF3
Targeting krüppel-like factor 3
- LPS
Lipopolysaccharide
- MDR1
Multidrug resistance-1
- MDSC
Myeloid-derived suppressor cells
- MHC
Major histocompatibility complex
- MMP1
Matrix metalloproteinase 1
- MMP9
Matrix metalloproteinase 9
- MPO
Myeloperoxidase
- MSCs
Mesenchymal stem cells
- MVBs
Multivesicular bodies
- ncRNA
Non-coding RNA
- NK cells
Natural killer cells
- OGT
O-glcnac transferase
- OPN
Osteopontin
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- P4HA1
Prolyl 4-hydroxylase, alpha polypeptide 1
- PTEN
Phosphatase and tensin homologs
- PTX
Paclitaxel
- TAMs
Tumor-associated macrophages
- TGF-β
Transforming growth factor β
- TGN
Trans-golgi network
- TLR7
Toll-like receptor 7
- TME
Tumor microenvironment
- TNBC
Triple-negative breast cancer
- TNC
Tenascin-c
- TNF-α
Tumor necrosis factor-α
- TXNIP
Inhibiting thioredoxin-interacting protein
- YAP1
Yes-associated protein 1
- YB-1
Y-box binding protein 1
Author contributions
Q.L. conceptualized and drafted the initial manuscript and designed all figures. G.H. and Y.Y. participated in the writing of part manuscript and the creation of figures. Y.Y. and G.H. arranged all tables and Q.L. finalized all tables. G.H., Y.Y., X.L. X.P., and L.Y. discussed the initial manuscript and provided significant advice. Q.L. revised and finalized the manuscript. G.H., X.L., X.P. and L.Y. provided financial support and supervision. All authors have read the article and approved the article for submission.
Funding
This study was supported by the National Natural Science Foundation of China (No.82372919; No.82003040; No.82303373), China Postdoctoral Science Foundation (2021M703602), Natural Science Foundation of Liaoning Province (2022-BS-137), Scientific Research Funding of Liaoning Province Education Department (LJKMZ20221210; LJ212410159099; LJ212410159084), Science and Technology Projects of Shenyang (22-321-31-02), Special programme for cultivating young and middle-aged talents in science and technology in Shenyang(RC230600), Support plan for young and middle-aged scientific and technological talents in Shenyang (RC220032).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qi Li, Guangpeng He, and Yifan Yu.
Contributor Information
Xueqiang Peng, Email: xqpeng@cmu.edu.cn.
Liang Yang, Email: 20191264@cmu.edu.cn.
References
- 1.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–30. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 3.Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, et al. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 2020;10(19):8721–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. [DOI] [PubMed] [Google Scholar]
- 6.Bhat GR, Sethi I, Sadida HQ, Rah B, Mir R, Algehainy N, et al. Cancer cell plasticity: from cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024;43(1):197–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saw PE, Liu Q, Wong PP, Song E. Cancer stem cell mimicry for immune evasion and therapeutic resistance. Cell Stem Cell. 2024;31(8):1101–12. [DOI] [PubMed] [Google Scholar]
- 8.Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014;54(5):716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8. [DOI] [PubMed] [Google Scholar]
- 10.Peng C, Xu Y, Wu J, Wu D, Zhou L, Xia X. TME-Related biomimetic strategies against Cancer. Int J Nanomed. 2024;19:109–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the Tumor Microenvironment. Cancer Discov. 2021;11(4):933–59. [DOI] [PubMed] [Google Scholar]
- 12.Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Théry C, SnapShot. Extracell Vesicles Cell. 2020;182(1):262–e1. [DOI] [PubMed] [Google Scholar]
- 13.He G, Peng X, Wei S, Yang S, Li X, Huang M, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022;21(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Z, Wang L, Zhou Y, Dong L, Ma W, Lv L, et al. Glioblastoma stem cell-derived Exosomes Enhance Stemness and Tumorigenicity of Glioma cells by transferring Notch1 protein. Cell Mol Neurobiol. 2020;40(5):767–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Hou J, Liu K, Liu Q, Shen L, Liu B, et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces nanog expression in their differentiated progenies and confers regorafenib resistance. J Gastroenterol Hepatol. 2021;36(12):3429–37. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Zhang L, Guo B, Deng J, Wu S, Li F, et al. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol Cancer. 2019;18(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang WL, Yang MH. Numb is involved in the non-random segregation of subcellular vesicles in colorectal cancer stem cells. Cell Cycle. 2016;15(20):2697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai W, Jin X, Han L, Huang H, Ji Z, Xu X, et al. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/β-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020;11(9):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han T, Chen L, Li K, Hu Q, Zhang Y, You X, et al. Significant CircRNAs in liver cancer stem cell exosomes: mediator of malignant propagation in liver cancer? Mol Cancer. 2023;22(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 2015;153:107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geindreau M, Bruchard M, Vegran F. Role of cytokines and chemokines in Angiogenesis in a Tumor Context. Cancers (Basel). 2022;14(10). [DOI] [PMC free article] [PubMed]
- 22.Collet G, El Hafny-Rahbi B, Nadim M, Tejchman A, Klimkiewicz K, Kieda C. Hypoxia-shaped vascular niche for cancer stem cells. Contemp Oncol (Pozn). 2015;19(1a):A39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZF, Liao F, Wu H, Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J Exp Clin Cancer Res. 2019;38(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Ma X, Wang J, Zhao Y, Wang Y, Bihl JC, et al. Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget. 2017;8(22):36137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. 2017;6(1):1359479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindoso RS, Collino F, Camussi G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget. 2015;6(10):7959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. [DOI] [PubMed] [Google Scholar]
- 28.Kumari A, Kashyap D, Garg VK. Osteopontin in cancer. Adv Clin Chem. 2024;118:87–110. [DOI] [PubMed] [Google Scholar]
- 29.Khalil A, Medfai H, Poelvoorde P, Kazan MF, Delporte C, Van Antwerpen P, et al. Myeloperoxidase promotes tube formation, triggers ERK1/2 and akt pathways and is expressed endogenously in endothelial cells. Arch Biochem Biophys. 2018;654:55–69. [DOI] [PubMed] [Google Scholar]
- 30.Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol. 2017;44:10–24. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Hu Z, Horta CA, Yang J. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics. Semin Cancer Biol. 2023;88:46–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana R, Mestre-Farrera A, Yang J. Update on epithelial-mesenchymal plasticity in Cancer Progression. Annu Rev Pathol. 2024;19:133–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AQ, Hasan A, Mir SS, Rashid K, Uddin S, Steinhoff M. Exploiting transcription factors to target EMT and cancer stem cells for tumor modulation and therapy. Semin Cancer Biol. 2024;100:1–16. [DOI] [PubMed] [Google Scholar]
- 34.Hardin H, Helein H, Meyer K, Robertson S, Zhang R, Zhong W, et al. Thyroid cancer stem-like cell exosomes: regulation of EMT via transfer of lncRNAs. Lab Invest. 2018;98(9):1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, Saleh AA, Saadeldin IM, El-Shetry ES, et al. Potential effect of Exosomes Derived from Cancer Stem cells and MSCs on progression of DEN-Induced HCC in rats. Stem Cells Int. 2018;2018:8058979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, He Y, Liu X, Luo F, Jiang Y, Xiang M, et al. Cancer stem cell-like cells-derived exosomal lncRNA CDKN2B-AS1 promotes biological characteristics in thyroid cancer via miR-122-5p/P4HA1 axis. Regen Ther. 2023;22:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, He J, Hu H, Tu L, Sun Z, Liu Y, et al. Lung CSC-derived exosomal mir-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J Cell Mol Med. 2020;24(11):6324–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Yang G, Zhao D, Wang J, Bai Y, Peng Q, et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol Cancer. 2019;18(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D, Li X, Zeng B, Zhao Q, Chen H, Zhang Y, et al. Exosomal microRNA-4535 of Melanoma Stem cells promotes metastasis by inhibiting Autophagy Pathway. Stem Cell Rev Rep. 2023;19(1):155–69. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Liu D, Chen H, Zeng B, Zhao Q, Zhang Y, et al. Melanoma stem cells promote metastasis via exosomal miR-1268a inactivation of autophagy. Biol Res. 2022;55(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Huang S, Chen S, Chen J, Wang Z, Wang Y, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021;12(5):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patnam S, Majumder B, Joshi P, Singh AD, Nagalla B, Kumar D, et al. Differential expression of SRY-Related HMG-Box transcription factor 2, Oligodendrocyte Lineage Transcription Factor 2, and Zinc Finger E-Box binding Homeobox 1 in serum-derived extracellular vesicles: implications for Mithramycin Sensitivity and targeted therapy in high-Grade Glioma. ACS Pharmacol Transl Sci. 2024;7(1):137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi L, Li B, Zhang Y, Chen Y, Tan J, Chen Y, et al. Exosomal lncRNA Mir100hg derived from cancer stem cells enhance glycolysis and promote metastasis of lung adenocarcinoma through mircroRNA-15a-5p/31-5p. Cell Commun Signal. 2023;21(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji W, Bai J, Ke Y. Exosomal ZFPM2-AS1 contributes to tumorigenesis, metastasis, stemness, macrophage polarization, and infiltration in hepatocellular carcinoma through PKM mediated glycolysis. Environ Toxicol. 2023;38(6):1332–46. [DOI] [PubMed] [Google Scholar]
- 45.Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. 2019;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Callejo P, Guo Z, Ziglari T, Claudio NM, Nguyen KH, Oshimori N, et al. Cancer stem cell-derived extracellular vesicles preferentially target MHC-II-macrophages and PD1 + T cells in the tumor microenvironment. PLoS ONE. 2023;18(2):e0279400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ning J, Hou X, Hao J, Zhang W, Shi Y, Huang Y, et al. METTL3 inhibition induced by M2 macrophage-derived extracellular vesicles drives anti-PD-1 therapy resistance via M6A-CD70-mediated immune suppression in thyroid cancer. Cell Death Differ. 2023;30(10):2265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang S, Zhang P, Yin N, Xu Z, Liu X, Wu A, et al. Glioblastoma stem cell-derived exosomal miR-374b-3p promotes tumor angiogenesis and progression through inducing M2 macrophages polarization. iScience. 2024;27(3):109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bied M, Ho WW, Ginhoux F, Blériot C. Roles of macrophages in tumor development: a spatiotemporal perspective. Cell Mol Immunol. 2023;20(9):983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan P, Xu X, Hu D, Chen Y, Fan J, Yao S, et al. Highly sensitive imaging of Tumor Metastasis based on the Targeting and polarization of M2-like macrophages. J Am Chem Soc. 2023;145(14):7941–51. [DOI] [PubMed] [Google Scholar]
- 52.Rouzbahani E, Majidpoor J, Najafi S, Mortezaee K. Cancer stem cells in immunoregulation and bypassing anti-checkpoint therapy. Biomed Pharmacother. 2022;156:113906. [DOI] [PubMed] [Google Scholar]
- 53.Gabrusiewicz K, Li X, Wei J, Hashimoto Y, Marisetty AL, Ott M, et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7(4):e1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Ye S, Yao Y, Zhang C, Liu W. Oral Cancer stem cell-derived small extracellular vesicles promote M2 macrophage polarization and suppress CD4(+) T-Cell activity by transferring UCA1 and targeting LAMC2. Stem Cells Int. 2022;2022:5817684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan Y, Wang L, Ge D, Tan L, Cao B, Fan H, et al. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8(+) T cells. Cancer Lett. 2021;500:98–106. [DOI] [PubMed] [Google Scholar]
- 57.Cheng WC, Liao TT, Lin CC, Yuan LE, Lan HY, Lin HH, et al. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int J Cancer. 2019;145(8):2209–24. [DOI] [PubMed] [Google Scholar]
- 58.Mirzaei R, Sarkar S, Dzikowski L, Rawji KS, Khan L, Faissner A, et al. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. Oncoimmunology. 2018;7(10):e1478647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naseri M, Zöller M, Hadjati J, Ghods R, Ranaei Pirmardan E, Kiani J, et al. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. J Cell Mol Med. 2021;25(7):3312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H, Liu D, Dong S, Zeng L, Wu Z, Zhao P, et al. Epitranscriptomics and epiproteomics in cancer drug resistance: therapeutic implications. Signal Transduct Target Ther. 2020;5(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (Dordr). 2020;43(1):123–36. [DOI] [PubMed] [Google Scholar]
- 62.Santos JC, Lima NDS, Sarian LO, Matheu A, Ribeiro ML, Derchain SFM. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8(1):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, Sun G, Hu Y, Yang J, Shi Y, Liu H, et al. Extracellular vesicle lncRNA Metastasis-Associated Lung Adenocarcinoma transcript 1 released from glioma stem cells modulates the inflammatory response of Microglia after Lipopolysaccharide Stimulation through regulating miR-129-5p/High mobility Group Box-1 protein Axis. Front Immunol. 2019;10:3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo Z, Guo A, Zhou C. Breast Cancer stem cell-derived ANXA6-Containing exosomes sustain Paclitaxel Resistance and Cancer aggressiveness in breast Cancer. Front Cell Dev Biol. 2021;9:718721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao W, Guo P, Mu Q, Wang Y. Exosome-derived Circ-PVT1 contributes to Cisplatin Resistance by regulating Autophagy, Invasion, and apoptosis Via miR-30a-5p/YAP1 Axis in Gastric Cancer cells. Cancer Biother Radiopharm. 2021;36(4):347–59. [DOI] [PubMed] [Google Scholar]
- 66.Lu T, Li Z, Yang Y, Ji W, Yu Y, Niu X, et al. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett. 2018;423:36–46. [DOI] [PubMed] [Google Scholar]
- 67.Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21(1):5–21. [DOI] [PubMed] [Google Scholar]
- 68.Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang J. Hedgehog signaling mechanism and role in cancer. Semin Cancer Biol. 2022;85:107–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadrkhanloo M, Entezari M, Orouei S, Ghollasi M, Fathi N, Rezaei S, et al. STAT3-EMT axis in tumors: modulation of cancer metastasis, stemness and therapy response. Pharmacol Res. 2022;182:106311. [DOI] [PubMed] [Google Scholar]
- 71.Yang Q, Zhao S, Shi Z, Cao L, Liu J, Pan T, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J Exp Clin Cancer Res. 2021;40(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang YH, Wang WL, Wang PH, Lee HT, Chang WW. EXOSC5 maintains cancer stem cell activity in endometrial cancer by regulating the NTN4/integrin β1 signalling axis. Int J Biol Sci. 2024;20(1):265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung WM, Molony RD, Lee YF. Non-stem bladder cancer cell-derived extracellular vesicles promote cancer stem cell survival in response to chemotherapy. Stem Cell Res Ther. 2021;12(1):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chhabra Y, Weeraratna AT. Fibroblasts in cancer: Unity in heterogeneity. Cell. 2023;186(8):1580–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo H, Xia X, Huang LB, An H, Cao M, Kim GD, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun. 2022;13(1):6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhuang J, Shen L, Li M, Sun J, Hao J, Li J, et al. Cancer-Associated fibroblast-derived miR-146a-5p generates a Niche that promotes bladder Cancer Stemness and Chemoresistance. Cancer Res. 2023;83(10):1611–27. [DOI] [PubMed] [Google Scholar]
- 77.Liu L, Zhang Z, Zhou L, Hu L, Yin C, Qing D, et al. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp Cell Res. 2020;391(2):111956. [DOI] [PubMed] [Google Scholar]
- 78.Hasegawa T, Yashiro M, Nishii T, Matsuoka J, Fuyuhiro Y, Morisaki T, et al. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-β signaling. Int J Cancer. 2014;134(8):1785–95. [DOI] [PubMed] [Google Scholar]
- 79.Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8(14):3932–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owusu BY, Galemmo R, Janetka J, Klampfer L. Hepatocyte Growth Factor, a key tumor-promoting factor in the Tumor Microenvironment. Cancers (Basel). 2017;9(4). [DOI] [PMC free article] [PubMed]
- 81.Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heenatigala Palliyage G, Samart P, Bobbala S, Rojanasakul LW, Coyle J, Martin K, et al. Chemotherapy-induced PDL-1 expression in cancer-associated fibroblasts promotes chemoresistance in NSCLC. Lung Cancer. 2023;181:107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Wang R, Xiong S, Wang X, Zhao Z, Bai S, et al. Cancer-associated fibroblasts promote the stemness of CD24(+) liver cells via paracrine signaling. J Mol Med (Berl). 2019;97(2):243–55. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Hua F, Zhan Y, Yang Y, Xie J, Cheng Y, et al. Carcinoma associated fibroblasts small extracellular vesicles with low miR-7641 promotes breast cancer stemness and glycolysis by HIF-1α. Cell Death Discov. 2021;7(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhan Y, Du J, Min Z, Ma L, Zhang W, Zhu W, et al. Carcinoma-associated fibroblasts derived exosomes modulate breast cancer cell stemness through exonic circHIF1A by mir-580-5p in hypoxic stress. Cell Death Discov. 2021;7(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225–37. [DOI] [PubMed] [Google Scholar]
- 87.Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lyu T, Wang Y, Li D, Yang H, Qin B, Zhang W, et al. Exosomes from BM-MSCs promote acute myeloid leukemia cell proliferation, invasion and chemoresistance via upregulation of S100A4. Exp Hematol Oncol. 2021;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Y, Liu H, Xiao X, Yu Q, Deng R, Hua L et al. Bone marrow mesenchymal stem cell-derived Exosomes Inhibit Triple-negative breast Cancer Cell Stemness and Metastasis via an ALKBH5-Dependent mechanism. Cancers (Basel). 2022;14(24). [DOI] [PMC free article] [PubMed]
- 90.Gu H, Yan C, Wan H, Wu L, Liu J, Zhu Z, et al. Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Hum Cell. 2021;34(6):1812–29. [DOI] [PubMed] [Google Scholar]
- 91.Gao X, Zhou J, Wang J, Dong X, Chang Y, Jin Y. Mechanism of exosomal miR-155 derived from bone marrow mesenchymal stem cells on stemness maintenance and drug resistance in myeloma cells. J Orthop Surg Res. 2021;16(1):637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H, Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br J Cancer. 2018;119(6):744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao X, Mao Y, Wu D, Zhu Y, Lu J, Huang Y, et al. Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging mir-338-5p and targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 2021;512:38–50. [DOI] [PubMed] [Google Scholar]
- 94.You B, Jin C, Zhang J, Xu M, Xu W, Sun Z, et al. MSC-Derived Extracellular vesicle-delivered L-PGDS inhibit gastric Cancer progression by suppressing Cancer Cell Stemness and STAT3 phosphorylation. Stem Cells Int. 2022;2022:9668239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W, Xin X, Li X, Geng J, Sun Y. Exosomes secreted by M2 macrophages promote cancer stemness of hepatocellular carcinoma via the miR-27a-3p/TXNIP pathways. Int Immunopharmacol. 2021;101(Pt A):107585. [DOI] [PubMed] [Google Scholar]
- 97.Chang J, Li H, Zhu Z, Mei P, Hu W, Xiong X, et al. microRNA-21-5p from M2 macrophage-derived extracellular vesicles promotes the differentiation and activity of pancreatic cancer stem cells by mediating KLF3. Cell Biol Toxicol. 2022;38(4):577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y, Yin K, Tian J, Xia X, Ma J, Tang X, et al. Granulocytic myeloid-derived suppressor cells promote the stemness of Colorectal Cancer cells through Exosomal S100A9. Adv Sci (Weinh). 2019;6(18):1901278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, et al. Myeloid-derived suppressor cells endow stem-like qualities to breast Cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 2016;76(11):3156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Babadag S, Altundag-Erdogan Ö, Akkaya-Ulum YZ, Çelebi-Saltik B. Evaluation of Tumorigenic properties of MDA-MB-231 Cancer stem cells cocultured with Telocytes and Telocyte-Derived Mitochondria following miR-146a inhibition. DNA Cell Biol. 2024. [DOI] [PubMed]
- 102.Rigg E, Wang J, Xue Z, Lunavat TR, Liu G, Hoang T, et al. Inhibition of extracellular vesicle-derived miR-146a-5p decreases progression of melanoma brain metastasis via notch pathway dysregulation in astrocytes. J Extracell Vesicles. 2023;12(10):e12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fabbri M. Natural killer cell-derived vesicular miRNAs: a New Anticancer Approach? Cancer Res. 2020;80(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J, Lee SA, Gu NY, Jeong SY, Byeon JS, Jeong DU, et al. Canine natural killer cell-derived exosomes exhibit Antitumor Activity in a mouse model of Canine Mammary Tumor. Biomed Res Int. 2021;2021:6690704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.García-Ortega MB, Aparicio E, Griñán-Lisón C, Jiménez G, López-Ruiz E, Palacios JL et al. Interferon-Alpha decreases Cancer Stem Cell properties and modulates exosomes in Malignant Melanoma. Cancers (Basel). 2023;15(14). [DOI] [PMC free article] [PubMed]
- 106.Chen J, Xu R, Xia J, Huang J, Su B, Wang S. Aspirin inhibits hypoxia-mediated lung cancer cell stemness and exosome function. Pathol Res Pract. 2019;215(6):152379. [DOI] [PubMed] [Google Scholar]
- 107.Chen JH, Wu ATH, Bamodu OA, Yadav VK, Chao TY, Tzeng YM et al. Ovatodiolide suppresses oral Cancer Malignancy by Down-regulating Exosomal Mir-21/STAT3/β-Catenin Cargo and Preventing Oncogenic Transformation of normal gingival fibroblasts. Cancers (Basel). 2019;12(1). [DOI] [PMC free article] [PubMed]
- 108.Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnol. 2018;16(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu L, Gui S, Liu Y, Qiu X, Zhang G, Zhang X, et al. Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging. 2019;11(15):5300–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11(9):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao X, Wu X, Qian M, Song Y, Wu D, Zhang W. Knockdown of TGF-β1 expression in human umbilical cord mesenchymal stem cells reverts their exosome-mediated EMT promoting effect on lung cancer cells. Cancer Lett. 2018;428:34–44. [DOI] [PubMed] [Google Scholar]
- 112.Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. [DOI] [PubMed] [Google Scholar]
- 113.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghorbaninezhad F, Alemohammad H, Najafzadeh B, Masoumi J, Shadbad MA, Shahpouri M, et al. Dendritic cell-derived exosomes: a new horizon in personalized cancer immunotherapy? Cancer Lett. 2023;562:216168. [DOI] [PubMed] [Google Scholar]
- 116.Li J, Li J, Peng Y, Du Y, Yang Z, Qi X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J Control Release. 2023;353:423–33. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Q, Wei S, Wang H, Li Y, Fan S, Cao Y, et al. T cell-derived exosomes in tumor immune modulation and immunotherapy. Front Immunol. 2023;14:1130033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu T, Chen Z, Jiang G, Huang X. Sequential targeting hybrid nanovesicles composed of chimeric Antigen receptor T-Cell-derived exosomes and liposomes for enhanced Cancer Immunochemotherapy. ACS Nano. 2023;17(17):16770–86. [DOI] [PubMed] [Google Scholar]
- 119.Si C, Gao J, Ma X. Natural killer cell-derived exosome-based cancer therapy: from biological roles to clinical significance and implications. Mol Cancer. 2024;23(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y, Gong L, Cao Y, Liu Z, Wang Y, Cheng H, et al. Reprogramming tumor-associated macrophages by a dually targeted milk exosome system as a potent monotherapy for cancer. J Control Release. 2024;366:395–409. [DOI] [PubMed] [Google Scholar]
- 121.Kim H, Park HJ, Chang HW, Back JH, Lee SJ, Park YE, et al. Exosome-guided direct reprogramming of tumor-associated macrophages from protumorigenic to antitumorigenic to fight cancer. Bioact Mater. 2023;25:527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8(2):eabj8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qiu H, Liang J, Yang G, Xie Z, Wang Z, Wang L, et al. Application of exosomes in tumor immunity: recent progresses. Front Cell Dev Biol. 2024;12:1372847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He C, Jaffar Ali D, Qi Y, Li Y, Sun B, Liu R, et al. Engineered extracellular vesicles mediated CRISPR-induced deficiency of IQGAP1/FOXM1 reverses sorafenib resistance in HCC by suppressing cancer stem cells. J Nanobiotechnol. 2023;21(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ishiguro K, Yan IK, Lewis-Tuffin L, Patel T. Targeting Liver Cancer Stem cells using Engineered Biological nanoparticles for the treatment of Hepatocellular Cancer. Hepatol Commun. 2020;4(2):298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10(1):3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li L, Xiong Y, Wang N, Zhu M, Gu Y. Breast Cancer stem cells-derived Extracellular vesicles affect PPARG expression by delivering MicroRNA-197 in breast Cancer cells. Clin Breast Cancer. 2022;22(5):478–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.