Abstract
Purpose
This study aimed to investigate the effects of 12 weeks of resistance exercise training (RT) on oxidative status, muscle strength, functional capacity, quality of life (QoL), and fatigue in women with Multiple Sclerosis (MS).
Methods
In this randomized control trial (ethical code: SSRI.REC-1402-101; IRCT registration code: IRCT20120912010824N3, 07.09.2023), Iran) twenty-five women with relapsing- remitting MS (aged 18–45 years and expanded disability status scale (EDSS) ≤ 4) were randomly divided in two groups MS without resistance exercise (MS + non-RT; n = 13) and with RT (12 weeks/3 times per week/ 60–80% of 1RM) (MS + RT; n = 12). “Informed” consent was obtained from all participants. Then, fifteen healthy aged-matched women participated as a control group (HCON; n = 15). Blood serum levels of oxidative stress [malondialdehyde (MDA)] and antioxidant capacity [superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity] were obtained pre and post intervention. In addition, muscle strength by 5-RM test, functional capacity (for lower limb T25FWT, 2MWT, and 5STS tests and for Upper limb Manual dexterity of both hands with the (9-HPT) test and MSWS-12 questionnaire were also assessed over the same period. Also, Quality of life and fatigue were assessed pre- and post- intervention with by 31-MusiQoL questionnaire and FSMC questionnaire.
Results
RT led to improvements in muscle strength for leg extension, lying leg curl, bench press movements (P < 0.001, P < 0.001, P < 0.001, respectively). Moreover, compared with the MS + non-RT group, RT demonstrated increased functional capacity (Timed 25 ft Walk Test, Two-Minute Walk Test, 5-Time Sit-To-Stand Test, Twelve Item MS Walking Scale (P < 0.001, P < 0.001, P < 0.001, P < 0.001, respectively). Dexterity of the left hand but not the right hand also improved (P < 0.01, P = 0.057, respectively). Improvements were also found for fatigue and QoL (P < 0.01, P < 0.05). However, the mean changes of MDA, SOD and GPx noted in RT group were not statistically significant (P˃0.05, P˃0.05, P˃0.05, respectively).
Conclusions
RT has positive effects on muscle strength, functional capacity, and quality of life while reducing fatigue in this population. However, markers of oxidative stress were not affected. When we consider the clear role in MS pathogenesis and progression, antioxidant increases in relation to a reduction in pro-oxidant capacity would have provided a positive and important clinical development for people with MS.
Keywords: Oxidative stress markers, Muscle strength, Functional capacity, Fatigue, Multiple sclerosis, Resistance training
Introduction
Multiple sclerosis (MS) is a multifactorial autoimmune disease that is characterized by chronic inflammation, demyelination, axonal injury, and oxidative stress [1, 2]. This disease is the most common non-traumatic cause of disability in younger adults reaching 2.8 million affected worldwide [3, 4]. The exact etiology of MS remains unclear, likely due to genetic, environmental, and immune factors [5]. Studies have reported that oxidative stress plays a major role in MS pathogenesis or progression by contributing to brain blood barrier (BBB) dysfunction, alterations in tight junctions and extravasation of leucocytes into the central nerve system (CNS), demyelination and neurodegeneration [1, 5–8].
Oxidative stress refers to a pathological condition that causes various types of toxic effects on cells due to an imbalance between the production of reactive oxygen species (ROS) and the scavenger system [8]. In this context, Aydin et al. demonstrated the presence of oxidative stress in all stages of MS suggesting a dynamic production of ROS in MS patients [9]. Intensification of ROS production may lead to intra- and extra-cellular damage-induced malfunction of molecular mechanisms and chronic inflammatory conditions [10]. Studies have shown that blood levels of malondialdehyde (MDA), a marker of lipid peroxidation, are significantly increased in patients with MS (pwMS) compared to healthy individuals. This suggests that lipid damage in pwMS may contribute to disease-induced neurodegeneration [3]. On the other hand, superoxide dismutase (SOD) and glutathione peroxidase (GPx) are important physiological antioxidant enzymes and protect cells against oxidative damage and neuroinflammation [6, 11]. Clinical studies have noted reduced levels of peripheral antioxidants, including vitamin E, GPx, together with an increase in lipid oxidation in pwMS [1, 5, 7] and is observed prior to relapse [1].
Oxidative stress plays a prominent role not only in the pathophysiology of MS but also in other symptoms, such as fatigue and Quality of life [12]. Due to the symptoms caused by MS, these patients experience functional limitations including impaired manual dexterity and walking ability [13] which impeding family relations, work and social dynamics and ultimately causes a decrease in quality of life (QoL) in these patients compared to the general population [14]. Furthermore, longitudinal studies reported that fatigue is highly associated with decreased QoL [14]. Two-thirds of patients describe fatigue as one of their worst symptoms [15].
Previous studies have shown that pwMS benefit from a variety of exercise modalities with regard to symptom management and performance [16, 17]. Also, physical exercise has been associated with reduced oxidative stress in observational studies and clinical trials in different diseases of the CNS [18]. In this context, Schreibelt et al. suggested that physical exercise in MS preserved tight-junction proteins such as occludin in the spinal cord of mice, by inhibiting ROS and oxidative stress [19]. Also, data from a study suggested that physical exercise inhibits progression of experimental autoimmune encephalomyelitis (EAE) in mice, likely through its immunomodulatory and antioxidant effects [18]. Two human studies indicated an improvement in the redox balance after resistance training [20, 21]. Regardless of intensity, volume, type of exercise and studied population, antioxidant parameters seem to increase and while pro-oxidant indicators decrease after physical training [22].
To date, however, little is known about the potential effects of physical exercise on the underlying disease mechanisms. Interesting, considering the important role of oxidative stress in the pathology of MS, no study to date has investigated the role of structured exercise to improve oxidative status in pwMS particularly females. This is an important development when we consider the widespread prevalence of this disease in women compared to men. Also, compared to the endurance exercise, resistance training in heat sensitive patients less frequently cause symptom exacerbations due to increased body temperature. Despite the fact that MS, fatigue and Qol share pathophysiological mechanisms associated with oxidative stress, the studies carried out thus far are scarce. The aim of this study, therefore, was to investigate the effects of 12 weeks of resistance exercise training (RT) on oxidative stress status, muscle strength, functional capacity, QoL, and fatigue in MS. We hypothesized that RT using a moderate intensity would result in a decreased concentration of oxidative stress biomarkers including (MDA) and enhanced antioxidant enzymes activity (SOD and GPx), muscle strength, functional capacity, QoL, and Fatigue in MS.
Methods
Research design and participants
This study was a RCT (parallel-group) using a quasi-experimental design with pre- and post-testing which was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Ethics in Research Working Group at the Sports Sciences Research Institute (ethical code: SSRI.REC-1402-101; IRCT registration code: IRCT20120912010824N3, 07.09.2023), Iran. “Informed” consent was obtained from all participants. The full trial was conducted from July, 2021 to February, 2022.
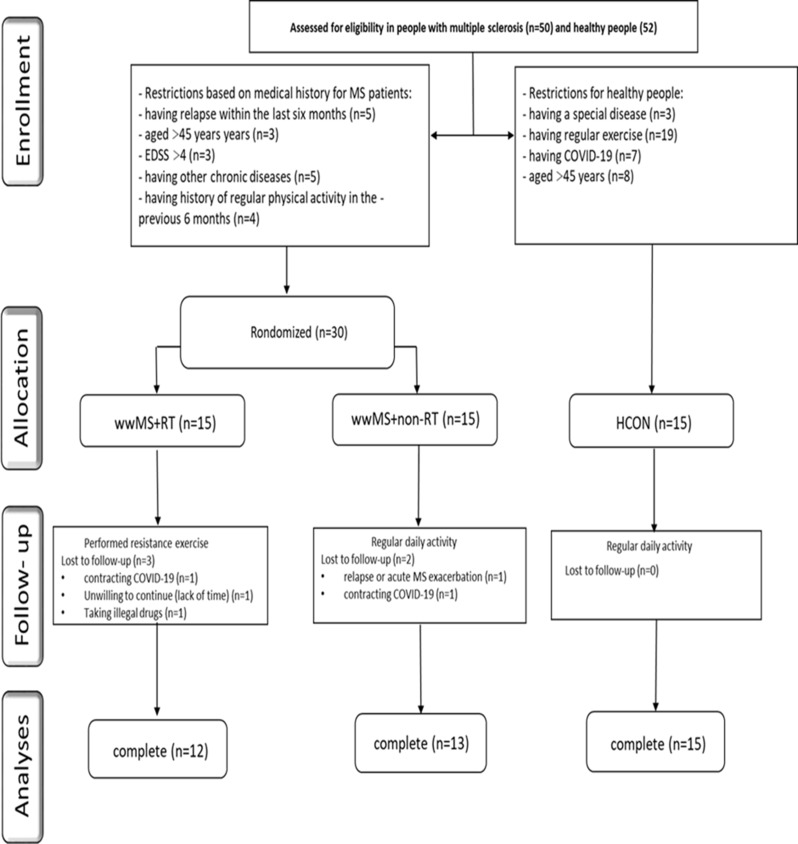
As outlined in Fig. 1, pwMS (MS) (relapsing-remitting MS (RRMS)) were recruited through advertising at the Kermanshah Multiple Sclerosis Center, Iran. Fifty MS (RRMS) volunteered to participate in the study. Subsequently, thirty eligible MS (RRMS) were considered eligible for the RCT based on inclusion/exclusion criteria (Fig. 1). The inclusion criteria were as follows: (a) history of at least two-year diagnosed MS; (b) no relapse or acute MS exacerbation within the last six months; (c) aged between 18 and 45 years; (d) expanded disability status scale (EDSS) ≤ 4; (e) no other chronic diseases (metabolic, cardiovascular, renal, …); (f) no history of regular physical activity in the previous 6 months. The patients’ diagnoses were confirmed by the MS committee of Kermanshah University of Medical Sciences, Iran according to the McDonald criteria. Exclusion criteria comprised: (a) severe relapses during the study period; (b) participation in any extra exercise training programs; (c) smoking and consuming other drugs (except MS medications); (d) lack of regular attendance in the intervention; (e) COVID-19 infection. Finally, Participants were randomly (using a random number table by sport specialist) divided in two (a) MS + non-resistance training (MS + non-RT; n = 15, age = 35.15 ± 7.80, body mass = 70.36 ± 12.99, body mass index (BMI) = 26.54 ± 4.23, body fat percentage = 24.54 ± 7.14) and (b) MS + resistance training (MS + RT; n = 15, age = 34.08 ± 8.90, body mass = 65.37 ± 17.69, BMI = 24.40 ± 5.74, body fat percentage = 22.00 ± 9.67) groups. Moreover, fifteen age-matched healthy women were recruited as a healthy control group (HCON; n = 15, age = 30.00 ± 6.44, body mass = 68.89 ± 7.52, BMI = 24.10 ± 4.42, body fat percentage = 29.89 ± 3.36) and these data have been published in our study in the Journal of BMC Neuroscience and this study is the second section of our study (Ph.D. thesis) [23]. Participants in MS + non-RT and HCON groups were instructed to keep their daily activity during the trial period without any exercise training.
Fig. 1.
Participant recruitment flow chart. MS + RT: Women with MS + resistance training; MS + non-RT: Women with MS + non-resistance training; HCON: Healthy control
The MS + RT group performed the RT program. The MS + non-RT and MS + RT groups received their drug treatment supervised by a specialist neurologist. It should be mentioned here that 5 participants were excluded from the study due to contracting COVID-19, relapse, unwillingness to continue cooperation, and taking illegal drugs.
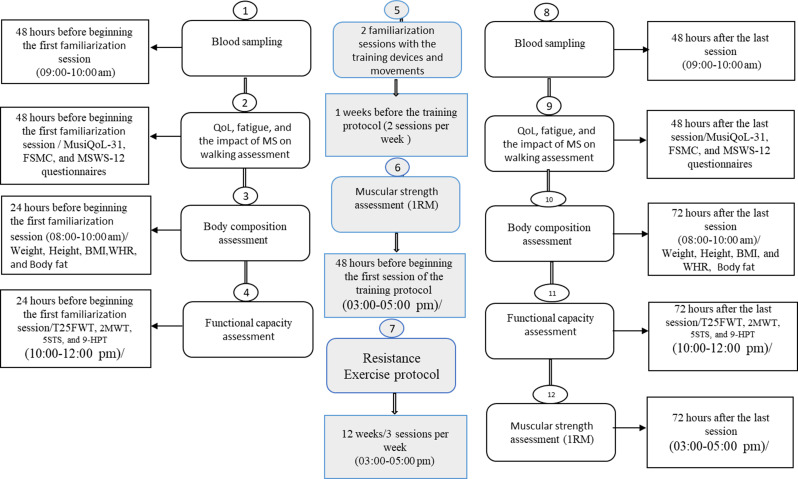
As detailed in Fig. 2, the experiment lasted for 13 weeks: 1 week for familiarization with the training devices and movements and 12 weeks for resistance training (MS + RT) or daily activity (for MS + non-RT and HCON). In summary, forty-eight hours before beginning the first familiarization session, QoL using the Multiple Sclerosis International Quality of Life Questionnaire (MusiQoL) with 31 items [24], Fatigue using the two-dimensional Fatigue Scale for Motor and Cognitive functions questionnaire (FSMC) [25], Impact of MS on walking ability using the 12-Item MS Walking Scale questionnaire (MSWS-12) were all completed under supervision of the research team [26]. Twenty-four hours before beginning the first familiarization session, body composition indices (height, age, weight, body fat percentage) (InBody; Model: Zuse 9.9, South Korea), BMI, waist-to-hip ratio (WHR) were also measured. In addition, functional capacity (for lower limb T25FWT, 2MWT [26], 5STS tests [17], and for Upper limb Manual dexterity of both hands with the nine-hole peg test (9-HPT) were also recorded [13].
Fig. 2.
Outline of experimental design
Following this, familiarization sessions and assessment of muscle strength (lower body and upper body) via the five-repetition maximum (5RM) test [27] were performed before starting the training protocol following previous instructions from Landers et al. [28]. Then, MS + RT group performed 12 weeks of resistance training and MS + non-RT and HCON groups followed daily activities. All pre-test processes were performed 48 to 72 h after the last training sessions (as presented in Fig. 2) as post-test values.
From all participants, 48 h before beginning the first familiarization session and 48 h after the last training sessions, blood samples (8 cc) were collected from an antecubital vein at the same time of day in the morning following a 12 h fasting state. All samples were collected with patients in a relaxed rested state. Following blood collection, samples were centrifuged at a temperature of 4 °C and 3000 rpm for 10 min [29]. Thereafter, the serum was separated and kept at − 80 °C until further analysis. Serum levels of MDA and Serum SOD and GPx activity were measured by commercially available enzyme-linked immunosorbent assay kits [20, 30] (Zellbio GmbH, Germany, with catalog number Cat No. ZB-MDA-96 A for MDA); (Zellbio GmbH, Germany, with Catalog number Cat No.ZB-SOD-96 A for SOD); (Zellbio GmbH, Germany, with Catalog number Cat. No: ZB-GPX -A96 for GPx). For all blood biochemistry measures, intra- CV was 5.8% and inter- CV was 7.6% [31].
Functional capacity
Walking performance was measured as both a short (Timed 25 ft Walk Test (T25FWT)) and a long (Two-Minute Walk Test (2MWT)) walking test [26]. T25FWT executed with a static start where the subjects were instructed to walk as fast as possible on a 25 foot track marked by two cones. Time to walk the distance was measured by the assessor using a handheld electronic stopwatch. The test was performed twice, and the best trial was used for further analyses [32].
2MWT as recommended by Gijbels et al. (2012). The subjects were instructed to walk as far as possible for two minutes back and forth a 30 m track marked by cones at each end. Subjects were informed when 60, 30 and 10 s remained, and there was a countdown from 3 s until the subjects were stopped and total distance was measured [32]. 5STS is a measure of the time taken to complete five repetitions of the STS movement. The test is performed using a chair without armrest and with a height of 45 cm and a depth of 41 cm. Prior to every test session, a standardized instruction was given. The assessor explained how the participant should move from a sitting (i.e. seated with full weight on the chair and arms folded across the chest) towards a standing position with both legs stretched five times. After completion of five repetitions the test ends when the participants touches the seat. 5STS was performed twice [33]. For all measures, the best trial was used for further analyses. 9-HPT requires participants to repeatedly place and then remove nine pegs into nine holes, one at a time, as quickly as possible [13].
Additionally, the MSWS-12 was completed and transformed to a 0–100 scale [26].
Patient-reported outcomes
Fatigue was assessed with the two-dimensional Fatigue Scale for Motor and Cognitive functions (FSMC). The FSMC has defined cut-off scores to differentiate between mildly (≥ 43 sum score), moderately (≥ 53 sum score) and severely (≥ 63 sum score) fatigued patients using 20 items with ten items for each dimension [25]. QOL was evaluated with the Multiple Sclerosis International Quality of Life Questionnaire (MusiQoL) by describing nine dimensions with 31 items in 9 dimensions. The index score computed as the mean of these subscale scores. All 9 dimensions and the index score were linearly transformed and standardized on a 0 to 100 scale, where 0 indicates the worst possible level of QoL and 100 indicates the best level [34].
Sample size calculation
To estimate the sample size in the present study, G*Power software [14] was used based on statistical power of 80% with a significance level of 0.5, effect size of 6.0, standard deviation (SD) 0.5 based on previous studies. A sample size of 13 achieved with an estimated drop-out of 15% we included 15 participants per group.
Resistance exercise training (RT) program
Based on the recommendations for RT for pwMS [35], the RT program included 12 weeks, 3 sessions/week, 60–80% 1RM for 60–90 min/session. The RT program consisted of three exercises for the lower extremity (leg press, lunges, and deadlift), and three exercises for the upper extremity (bench press, wide grip lat pulldown and front dumbbell raise) (Table 1 outlines RT details [36]).
Table 1.
Resistance exercise training (RT) program
| Weeks | Load (%1RM) | Rep*Sets | Rest (minutes) |
|---|---|---|---|
| 1 | 60 | 10*3 | 2 |
| 2 | 60 | 12*3 | 2 |
| 3 | 70 | 8*3 | 2 |
| 4 | 70 | 10*3 | 2 |
| 5(1st session) | 1RM Assessment | ||
| 5 (2nd and 3rd sessions) | 75 | 8*3 | 2 |
| 6 | 75 | 8*4 | 2 |
| 7 | 65 | 10*4 | 2 |
| 8 | 65 | 12*4 | 2 |
| 9 (1st session) | 1RM Assessment | ||
| 9 (2nd and 3rd sessions) | 75 | 8*4 | 2 |
| 10 | 75 | 10*4 | 2 |
| 11 | 80 | 5*4 | 3 |
| 12 | 80 | 6 *4 | 3 |
Rep: A repetition is one complete exercise movement (repetitions in each set); Set: A “set” is a group of consecutive reps; 1RM: one-repetition maximum
Statistical analysis
Distribution of data was assessed for normality using the Shapiro-Wilk test. A repeated-measures (RM) ANOVA 2 * 3 was used to evaluate the pre-post results of the three groups and to analyze the effect of time (pre–post) vs. the group (HCON, MS + non-RT, and MS + RT). In addition, Bonferroni post hoc analysis was undertaken to compare the pairs. The effect size (ES) was also calculated as the change score divided by the SD of the change score to examine the magnitude of differences while controlling for the influence of the sample size, with 0.2 considered as a small ES, 0.5 as a moderate ES and > 0.8 as a large ES. The differences were accepted as significant if P ≤ 0.05, P ≤ 0.01, or P ≤ 0.001. Data were expressed as mean ± SD.
Statistical analysis was done by SPSS software version 24 (IBM Corporation, USA).
Results
Biological indexes
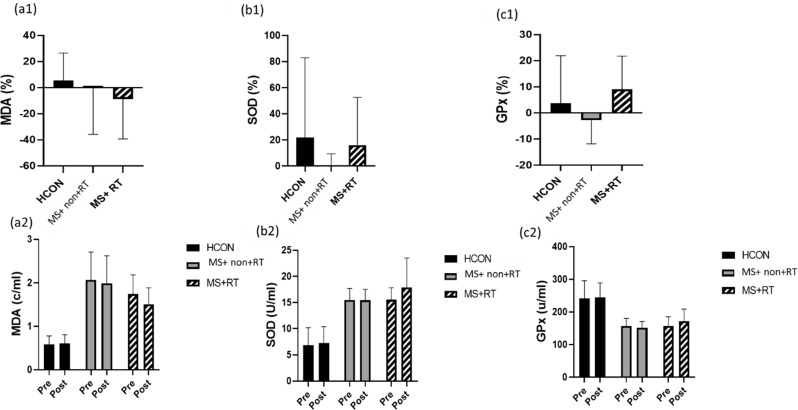
In the analysis of MDA, a 2 (Time) x 3 (Group) mixed-model ANOVA showed no significant differences over Time (F (1, 37) = 1.708, P = 0.199, Eta-Squared = 0.044). In addition, the main effect for the group was significant (F (2, 37) = 54.668, P < 0.001, Eta-squared = 0.747). Thus, there was an overall difference between the three groups. However, a non-significant Time × Group interaction effect was obtained (F (2, 37) = 0.962, p = 0.391, Eta-squared = 0.049) (Fig. 3 (a1, a 2).
Fig. 3.
percentage changes presented in: a1) MDA: Malondialdehyde content; b1) SOD: Superoxide dismutase activity; c1) GPx: Glutathione peroxidase activity, in three groups; and variables changes presented for: a2) MDA content; b2) SOD activity; c2) GPx activity from pre to post-training in three groups; HCON: Healthy control; MS + non-RT: Women with MS + non-resistance training; MS + RT: Women with MS + resistance training. *Significant difference from pre- to post-test
In addition, in the analysis of SOD, there was no main effect over Time (F (1, 37) = 2.433, P = 0.127, Eta-Squared = 0.062). Also, no significant Time × Group interaction effects were noted (F (2, 37) = 1.572, p = 0.221, Eta-squared = 0.078). However, the main effects for the group was significant (F (2, 37) = 50.385, P < 0.001, Eta-squared = 0.731). Thus, there was an overall difference between the three groups (Fig. 3 (b1, b2)).
In the analysis of GPX, there was no main effect over Time (F (1, 37) = 0.786, P = 0.381, Eta-Squared = 0.021). Also, no significant Time×Group interaction effects were noted (F (2, 37) = 1.430, P = 0.252, Eta-squared = 0.072) (Table 2; Fig. 3 (c1, c 2)). However, the main effect for group was significant (F (2, 37) = 27.990, P < 0.001, Eta-squared = 0.602). The pairwise comparison test showed that the GPX in MS + non-RT and MS + RT groups was significantly lower than the HCON group (P < 0.001) (Fig. 3 (c1, c 2)) (see Table 2, supplemental content, biological indices data from pre- to post-test).
Table 2.
The comparison of Biological indexes in in pre and post-exercise (Time effects) and between three groups (Time X Group interaction effects) (mean ± SD)
| Variables | Time | HCON (n = 15) |
MS + non-RT (n = 13) | MS + RT | Time effects p-value |
Interaction effect (time*group) P-value |
|---|---|---|---|---|---|---|
| MDA (c/ml) | Pre | 0.57 ± 0.19 | 2.06 ± 0.64 | 1.74 ± 0.43 |
P = 0.199, Eta-squared = 0.044 |
P = 0.391 Eta-squared = 0.049 |
| Post | 0.59 ± 0.20 | 1.98 ± 0.63 | 1.50 ± 0.37 | |||
| Pre-post P value | P = 0.535 | P = 0.657 | P = 0160 | |||
| SOD (U/ml) | Pre | 6.81 ± 3.37 | 15.51 ± 2.15 | 15.53 ± 2.27 |
P = 0.127, Eta-squared = 0.062 |
P = 0.221 Eta-squared = 0.078 |
| Post | 7.22 ± 3.18 | 15.44 ± 2.06 | 17.87 ± 5.60 | |||
| Pre-post P value | P = 0.630 | P = 0.850 | P = 0.156 | |||
| GPx (U/ml) | Pre | 241.39 ± 54.05 | 157.02 ± 23.59 | 157.15 ± 28.08 |
P = 0.381, Eta-squared = 0.021 |
P = 0.252 Eta-squared = 0.072 |
| Post | 244.69 ± 44.59 | 151.69 ± 19.05 | 171.39 ± 37.06 | |||
| Pre-post P value | P = 0.764 | P = 0.218 | P = 0.025 |
HCON: Health control group; MS + non-RT: Multiple Sclerosis + non-resistance training group; MS + non-RT: Multiple Sclerosis + non- resistance training group; MDA: Malondialdehyde; SOD: Superoxide dismutase; GPX: Glutathione peroxidase. *Significant difference from pre- to post-test. # Significant time effect $ Significant Time*Group interaction effect
Muscle strength, functional capacity
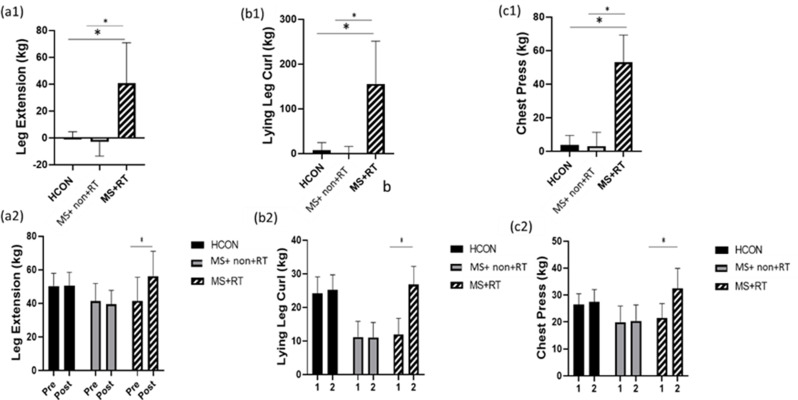
In the analysis of leg extension, data analysis revealed that the main effect over time was significant (F (1, 37) = 27.253, P < 0.001, Eta-squared = 0.424). The pairwise comparison test showed that the leg extension in the post-test significantly increased compared to pre-test values (P < 0.01) for MS + RT group (percentage increase 40/7); in addition, the main effect for the group was significant (F (2, 37) = 3.622, P < 0.05, Eta-squared = 0.164). The pairwise comparison test showed that the leg extension between MS and healthy groups were significantly different (P < 0.05). Also, Time×group interaction effects were also significant (F (2, 37) = 37.300, P < 0.001, Eta-squared = 0.668). Comparison of means indicated that, in comparison to MS + non-RT, MS + RT group had higher leg extension after resistance training compared to pre-test (Fig. 4 (a1, a 2)).
Fig. 4.
a1) leg Extension; b1) Lying leg Curl; c1) Chest Press percentage changes in three groups; and a2) leg Extension; b2) Lying leg Curl; c2) Chest Press values from pre to post-training in three groups; HCON: Healthy control; MS + non-RT: Women with MS + non-resistance training; MS + RT: Women with MS + resistance training. *Significant difference from pre- to post-test
In the analysis of lying leg curl the main effect over time was significant (F (1, 37) = 88.972, P < 0.001, Eta-squared = 0.706). The pairwise comparison test showed that, for MS + RT group, the lying leg curl in the post-test significantly increased compared to the pre-test (P < 0.001) (percentage increase 155/7);. In addition, the main effect for group was significant (F (2, 37) = 32.341, P < 0.001, Eta-squared = 0.636). The pairwise comparison test showed that the lying leg curl in MS + non-RT and MS + RT were lower than HCON group (P < 0.01). Also, Time × group interaction effects was significant (F (2, 37) = 69.102, P < 0.001, Eta-squared = 0.789). Comparison of means indicated that, in comparison to HCON and MS + non-RT, lying leg curl increased after resistance training (Fig. 4 (b1, b 2)).
In the analysis of bench press, data analysis revealed that the main effect over time was significant (F (1, 37) = 131.642, P < 0.001, Eta-squared = 0.781). The pairwise comparison test showed that, for MS + RT group, the bench press in the post-test significantly increased compared to the pre-test (P < 0.001) (percentage increase 53/02);. In addition, the main effect for the group was significant (F (2, 37) = 7.002, P < 0.01, Eta-squared = 0.275). The pairwise comparison test showed that the bench press in MS + non-RT and MS + RT were lower than HCON group (P < 0.01). Also, Time × group interaction effects were significant (F (2, 37) = 83.931, P < 0.001, Eta-squared = 0.819). Comparison of means indicated that, in comparison to HCON and MS + non-RT, bench press increased after resistance training (Fig. 4 (c1, c 2)) (see Table 3, supplemental content, muscle strength and functional capacity from pre- to post-test).
Table 3.
The comparison of muscle strength, functional capacity of lower and upper limbs in pre and post-exercise (Time effects) and between three groups (Time X Group interaction effects) (mean ± SD)
| Variables | Time | HCON (n=15) (mean±SD) |
MS+non-RT (n=13) (mean±SD) |
MS+RT (n=12) (mean±SD) |
Time effects p-value |
Interaction effect (Time x Group) P-value |
|---|---|---|---|---|---|---|
| Leg Extension (kg) | Pre | 50.28±7.59 | 41.28±10.55 | 41.49±14.07 |
#P<0.001, Eta-squared:0.424 |
$P<0.001 Eta-squared=0.668 |
| Post | 50.46±8.02 | 39.41±8.30 | 56.17±14.86 | |||
| Pre-post P value | P=0.742 | P=0.260 | *P<0.001 | |||
| Lying leg curl (kg) | Pre | 24.10±4.98 | 11.18±4.68 | 11.96±4.81 |
#P<0.001, Eta-squared:0.706 |
$P<0.001 Eta-squared=0.789 |
| Pos | 25.26±4.41 | 11.05±4.46 | 26.77±5.44 | |||
| Pre-post P value | P=0.277 | P=0.074 | *P<0.001 | |||
| Bench Press (kg) | Pre | 26.46±4.00 | 19.90±5.99 | 21.53±5.27 |
#P<0.001, Eta-squared:0.781 |
$P<0.001 Eta-squared=0.819 |
| Post | 27.49±4.56 | 20.38±5.93 | 32.52±7.38 | |||
| Pre-post P value | *P<0.05 | P=0.836 | *P<0.001 | |||
| T25FWT (m/s) | Pre | 2.05±0.28 | 1.63±0.18 | 1.57±0.14 |
#P<0.001, Eta-squared:0.307 |
$P<0.001 Eta-squared=0.456 |
| Post | 2.11±0.20 | 1.58±0.20 | 1.90±0.04 | |||
| Pre-post P value | P=0.329 | P=0.133 | *P<0.001 | |||
| 2-MWT (m/s) | Pre | 1.79±0.12 | 1.59±0.09 | 1.53±0.20 |
#P<0.001, Eta-squared:0.375 |
$P<0.001 Eta-squared=0.503 |
| Post | 1.80±0.11 | 1.58±0.09 | 1.77±0.13 | |||
| Pre-post P value | P=0.329 | P=0.133 | *P<0.001 | |||
| 5-STS (S) | Pre | 5.72±0.90 | 8.82±2.14 | 8.24±1.33 |
#P<0.001, Eta-squared:0.283 |
$P<0.001 Eta-squared=0.703 |
| Post | 5.81±0.99 | 9.51±2.39 | 5.91±0.69 | |||
| Pre-post P value | P=0.542 | *P<0.01 | *P<0.001 | |||
| 9HPT (R) (S) | Pre | 16.76±1.53 | 17.98±1.98 | 18.42±2.27 |
P=0.104, Eta-squared:0.070 |
P=0.057 Eta-squared=0.143 |
| Post | 16.43±0.92 | 18.30±2.03 | 17.23±1.86 | |||
| Pre-post P value | P=0.224 | P=0.522 | P=0.058 | |||
| 9HPT (L) (S) | Pre | 17.86±1.59 | 19.50±1.72 | 20.72±3.36 |
#P<0.001, Eta-squared:0.378 |
$P<0.01 Eta-squared=0.332 |
| Post | 17.22±1.31 | 19.37±1.91 | 18.28±2.43 | |||
| Pre-post P value | P=0.100 | P=0.730 | *P<0.001 |
HCON: Health control group; MS + non-RT: Multiple Sclerosis + non-resistance exercise training group; MS + RT: Multiple Sclerosis + resistance exercise training group; 2-MWT: Two-Minute Walk Test; 5-STS: 5-Time Sit-To-Stand Test; T25FWT: Timed 25 ft Walk Test; 9HPT (R): Nine-hole peg Test (Right hand); 9HPT (L): Nine-hole peg Test (Left hand). *Significant difference from pre- to post-test # Significant Time effect $ Significant Time*Group interaction effect
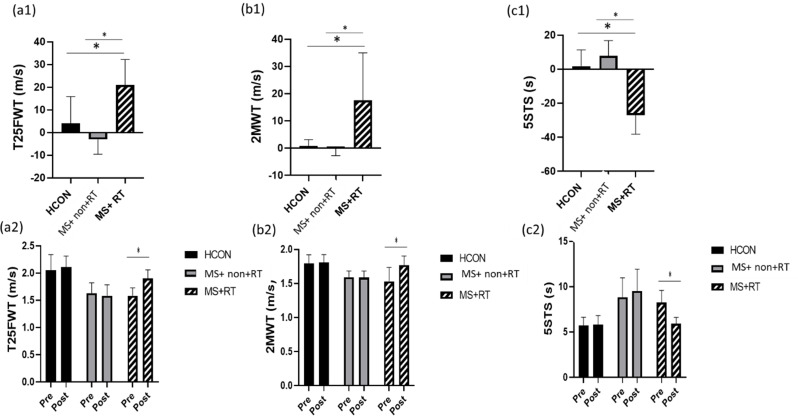
In the analysis of T25FWT, the main effect over time was significant (F (1, 37) = 16.374, P < 0.001, Eta-squared = 0.307). The pairwise comparison test showed that, for the MS + RT group, the T25FWT in the post-test significantly increased compared to the pre-test (P < 0.001) (percentage increase 21/02);. In addition, the main effect for group was significant (F (2, 37) = 24.512, P < 0.001, Eta-squared = 0.570). The pairwise comparison test showed that the T25FWT in MS + non-RT and MS + RT groups was lower than the HCON group (P < 0.001). Also, Time × group interaction effects were significant (F (2, 37) = 15.479, P < 0.001, Eta-squared = 0.456). Comparison of means indicated that, in comparison to HCON and MS + non-RT, T25FWT speed was increased after resistance training (Fig. 5 (a1, a 2)) (See Table 3, supplemental content, muscle strength and functional capacity from pre- to post-test).
Fig. 5.
a1) T25FWT: Timed 25 ft Walk Test; b1) 2-MWT: Two-Minute Walk Test; c1) 5-STS: 5-Time Sit-To-Stand Test percentage changes in three groups; and a2) T25FWT; b2) 2MWT; c2) 5STS values (m/s, m/s, s) in three groups in pre and post-training; HCON: Healthy control; MS + non-RT: Women with MS + non-resistance training; MS + RT: Women with MS + resistance training.*Significant difference from pre- to post-test
In the analysis of 2MWT, the main effect over time was significant (F (1, 37) = 22.192, P < 0.001, Eta-squared = 0.375). The pairwise comparison test showed that, for MS + RT group, the 2MWT in the post-test significantly increased compared to the pre-test (P < 0.001) (percentage increase 17/51);. In addition, the main effect for group was significant (F (2, 37) = 12.279, P < 0.001, Eta-squared = 0.399). The pairwise comparison test showed that the 2MWT in MS + non-RT and MS + RT were lower than the Healthy group (P < 0.01; P < 0.001). Also, Time × group interaction effects were significant (F (2, 37) = 18.736, P < 0.001, Eta-squared = 0.503). Comparison of means indicated that, in comparison to HCON and MS + non-RT, 2MWT increased after resistance training (Fig. 5 (b1, b2)).
In the analysis of 5-STS, the main effect over time was significant (F (1, 37) = 14.593, P < 0.001, Eta-squared = 0.283). The pairwise comparison test showed that, for MS + RT group, the 5-STS in the post-test significantly decreased compared to the pre-test (P < 0.001) (percentage increase − 27/02);, whereas in MS + non-RT there was an increase. In addition, the main effect for group was significant (F (2, 37) = 18.665, P < 0.001, Eta-squared = 0.502). The pairwise comparison test showed that the 5-STS in MS was higher than Healthy group (P < 0.01). Also, Time × group interaction effects were significant (F (2, 37) = 43.893, P < 0.001, Eta-squared = 0.703). Comparison of means indicated that, in comparison to HCON and MS + non-RT, 5-STS was decreased after resistance training (Fig. 5 (c1, c2)) (see Table 3, supplemental content, muscle strength and functional capacity from pre- to post-test).
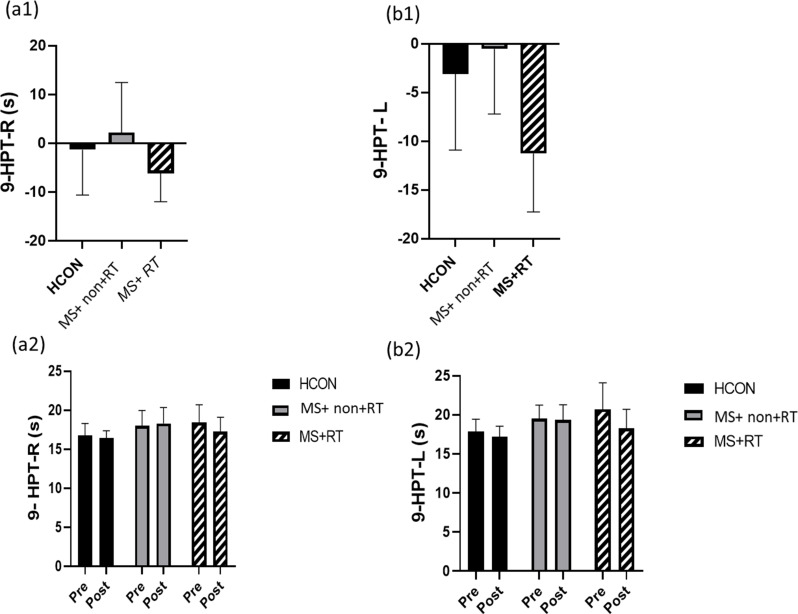
In the analysis of 9-HPT(R), there was no significant main effect over time (F (1, 37) = 2.783, P = 0.104, Eta-squared = 0.070). The main effect for group was significant (F (2, 37) = 3.588, P < 0.05, Eta-squared = 0.162). The pairwise comparison test showed that the 9HPT(R) in MS was higher than the Healthy group (P < 0.05). Also, Time × group interaction effects were non-significant (F (2, 37) = 3.096, p = 0.057, Eta-squared = 0.143) (Fig. 6 (a1, a 2)).
Fig. 6.
a1) 9-HPT (R): Nine-hole peg Test (Right hand); b1) 9-HPT (L): Nine-hole peg Test (Left hand) percentage changes in three groups; and a2) 9-HPT (R); b2) 9-HPT (L) values from pre to post-training in three groups; HCON: Healthy control; MS + non-RT: Women with MS + non-resistance training; MS + RT: Women with MS + resistance training. *Significant difference from pre- to post-test
In the analysis of 9-HPT (L), the main effect over time was significant (F (1, 37) = 22.523, P < 0.001, Eta-squared = 0.378). The pairwise comparison test showed that, for MS + RT group, the 9HPT (L) in the post-test significantly decreased compared to the pre-test (P < 0.001) (percentage increase − 11/25); In addition, the main effect for group was significant (F (2, 37) = 4.401, P < 0.05, Eta-squared = 0.192). The pairwise comparison test showed that the 9HPT (L) in MS were higher than the Healthy group (P < 0.05). Also, Time × group interaction effects were significant (F (2, 37) = 9.189, P < 0.01, Eta-squared = 0.332). Comparison of means indicated that, in comparison to HCON and MS + non- RT, 9HPT (L) was decreased after resistance training (Fig. 6 (b1, b 2)) (see Table 3, supplemental content, muscle strength and functional capacity from pre- to post-test).
Questionnaire variables
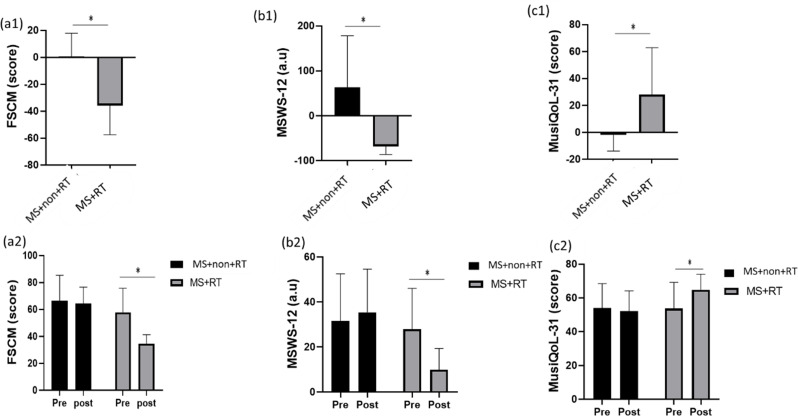
In the analysis of FSCM, there was a main effect over Time (F (1, 23) = 20.793, P < 0.001, Eta-Squared = 0.475). The pairwise comparison test showed that, for MS + RT group, the FSCM level in the post-test was significantly decreased compared to the pre-test (P < 0.01). In addition, the main effect for group was significant (F (1, 23) = 13.24, P < 0.01, Eta-squared = 0.748). Moreover, a significant Time × Group interaction effects was also obtained F (1, 23) = 14.763, P < 0.01, Eta-squared = 0.391). Comparison of means indicated that, in comparison to HCON and MS + non-RT, MS + RT group had lower FSCM levels after resistance training rather than pre-test (Fig. 7 (a1, a2)).
Fig. 7.
a1) FSCM: Fatigue Scale for Motor and Cognitive functions; b1) MSWS-12: Multiple Sclerosis Walking Scale-12; c1) MusiQoL-31: International Quality of Life Questionnaire (31 items) percentage changes in three groups; and a2) FSCM; b2) MSWS-12; c2) MusiQoL-31 scores from pre to post-training in three groups; HCON: Healthy control; MS + non-RT: Women with MS + non-resistance training; MS + RT: Women with MS + resistance training. *Significant difference from pre- to post-test
In the analysis of MSWS-12, there were significant pre-post differences over Time (F (1, 23) = 13.343, P < 0.01, Eta-Squared = 0.367). The pairwise comparison test showed that, for MS + RT group, the MSWS-12 level in the post-test significantly decreased compared to the pre-test (P < 0.01). In addition, the main effect for group was non-significant (F (1, 23) = 4.259, P = 0.51, Eta-squared = 0.156). A significant Time × Group interaction effect was also obtained F (1, 23) = 31.708, p < 0.001, Eta-squared = 0.580). Comparison of means indicated that, in comparison to MS + non-RT, MS + RT group had lower MSWS-12 levels after resistance training rather than pre-test (Fig. 7 (b1, b2)).
Moreover, in the analysis of MusiQoL-31, there was a main effect over Time (F (1, 23) = 5.656, P < 0.05, Eta-Squared = 0.197). The pairwise comparison test showed that, for MS + RT group, the MusiQoL-31was significantly increased in the post-test (P < 0.05). In addition, the main effect for group was not significant (F (1, 23) = 1.608, P = 0.217, Eta-squared = 0.065). Moreover, significant Time × Group interaction effects was also obtained (F (1, 23) = 11.269, P < 0.05, Eta-squared = 0.329) (Table 4). Comparison of means indicated that, in comparison to MS + non-RT, MS + RT group had higher MusiQoL-31 after resistance training rather than pre-test (Fig. 7 (c1, c 2)) (see Table 4, supplemental content, for Questioner variables).
Table 4.
The comparison of questioner variables in pre and post-exercise (Time effects) and between two groups (Time X Group interaction effects) (mean ± SD)
| Variables | Time | HCON (n = 15) (mean ± SD) |
MS + non-RT (n = 13) (mean ± SD) |
MS + RT (n = 12) (mean ± SD) |
Time effects p-value |
Interaction effect (Time x Group) P-value |
|---|---|---|---|---|---|---|
| FSCM (score) | Pre | ----------- | 66.30 ± 19.05 | 57.91 ± 17.78 |
#P < 0.001, Eta-squared:0.475 |
$P < 0.01 Eta-squared = 0.391 |
| Post | ----------- | 64.30 ± 12.22 | 34.50 ± 6.77 | |||
| Pre-post P value | P = 0.515 | #P < 0.01 | ||||
| MSWS-12(a.u) | Pre | ----------- | 31.57 ± 20.95 | 27.95 ± 18.8 |
#P < 0.01, Eta-squared:0.367 |
$P < 0.001 Eta-squared = 0.580 |
| Pos | ----------- | 35.41 ± 19.20 | 9.89 ± 9.40 | |||
| Pre-post P value | P = 0.142 | *P < 0.001 | ||||
| MusiQoL-31 (score) | Pre | ----------- | 54.03 ± 14.44 | 53.72 ± 15.49 |
#P < 0.05, Eta-squared:0.197 |
$P < 0.05 Eta-squared = 0.329 |
| Post | ----------- | 52.15 ± 12.00 | 64.78 ± 9.34 | |||
| Pre-post P value | P = 0.690 | *P < 0.05 |
HCON: Health control group; MS + non-RT: Multiple Sclerosis + non-resistance exercise training group; MS + RT: Multiple Sclerosis + resistance exercise training group; FSCM: Fatigue Scale for Motor and Cognitive functions; MSWS-12: Multiple Sclerosis Walking Scale-12; MusiQoL-31: International Quality of Life Questionnaire (31 items). *Significant difference from pre- to post-test. # Significant time effect $ Significant Time*Group interaction effect
Discussion
The main purpose of the current study was to investigate the effects of 12 weeks of resistance exercise training (RT) on oxidative status (MDA, SOD, GPx), muscle strength, functional capacity, quality of life (QoL) and fatigue in MS. The main findings of the study were improvements in functional capacity, muscle strength, QoL, and fatigue in MS + RT compared to the MS + non-RT and HCON groups. In contrast, no significant change was also obtained for serum MDA and both SOD and GPx activity over 12 weeks.
Our results showed that baseline MDA serum levels, an important lipid peroxidation marker, were higher in the MS + non- and MS + RT groups than in the HCON group and this agrees with the results of Ghonimi et al. [37] and Juybari et al. [7] in RRMS patients in comparison with control group subjects. These findings indicate peripheral oxidative stress in RR-MS patients and support the role of oxidative stress in MS pathogenesis. Several studies have reported that oxidative stress may be involved in inflammation, demyelination, and axonal injury occurring in MS [38]. ROS as mediators of oxidative damage cause initiation of a process called lipid peroxidation (LPO) which results in destruction of lipid-rich areas such as cell membranes or myelin sheaths and the generation of highly reactive aldehydes such as MDA [37, 38]. In MS, MDA is present in active inflammatory lesions as well as neurons undergoing axonal degeneration, in keeping with pathogenicity [38]. In a rat EAE model, inhibition of MDA production was shown to ameliorate neurological deficits, supporting a pathogenic role in MS [38]. MDA can cause cross-linking polymerization of proteins, nucleic acids, and other life macromolecules, leading to cytotoxicity [5].
However, we did not detect any significant decrease following 12 weeks of RT program in the MDA serum level as an oxidant marker. Data from a meta-analysis confirmed a significant decrease in pro-oxidant parameters and an increase in antioxidant capacity as a result of physical exercise [22]. Also, four weeks of voluntary exercise reduces oxidative stress and disease severity during the chronic period of the disease in mice with EAE [39]. Souza et al. have noted reduced levels of lipid peroxidation and protein oxidation in the EAE group with physical training [18]. Furthermore, Bloomer et al. assessed the effects of RT on oxidative damage in persons with Parkinson disease (PD) and have noted reduced levels of MDA and H2O2 [16].
Data show that both strength and endurance training protocols consistently prevented clinical signs of EAE and decreased oxidative stress, an effect which was likely due to improving genomic antioxidant defense—nuclear factor erythroid 2-related factor (Nrf2)/antioxidant response elements (ARE) pathway—in the CNS [18].
Furthermore, clinical studies have reported increased oxidative stress caused by dysregulated antioxidant enzymes such as SOD and GPx in the blood of MS patients [1]. We noted that the activity of SOD and GPx was higher and lower, respectively, in MS patients compared to healthy subjects. Similar findings are reported in other studies [1, 40, 41]. However, a further study showed the opposite results between MS patients and controls for SOD activity [42]. Since SODs are part of a physiological response to oxidative stress, suppressing their activity increases tissue damage and their overexpression reduces damage by reducing disability in the EAE mouse model [38]. It is well known that MS sufferers undergo unpredictable and debilitating neurological symptoms [1, 2]. Thus, treatment with antioxidants might prevent propagation of tissue damage and improve both survival and neurological outcome [43]. There is evidence that one of the adaptations resulting from exercise is a strengthening of the body’s antioxidant defenses, particularly the glutathione system, to regulate increased oxidative stress.
However, in our study, the mean increases in SOD and GPx activity noted in the MS + RT group were not statistically significant in MS. Consistent with our study, Bloomer et al. reported no significant changes in GPx and SOD activity in subjects with PD as a result of 8 weeks of RT [16]. However, Souza et al. found significantly higher changes in the activity of GPx but not SOD in the exercise group than in the EAE [18]. Increased SOD activity, a major enzyme in ROS detoxification, leads to an increase of hydrogen peroxide levels, which is further eliminated by CAT. The activity of both enzymes is controlled by the nuclear factor erythroid 2-related factor 2 (Nrf2) regulatory pathway which plays an essential role in cell and tissue protection from oxidative stress [44]. The beneficial influence of exercise on oxidative stress parameters likely depends on the mode, intensity, duration of the exercise and mechanical stress imposed on the tissues [45]. Although, in our study, the mean changes of oxidative parameters were not significant due to RT program, but considering the clear role of oxidative stress in the pathogenesis and progression of MS, any increase in antioxidant defenses and reduction in pro-oxidant (to improve cerebral mitochondrial functions) may be considered positive. Such an effect over several years may have important clinical relevance for pwMS.
One of the most critical symptoms in pwMS is muscle weakness and impaired mobility [17, 46]. Also, the existence of a relationship between muscle strength and performance in daily activities such as walking has been confirmed in these people [17]. For example, lower body muscle strength is a well-established predictor of walking speed in pwMS [46]. In our study, after 12 weeks of RT, muscle strength (in leg extension, bench press, lying leg curl movements) and functional capacity (2MWT test, T25FWT test, 5STS test, MSWS-12) were significantly improved in the MS + RT group compared to the MS + non-RT MS and HCON. Increases in muscle strength, due to RT, might lead to more permanent changes in daily physical activities that might lead to the maintenance of strength and other beneficial outcomes [46]. In a study, high volume progressive- RT (24 weeks) efficiently improves functional capacity with concomitant neuromuscular adaptations in mild to moderately impaired in adults with RR-MS and also improved MSWS-12 [17]. Another RCT study reported significant effects of a 12-week supervised RT program on muscle function and functional outcomes including walking [47]. Contrary to our results, other study have not consistently suggested improvements in functional capacity [46] after progressive RT. It is possible that the PRT progression model used in the previous randomized controlled trial which applied training intensities of 10–12 RM (higher training intensities of up to 8RM), and less training volumes of two sets of each exercise (compared with our four sets) over a slightly shorter duration (10 weeks versus our 12 weeks) than our program could perhaps explain the different findings.
While the clinical and functional impairment of the lower limbs in people with MS has been extensively studied, the upper limbs are also commonly affected [4]. Impaired manual dexterity is a frequently reported disability in pwMS and is increasingly prevalent with worsening disease [13]. Our observations demonstrated that the 9HPT(R) and 9HPT (L) time was higher in MS in the MS + non-RT and MS + RT groups than in the HCON group. Studies have suggested that the 9-HPT is discriminates between healthy subjects and pwMS [13]. A combination of predominantly motor and sensory symptoms causes upper limb disability, which hampers the ability to perform activities of daily living resulting in decreased independence and QoL [13]. Therefore, management strategies for this disorder are important. According to our results, after 12 weeks of RT, 9HPT (L) time decreased in MS + RT compared to HCON and MS + non-RT, while no changes were observed in the 9HPT(R) time as a result of RT. Hoang et al. suggested that step training for 12 weeks led to a significant improvement in the 9-HPT test in pwMS [16]. A study in patients with PD reported a significant improvement in the 9HPT test following low resistance interval cycling [48]. Due to the deficit of studies, further research is needed on the effects of exercise and impaired manual dexterity.
According to the study by Severijins et al. [49], pwMS who scored less than 6 on the EDSS had lower maximum grip strength and increased fatigue during static contractions compared with healthy people. Fatigue is one of the most common and disabling symptoms in pwMS, which is associated with an increase in cognitive impairments and has a negative impact on the individual’s collaborative roles and the quality of life of patients [46, 50]. Fatigue is a subjective sensation of exhausted physical and/or mental energy reserves [51]. Both central and peripheral mechanisms have been proposed as factors involved in fatigue [52]. Also, previous neuroimaging research has reported the caudate nucleus, one of the brain structures that make up the basal ganglia, to be largely implicated in MS-related fatigue [50]. Soluble inflammatory mediators such as pro-inflammatory cytokines like tumor necrosis factor (TNF) and Interleukin-6 (IL-6) have also been proposed to be involved in the pathophysiology of MS fatigue [52]. Progressive RT has been reported as a promising strategy to reduce fatigue in pwMS [46, 50]. The mechanism by which this reduction occurs could be the result of functional changes involving the caudate [50]. Our results showed that 12 weeks of resistance training decreased self-reported fatigue (FSMC) in women with MS. This results are consistent with previous studies that high-intensity RT (HIRT) over 12-weeks can reduce self-reported fatigue (FSMC) in fatigued MS [52]. In addition, Kierkegaard et al. reported that HIRT (twice a week for 12 weeks) in RRMS with low disability reduced TNF levels and led to clinically relevant improvements in measures of fatigue and health-related QoL [53]. Therefore, progressive RT can induce physiological and psychological changes that may counter the mechanisms of fatigue and reduce it in pwMS [54]. However, there are Insufficient Exploration of Fatigue and its role warrants further investigation.
This wide range of symptoms and disease progression patterns, with a significant potential impact on the quality of life of individuals [4]. Several authors have suggested that impairment of muscle strength and manual dexterity is directly related to activities of daily living, which are closely associated with independence and quality of life in pw MS [4, 55]. While pwMS often report lower QoL than healthy individuals, improving QoL of the MS population has become an important goal of researchers [56]. Cross-sectional studies suggested regular physical activity for the solution of problems associated with inactivity and for improvements in QoL [56]. In accordance with these studies, we have determined that after 12 weeks of RT, QoL improved in the MS + RT group compared to MS-non-RT. Also, Ertekin et al. observed a significant improvement in QoL and fatigue of pwMS following 12 weeks of home-based exercise training [56]. Two studies reported an increase in QoL because of progressive RT exercises over 10 and 12 weeks [47, 57]. Also, another study observed positive effects of yoga and clinical Pilates training in improving walking speed, and QoL in these patients [57]. Considering that increasing the level of physical fitness of MS patients helps to achieve lower fatigue levels, higher activity levels, increased functional ability and self-confidence, improving QoL of these patients with RT is a positive outcome [57]. Therefore, physical exercise structured according to the physical disabilities of patients can be a very useful strategy to increase strength, reduce distal upper limb dysfunction, reduce fatigue and ultimately functional autonomy and improve the QoL in patients [58].
Study limitations and strengths
The main limitation of the study is its relatively small sample size and relatively short period. We had to form small groups due to the strict inclusion and exclusion criteria and the spread of the corona virus. Another limitation was the non-controlled diet, which could have influenced oxidative Stress status. One of the strengths of the study was that resistance training supervised for safety by an experienced staff. It has been shown that supervised is more effective than no supervised resistance training. Also, compared to the endurance exercise, resistance training in heat sensitive patients less frequently cause symptom exacerbations due to increased body temperature.
Conclusion
We showed a modest change in measured biological variables in the MS resistance training group, however, these changes were not statistically significant, likely due to small sample size. However, considering the clear role of oxidative stress in the pathogenesis and progression of MS, any increase in antioxidant defenses and reduction in pro-oxidant (to improve cerebral mitochondrial functions) may be considered positive. In addation, a moderate-intensity resistance training program according to the physical disabilities of pwMS can be a potential measure to increase strength, reduce distal upper limb dysfunction, reduce fatigue and ultimately improve the quality of life of pwMS. Future research with larger samples and different types of exercise needs to consider exercise intensity, duration and effects on oxidative stress parameters MS patients. In conclusion, these data provide evidence that resistance exercise has the potential to improve oxidative status in pwMS and led to clinically important changes to the better in measures of muscle strength, functional capacity, fatigue and quality of life.
Acknowledgements
The authors wish to thank all MS patients for their enthusiastic participation in this investigation.
Abbreviations
- 2MWT
Two-Minute Walk Test
- 1RM
one-repetition maximum
- 5RM
Five-repetition maximum
- 5STS
5-Time Sit-To-Stand Test
- 9-HPT
Nine-hole peg test
- BBB
Blood brain barrier
- BMI
body mass index
- CAT
catalase
- CNS
Central Nerve System
- EAE
Experimental autoimmune encephalomyelitis
- EDSS
Expanded Disability Status Scale
- FSMC
Fatigue Scale for Motor and Cognitive functions questionnaire
- GPx
Glutathione peroxidase
- HCON
Healthy control group
- HIRT
High-intensity resistance training
- IL-6
Interleukin-6
- MDA
Malondialdehyde
- MusiQoL
Multiple Sclerosis International Quality of Life Questionnaire
- MS
Multiple sclerosis
- MSWS-12
Twelve Item MS Walking Scale
- Nrf2
nuclear factor erythroid 2-related factor 2
- PD
Parkinson’s disease
- pwMS
patient with MS
- QoL
Quality of Life
- RONS
Reactive oxygen and nitrogen species
- RRMS
Patients with relapsing-remitting MS
- RT
Resistance exercise training
- ST
strength training
- SOD
Superoxide dismutases
- ROS
reactive oxygen species
- T25FWT
Timed 25 ft Walk Test
- TNF
Tumor necrosis factor
- WHR
waist-to-hip ratio
- MS
Women with MS
- MS + non-RT
Women with MS + non-resistance training
- MS + RT
Women with MS + resistance training
Author contributions
N. Niazi Nezhad involved in conceptualization, methodology and study design, formal analysis, data curation and writing original draft; AH. Parnow supervised the research project conceptualization, methodology and study design, validation, formal analysis, data curation, and writing-review and editing; K. Khamoushian involved in validation, data curation, writing-review and editing; R. Eslami contributed in formal analysis, data curation, software, and writing-review and editing; and J. S. Baker surprised and contributed in writing-review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Ethics in Research Working Group at the Sports Sciences Research Institute (ethical code: SSRI.REC-1402-101; IRCT registration code: IRCT20120912010824N3, 07.09.2023), Iran. “Informed” consent was obtained from all participants. The full trial was conducted from July, 2021 to February, 2022.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tasset I, Agüera E, Sánchez-López F, Feijóo M, Giraldo AI, Cruz AH, et al. Peripheral oxidative stress in relapsing–remitting multiple sclerosis. Clin Biochem. 2012;45(6):440–4. [DOI] [PubMed] [Google Scholar]
- 2.Pegoretti V, Swanson KA, Bethea JR, Probert L, Eisel UL, Fischer R. Inflammation and oxidative stress in multiple sclerosis: consequences for therapy development. Oxidative medicine and cellular longevity. 2020;2020. [DOI] [PMC free article] [PubMed]
- 3.Naseri A, Forghani N, Sadigh-Eteghad S, Shanehbandi D, Asadi M, Nasiri E, et al. Circulatory antioxidant and oxidative stress markers are in correlation with demographics but not cognitive functions in multiple sclerosis patients. Multiple Scler Relat Disorders. 2022;57:103432. [DOI] [PubMed] [Google Scholar]
- 4.Blázquez-Fernández A, López-Hazas-Jiménez G, Fernández-Vázquez D, Navarro-López V, Fernández-González P, Marcos-Antón S, et al. Effects of the powerball® system on muscle strength, coordination, fatigue, functionality and quality of life in people with multiple sclerosis. A randomized clinical trial. J Neuroeng Rehabil. 2024;21(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S-Y, Gui L-N, Liu Y-Y, Shi S, Cheng Y. Oxidative stress marker aberrations in multiple sclerosis: a meta-analysis study. Front NeuroSci. 2020;14:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira B, Mendes F, Osorio N, Caseiro A, Gabriel A, Valado A. Glutathione in multiple sclerosis. Br J Biomed Sci. 2013;70(2):75–9. [DOI] [PubMed] [Google Scholar]
- 7.Juybari KB, Ebrahimi G, Moghaddam MAM, Asadikaram G, Torkzadeh-Mahani M, Akbari M, et al. Evaluation of serum arsenic and its effects on antioxidant alterations in relapsing-remitting multiple sclerosis patients. Multiple Scler Relat Disorders. 2018;19:79–84. [DOI] [PubMed] [Google Scholar]
- 8.Song K, Li Y, Zhang H, An N, Wei Y, Wang L et al. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Medicine and Cellular Longevity. 2020;2020.
- 9.Aydin O, Ellidag HY, Eren E, Kurtulus F, Yaman A, Yılmaz N. Ischemia modified albumin is an indicator of oxidative stress in multiple sclerosis. Biochemia Med. 2014;24(3):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizwan S, ReddySekhar P, MalikAsrar B. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling; 2014. [DOI] [PMC free article] [PubMed]
- 11.Guy J, Ellis EA, Hope G, Rao NA. Antioxidant enzymes reduce loss of blood-brain barrier integrity in experimental optic neuritis. Arch Ophthalmol. 1989;107(9):1359–63. [DOI] [PubMed] [Google Scholar]
- 12.Piñar-Morales R, Duran-Ogalla R, Bautista A, Garcia MJ, Aliaga-Gaspar P, Vives-Montero F et al. Oxidative Stress and Symptoms Associated with Multiple Sclerosis. 2024.
- 13.Feys P, Lamers I, Francis G, Benedict R, Phillips G, LaRocca N, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Multiple Scler J. 2017;23(5):711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil-González I, Martín-Rodríguez A, Conrad R, Pérez-San-Gregorio MÁ. Quality of life in adults with multiple sclerosis: a systematic review. BMJ open. 2020;10(11):e041249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gümüş H. Fatigue can be objectively measured in multiple sclerosis: multipl sklerozda yorgunluk objektif olarak ölçülebilir. Archives Neuropsychiatry. 2018;55(Suppl 1):S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang P, Schoene D, Gandevia S, Smith S, Lord SR. Effects of a home-based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis–a randomized controlled trial. Multiple Scler J. 2016;22(1):94–103. [DOI] [PubMed] [Google Scholar]
- 17.Kjølhede T, Vissing K, de Place L, Pedersen BG, Ringgaard S, Stenager E, et al. Neuromuscular adaptations to long-term progressive resistance training translates to improved functional capacity for people with multiple sclerosis and is maintained at follow-up. Multiple Scler J. 2015;21(5):599–611. [DOI] [PubMed] [Google Scholar]
- 18.Souza PS, Gonçalves ED, Pedroso GS, Farias HR, Junqueira SC, Marcon R, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol Neurobiol. 2017;54:4723–37. [DOI] [PubMed] [Google Scholar]
- 19.Schreibelt G, Musters RJ, Reijerkerk A, de Groot LR, van der Pol S, Hendrikx EM, et al. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. 2006;177(4):2630–7. [DOI] [PubMed] [Google Scholar]
- 20.Bloomer RJ, Schilling BK, Karlage RE, Ledoux MS, Pfeiffer RF, Callegari J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc. 2008;40(8):1385–9. [DOI] [PubMed] [Google Scholar]
- 21.Azizbeigi K, Azarbayjani MA, Peeri M, Agha-Alinejad H, Stannard S. The effect of progressive resistance training on oxidative stress and antioxidant enzyme activity in erythrocytes in untrained men. Int J Sport Nutr Exerc Metab. 2013;23(3):230–8. [DOI] [PubMed] [Google Scholar]
- 22.de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG. The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med. 2017;47(2):277–93. [DOI] [PubMed] [Google Scholar]
- 23.Nezhad NN, Parnow A, Khamoushian K, Eslami R, Baker JS. Resistance training modifies of serum levels of matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinases in multiple sclerosis women-a randomized controlled trail. BMC Neurosci. 2024;25(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simeoni M, Auquier P, Fernandez O, Flachenecker P, Stecchi S, Constantinescu C, et al. Validation of the multiple sclerosis international quality of life questionnaire. Multiple Scler J. 2008;14(2):219–30. [DOI] [PubMed] [Google Scholar]
- 25.Penner I-K, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and cognitive functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Multiple Scler J. 2009;15(12):1509–17. [DOI] [PubMed] [Google Scholar]
- 26.Gijbels D, Dalgas U, Romberg A, de Groot V, Bethoux F, Vaney C, et al. Which walking capacity tests to use in multiple sclerosis? A multicentre study providing the basis for a core set. Multiple Scler J. 2012;18(3):364–71. [DOI] [PubMed] [Google Scholar]
- 27.Pescatello L, Arena R, Riebe D, Thompson P. ACSM’s Guidelines for exercise testing and prescription. 9th ed. Wolters Kluwer. Lippincott Williams & Wilkins: American College of Sports Medicine;; 2013. [DOI] [PubMed]
- 28.Lander J. Maximum based on reps. NSCA J. 1985;6(6):60–1. [Google Scholar]
- 29.Zimmer P, Bloch W, Schenk A, Oberste M, Riedel S, Kool J, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: a randomized controlled trial. Multiple Scler J. 2018;24(12):1635–44. [DOI] [PubMed] [Google Scholar]
- 30.Acar A, Ugur Cevik M, Evliyaoglu O, Uzar E, Tamam Y, Arıkanoglu A, et al. Evaluation of serum oxidant/antioxidant balance in multiple sclerosis. Acta Neurol Belgica. 2012;112:275–80. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Free radicals in diagnostic medicine: a systems approach to laboratory technology, clinical correlations, and antioxidant therapy. 1994:43–58. [DOI] [PubMed]
- 32.Kjølhede T, Vissing K, Langeskov-Christensen D, Stenager E, Petersen T, Dalgas U. Relationship between muscle strength parameters and functional capacity in persons with mild to moderate degree multiple sclerosis. Multiple Scler Relat Disorders. 2015;4(2):151–8. [DOI] [PubMed] [Google Scholar]
- 33.Møller AB, Bibby BM, Skjerbæk AG, Jensen E, Sørensen H, Stenager E, et al. Validity and variability of the 5-repetition sit-to-stand test in patients with multiple sclerosis. Disabil Rehabil. 2012;34(26):2251–8. [DOI] [PubMed] [Google Scholar]
- 34.TUNCAY F, KAYGISIZ F, BORMAN P, KURT EE. ERGÜN U. Quality of life in patients with multiple sclerosis: relationship with clinical variables. J Phys Med Rehabilitation Sci. 2017;20(1).
- 35.Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017;17(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gravesteijn A, Beckerman H, De Jong B, Hulst H, De Groot V. Neuroprotective effects of exercise in people with progressive multiple sclerosis (Exercise PRO-MS): study protocol of a phase II trial. BMC Neurol. 2020;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghonimi NA, Elsharkawi KA, Khyal DS, Abdelghani AA. Serum malondialdehyde as a lipid peroxidation marker in multiple sclerosis patients and its relation to disease characteristics. Multiple Scler Relat Disorders. 2021;51:102941. [DOI] [PubMed] [Google Scholar]
- 38.Ibitoye R, Kemp K, Rice C, Hares K, Scolding N, Wilkins A. Oxidative stress-related biomarkers in multiple sclerosis: a review. Biomark Med. 2016;10(8):889–902. [DOI] [PubMed] [Google Scholar]
- 39.Quchan AHSK, Kordi MR, Shabkhiz F. Voluntary Exercise reduces oxidative stress and Disease Severity in mice with multiple sclerosis, 2022.
- 40.Obradovic D, Andjelic T, Ninkovic M, Dejanovic B, Kotur-Stevuljevic J. Superoxide dismutase (SOD), advanced oxidation protein products (AOPP), and disease-modifying treatment are related to better relapse recovery after corticosteroid treatment in multiple sclerosis. Neurol Sci. 2021;42:3241–7. [DOI] [PubMed] [Google Scholar]
- 41.Jensen GE, Gissel-Nielsen G, Clausen J. Leucocyte glutathione peroxidase activity and selenium level in multiple sclerosis. J Neurol Sci. 1980;48(1):61–7. [DOI] [PubMed] [Google Scholar]
- 42.De Riccardis L, Buccolieri A, Muci M, Pitotti E, De Robertis F, Trianni G, et al. Copper and ceruloplasmin dyshomeostasis in serum and cerebrospinal fluid of multiple sclerosis subjects. Biochim et Biophys Acta (BBA)-Molecular Basis Disease. 2018;1864(5):1828–38. [DOI] [PubMed] [Google Scholar]
- 43.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251(3):261–8. [DOI] [PubMed] [Google Scholar]
- 44.Obradovic D, Andjelic T, Ninkovic M, Dejanovic B, Kotur-Stevuljevic J. Superoxide dismutase (SOD), advanced oxidation protein products (AOPP), and disease-modifying treatment are related to better relapse recovery after corticosteroid treatment in multiple sclerosis. Neurol Sci. 2021;42(8):3241–7. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira VNd, Bessa A, Jorge MLMP, Oliveira RJS, de Mello MT, De Agostini GG et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Applied Physiology, Nutrition, and Metabolism. 2012;37(2):334 – 44. [DOI] [PubMed]
- 46.Dodd KJ, Taylor N, Shields N, Prasad D, McDonald E, Gillon A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Multiple Scler J. 2011;17(11):1362–74. [DOI] [PubMed] [Google Scholar]
- 47.Dalgas U, Stenager E, Jakobsen J, Petersen T, Hansen H, Knudsen C, et al. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology. 2009;73(18):1478–84. [DOI] [PubMed] [Google Scholar]
- 48.Uygur M, Bellumori M, Knight CA. Effects of a low-resistance, interval bicycling intervention in Parkinson’s Disease. Physiother Theory Pract. 2017;33(12):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Severijns D, Lamers I, Kerkhofs L, Feys P. Hand grip fatigability in persons with multiple sclerosis according to hand dominance and disease progression. J Rehabil Med. 2015;47(2):154–60. [DOI] [PubMed] [Google Scholar]
- 50.Akbar N, Sandroff BM, Wylie GR, Strober LB, Smith A, Goverover Y, et al. Progressive resistance exercise training and changes in resting-state functional connectivity of the caudate in persons with multiple sclerosis and severe fatigue: a proof-of-concept study. Neuropsychological Rehabilitation. 2020;30(1):54–66. [DOI] [PubMed] [Google Scholar]
- 51.Sander C, Voelter H-U, Schlake H-P, Eling P, Hildebrandt H. Assessment of fatigue in multiple sclerosis. Neurol Int Open. 2017;1(02):E79–85. [Google Scholar]
- 52.Englund S, Piehl F, Kierkegaard M. High-intensity resistance training in people with multiple sclerosis experiencing fatigue: a randomised controlled trial. Multiple Scler Relat Disorders. 2022;68:104106. [DOI] [PubMed] [Google Scholar]
- 53.Kierkegaard M, Lundberg IE, Olsson T, Johansson S, Ygberg S, Opava C, et al. High-intensity resistance training in multiple sclerosis—An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J Neurol Sci. 2016;362:251–7. [DOI] [PubMed] [Google Scholar]
- 54.Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Reviews. 2015;(9). [DOI] [PMC free article] [PubMed]
- 55.Bertoni R, Lamers I, Chen CC, Feys P, Cattaneo D. Unilateral and bilateral upper limb dysfunction at body functions, activity and participation levels in people with multiple sclerosis. Multiple Scler J. 2015;21(12):1566–74. [DOI] [PubMed] [Google Scholar]
- 56.Ertekin Ö, Özakbaş S, İdiman E, Algün ZC. Quality of life, fatigue and balance improvements after home-based exercise program in multiple sclerosis patients. Archives Neupopsychiatry. 2012.
- 57.Abasıyanık Z, Yiğit P, Özdoğar AT, Kahraman T, Ertekin Ö, Özakbaş S. A comparative study of the effects of yoga and clinical pilates training on walking, cognition, respiratory functions, and quality of life in persons with multiple sclerosis: a quasi-experimental study. EXPLORE. 2021;17(5):424–9. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez-Lastra MA, Martínez-Aldao D, Molina AJ, Ayán C. Pilates for people with multiple sclerosis: a systematic review and meta-analysis. Multiple Scler Relat Disorders. 2019;28:199–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.