Abstract
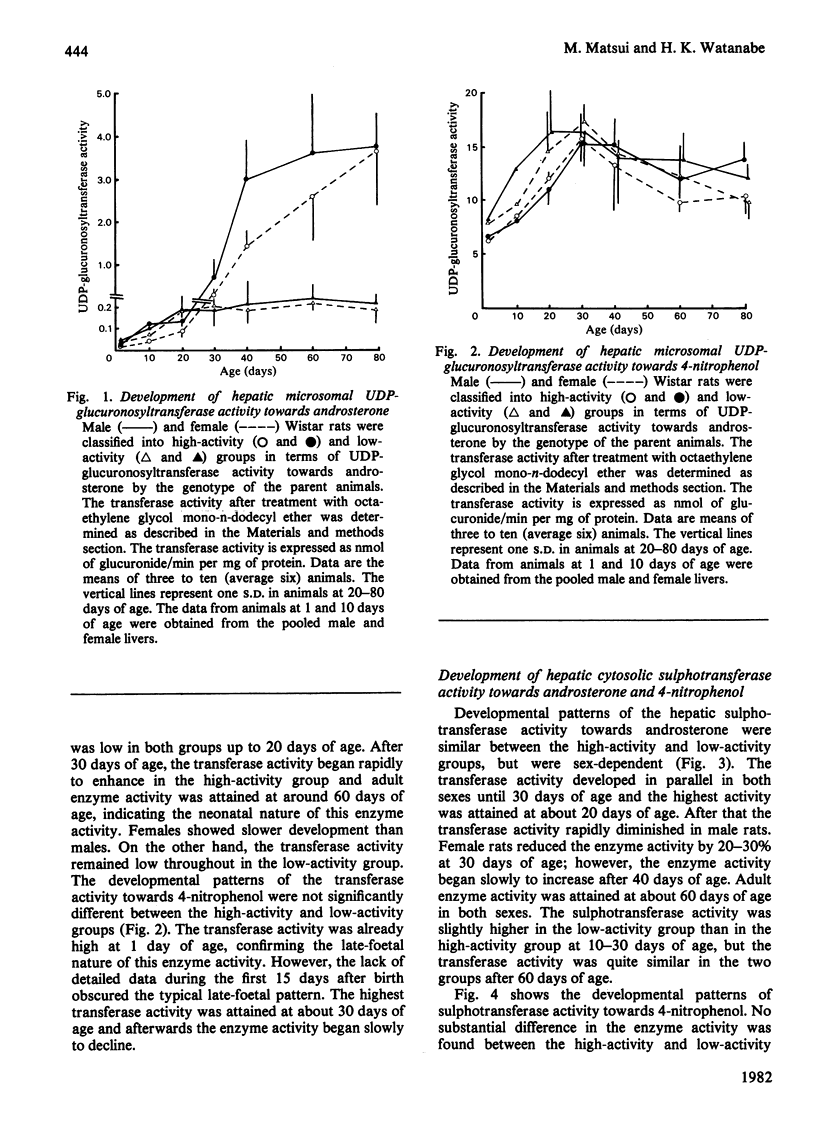
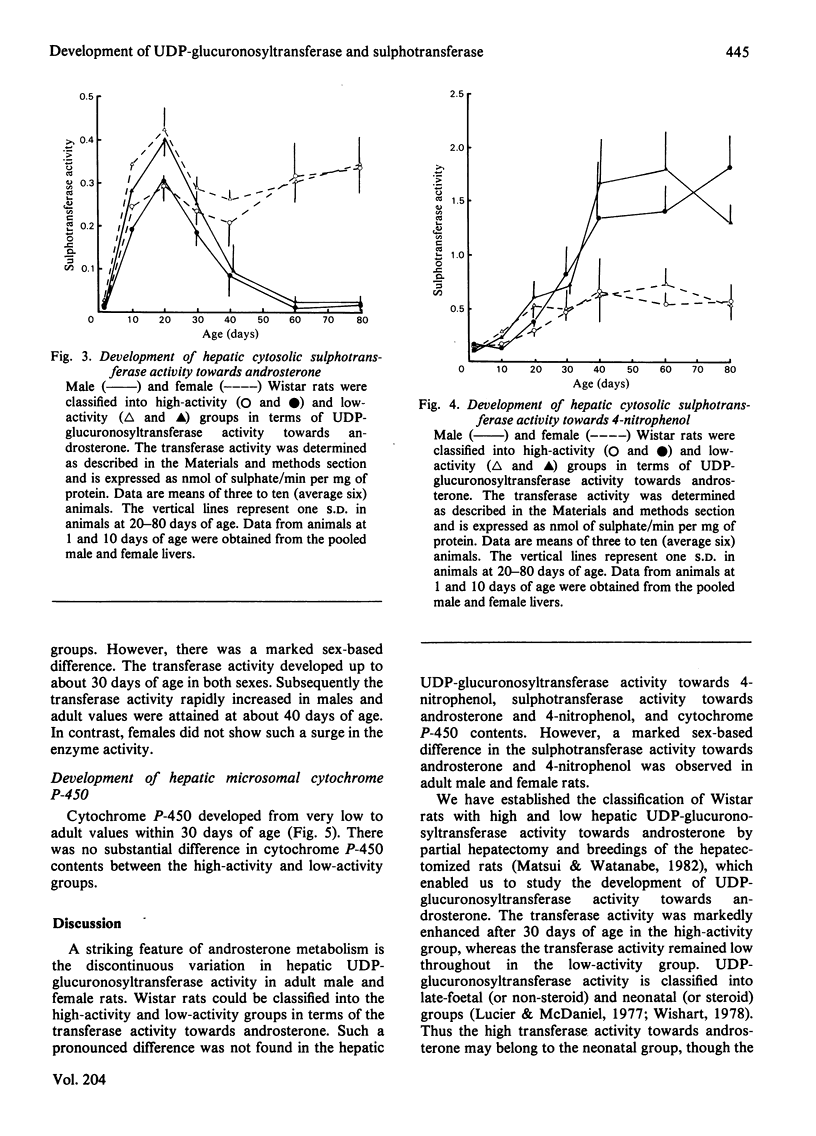
Postnatal development of hepatic UDP-glucuronosyltransferase and sulphotransferase activities towards androsterone and 4-nitrophenol as well as cytochrome P-450 contents was studied in male and female Wistar rats. The rats with high and low UDP-glucuronosyltransferase activity towards androsterone were classified by the genotype of the parent animals. UDP-glucuronosyltransferase activity towards androsterone began rapidly to enhance after 30 days of age in the high-activity group, whereas the transferase activity remained low throughout in the low-activity group. Such a striking difference was not observed in UDP-glucuronosyltransferase activity towards 4-nitrophenol, sulphotransferase activity towards androsterone and 4-nitrophenol, and cytochrome P-450 contents. Sex-based difference in the sulphotransferase activity was marked after 30 days of age. Sulphotransferase activity towards androsterone was much higher in adult females than in adult males, whereas higher sulphation activity towards 4-nitrophenol was found in adult males. The results also indicate that the low level of the UDP-glucuronosyltransferase activity did not lead to compensatory stimulation of the sulphotransferase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- Fuchs M., Rao G. S., Rao M. L., Breuer H. Studies on the properties of an enzyme forming the glucuronide of pregnanediol and the pattern of development of steroid glucuronyltransferases in rat liver. J Steroid Biochem. 1977 Mar;8(3):235–241. doi: 10.1016/0022-4731(77)90057-7. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., McDaniel O. S. Steroid and non-steroid UDP glucuronyltransferase: glucuronidation of synthetic estrogens as steroids. J Steroid Biochem. 1977 Aug;8(8):867–872. doi: 10.1016/0022-4731(77)90096-6. [DOI] [PubMed] [Google Scholar]
- Matsui M., Aoyagi S. Variability of androsterone metabolism in male Wistar rats. Biochem Pharmacol. 1979 Apr 1;28(7):1023–1028. doi: 10.1016/0006-2952(79)90298-3. [DOI] [PubMed] [Google Scholar]
- Matsui M., Hakozaki M. Discontinuous variation in hepatic uridine diphosphate glucuronyltransferase toward androsterone in Wistar rats. A regulatory factor for in vivo metabolism of androsterone. Biochem Pharmacol. 1979;28(3):411–415. doi: 10.1016/0006-2952(79)90107-2. [DOI] [PubMed] [Google Scholar]
- Matsui M., Hakozaki M. Variations in biliary metabolites of androsterone in female rats. J Steroid Biochem. 1977 Apr;8(4):319–322. doi: 10.1016/0022-4731(77)90027-9. [DOI] [PubMed] [Google Scholar]
- Matsui M., Nagai F., Aoyagi S. Strain differences in rat liver (UDP-glucuronyltransferase activity towards androsterone. Biochem J. 1979 Jun 1;179(3):483–487. doi: 10.1042/bj1790483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Watanabe H. K. Classification and genetic expression of Wistar rats with high and low hepatic microsomal UDP-glucuronosyltransferase activity towards androsterone. Biochem J. 1982 Jan 15;202(1):171–174. doi: 10.1042/bj2020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Sekura R. D., Jakoby W. B. Aryl sulfotransferase IV from rat liver. Arch Biochem Biophys. 1981 Oct 1;211(1):352–359. doi: 10.1016/0003-9861(81)90464-1. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Jakoby W. B. Phenol sulfotransferases. J Biol Chem. 1979 Jul 10;254(13):5658–5663. [PubMed] [Google Scholar]
- Singer S. S. Enzymatic sulfation of steroids. IV. Control of the hepatic glucocorticoid sulfotransferase activity and the individual glucocorticoid sulfotransferases from male and female rats by adrenal glands and corticosteroids. Endocrinology. 1978 Jul;103(1):66–73. doi: 10.1210/endo-103-1-66. [DOI] [PubMed] [Google Scholar]
- Singer S. S. Enzymatic sulfation of steroids. VI. A simple, rapid method for routine enzymatic preparation of 3'-phosphoadenosine-5'-phosphosulfate. Anal Biochem. 1979 Jul 1;96(1):34–38. doi: 10.1016/0003-2697(79)90550-5. [DOI] [PubMed] [Google Scholar]
- Singer S. S., Giera D., Johnson J., Sylvester S. Enzymatic sulfation of steroids: I. The enzymatic basis for the sex difference in cortisol sulfation by rat liver preparations. Endocrinology. 1976 Apr;98(4):963–974. doi: 10.1210/endo-98-4-963. [DOI] [PubMed] [Google Scholar]
- Singer S. S., Sylvester S. Enzymatic sulfation of steroids: II. The control of the hepatic cortisol sulfotransferase activity and of the individual hepatic steroid sulfotransferases of rats by gonads and gonadal hormones. Endocrinology. 1976 Nov;99(5):1346–1352. doi: 10.1210/endo-99-5-1346. [DOI] [PubMed] [Google Scholar]
- Wishart G. J. Functional heterogeneity of UDP-glucuronosyltransferase as indicated by its differential development and inducibility by glucocorticoids. Demonstration of two groups within the enzyme's activity towards twelve substrates. Biochem J. 1978 Aug 15;174(2):485–489. doi: 10.1042/bj1740485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. G., Straub K. D. Purification and characterization of N-hydroxy-2-acetylaminofluorene sulfotransferase from rat liver. J Biol Chem. 1976 Nov 10;251(21):6529–6536. [PubMed] [Google Scholar]


