Summary
Background
India has made exceptional advances in child immunisation, but subnational inequities in vaccination coverage impede attainment of key programmatic goals. Our study provides an up-to-date national portrait of local variations in child vaccination using a comprehensive set of indicators relevant to routine immunisation.
Methods
Indicators representing unvaccinated (zero-dose) children, incomplete basic immunisation, and vulnerability to measles and polio, were constructed from India’s 2019–2021 National Family Health Survey. We used four-level random effects logistic regression models to partition the total outcome variation over state, district and cluster levels, and produce precision-weighted estimates of prevalence across clusters. District-level prevalence and within-district variation using standard deviation measures were derived for each outcome. Boxplots graphically summarised the distribution of precision-weighted mean cluster prevalence by state.
Findings
The analysis included 87,622 children aged 12–36 months. Clusters accounted for 67.6% (var: 1.36; SE: 0.127) of the variation among zero-dose children, and more than 50% for all indicators. Districts with a higher prevalence of under-vaccination tended to have higher within-district heterogeneity, interpretable as greater within-district child vaccination inequities. For vaccines administered in the first year of life, the northeastern states and Uttar Pradesh had the highest median under-vaccination. Despite India’s high aggregate vaccine coverage, the distribution of small-area (cluster) mean prevalence highlighted pockets of low coverage in most states, suggesting ongoing vulnerability to measles and polio.
Interpretation
Achieving India’s vaccination goals requires a strategic shift towards identification and targeting of low-immunity clusters at the sub-district level.
Funding
Canadian Institutes of Health Research.
Keywords: Equity, Health inequalities, Immunization programs, Child health, Developing countries, India/epidemiology, Vaccination/∗statistics & numerical data, Vaccination coverage/∗statistics & numerical data
Research in context.
Evidence before this study
Large-area summaries of vaccination coverage often mask subnational heterogeneities, but analyses of fine-scale variation are rarely available to inform decisionmaking. To identify original research articles with quantitative empirical findings concerning small-area (below district-level) variations in child under-vaccination, we searched the PubMed database on July 6, 2023 (updated August 14, 2024), using the following search terms: ((“child” [MeSH] OR child∗ OR “pediatrics” [MeSH] OR pediatric∗ OR paediatric∗) AND ((“vaccin∗” [MeSH Terms] OR “immuniz∗” [MeSH Terms]) AND ((“incomplete” OR “non-vaccination” OR “under-vaccination” OR “under∗” OR “unreached” OR “unvaccinated”) OR (“zero-dose” OR “zero”)))) AND (“small area” OR “geospatial” OR “heterogeneity” OR “mapping” OR “map∗” OR “subnational”). No geographic, date or language restrictions were applied. In total, 20 relevant articles published between 2002 and 2023 were identified. Three studies included findings for India; however, all focussed on limited set of antigens, and two were multi-country studies incorporating data from India’s 2015–2016 National Family Health Survey (NFHS). In a precursor to this study, Rajpal and colleagues (2023) analysed all five rounds of India’s NFHS using multi-level logistic regression models to study spatiotemporal trends in non-receipt of diphtheria-tetanus-pertussis-containing vaccine. While the study described trends in variance partitioning, no sub-district analyses were conducted.
Added value of this study
To our knowledge, our study offers the third national analysis of local variations in child vaccination in India and the first that considers a comprehensive set of indicators relevant to routine immunisation.
Using India’s most recent national survey data from the 2019–2021 NFHS, we examined a range of under-vaccination indicators relevant to the Indian immunisation programme, Immunization Agenda 2030 (IA2030), and the WHO 2023 Big Catch-Up strategy, lending relevance to the findings. Our results are based on multi-level random intercept logistic regression modelling, including shrinkage methods to improve small-area estimation. We highlight four vital findings. First, in India, for all indicators of under-vaccination studied, the largest variations in vaccination coverage occurred at the small-area level, followed by states, and then districts. This indicates the need for a shift in strategic focus from the district to sub-areas within districts. Second, for all indicators, prevalence varied widely across districts, and districts with a higher prevalence of under-vaccination tended to have greater within-district child vaccination inequities. Third, exploratory analyses studying patterns of predicted cluster mean prevalence reveal persistent pockets of low immunity even in many high-performing states and UTs. Fourth, despite India’s high aggregate vaccine coverage, the distribution of predicted cluster mean prevalence suggests ongoing vulnerability to vaccine-preventable diseases with outbreak potential such as measles and polio, corroborated by large and disruptive measles outbreaks experienced in 2022 and 2023.
Implications of all the available evidence
Together with other studies exploring small-area variations in child vaccination, our research demonstrates that local geographic variations constitute a critical roadblock to achievement of key immunisation objectives, such as those outlined in IA2030, the global immunisation strategy to “leave no one behind” companion to the UN 2030 Agenda for Sustainable Development.
In India, where aggregate vaccine coverage levels are high, our findings support a fundamental tactical shift from a state and district focus towards interventions aimed at granular pockets of low immunity within districts to increase vaccination coverage, advance disease-control priorities, and reduce inequities. This shift will require rapid adoption of new strategies accompanied by implementation-oriented research to enable high-impact, precision approaches to vaccinate and close immunity gaps.
Introduction
Endorsed by the World Health Assembly in 2021, Immunization Agenda 2030 (IA2030) is the global immunisation strategy to “leave no one behind” companion to the UN 2030 Agenda for Sustainable Development.1 To ensure that everyone, everywhere, at every age fully benefits from vaccination, key IA2030 goals include preventing diseases by initiatives such as global polio eradication and elimination of measles and rubella transmission, promoting equity in immunisation by halving the number of “zero-dose” (ZD) children missing out on all routine immunisation services, and building strong immunisation programmes that deliver vaccination across the life course.1
With an annual birth cohort of 23 million,2 India’s success is decisive to that of IA2030. India has made exceptional progress in immunisation in recent decades, but subnational inequities in vaccination persist.3, 4, 5 Reflecting its large population, despite high aggregate immunisation coverage, India ranked first in 2021 and second in 2023 for the highest absolute number of ZD children globally and figures among the top 10 countries home to children missing out on measles vaccination worldwide.6 Like many countries, due to immunisation gaps exacerbated by the global COVID-19 pandemic, India recently experienced vaccine-preventable disease outbreaks, including flare-ups of measles and diphtheria.6
To address these challenges and achieve India’s aim to fully vaccinate every child, insights into the subnational patterning of vaccination coverage are required. While high quality information is now available at the district level, Indian districts are large administrative areas with an average population exceeding 1.5 million. District-level aggregates can conceal important local variations in immunisation coverage.7 Health management information system (HMIS) data are sometimes used for programme review at district and sub-district levels, but data quality may be uneven. In addition, HMIS data only include children who engage with immunisation and related services, thereby excluding the most vulnerable in hard-to-reach communities who should be the target of expanded strategies.
Two previous studies have provided limited information on local variations in child vaccination coverage in India. These studies used Bayesian geospatial regression to generate small-area and second administrative level estimates of vaccination coverage for multiple countries, including India; however, they discussed a restricted range of antigens limited to diphtheria-tetanus-pertussis-containing vaccine first-dose (DTP1) and third-dose (DTP3) and measles-containing vaccine first-dose (MCV1) coverage, and used older data from India’s 2015–2016 National Family Health Survey (NFHS).8,9 Up-to-date analyses of small-area variations in vaccination coverage suitable to inform strategy in India are not available.
Contributing to India’s success in achieving its vaccination goals represents an pivotal opportunity to improve health and wellbeing. Using the most recent nationally representative survey data, the aim of our study was to provide an up-to-date national portrait of local variations in child vaccination using a comprehensive set of indicators relevant to routine immunisation.
Methods
Study design, data source, and sample
We conducted a cross-sectional analysis of India’s most recent national survey, the NFHS-5,10 using multilevel analysis techniques to model small-area variation for priority vaccines. Conducted in 2019–21, the NFHS-5 provides representative data on child vaccinations for all of India’s 707 districts (as of March 31st, 2017), 28 states, and 8 Union Territories (UTs).10
India’s NFHS series forms part of the Demographic and Health Surveys Program and follows similar survey design, sampling, and quality assurance methods10 (Supplementary Appendix, 1.1). The NFHS-5 employed a two-stage design with stratified sampling for priority groups, including scheduled castes and tribes, and women with low literacy levels, and followed principles of random selection for inclusion of primary sampling units (PSUs), clusters, and households. In the first stage, India’s 2011 census provided the sampling frame for PSU selection. In the second stage, a household enumeration was conducted in each selected PSU, and large PSUs with more than 300 households were subdivided into segments of approximately 100–150 households. Thus, an NFHS-5 cluster is either a PSU, or a PSU segment. Finally, in every included cluster, 22 households were selected from the enumeration list for inclusion in the survey.10 Application of survey sampling weights is recommended to adjust for the multistage design and ensure that prevalence estimates are representative of the population.
NFHS-5 data were collected face-to-face via computer-assisted personal interviewing in two phases: June 17, 2019 to January 30, 2020, and January 2, 2020 to April 30, 2021.10 Administered by female surveyors, the woman’s questionnaire collected information on topics including child vaccination from all eligible women aged 15–49 years. The NFHS-5 household response rate was 97.5%, while 96.9% of eligible women completed the woman’s questionnaire.10
We constructed an analysis sample from the NFHS-5 children’s dataset. Based on standard age ranges used to assess vaccination coverage,11 to study vaccines administered in the first year of life, we included all children 12–23 months of age at the time of the survey. To study vaccines given in the second year of life, we included all children 24–35 months.
Variables, data sources, and measurement
We chose variables for analysis based on the priorities of the Indian government, the global IA2030 partnership,1 and the WHO Big Catch Up for post COVID-19 recovery.12
Primary outcomes
Zero-dose child: Operationally, IA2030 defines ZD children as those who fail to receive even a single dose of diphtheria, tetanus, pertussis (DTP)-containing vaccine.1 We constructed a binary variable designating all surviving children aged 12–23 months who failed to receive any DTP or pentavalent vaccine doses as “ZD”.
Non-receipt of measles-containing vaccines: To facilitate the WHO South-East Asia Region (SEARO) goal of measles and rubella elimination by 2023, India introduced measles-rubella (MR) vaccine into the routine immunization program, replacing monovalent formulations.13,14 To represent insufficient measles vaccination we created two binary variables, designating all surviving children 12–23 months of age who failed to receive any measles vaccination as “No MR-1″, and all surviving children 24–35 months of age who failed to receive a second measles-containing dose as “No MR-2”.
Basic immunisation incomplete: India prioritises full immunisation for all children and tracks a set of basic first year of life vaccines specified by the WHO Expanded Programme on Immunisation: one dose of Bacillus Calmette-Guérin (BCG) to protect against tuberculosis, three doses of DPT-containing vaccine, which protect against diphtheria, pertussis, and tetanus, three doses of oral polio vaccine (OPV), and one measles-containing vaccine. We created a binary variable assigning all surviving children 12–23 months of age who failed to receive all 8 recommended doses as “basic immunisation incomplete” (henceforth, “BI”).
Secondary outcome
To induce a favourable immunogenic response against polio, during the NFHS-5 data collection period, India offered a birth dose of bivalent OPV (bOPV), a primary series of 3 bOPV doses at 6, 10, and 14 weeks, and two intradermal fractional doses of inactivated polio vaccine (IPV) at ages 6 and 14 weeks.15 WHO recommends that all children receive at least one dose of IPV to eliminate the risks due to poliovirus type 2, against which bOPV offers no protection.15 We created a binary variable assigning all surviving children aged 12–23 months who failed to receive three bOPV doses as “No OPV3”. Although IPV is used in India, data are not available in the NFHS-5.
The NFHS-5 collected vaccination information from biological mothers of all children born in or after 2017 and alive at the time of the interview. Vaccination data were transcribed from the child’s vaccination record. If the card was not available at the time of the interview or the information was incomplete, vaccination data were taken by recall.10
Analysis
For all child under-vaccination outcomes, we analysed the NFHS-5 dataset to compute direct estimates of national, state/UT, and district mean prevalence and their 95% confidence intervals, applying the appropriate weights to balance the complex sampling design, and using subpopulation estimation to generate standard errors consonant with the data subset (living children 12–35 months).
Next, recognising that the NFHS-5 vaccination data subset includes strata that are small and unbalanced, we used multilevel modelling techniques to generate smoothed estimates of the underlying population characteristics. Exploiting methods from previous analyses of small-area variations using NFHS data,16 we developed multilevel logistic models to represent the random effects on binary child under-vaccination outcomes (Y) of membership in a hierarchy of nested spatial units at four levels: household-child i (level-1), cluster j (level-2), district k (level-3), and state/UT l (level-4). Specifically, we estimated the equation logit (Yijkl) = β0 + (u0jkl + v0kl + f0l), where Yijkl is the log odds of the outcome for individual i, and u0jkl, v0kl, f0l represent the residual error terms for cluster, district, and state, respectively. Residuals were assumed to be normally distributed with a mean of 0 and a variance of u0jkl ∼ N (0,σ2u0), v0kl ∼ N (0, σ2v0), and f0l ∼ N (0, σ2f0). The term σ2u0, therefore, denotes within-district, between-cluster variation; σ2v0 represents within-state, between-district variation; and σ2f0 is the between-state variation. For binary outcomes, level-1 variance is not freely estimated.17 To decompose the proportion of variation attributable to distinct geospatial levels, the variance estimate at a given level was divided by the total geographic variation in the outcomes.
For all multilevel analyses, we employed Markov Chain Monte Carlo (MCMC) simulation methods using starting values from Iterative Generalized Least Squares (IGLS) estimations. To ensure stability of estimates, for all outcomes, we specified a sufficient number of iterations for the burn-in period [burnin (500)] necessary for the MCMC to establish a stationary distribution and used a monitoring chain length of 5000 iterations.16 Further, we followed the best practice of looking for adequate iterative and independent chains using postestimation commands available in Stata’s runmlwin module.18
To extrapolate small-area variations in child under-vaccination, for each under-vaccination outcome, we used the corresponding fitted multilevel model to generate cluster-specific mean estimates of child under-vaccination prevalence.16 As area-specific estimates may be imprecise, we used empirical Bayes “precision-weighted” estimation to downplay the influence of clusters with lower reliability and shrink their influence towards the pooled mean. The probability of each under-vaccination outcome Y for each cluster was calculated as: exp (β0 + u0jkl + v0kl + f0l)/(1 + exp (β0 + u0jkl + v0kl + f0l)). In addition, we computed the precision-weighted standard deviations of the predicted cluster under-vaccination indicators by district.16 These values can be interpreted as the within-district and between-cluster variations in individual child vaccination status, and hence, as measures of within-district heterogeneity indicative of local child vaccination inequities. For each under-vaccination outcome, we mapped the precision-weighted district means and standard deviations from the multilevel model.
To stimulate insights into patterns of under-vaccination, we performed exploratory data analysis using two techniques to study the outcomes predicted by the multilevel models. First, we calculated the Pearson correlation coefficient to evaluate the linear relationship between the predicted district means for pairs of under-vaccination measures. To provide additional information at the small-area level, we repeated correlation analyses using the predicted cluster means.16 Second, for each outcome, we present boxplots to graphically summarise the distribution of precision-weighted mean cluster prevalence by State/UT. Boxplots provide information in a compact format on central tendency, skewness and spread, and highlight outliers, without imposing additional parametric assumptions.
Finally, we conducted sensitivity analyses to examine the robustness of findings in relation to three issues: (1) As cluster size may influence results and singleton clusters experience greater shrinkage towards the mean, we re-ran all analyses on data subsamples restricted to clusters of size 2 or more, and size 5 or more; (2) As BI is a composite indicator, to understand underlying drivers, we also present analyses for its subcomponents. (3) The WHO Big Catch-Up promotes delivery of vaccines up to age 5 for children who have missed out on vaccination. To understand whether doses were delayed or missed, we conducted a sensitivity analysis extending the age range for the first year of life vaccines from 23 to 35 months.
The NFHS-5 dataset contains no missing values for child vaccination outcomes in the age groups studied. Analyses were executed by SR and validated by MJ using Stata statistical software (Version 16).19 Analysis code is publicly available.20 Multilevel modelling was performed using the MLwiN 3.0 software program21 accessed via Stata’s runmlwin command.18 The choropleth mapping process used a verified 707-district shapefile of India.22,23 The external boundary for India provided by the Survey of India was used to modify the DHS shape file.24 Vaccination coverage projections derived from multilevel models were added to the ArcGIS project25 and joined with the 707-district shapefile using NFHS-5 District Codes as the common identifier for data joining.22
Research ethics
This study is based on secondary use of publicly available, anonymised data, and does not generate information that would enable identification of individuals. It is thus exempt from the requirement for research ethics review. Respondents to the NFHS-5 household and women’s questionnaires provided verbal informed consent.
Role of the funding source
This study was funded by the Canadian Institutes for Health Research (FRN-130280). The funding agency played no role in study design; in the collection, analysis, and interpretation of data; in writing the report; or in the decision to submit for publication.
Results
Sample characteristics
We screened 232,920 children less than 5 years of age for eligibility and excluded 145,298 outside the target age ranges. The analysis included 87,622 children (Appendix Fig. S1) from all of India’s 36 states/UTs and 707 districts, representing a population of approximately 46 million children between one and three years of age.2 (Table 1). For vaccines administered in the first year of life, analyses comprised 43,436 children residing in 22,061 clusters. Analyses of MR-2 comprised 44,186 children in 22,349 clusters. Table 1 provides estimates of mean prevalence and their 95% confidence intervals nationally and by state/UT for four primary child under-vaccination outcomes. Information on model convergence is provided (Appendix Figs. S2 and S3 and Tables S1–S3).
Table 1.
Prevalence of child under-vaccination across states and Union Territories, India, National Family Health Survey (NFHS-5) 2019–2021.a
| State/UTb | # Districts | Children 12–23 months |
Children 24–35 months |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZDc |
BId |
No MR-1e |
No MR-2f |
||||||||||
| # Clusters | n (Sample) | (%) | 95% CI | (%) | 95% CI | (%) | 95% CI | # Clusters | n (Sample) | (%) | 95% CI | ||
| All | 707 | 21,771 | 43,436 | 6.1 | [5.76; 6.48] | 23.40 | [22.83; 24.01] | 12.15 | [11.66; 12.63] | 44,186 | 22,068 | 41.31 | [40.59; 42.03] |
| Andaman & Nicobar Island | 3 | 60 | 94 | 1.83 | [0.57; 3.10] | 22.22 | [12.18; 32.25] | 17.95 | [8.66; 27.23] | 89 | 68 | 22.58 | [13.67; 31.50] |
| Andhra Pradesh | 13 | 327 | 541 | 6.78 | [4.43; 9.12] | 26.98 | [23.01; 30.96] | 12.93 | [10.01; 15.84] | 516 | 328 | 42.46 | [37.60; 47.31] |
| Arunachal Pradesh | 20 | 541 | 970 | 13.01 | [10.06; 15.96] | 35.09 | [31.09; 39.10] | 19.28 | [15.86; 22.70] | 1110 | 601 | 48.09 | [44.44; 51.75] |
| Assam | 33 | 1023 | 1965 | 9.02 | [7.46; 10.57] | 33.53 | [31.09; 35.97] | 17.22 | [15.04; 19.40] | 2113 | 1036 | 64.48 | [61.64; 67.31] |
| Bihar | 38 | 1546 | 3977 | 6.11 | [5.18; 7.04] | 28.67 | [26.98; 30.36] | 14.27 | [12.95; 15.59] | 3928 | 1524 | 44.33 | [42.36; 46.30] |
| Chandigarh | 1 | 20 | 28 | 6.58 | [−2.56; 15.72] | 19.07 | [5.01; 33.14] | 12.10 | [0.90; 23.31] | 33 | 21 | 24.46 | [8.74; 40.19] |
| Chhattisgarh | 27 | 855 | 1604 | 4.55 | [3.19; 5.90] | 20.19 | [17.42; 22.97] | 9.76 | [7.56; 11.96] | 1623 | 858 | 43.75 | [39.88; 47.62] |
| Goa | 2 | 48 | 68 | 2.11 | [−1.97; 6.20] | 18.06 | [8.62; 27.51] | 7.10 | [0.84; 13.35] | 67 | 46 | 67.00 | [51.61; 82.40] |
| Gujarat | 33 | 974 | 1864 | 7.25 | [5.74; 8.76] | 23.35 | [20.92; 25.78] | 13.18 | [11.17; 15.20] | 1887 | 1002 | 49.22 | [45.99; 52.45] |
| Haryana | 22 | 666 | 1313 | 5.75 | [4.46; 7.04] | 23.02 | [20.27; 25.77] | 10.62 | [8.70; 12.54] | 1316 | 652 | 43.26 | [40.01; 46.51] |
| Himachal Pradesh | 12 | 332 | 533 | 1.81 | [0.34; 3.29] | 10.75 | [7.59; 13.91] | 4.07 | [2.07; 6.06] | 453 | 293 | 30.85 | [24.73; 36.97] |
| Jammu & Kashmir | 20 | 566 | 1020 | 5.20 | [3.55; 6.86] | 13.61 | [10.98; 16.24] | 8.30 | [6.16; 10.43] | 1192 | 625 | 26.38 | [22.86; 29.90] |
| Jharkhand | 24 | 868 | 1923 | 7.47 | [5.73; 9.21] | 26.09 | [23.60; 28.58] | 13.33 | [11.25; 15.42] | 1844 | 856 | 40.17 | [37.33; 43.00] |
| Karnataka | 30 | 865 | 1585 | 4.06 | [2.59; 5.53] | 15.84 | [13.12; 18.56] | 8.78 | [6.69; 10.87] | 1583 | 880 | 37.6 | [34.25; 40.94] |
| Kerala | 14 | 316 | 487 | 4.03 | [2.15; 5.91] | 21.85 | [17.49; 26.21] | 11.66 | [8.48; 14.84] | 545 | 343 | 68.15 | [63.37; 72.94] |
| Ladakh | 2 | 50 | 83 | 0.94 | [−0.91; 2.79] | 11.78 | [2.26; 21.30] | 7.14 | [1.05; 13.23] | 105 | 60 | 25.81 | [15.40; 36.22] |
| Lakshadweep | 1 | 26 | 45 | 9.02 | [1.44; 16.59] | 13.86 | [4.67; 23.04] | 9.02 | [1.44; 16.59] | 52 | 32 | 87.55 | [78.30; 96.79] |
| Madhya Pradesh | 51 | 1561 | 3109 | 5.71 | [4.74; 6.67] | 22.79 | [20.91; 24.68] | 12.01 | [10.62; 13.39] | 3011 | 1548 | 36.06 | [34.06; 38.05] |
| Maharashtra | 36 | 1006 | 1812 | 7.50 | [4.92; 10.08] | 26.51 | [23.09; 29.92] | 15.30 | [12.01; 18.59] | 1814 | 1026 | 49.70 | [45.65; 53.76] |
| Manipur | 9 | 285 | 567 | 6.51 | [4.26; 8.75] | 31.17 | [26.16; 36.19] | 23.41 | [18.91; 27.90] | 600 | 291 | 74.19 | [69.45; 78.92] |
| Meghalaya | 11 | 360 | 1136 | 15.83 | [12.39; 19.28] | 36.10 | [32.18; 40.02] | 27.52 | [23.75; 31.29] | 1216 | 391 | 74.07 | [70.03; 78.10] |
| Mizoram | 8 | 238 | 486 | 14.25 | [9.49; 19.00] | 27.48 | [20.20; 34.77] | 19.08 | [12.92; 25.23] | 453 | 242 | 51.30 | [45.07; 57.52] |
| Nagaland | 11 | 304 | 560 | 15.14 | [10.97; 19.31] | 42.12 | [36.44; 47.81] | 26.20 | [21.35; 31.05] | 585 | 304 | 58.86 | [53.66; 64.05] |
| D&N Haveli; D & Diu | 3 | 87 | 151 | 2.15 | [0.13; 4.17] | 5.12 | [1.74; 8.50] | 3.80 | [0.72; 6.88] | 156 | 87 | 15.85 | [9.08; 22.61] |
| NCT of Delhi | 11 | 301 | 570 | 6.32 | [3.78; 8.87] | 24.01 | [19.83; 28.20] | 9.90 | [7.11; 12.68] | 595 | 335 | 26.55 | [22.15; 30.95] |
| Odisha | 30 | 882 | 1566 | 2.67 | [1.63; 3.72] | 9.54 | [7.60; 11.49] | 4.13 | [2.74; 5.52] | 1652 | 902 | 21.13 | [18.37; 23.89] |
| Puducherry | 4 | 98 | 157 | 0.63 | [−0.12; 1.37] | 18.00 | [8.33; 27.66] | 4.44 | [1.08; 7.80] | 145 | 95 | 15.37 | [6.65; 24.10] |
| Punjab | 22 | 610 | 1060 | 5.66 | [4.01; 7.30] | 23.80 | [20.67; 26.94] | 11.87 | [9.59; 14.14] | 1103 | 634 | 43.26 | [39.50; 47.03] |
| Rajasthan | 33 | 1180 | 2548 | 5.09 | [4.06; 6.13] | 19.27 | [17.42; 21.12] | 8.85 | [7.49; 10.21] | 2801 | 1246 | 47.74 | [45.10; 50.38] |
| Sikkim | 4 | 79 | 114 | 4.90 | [0.30; 9.51] | 19.43 | [7.56; 31.30] | 9.52 | [0.74; 18.29] | 131 | 93 | 64.27 | [51.80; 76.74] |
| Tamil Nadu | 32 | 835 | 1291 | 2.42 | [1.49; 3.34] | 10.60 | [8.59; 12.62] | 4.20 | [2.86; 5.54] | 1230 | 793 | 25.04 | [22.01; 28.06] |
| Telangana | 31 | 816 | 1443 | 7.14 | [5.12; 9.16] | 20.88 | [17.93; 23.82] | 9.42 | [7.21; 11.63] | 1360 | 824 | 36.87 | [32.96; 40.77] |
| Tripura | 8 | 227 | 391 | 5.27 | [2.82; 7.73] | 30.05 | [24.97; 35.14] | 13.68 | [10.04; 17.33] | 368 | 228 | 50.47 | [44.85; 56.09] |
| Uttar Pradesh | 75 | 2828 | 6553 | 8.85 | [7.98; 9.72] | 30.27 | [28.86; 31.68] | 16.69 | [15.52; 17.86] | 6701 | 2815 | 41.23 | [39.78; 42.67] |
| Uttarakhand | 13 | 404 | 714 | 4.58 | [2.52; 6.64] | 18.93 | [14.18; 23.67] | 9.44 | [6.26; 12.61] | 705 | 388 | 28.70 | [23.85; 33.54] |
| West Bengal | 20 | 587 | 1108 | 2.09 | [1.09; 3.09] | 12.19 | [10.09; 14.28] | 5.61 | [4.07; 7.16] | 1104 | 601 | 27.40 | [24.01; 30.79] |
Survey-weighted estimates from the NFHS-5.
UT–Union Territory.
ZD–“Zero-dose”, defined as all surviving children aged 12–23 months who did not receive the first dose of diphtheria, tetanus, and pertussis -containing vaccine (i.e., no diphtheria-tetanus- pertussis (DPT1) or pentavalent (PENTA1) vaccine).
BI–“Basic incomplete”, defined as all surviving children aged 12–23 months who did not receive 8 vaccine doses recommended in the first year of life: 1 dose of Bacillus Calmette-Guérin (BCG) vaccine, 3 doses of pentavalent vaccine, 3 doses of oral polio vaccine, 1 dose of measles-rubella vaccine.
No MR-1–All surviving children aged 12–23 months who did not receive the first dose of measles-rubella-containing vaccine.
No MR-2–All surviving children aged 24–35 months who did not receive the second dose of measles-rubella-containing vaccine.
Variance partitioning
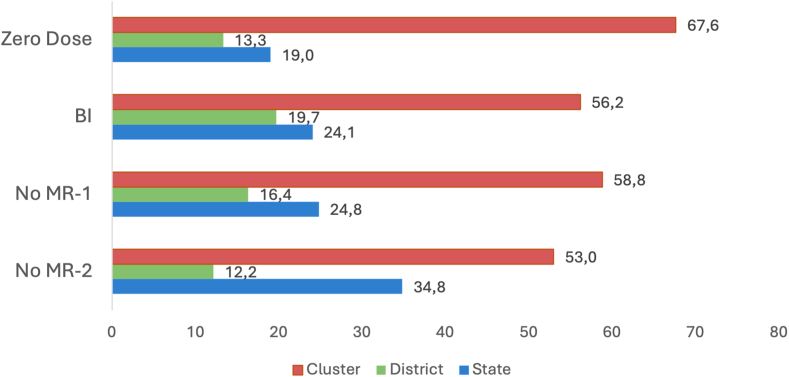
For all four primary measures of under-vaccination, small-area clusters accounted for the largest share (more than 50%) of the total variation in the outcome, followed by states, and then districts (Fig. 1; Appendix Table S3). The share of variation attributable to clusters was most important for the ZD indicator (67.6%; (var: 1.36; SE: 0.127)), followed by no MR-1 (58.8%; (var: 1.02; SE: 0.061)), BI (56.2% (var: 0.80; SE: 0.054)), and no MR-2 (53.0% (var: 1.13; SE: 0.062)), underscoring that small-area variation is progressively more important as vaccination coverage increases.
Fig. 1.
Geographic variance partitioning (%) by clusters, districts and states for four indicators of child under-vaccination, National Family Health Survey 2019–2021 (NFHS-5), India. “Zero Dose”–All surviving children aged 12–23 months who did not receive the first dose of diphtheria, tetanus, and pertussis -containing vaccine. “BI”–Basic incomplete: All surviving children aged 12–23 months who did not receive 8 basic vaccine doses recommended in the first year of life: 1 dose of Bacillus Calmette-Guérin (BCG) vaccine, 3 doses of pentavalent vaccine, 3 doses of oral polio vaccine, 1 dose of measles-rubella vaccine. “No MR-1”–All surviving children aged 12–23 months who did not receive the first dose of measles-rubella-containing vaccine. “No MR-2”–All surviving children aged 24–35 months who did not receive the second dose of measles-rubella-containing vaccine.
Correlations between measures of under-vaccination
The four measures of under-vaccination exhibited positive linear correlations at the district level, suggesting that problems of under-vaccination tend to be geographically co-located within districts (Appendix Fig. S4). There was a strong positive relationship between the district means for the ZD, BI, and no MR-1 indicators, and a moderate positive relationship between the district means for non-receipt of MR-1 and MR-2 (p < 0.001 for all). Exploratory analysis of the sample excluding singleton clusters identified positive linear correlations between measures of under-vaccination at the cluster level, suggesting that problems of under-vaccination may also be geographically co-located within clusters (Appendix Fig. S5).
District mean prevalence and within-district heterogeneity in under-vaccination
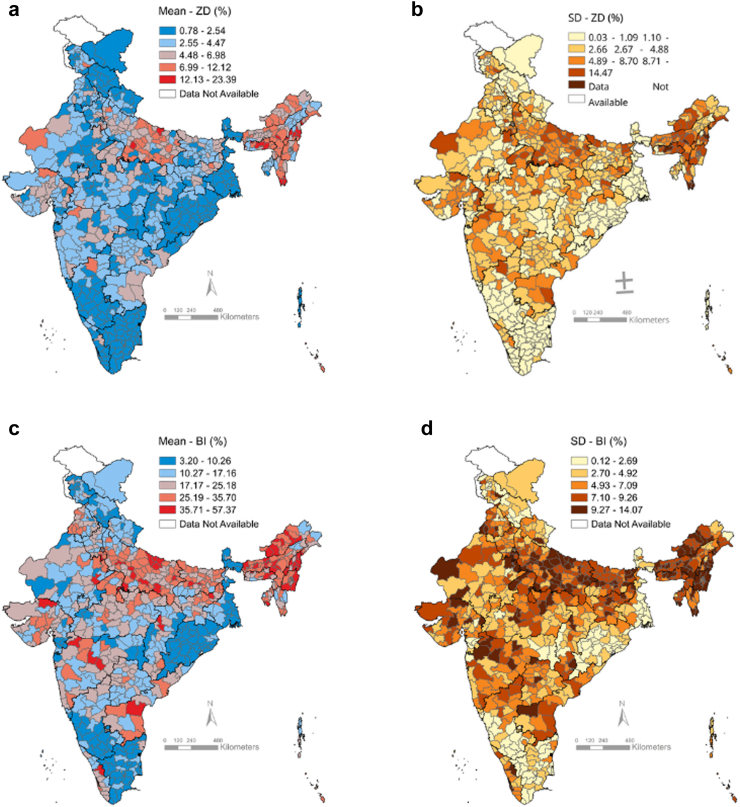
Over India’s 707 districts, the precision-weighted mean prevalence of ZD children ranged from 0.78% (standard deviation, henceforth “SD” 0.03) to 23.39% (SD 11.73), while the mean prevalence of BI children ranged from 3.20% (SD 0.14) to 57.37 (SD 12.32) (Fig. 2, Appendix Table S4). For both indicators, districts with the highest prevalence of under-vaccination and the highest within-district heterogeneity (indicative of within-district child vaccination inequities) were concentrated in the north-eastern states and in the populous north–central state of Uttar Pradesh. Notwithstanding, there were high-burden and high-inequity districts scattered across the country, including in states with high vaccination coverage.
Fig. 2.
Maps of district-level prevalence and within-district small-area variations in the prevalence of zero-dose (ZD) children and children with basic immunisation incomplete (BI), National Family Health Survey 2019–2021, India. (a) District-level mean and (b) within-district between-cluster standard deviation (SD) for zero-dose child prevalence; (c) District-level mean and (d) within-district between-cluster SD for child prevalence with basic immunisation incomplete.
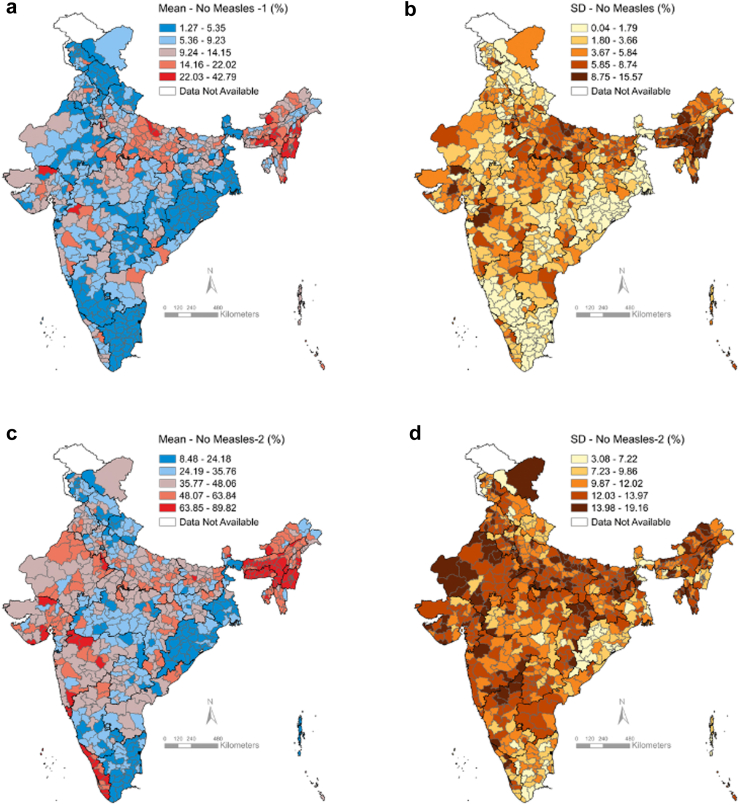
The mean prevalence of no-MR1 children over India’s 707 districts ranged from 1.27% (SD 0.05) to 42.79% (SD 15.57), with patterns similar to those for ZD and BI (Fig. 3, Appendix Table S4). The mean prevalence of no-MR2 children ranged from 8.48% (SD 3.9) to 89.82% (SD 4.6). Patterns for no MR-2 were distinct, with high-burden districts concentrated in the northeast, and in otherwise high-performing states such as Maharashtra, Gujarat, and Kerala.
Fig. 3.
Maps of district-level prevalence and within-district small-area variations in the prevalence of non-receipt of measles-rubella (MR) vaccination, National Family Health Survey 2019–2021, India. (a) District-level prevalence and (b) within-district between-cluster standard deviation (SD) for non-receipt of MR first dose; (c) District-level prevalence and (d) within-district between-cluster SD for non-receipt of MR second dose.
For all indicators of under-vaccination, we found a positive association between district mean prevalence and within-district between-cluster standard deviations, revealing that districts with higher burden of under-vaccination generally had greater within-district inequities (Fig. 2, Fig. 3, Appendix Table S5). Within-district heterogeneities in vaccination coverage were smallest for the ZD indicator, followed by no MR-1, BI, and no MR-2 (Appendix Fig. S6).
Distribution of under-vaccination across states and UTs
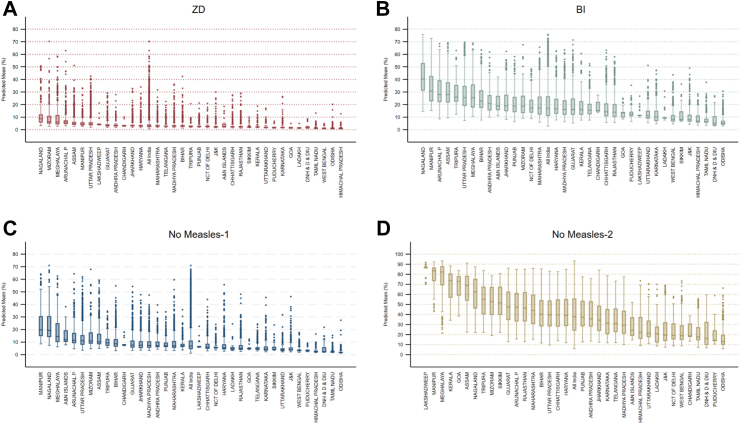
For first-year-of-life (FYL) vaccines ZD, BI, No-MR-1, the northeastern states and Uttar Pradesh consistently had the highest median under-vaccination, but the distributions of predicted cluster mean prevalences for all States and UTs were positively skewed, with more extreme values on the high end and numerous outliers (Fig. 4; Appendix Fig. S7). For all indicators of under-vaccination, States and UTs with a higher prevalence of under-vaccination tended to have greater variability. However, the patterning of cluster means highlights pockets of low immunity even in high-performing states and UTs.
Fig. 4.
Box plots of predicted small-area cluster1 mean prevalence for four indicators of under-vaccination across states and Union Territories, National Family Health Survey 2019–2021, India. Note: for each plot, the middle bar represents the median, the box represents the interquartile (25th to 75th percentile) range (IQR), and the upper (lower) whiskers are located at the 75th (25th) percentile plus (minus) 1.5 times the IQR. 1For panels A, B, and C, analyses include 21,771 clusters. For panel D, analyses include 22,068 clusters. (A) “Zero Dose”–All surviving children aged 12–23 months who did not receive the first dose of diphtheria, tetanus, and pertussis-containing vaccine. (B) “BI”–Basic incomplete: All surviving children aged 12–23 months who did not receive 8 basic vaccine doses recommended in the first year of life: 1 dose of Bacillus Calmette-Guérin (BCG) vaccine, 3 doses of pentavalent vaccine, 3 doses of oral polio vaccine, 1 dose of measles-rubella vaccine. (C) “No MR-1”–All surviving children aged 12–23 months who did not receive the first dose of measles-rubella-containing vaccine. (D) “No MR-2”–All surviving children aged 24–35 months who did not receive the second dose of measles-rubella-containing vaccine.
For the ZD indicator, the all-India median prevalence was 3.21% (IQR: 2.83; min 0.77, max 23.38). Most states had a very compact distribution, with the top whisker (located at the 75th percentile plus 1.5 times the interquartile range) entirely below 5%. Notwithstanding, distributions for most states were positively skewed. For the BI indicator, the all-India median prevalence was 18.34% (IQR: 14.62; 3.19, 57.63), and distributions for virtually all states were positively skewed. For no MR-1, the all-India median prevalence was 7.96% (IQR: 7.02; min 1.26%, max 42.78%]. HIgh-performing states such as Odisha, Tamil Nadu, Himachal Pradesh, Pudicherry, Goa, and Lakshadweep had low prevalence and low variability of measles-rubella first dose non-vaccination consistent with uniform MR-1 coverage of 95%; however, the remaining states exceeded these levels, and the northeastern states and Uttar Pradesh had median prevalences of non-vaccination ranging from 12 to 20% and high variability. For no MR-1, virtually all states revealed positively skewed distributions and numerous outliers. The all-India median prevalence of non-receipt of measles-rubella second dose was 38.97% (IQR 22.75; min 8.48, max 89.81), and all states had median prevalences of non-vaccination in excess of 12%.
Sensitivity analyses
(1) The proportion of singleton clusters in the dataset was 44% (Appendix Table S6). Notwithstanding, analyses excluding singleton clusters (Appendix Figs. S8–S12 and Tables S5 and S6) and clusters with fewer than five children (Appendix Fig. S11 and Table S7) revealed patterns of variance partitioning and distribution of cluster mean prevalences similar to the main analysis and support identical qualitative inferences. As compared to all clusters, estimates of all-India median prevalence of under-vaccination were uniformly higher for clusters with 5 or more children: zero-dose 5.92% (IQR 4.46; min 2.19, max 65.9), BI 25.97% (IQR 19.17; min 7.26, max 69.88), non-receipt of measles-rubella first dose 13.31% (IQR 8.99; min 3.88, max 70.08), non-receipt of measles-rubella second dose 46.96% (IQR 37.72; min 7.86, max 92.15), suggesting that high-natality areas may be at greater risk of sub-optimal vaccination. (2) With respect to decomposition of the BI indicator, patterns for children not receiving 3 doses of OPV or 3 doses of DTP-containing vaccine were very similar to those for No MR-1 and BI (Appendix Fig. S14–S19 and Table S10), suggesting coherence among BI sub-components. Analyses for non-receipt of Bacillus Calmette–Guérin (BCG) vaccine failed to converge. (3) For each FYL vaccine dose, the estimated mean prevalence of under-vaccination at ages 12–23 months and 24–35 months were similar, suggesting that doses were largely missed rather than delayed (Appendix Table S11).
Discussion
Principal findings
To our knowledge, our study offers the third national analysis of local variations in child vaccination in India, the first analysis to use the most recent NFHS-5 dataset, and the first to consider a comprehensive set of indicators relevant to routine immunisation. We highlight four programmatically-relevant findings.
First, in India, the largest variations in vaccination coverage now occur at the small-area level, indicating the need for a shift in strategic focus from the district to sub-areas within districts. Analyses of variance partitioning derived from multilevel models demonstrated that, for all indicators of under-vaccination, small-area clusters accounted for the majority of total outcome variation, followed by states, and then districts. Indicators with higher overall coverage displayed a greater concentration of variation at the small-area level. Positive linear correlations between measures of under-vaccination at the district level signify that problems of under-vaccination tend to be geographically co-located within districts. In addition, exploratory analysis between measures of under-vaccination at the cluster level also identified positive linear correlations, suggesting that problems of under-vaccination may also be geographically co-located within clusters.
Second, for all indicators, districts with a higher prevalence of under-vaccination tended to be more inequitable. The prevalence of under-vaccination varied widely across India’s 707 districts. Districts with a higher prevalence of under-vaccination tended to have higher within-district heterogeneity, interpretable as greater within-district child vaccination inequities. For first year-of-life vaccines (ZD, BI, No-MR-1, DPT3, OPV3), these high-burden districts were concentrated in the north-eastern states and in Uttar Pradesh.
Third, exploratory analyses studying patterns of predicted cluster mean prevalence reveal persistent pockets of low immunity even in many high-performing states and UTs. For all indicators of under-vaccination, States and UTs with a higher prevalence of under-vaccination tended to have greater variability. For first year of life vaccines, distributions of cluster mean prevalence were positively skewed, with more extreme values towards the high end and numerous outliers.
Fourth, despite India’s high aggregate vaccine coverage, the distribution of predicted cluster mean prevalence suggests ongoing vulnerability to vaccine-preventable diseases with outbreak potential such as measles, diphtheria, pertussis, and polio. Measles is a global priority disease due to its impact on child mortality.26 WHO recommends that countries seeking to eliminate measles deliver at least 95% coverage with two measles-containing vaccine doses (equivalent to at most 5% prevalence of non-receipt of MR-1 and MR-2) equitably to all children.26 While, in 2023, official mean estimates of India’s national measles-rubella first dose and second dose coverage were 93% and 90% respectively,6 we found that only the highest performing states such as Odisha, Tamil Nadu, Himachal Pradesh and Goa had distributions of first dose measles vaccination coverage across clusters consistent with measles elimination, and none had achieved elimination-level second dose coverage. A majority of states demonstrated substantial variation in measles coverage with more extreme values on the high end and numerous outliers. These findings suggest persistent vulnerability to measles outbreaks in multiple geographies, corroborated by India’s reported 65,150 measles cases–roughly ten percent of the global total–in 2023.6 Although India and the SEARO region were declared polio free in 2014, the country remains at risk for importation and spread of poliovirus due to endemic polio in neighbouring countries and risk of vaccine-derived polio in low-coverage geographies.27 While national coverage of the third dose of oral polio vaccine and DTP-containing vaccine stand at 91%,6 descriptive exploration of patterns of predicted cluster mean prevalence of failure to receive 3 doses of these vaccines revealed important gaps in protection in many areas of the country, suggesting possible vulnerability to diphtheria, pertussis, and polio.
Strengths and limitations
Study strengths include use of a rigorously conducted, up-to-date national household survey representing approximately 46 million Indian children, consideration of a comprehensive set of immunisation indicators to inform strategic actions, and appropriate statistical methods enabling us to quantify within-district inequities and flag districts with higher inequities for intervention. Several limitations should be acknowledged. First, recall-based vaccination estimates may be less accurate. Moreover, for data collected by recall, best practice guidelines followed by the NFHS-5 stipulates that, if the response of a caregiver concerning a specific vaccine is “don’t know”, it should be coded as a “no”.11 This could result in some inaccuracy and underestimation of the true vaccination coverage. However, a high proportion (86%) of NFHS-5 respondents had a vaccination card that was seen. Second, compounded by use of historical census data to set the sampling frame, survey sampling procedures may systematically under-represent groups whose children are less likely to be vaccinated, such as conflict-affected and mobile populations. Third, the analysis dataset included only one child per household for more than 97% of households. As the paper objective was to estimate variance at the smallest administrative level, we did not attempt to model intra-household variation for the very small fraction of children who belonged to a single household. Fourth, although NFHS-5 vaccination estimates are representative at the district level, confidence intervals are wide. Extrapolation to the cluster level introduces additional uncertainty due to the small number of children contributing to each cluster estimate. We address the impact of uncertainty on our results through appropriate methodology, extensive sensitivity analysis, and cautious interpretation. Multilevel modelling approaches are statistical techniques to analyse a hierarchical (in our case, nested) data structure. A key assumption is that individuals who share membership in a specific level (e.g., state, district, clusters) may share characteristics relevant to vaccination outcomes, which result in outcomes for individuals in a similar geographic area being correlated. Based on the assumed data structure, empirical Bayes estimation systematically pulls outliers towards the population mean, with greater shrinkage where uncertainty is larger, due, for example, to small cluster size. This enables estimates for more uncertain clusters to borrow strength from others, yielding more stable estimates than reliance on the raw observed data. The approach is conservative as the shrunken estimates are always equal to or smaller than those obtained by direct estimation. In addition, we note that the number of clusters is large (more than 22,000 per analysis), and the estimates are not expected to be systematically biased (as they are drawn from a random sample). A specific challenge for our study relates to small cluster sizes, especially singleton clusters with only one child that experience greater shrinkage. Sensitivity analyses excluding singleton clusters and clusters with fewer than five children yielded very similar insights to those from the main analysis, demonstrating that general inferences are robust. While supporting cautious interpretation of point estimates, we believe that descriptive exploration of sub-district patterning from multilevel models can yield valuable insights and are confident in the overall patterns identified. That said, we acknowledge that results are dependent on unverified underlying distributional assumptions (conditional normality on the log odds scale). Fifth, we were unable to study protection against poliomyelitis adequately as data on inactivated polio vaccine were unavailable. However, as IPV is co-delivered with oral polio drops and requires delivery via injection, it is improbable that it has better coverage than OPV. Moreover, IPV was introduced relatively recently and coverage is still catching up. Our findings are thus unlikely to overstate polio-related immunity gaps. Finally, the NFHS-5 data offer a temporal snapshot of an evolving situation.6,13
Strengths and weaknesses in relation to other literature–differences in results
A multi-country study used Bayesian geospatial regression to generate globally comparable local estimates of routine first-dose measles-containing vaccine (MCV1) coverage for 101 LMICs from 2000 to 2019.8 Incorporating data from India’s 2015–2016 NFHS, it found that India had exemplary performance, achieving very high coverage and reducing geographic inequalities over time, and that a substantial proportion of India’s districts had achieved 95% MCV1 coverage.8 To advance learning by considering district and sub-district levels, we used multilevel models to produce smoothed estimates of sampled clusters and analysed their geospatial distribution. Our results affirm that a high proportion of India’s districts have achieved 95% MCV1 coverage; however, we found considerable sub-district variation. The coexistence of pockets of high and low coverage may permit subnational chains of infection to continue even when overall coverage is high.28 Corroborated by India’s large and disruptive measles outbreaks in 2022 and 2023,6,13 our findings support the hypothesis that subnational chains of infection can fuel outbreaks despite high aggregate coverage and are particularly important at near-elimination coverage levels.28 India has developed a new measles and rubella elimination response with a focus on district level implementation, tracking, and programme review.13 By contrast, our results suggest that a sub-district focus will be required for measles elimination.
Interpretation & conclusions
In India, where aggregate vaccine coverage levels are high, our findings support a fundamental strategic shift towards sub-district level strategies aimed at granular pockets of low immunity to increase vaccination coverage, advance disease-control priorities, and reduce inequities. India’s recently created “Ayushman Arogya Mandirs” (Health and Wellness Centres), with a territorial responsibility to deliver primary health care, may provide a natural niche for localised approaches.29
This tactical shift will require rapid adoption of new strategies accompanied by implementation-oriented research, to enable high-impact, precision approaches to close immunity gaps. Several approaches warrant consideration. Geospatial analyses, visualisations, and data triangulation can inform real-time strategic actions to improve vaccination coverage at precise spatial scales,30 requiring innovations in survey-based analyses and investments in digital data systems, such as India’s adoption of the U-WIN electronic immunisation registry in 2023. Given the low proportion of zero-dose children and substantial proportion of children with incomplete basic immunisation, a focus on reducing missed opportunities for vaccination is needed, with strategies tailored to local context. In addition to strengthening roll-out of the recently introduced 3rd dose of fractional IPV, hexavalent vaccine, which delivers protection against six diseases, including polio, in a single injection, and recently prequalified novel oral polio vaccine type 2 (nOPV2) should be considered for roll out to boost polio immunity and limit emergence of circulating vaccine-derived polioviruses. To further measles elimination efforts, as supported by India’s current measles-rubella elimination plan, MR-2 coverage should be strengthened.12 Once available, new measles-specific immunoglobulin M (IgM) Rapid Diagnostic Tests should be considered to facilitate agile surveillance and outbreak response31,32 and measles micro-array patches to expand vaccination coverage.33 Finally, as child under-vaccination reflects the interplay of numerous supply- and demand-related factors34 and is correlated with multiple deprivations,4 social scientific research is required to better understand and intervene on the complex factors underlying geographies of persistent vulnerability.
Contributors
Mira Johri: Conceptualization, Validation, Writing—original draft, Writing—review & editing Funding Acquisition. Sunil Rajpal: Formal analysis, Visualisation, Writing—review & editing. Rockli Kim, SV Subramanian: Conceptualization, Methodology, Writing—review & editing, Supervision. MJ and SR verified the underlying data. All authors had access to the study data and accept responsibility for the decision to submit.
Data sharing statement
This study is based on publicly available data from India’s National Family Health Survey. The datasets that support the findings of this study are available for download through the Demographic and Health Services (DHS) program’s data distribution system www.DHSprogram.com.
Declaration of interests
The authors declare that they have no competing interests, financial or otherwise, in completion of this work.
Acknowledgements
Financial support for this work was provided by the Canadian Institutes of Health Research (FRN #130280). The authors are grateful to Mohit Chaurasiya for his assistance in producing the choropleth maps. We would like to acknowledge the inputs of Dr. George Leckie, a professor of statistics faculty at the Centre for Multilevel Modelling at the University of Bristol, in responding to reviews. The authors would also like to thank the Demographic and Health Surveys program for making the National Family Health Survey data freely accessible.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2024.100504.
Appendix ASupplementary data
References
- 1.Immunization Agenda 2030 - framework for action (living document) 2020. 2023. https://www.immunizationagenda2030.org/framework-for-action [Google Scholar]
- 2.United Nations Department of Economic and Social Affairs Population Division . Online Edition. United Nations; New York, USA: 2024. World population prospects 2024. [Google Scholar]
- 3.Rajpal S., Kumar A., Johri M., Kim R., Subramanian S.V. Patterns in the prevalence of unvaccinated children across 36 states and union Territories in India, 1993-2021. JAMA Netw Open. 2023;6(2) doi: 10.1001/jamanetworkopen.2022.54919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johri M., Rajpal S., Subramanian S.V. Progress in reaching unvaccinated (zero-dose) children in India, 1992-2016: a multilevel, geospatial analysis of repeated cross-sectional surveys. Lancet Glob Health. 2021;9(12):e1697–e1706. doi: 10.1016/S2214-109X(21)00349-1. [DOI] [PubMed] [Google Scholar]
- 5.Gurnani V., Haldar P., Aggarwal M.K., et al. Improving vaccination coverage in India: lessons from Intensified Mission Indradhanush, a cross-sectoral systems strengthening strategy. BMJ. 2018;363 doi: 10.1136/bmj.k4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO/UNICEF . 2024. 2023 WHO/UNICEF estimates of national immunization coverage (WUENIC) (data as of 1 July 2024; estimates as of 15 July 2024)https://immunizationdata.who.int/ [Google Scholar]
- 7.Mosser J.F., Gagne-Maynard W., Rao P.C., et al. Mapping diphtheria-pertussis-tetanus vaccine coverage in Africa, 2000-2016: a spatial and temporal modelling study. Lancet. 2019;393(10183):1843–1855. doi: 10.1016/S0140-6736(19)30226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Local Burden of Disease Vaccine Coverage Collaborators Mapping routine measles vaccination in low- and middle-income countries. Nature. 2021;589(7842):415–419. doi: 10.1038/s41586-020-03043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utazi C.E., Chan H.M.T., Olowe I., et al. A zero-dose vulnerability index for equity assessment and spatial prioritization in low- and middle-income countries. Spatial Statistics. 2023;57 [Google Scholar]
- 10.International Institute for Population Sciences II, ICF . IIPS and ICF; Mumbai, India: 2022. India national family health survey NFHS-5 (2019-21) [Google Scholar]
- 11.World Health Organization . World Health Organization; Geneva: 2018. Vaccination coverage cluster surveys: reference manual (WHO/IVB/18.09). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 12.World Health Organization United Nations Children’s Fund (UNICEF) and Gavi the Vaccine Alliance . WHO, UNICEF and Gavi, the vaccine Alliance; New York: New York: 2023. The Big catch-up: an essential immunization recovery plan for 2023 and beyond. [Google Scholar]
- 13.World Health Organization . No. 2. 98 ed. World Health Organization; Geneva: 2023. Progress towards measles and rubella elimination – India, 2005–2021 - Jan 2023. Weekly epidemiologic record; pp. 19–28. [Google Scholar]
- 14.Moss W.J., Shendale S., Lindstrand A., et al. Feasibility assessment of measles and rubella eradication. Vaccine. 2021;39(27):3544–3559. doi: 10.1016/j.vaccine.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; Geneva: 2016. Polio vaccines: WHO position paper - March 2016. Weekly epidemiologic record, No.12; pp. 145–168. [Google Scholar]
- 16.Rajpal S., Kim J., Joe W., Kim R., Subramanian S.V. Small area variation in child undernutrition across 640 districts and 543 parliamentary constituencies in India. Sci Rep. 2021;11(1):4558. doi: 10.1038/s41598-021-83992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim R., Mohanty S.K., Subramanian S.V. Multilevel geographies of poverty in India. World Dev. 2016;87:349–359. [Google Scholar]
- 18.Leckie G., Charlton C. Runmlwin - a program to run the MLwiN multilevel modelling software from within Stata. J Stat Softw. 2013;52(11):1–40. [Google Scholar]
- 19.Stata Corporation . 2019. Stata release 16. College Station, TX. [Google Scholar]
- 20.Rajpal S., Johri M. Open Science Foundation (OSF); 2024. Small-area variation in child under-vaccination in India: a multilevel analysis of 36 states and Union Territories, 707 districts, and 22,349 small-area clusters (Analysis Files)https://osf.io/c6k5v/?view_only=838b78f7e1204a7283bb65cf0f8d2fc3 [Google Scholar]
- 21.Rasbash J., Charlton C., Browne W.J., Healy M., Cameron B. Centre for Multilevel Modelling, University of Bristol; 2009. MLwiN version 2.1. [Google Scholar]
- 22.Subramanian S.V., Ambade M., Kumar A., et al. Progress on sustainable development goal indicators in 707 districts of India: a quantitative mid-line assessment using the national family health surveys, 2016 and 2021. Lancet Reg Health Southeast Asia. 2023;13 doi: 10.1016/j.lansea.2023.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demographic and Health Surveys Program . The DHS Program; 2022. Spatial data repository: India. [Google Scholar]
- 24.Survey of India . 2022. Survey of India external boundary. [Google Scholar]
- 25.ESRI . 3 ed. Environmental Systems Research Institute; Redlands, CA: 2022. ArcGIS pro 3.0. [Google Scholar]
- 26.World Health Organization . 84 ed. World Health Organization; Geneva: 2009. Measles vaccines - WHO position paper. Weekly epidemiologic record, No. 35; pp. 349–360. [Google Scholar]
- 27.Polio eradication strategy 2022–2026: delivering on a promise. World Health Organization; Geneva: 2021. [Google Scholar]
- 28.Truelove S.A., Graham M., Moss W.J., Metcalf C.J.E., Ferrari M.J., Lessler J. Characterizing the impact of spatial clustering of susceptibility for measles elimination. Vaccine. 2019;37(5):732–741. doi: 10.1016/j.vaccine.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahariya C. Health & wellness centers to strengthen primary health care in India: concept, progress and ways forward. Indian J Pediatr. 2020;87(11):916–929. doi: 10.1007/s12098-020-03359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavi . 2020. Maintaining, Restoring & strengthening immunisation - gavi innovation catalogue Geneva, Switzerland: gavi, the vaccine alliance. [Google Scholar]
- 31.Gavi . Gavi, the Vaccine Alliance; Geneva, Switzerland: 2023. GAVI measles rapid diagnostic test deployment pilot study 042-2023-GAVI-RFP issue date 7 March 2023. [Google Scholar]
- 32.Brown D.W., Warrener L., Scobie H.M., et al. Rapid diagnostic tests to address challenges for global measles surveillance. Curr Opin Virol. 2020;41:77–84. doi: 10.1016/j.coviro.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasso-Agopsowicz M., Crowcroft N., Biellik R., et al. Accelerating the development of measles and rubella microarray patches to eliminate measles and rubella: recent progress, remaining challenges. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.809675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . 97 ed. World Health Organization; Geneva: 2022. Understanding the behavioural and social drivers of vaccine uptake WHO position paper – may 2022. Weekly epidemiologic record, No. 20; pp. 209–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.