Abstract
Background
Autistic individuals often have difficulty flexibly adjusting their behavior. However, laboratory experiments have yielded inconsistent results, potentially due to various influencing factors, which need to be examined in detail. This study aimed to investigate the hypothesis that the social content of stimuli could play a specific role in some of the flexibility challenges faced by autistic individuals. The second aim was to explore sex differences in this context.
Methods
We analyzed data from 256 adult participants (124 with autism), matched on age, gender, and sex, who performed an emotional shifting task involving unpredictable shifts between positive and negative stimuli. Additionally, the task included both social and non-social conditions.
Results
Our results revealed a larger switch cost in the social than in the non-social condition, and this was more pronounced in autistic than in non-autistic individuals. Furthermore, we observed that autistic females differed from autistic males in the non-social condition and from non-autistic females in the social condition.
Limitations
The online nature of the study reduced the control over participant conditions. In addition, further studies are needed to investigate whether these results apply to the broader autism spectrum.
Conclusions
Building on previous research demonstrating a greater switch cost in autistic than non-autistic individuals for socio-emotional stimuli, our study further extends these findings by highlighting that the social context, rather than the emotional nature of the stimuli alone, may play a significant role in the flexibility challenges faced by autistic individuals. Our findings also contribute to the literature on sex differences in autism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13229-024-00622-4.
Keywords: Autism, Social processing, Emotional processing, Flexibility, Predictive coding, Sex differences, Gender differences
Background
Some characteristics associated with autism, such as stereotyped behaviors, intolerance to uncertainty, and resistance to change, have been linked to difficulties in cognitive flexibility [1, 2]. Cognitive flexibility is a cognitive ability that encompasses the capacity to change perspectives, adjust behavior in response to changes, and shift between tasks and mental sets. For a broader conceptualization and more definitions, see [3]. It is a critical adaptive skill, usually evaluated through task shifting (or task switching), and involves other executive functions such as inhibition (of the prior task). Responses after a task switch are slower and usually more error-prone due to the associated cost, known as the “switch cost” [4].
Cognitive flexibility is often impaired in autistic individuals. Real-life assessments of flexibility using explicit questionnaires, such as the Behavioral Rating Inventory of Executive Function (BRIEF) [5, 6], highlight cognitive flexibility challenges in autism [7, 8]. However, studies employing cognitive flexibility tasks have yielded inconsistent findings [for reviews, see 9, 10]. Some tasks have not found differences between autistic and non-autistic individuals, while others indicate larger switch costs in autism after task switching, evidenced by increased response times and/or higher error rates [for a review, see 10]. These discrepancies are possibly due to task heterogeneity [11, 12]. Tasks involving unpredictable shifts, implicit rules, or social content may be more ecologically valid and better suited to highlighting the flexibility difficulties encountered by autistic individuals [13–15]. Autistic individuals may still adapt to predictable and explicit shifts [9].
Cognitive flexibility challenges in autism might contribute to impairments in social interactions, which is the second major domain of difficulties in autism [16]. Social interactions and facial emotion recognition, which are challenging for autistic individuals [17–19], require flexible adjustments to the situation and context. Interestingly, studies have shown a positive correlation between cognitive flexibility and facial emotion recognition [20], particularly in context [21], in autistic children. In addition, higher cognitive flexibility in non-autistic youths has been linked to greater social competence in interacting with others [22]. While these results suggest that these two abilities are linked, they have predominantly been investigated in independent tasks, making it difficult to precisely understand cognitive flexibility in autism during socio-emotional situations. Although one study has combined the two tasks [11], the results were inconclusive, possibly due to a lack of complexity arising from the predictability of the task or its lack of ecological validity.
A recent study compared the performance of autistic and non-autistic participants on a new task [23], called the Emotional Shifting Task (EST) [24]. Participants were asked to evaluate the valence of an emotional face, first out of and then in context. This context could either confirm (non-shift condition) or contradict (shift condition) the initial evaluation. In the latter case, participants had to adjust their initial prediction based on the context. While this task involved unpredictable shifts with implicit rules (as the potential shift has to be inferred by the participant according to the context) and socio-emotional content, participants also performed the Task Switching Task (TST) [25] in the same study. This latter task featured predictable shifts with explicit rules and non-social/non-emotional content. In line with their hypotheses, the authors observed a fall-off in correct responses and increased response times in autistic participants compared to non-autistic participants in the shift condition of the EST (i.e., increased switch cost in autism). This was not observed in the TST. While these findings suggest that flexibility difficulties in autism may be particularly pronounced in complex situations involving unpredictable and implicit shifts of socio-emotional stimuli, they do not tell us which specific factor (unpredictability, implicitness, social content, or emotional content) contributes most to the flexibility challenges of autistic individuals. More specifically, given the social difficulties encountered by autistic individuals, the results might be explained by the social content of the scenes and might be less pronounced or disappear altogether if emotional but non-social scenes were used. Little research has explored the behavioral or neural response to emotional non-social stimuli in autism, but those that have done so have tended to show preserved abilities [26]. This question will therefore have to be addressed.
Furthermore, the findings of Lacroix et al., [23] revealed sex differences, which were particularly evident on the EST. Autistic females exhibited an intermediate profile of emotion recognition in context, being faster than autistic males but slower than non-autistic females, on the EST and performing at the same level as non-autistic males. However, this effect did not interact with the shift condition. This result suggests that, rather than exhibiting superior cognitive flexibility, autistic females may simply process complex socio-emotional stimuli more effectively than autistic males. However, the results reported in the literature on this subject are mixed [27–29]. This finding is consistent with a growing body of research highlighting sex and gender differences in the phenotypes of autistic males and females, indicating that autistic females have better social abilities than autistic males for a comprehensive review and meta-analysis, refer to [30]. These differences might mirror sex differences found in the general population [31, 32]. While these results may find support in neurophysiological sex differences in face processing in autism [33, 34], the evidence remains limited and requires replication.
The aim of the current study was to better investigate the role of social content in explaining the results reported by Lacroix et al. [23] and, more broadly, to understand the role of social content in flexibility difficulties in autism during emotional situations. To this end, we developed a new task based on the EST (referred to as EST-2) that includes both social and non-social emotional stimuli. Our main hypothesis is that autistic individuals will find it more difficult to shift perspective (adjusting their predictions) than non-autistic individuals, particularly in response to social stimuli. This would translate into fewer correct responses and/or longer response times. Additionally, we anticipated that autistic females would experience fewer difficulties in this regard than autistic males (i.e., increased number of correct responses/shorter response times). Finally, the literature makes it clear that negative stimuli are more difficult to process than positive stimuli [35], especially for autistic participants [18, 36, 37]. Since our task comprised both positive and negative stimuli, we controlled for this factor and made the complementary hypothesis that lower performances would be observed for negative than positive stimuli, particularly in the autistic group. Such an observation would be consistent with the findings of Lacroix et al. [23]. These hypotheses were preregistered and a more detailed version of the expected main effects and interaction effects is provided in the pre-registration document.
Method
Participants
A total of 274 participants, aged between 18 and 45 years old, completed the entire study. The decision to choose participants aged 18 to 45 takes account of the late maturation and early decline of executive function. In accordance with our exclusion criteria outlined in the preregistration document, 5 non-autistic participants were excluded because they had an autism quotient over 32. Additionally, 7 autistic participants and 1 non-autistic participant were excluded because their correct response score for emotion recognition without context was below 60%. Considering the investigation of sex and gender differences, the 5 autistic transgender individuals (female-to-male) were also excluded from the analyses as there were not enough of them to perform group comparisons. Thus, participants were matched on sex and gender and were all cisgender. The final sample comprised 124 autistic participants (63 females) and 132 non-autistic participants (70 females). Autistic participants were recruited with the assistance of specialized centers, physicians, autism-focused associations, and social networks. Participants recruited via the latter two channels had to contact us to receive the study link, allowing us to verify their formal diagnosis. Non-autistic participants were recruited through social networks and, similarly to autistic participants, they first had to send us an email, allowing us to verify that they met the inclusion criteria. Non-autistic participants were not enrolled in the study if they suspected themselves of being autistic or if they had a close family member (child, parent, or sibling) on the autism spectrum. Participants were not included if they were undergoing medical treatment that could alter their cognitive function.
Material and procedure
The study was conducted online using the PsyToolkit platform [38, 39]. At the beginning of the study, participants were presented with an informed consent form, which they had to approve before proceeding. They were also instructed to ensure they were in a quiet and undisturbed environment during the tasks. They were asked to provide demographic information including sex, age, and diagnoses (i.e., autism diagnosis and other psychiatric/neurological diagnoses or other neurodevelopmental diagnoses). Following this, participants performed two tasks: the EST-2 and another task for a separate research question. The tasks were presented in a randomized order. After this, the participants were asked to complete the Autism Spectrum Questionnaire. Finally, to assess the validity of the experimental condition, participants were asked whether they had been disturbed in any way during the tasks and invited to provide any comments.
The emotional shifting Task-2 (EST-2)
This task, lasting 10 min, was composed of 72 pairs of emotional stimuli, half of which were social (i.e., involving humans), and the other half not social (i.e., not involving humans). In addition, in each condition, half of the stimuli had a positive valence and the other half had a negative valence. The stimuli used were selected from the following databases: Natural Disasters Picture System [40], International Affective Picture System [41], Geneva Affective Picture Database [42], Nencki Affective Picture System [43], and Open Affective Standardized Image Set [44]. Further images were also taken from the internet (copyright-free images). The images were selected after a series of pretests regarding their valence and arousal, as described in Supplementary Materials. There was no significant difference in arousal between social and non-social stimuli.
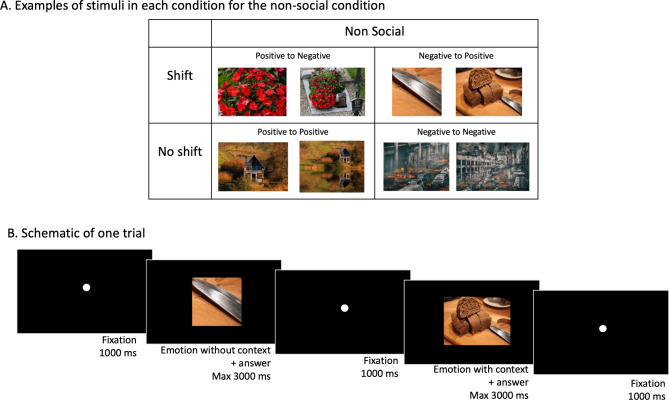
During the EST-2, each trial started with the display of a cropped image. For the social stimuli, the image was cropped around a human body part (usually the face), and for the non-social stimuli, around a meaningful part of the image (See Fig. 1. A). Participants were asked to evaluate the valence of these cropped stimuli as negative or positive. The image then appeared uncropped and the participants were asked to evaluate the valence of the uncropped image. In half of the trials in each condition (i.e., social and non-social), the valence of the cropped and uncropped images were congruent, corresponding to the non-shift condition. In the other half, they were incongruent, corresponding to the shift condition. Hence, the plan included 9 stimulus pairs x 2 social conditions x 2 emotions x 2 shift conditions (see Fig. 1. A for example stimuli in the non-social condition). Figure 1. B presents a trial in schematic form. All stimulus pairs were randomized.
Fig. 1.
(A) Stimuli. Examples of pairs of stimuli in each condition for non-social stimuli for the Emotional Shifting Task, i.e., shift vs. nonshift x positive vs. negative (for the second image). (B) Procedure. Schematic presentation of one trial. All stimulus images are freely available for use, with a CC0 public domain license for the shift images and a Pixabay content license for the non-shift images
The Autism Spectrum Quotient [AQ − 45]. The AQ is a 50-item scale related to autistic traits. Five areas are evaluated and include 10 questions each: social skills, attention switching, attention to detail, communication, and imagination. Participants rate their agreement with statements on a 4-point Likert scale, going from “definitely agree” to “definitely disagree”. Each question is rated 0 or 1. A score of 32 or above is associated with high autistic traits.
Analyses
All analyses were conducted using R [46] and RStudio [47] version 2023.12.1 + 402. We compared the four subgroups of our sample (autistic males, autistic females, non-autistic males, and non-autistic females) using Pearson’s Chi-square test with Yale’s correction for categorical variables and linear models for continuous variables. For post-hoc comparisons of significant differences in categorical and continuous variables, we employed the Chi-square test of association and Tukey’s HSD, respectively.
We used the lme4 package [48] to analyze the EST-2 on the basis of accuracy, defined as the number of correct responses, and response time (RT) for correct responses on the uncropped images, when participants had provided a correct answer on the cropped images. The analysis of accuracy in response to the cropped images is presented in Supplementary Materials. A mixed-effects logistic regression was employed for the analysis of accuracy, given the binomial nature of this dependent variable, while a generalized mixed-effects model with an inverse Gaussian function was used for RT analyses [49]. Results tables are presented in Supplementary Materials. The models included group (autism vs. no autism), sex (males vs. females), shift condition (non-shift vs. shift), social condition (non-social vs. social), and emotion (negative vs. positive) as fixed effects, along with psychiatric/neurological and neurodevelopmental diagnoses other than autism as covariates, as some of the associated conditions can impair executive functions (e.g., ADHD).
The random effects structure was kept maximal and was reduced in the case of model non-convergence [50]. Due to the absence of a standardized approach for computing effect sizes in mixed models, unstandardized effect sizes are presented [51]. They correspond to 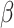 coefficient values, as each factor within the model involved dichotomous variables contrast-coded as -0.5 and 0.5, thus making their interpretation straightforward. Significant interactions are plotted and described with the emmeans package [52], using estimated marginal means (i.e., the estimation of each effect devoid of the influence of other factors accounted for in the statistical model) and 95% confidence intervals [53, 54]. Pairwise comparisons using estimated marginal means and error were also conducted, employing Tukey adjustment to estimate differences (
coefficient values, as each factor within the model involved dichotomous variables contrast-coded as -0.5 and 0.5, thus making their interpretation straightforward. Significant interactions are plotted and described with the emmeans package [52], using estimated marginal means (i.e., the estimation of each effect devoid of the influence of other factors accounted for in the statistical model) and 95% confidence intervals [53, 54]. Pairwise comparisons using estimated marginal means and error were also conducted, employing Tukey adjustment to estimate differences (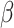 values). As pre-registered, correlations with AQ were performed as complementary analyses. They are presented in Supplementary Materials.
values). As pre-registered, correlations with AQ were performed as complementary analyses. They are presented in Supplementary Materials.
Results
Participants
Demographics of the final sample are summarized in Table 1. There were no significant differences in age between groups. However, disparities emerged in educational attainment, with autistic males exhibiting lower levels than both non-autistic males (p < 0.001) and females (p = 0.03). As anticipated, significant differences were found in AQ scores, with autistic individuals scoring higher than non-autistic counterparts (all p < 0.001). Furthermore, non-autistic males had higher AQ scores than non-autistic females (p = 0.03). Autistic males received their diagnosis at a younger age than autistic females (p < 0.001). Lastly, autistic participants presented with a greater number of comorbid diagnoses than non-autistic participants (all p < 0.001).
Table 1.
Demographics. Mean value, standard deviation (SD), and range for age, education, AQ scores, age at the diagnosis of autism, as well as the percentage of participants with a diagnosis other than autism for each group, and group comparison. AQ = autism quotient, N-miss = number of missing data
| Autistic Females (N = 63) | Autistic Males (N = 61) | Non-Autistic Females (N = 70) | Non-Autistic Males (N = 62) | p value | |
|---|---|---|---|---|---|
| Age | 0.066 | ||||
| Mean (SD) | 35.4 (6.8) | 32.1 (7.7) | 34.1 (7.7) | 35.0 (7.1) | |
| Range | 19.0–45.0 | 18.0–45.0 | 19.0–45.0 | 20.0–45.0 | |
| Educational Attainment | 0.002 | ||||
| Mean (SD) | 14.5 (1.9) | 13.7 (2.0) | 14.5 (1.1) | 14.9 (1.6) | |
| Range | 9.0–17.0 | 9.0–17.0 | 12.0–17.0 | 9.0–17.0 | |
| AQ | < 0.001 | ||||
| Mean (SD) | 38.2 (5.3) | 36.7 (5.8) | 14.3 (7.6) | 17.5 (7.5) | |
| Range | 24.0–49.0 | 25.0–50.0 | 1.0–32.0 | 1.0–32.0 | |
| Diagnostic age | |||||
| N-Miss | 1 | 0 | < 0.001 | ||
| Mean (SD) | 34.9 (6.7) | 28.7 (10.2) | |||
| Range | 19.0–46.0 | 4.0–47.0 | |||
| Psychiatric/neurological Diagnosis | < 0.001 | ||||
| No | 46 (73.0%) | 47 (77.0%) | 70 (100.0%) | 62 (100.0%) | |
| Yes | 17 (27.0%) | 14 (23.0%) | 0 (0.0%) | 0 (0.0%) | |
| Neurodevelopmental Diagnosis (other than autism) | < 0.001 | ||||
| No | 48 (76.2%) | 43 (70.5%) | 66 (94.3%) | 59 (95.2%) | |
| Yes | 15 (23.8%) | 18 (29.5%) | 4 (5.7%) | 3 (4.8%) |
EST-2
Analyses on accuracy
An analysis of accuracy based on rated valence of the picture with context, following a correct answer on the picture without context, revealed lower odds of correct responses in the shift than in the non-shift condition (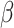 = -2.9, 95% CI [-3.45, -2.34], p < 0.001), and in the social than in the non-social condition (
= -2.9, 95% CI [-3.45, -2.34], p < 0.001), and in the social than in the non-social condition (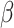 = -0.66, 95% CI [-1.2, -0.13], p = 0.016). In addition, autistic participants had lower odds of making a correct response (
= -0.66, 95% CI [-1.2, -0.13], p = 0.016). In addition, autistic participants had lower odds of making a correct response (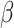 = 0.3, 95% CI [0.06, 0.54], p = 0.013) than non-autistic participants.
= 0.3, 95% CI [0.06, 0.54], p = 0.013) than non-autistic participants.
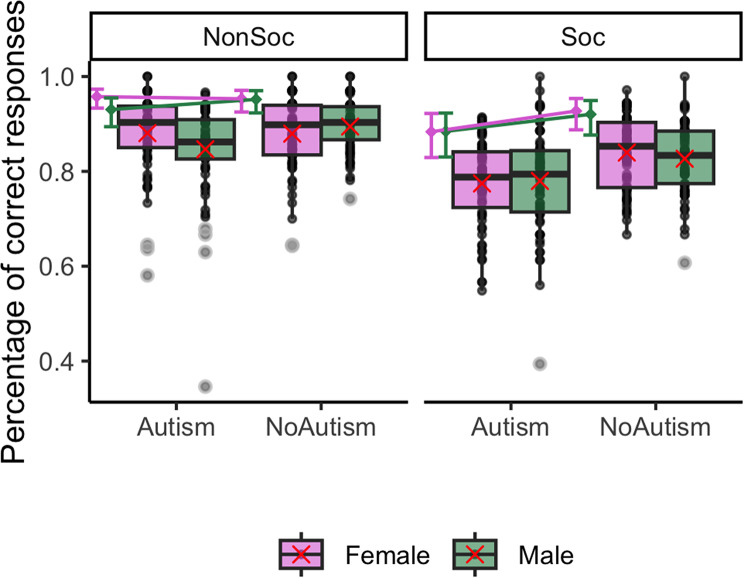
These main effects were further qualified by an interaction effect between group, sex and the social nature of the stimuli on the odds of correct responses (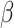 = 0.61, 95% CI [0.06, 1.15], p = 0.029). Post-hoc comparisons revealed a significantly higher odds of correct responses in autistic females than autistic males in the non-social condition (
= 0.61, 95% CI [0.06, 1.15], p = 0.029). Post-hoc comparisons revealed a significantly higher odds of correct responses in autistic females than autistic males in the non-social condition (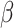 = -0.52, 95% CI [-1.02, -0.03], p = 0.035), not observed in the social condition (
= -0.52, 95% CI [-1.02, -0.03], p = 0.035), not observed in the social condition (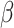 = 0.01, 95% CI [-0.42, 0.44], p = 1) and not observed in the non autistic group (non-autistic females vs. non autistic males in the non-social condition:
= 0.01, 95% CI [-0.42, 0.44], p = 1) and not observed in the non autistic group (non-autistic females vs. non autistic males in the non-social condition: 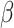 = -0.03, 95% CI [-0.52, 0.47], p = 0.999; in the social condition:
= -0.03, 95% CI [-0.52, 0.47], p = 0.999; in the social condition: 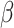 = -0.1, 95% CI [-0.55, 0.35], p = 0.943). Post-hoc comparisons also revealed a lower odds of correct responses in autistic females than non-autistic females in the social condition (
= -0.1, 95% CI [-0.55, 0.35], p = 0.943). Post-hoc comparisons also revealed a lower odds of correct responses in autistic females than non-autistic females in the social condition (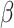 = -0.52, 95% CI [-0.98, -0.06], p = 0.02), not observed in the non-social condition (
= -0.52, 95% CI [-0.98, -0.06], p = 0.02), not observed in the non-social condition (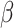 = 0.11, 95% CI [-0.42, 0.64], p = 0.955). This was not observed in males (autistic vs. non-autistic males in the non-social condition:
= 0.11, 95% CI [-0.42, 0.64], p = 0.955). This was not observed in males (autistic vs. non-autistic males in the non-social condition: 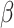 = -0.39, 95% CI [-0.9, 0.12], p = 0.196; in the social condition:
= -0.39, 95% CI [-0.9, 0.12], p = 0.196; in the social condition: 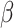 = -0.41, 95% CI [-0.88, 0.06], p = 0.116). This interaction is depicted in Fig. 2.
= -0.41, 95% CI [-0.88, 0.06], p = 0.116). This interaction is depicted in Fig. 2.
Fig. 2.
Interaction between group, sex, and social content for correct responses. The box plots represent the observed median correct response rates for emotion recognition with context, with the interquartile range and individual data points for each condition. The red crosses represent the observed means. In addition, the colored points represent the estimated marginal means with their 95% CI
Analyses on RT
Analyses showed longer RT in the shift than in the non-shift condition (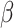 = 158, 95% CI [137, 178], p < 0.001) and in the social than in the non-social condition (
= 158, 95% CI [137, 178], p < 0.001) and in the social than in the non-social condition (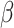 = 61, 95% CI [12, 111], p = 0.016). Autistic participants were slower than non-autistic participants (
= 61, 95% CI [12, 111], p = 0.016). Autistic participants were slower than non-autistic participants (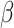 = -90, 95% CI [-110, -69], p < 0.001). Additionally, RT were longer for negative than positive stimuli (
= -90, 95% CI [-110, -69], p < 0.001). Additionally, RT were longer for negative than positive stimuli (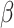 = -66, 95% CI [-85, -47], p < 0.001) and for females than males (
= -66, 95% CI [-85, -47], p < 0.001) and for females than males (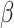 = 19, 95% CI [2, 37], p = 0.031).
= 19, 95% CI [2, 37], p = 0.031).
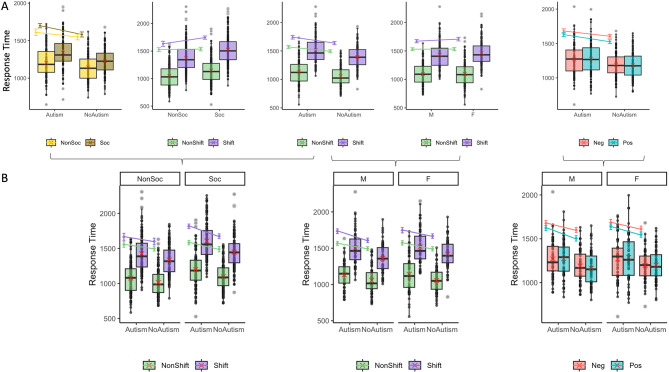
The main effects on RT were qualified by significant two-way and three-way interaction effects, which are presented both here and in Fig. 3. First, the interaction effect between group and shift (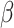 = -27, 95% CI [-43, -10], p = 0.001) revealed that the difference between the shift and non-shift condition was larger in autism (
= -27, 95% CI [-43, -10], p = 0.001) revealed that the difference between the shift and non-shift condition was larger in autism (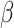 = -171, 95% CI [-199, -144], p < 0.001) than in non-autistic individuals (
= -171, 95% CI [-199, -144], p < 0.001) than in non-autistic individuals (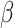 = -144, 95% CI [-174, -114], p < 0.001). In addition, the interaction effect between the group and the social nature of the stimuli (
= -144, 95% CI [-174, -114], p < 0.001). In addition, the interaction effect between the group and the social nature of the stimuli (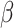 = -55, 95% CI [-74, -36], p < 0.001) indicated that the difference in RT between social and non-social stimuli was also larger in autism (
= -55, 95% CI [-74, -36], p < 0.001) indicated that the difference in RT between social and non-social stimuli was also larger in autism (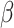 = -89, 95% CI [-153, -24], p = 0.002) than in non-autistic participants (
= -89, 95% CI [-153, -24], p = 0.002) than in non-autistic participants (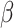 = -34, 95% CI [-102, 34], p = 0.579). Finally, the interaction between the shift and the social nature of the stimuli (
= -34, 95% CI [-102, 34], p = 0.579). Finally, the interaction between the shift and the social nature of the stimuli (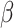 = 103, 95% CI [85, 121], p < 0.001) revealed that the RT difference between social and non-social stimuli was larger in the shift (
= 103, 95% CI [85, 121], p < 0.001) revealed that the RT difference between social and non-social stimuli was larger in the shift (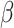 = -113, 95% CI [-181, -44], p < 0.001) than in the non-shift condition (
= -113, 95% CI [-181, -44], p < 0.001) than in the non-shift condition (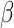 = -10, 95% CI [-73, 54], p = 0.979). However, these three interactions were qualified by a three-way interaction between group, shift and the social nature of the stimuli (
= -10, 95% CI [-73, 54], p = 0.979). However, these three interactions were qualified by a three-way interaction between group, shift and the social nature of the stimuli (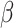 = -42, 95% CI [-59, -25], p < 0.001) indicating that while the difference between the shift and non-shift conditions was larger in the autistic than in the non-autistic individuals, this difference was more pronounced in the social than in the non-social condition (shift vs. no shift in autism and non-social condition:
= -42, 95% CI [-59, -25], p < 0.001) indicating that while the difference between the shift and non-shift conditions was larger in the autistic than in the non-autistic individuals, this difference was more pronounced in the social than in the non-social condition (shift vs. no shift in autism and non-social condition: 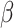 = -109, 95% CI [-147, -72], p < 0.001; in autism and social condition:
= -109, 95% CI [-147, -72], p < 0.001; in autism and social condition: 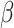 = -233, 95% CI [-267, -199], p < 0.001; in no-autim and non-social condition:
= -233, 95% CI [-267, -199], p < 0.001; in no-autim and non-social condition: 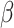 = -103, 95% CI [-145, -62], p < 0.001; in no-autism and social condition:
= -103, 95% CI [-145, -62], p < 0.001; in no-autism and social condition: 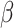 = -185, 95% CI [-221, -150], p < 0.001).
= -185, 95% CI [-221, -150], p < 0.001).
Fig. 3.
Significant interactions on RT. The box plots represent the observed median response time (RT) for emotion recognition with context, with the interquartile range and individual data points for each condition. The red crosses represent the observed means. In addition, the colored points represent the estimated marginal means with their 95% CI. The first line (A) represents the significant two-way interactions and the second line (B) represents the significant three-way interactions (relative to the corresponding two-way interactions). M = Males; F = Females; Neg = Negative stimuli (with context); Pos = Positive stimuli (with context); Soc = Social stimuli, NonSoc = Non-social stimuli
In addition, a significant interaction between group and emotion (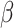 = -28, 95% CI [-50, -6], 95% CI [-50, -6], p = 0.012) showed that the difference in RT between negative and positive stimuli was more pronounced in non-autistic (
= -28, 95% CI [-50, -6], 95% CI [-50, -6], p = 0.012) showed that the difference in RT between negative and positive stimuli was more pronounced in non-autistic (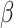 = 80, 95% CI [50, 110], p < 0.001) than in autistic individuals (
= 80, 95% CI [50, 110], p < 0.001) than in autistic individuals (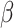 = 52, 95% CI [25, 79], p < 0.001). This interaction was qualified by a three-way interaction between group, sex and emotion (
= 52, 95% CI [25, 79], p < 0.001). This interaction was qualified by a three-way interaction between group, sex and emotion (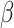 = 24, 95% CI [0, 49], p = 0.05), indicating that this slower response time for negative than positive stimuli in non-autistic individuals was more pronounced in males (positive vs. negative stimuli in non-autistic males:
= 24, 95% CI [0, 49], p = 0.05), indicating that this slower response time for negative than positive stimuli in non-autistic individuals was more pronounced in males (positive vs. negative stimuli in non-autistic males: 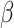 = 95, 95% CI [58, 133], p < 0.001, autistic males:
= 95, 95% CI [58, 133], p < 0.001, autistic males: 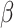 = 55, 95% CI [18, 93], p < 0.001, non-autistic females:
= 55, 95% CI [18, 93], p < 0.001, non-autistic females: 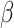 = 65, 95% CI [22, 108], p < 0.001, autistic females:
= 65, 95% CI [22, 108], p < 0.001, autistic females: 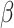 = 49, 95% CI [14, 84], p = 0.001).
= 49, 95% CI [14, 84], p = 0.001).
Finally, there was an interaction between shift and sex (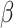 = 34, 95% CI [13, 55], p = 0.002), indicating that the difference in RT between the shift and non-shift conditions was smaller in males (
= 34, 95% CI [13, 55], p = 0.002), indicating that the difference in RT between the shift and non-shift conditions was smaller in males (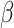 = -141, 95% CI [-171, -111], p < 0.001) than females (
= -141, 95% CI [-171, -111], p < 0.001) than females (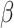 = -175, 95% CI [-204, -145], p < 0.001). However, this interaction, along with the aforementioned interaction between group and shift, was further qualified by a three-way interaction involving group, shift, and sex (
= -175, 95% CI [-204, -145], p < 0.001). However, this interaction, along with the aforementioned interaction between group and shift, was further qualified by a three-way interaction involving group, shift, and sex (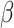 = 56, 95% CI [39, 73], p < 0.001). This revealed that the reduced difference in RT between the shift and the non-shift conditions in males compared to females was more pronounced in the non-autistic group than it was in the autistic group (shift vs. no shift in ASD Females:
= 56, 95% CI [39, 73], p < 0.001). This revealed that the reduced difference in RT between the shift and the non-shift conditions in males compared to females was more pronounced in the non-autistic group than it was in the autistic group (shift vs. no shift in ASD Females: 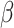 = -174, 95% CI [-212, -136], p < 0.001; ASD Males:
= -174, 95% CI [-212, -136], p < 0.001; ASD Males: 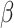 = -168, 95% CI [-206, -131], p < 0.001; non-autistic females:
= -168, 95% CI [-206, -131], p < 0.001; non-autistic females: 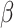 = -175, 95% CI [-213, -137], p < 0.001; non-autistic males:
= -175, 95% CI [-213, -137], p < 0.001; non-autistic males: 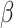 = -113, 95% CI [-153, -74], p < 0.001).
= -113, 95% CI [-153, -74], p < 0.001).
Discussion
The main goal of the study was to explore whether the social nature of stimuli significantly influences the performance of autistic individuals during a socio-emotional flexibility task. We also aimed to investigate sex differences within this context. In line with our hypothesis, our results indicated that autistic individuals find it more difficult than non-autistic individuals to adjust their predictions to emotional contexts, particularly when the context is social. Additionally, we found sex differences: autistic females performed more accurately than autistic males on non-social stimuli (and did not differ from non-autistic females), but performed less accurately than non-autistic females on social stimuli (and did not differ from autistic males). These findings have implications for understanding cognitive flexibility in autism and the unique profile of autistic females.
In the current study, participants were asked to first evaluate the emotional valence of a cropped image and then to reassess the same image within its contextual setting. Our analyses revealed that participants experienced significant difficulty in accurately and swiftly reassessing the emotional valence when it differed from their initial evaluation, indicating that this re-evaluation was associated with a significant switch cost. This suggests that the task effectively captured the demanded cognitive flexibility. Additionally, we found a significant interaction between shift condition and participant group in RT, with autistic individuals showing a more pronounced switch cost than non-autistic individuals. Autistic participants found it more challenging to adjust to unexpected socio-emotional contexts, as evidenced by their longer RTs. These findings replicate earlier research on both non-autistic [24, 55] and autistic participants [23] using similar tasks, but with new stimuli and participants. This underscores the robustness of the Emotional Shifting Task (EST) and its extended version (EST-2), as well as the reliability of the observed results.
As outlined previously, the primary goal of this study was to examine the impact of the social nature of stimuli on our results. Our findings revealed that emotional stimuli with social content significantly reduced accuracy and increased RT. This observation is consistent with previous research showing slower RTs for social versus non-social affective scenes, as reported in the COMPASS database [56]. However, our study extends these findings by demonstrating an interaction between the shift condition and the social nature of the stimuli. More specifically, social stimuli led to a greater switch cost than non-social stimuli, as reflected by the RT, even though arousal levels were balanced between the two types of stimuli. Our results, which were obtained using naturalistic stimuli, provide additional evidence that social stimuli can convey more ambiguous emotional signals than non-social stimuli [57], particularly when the context deviates from the initially predicted emotion. Alternatively, this might also suggest that social information captures attention more strongly, making it more costly to disengage attention and, consequently, leading to slower RTs [56].
In line with our preregistered hypotheses, the results revealed significantly longer RTs for social stimuli than for non-social stimuli, particularly in autistic rather than non-autistic individuals, as is indicated by a significant interaction between group and the social nature of the stimuli. This increased difficulty in processing social stimuli is consistent with a range of challenges faced by autistic individuals when processing social situations. These include reduced attention to faces and eyes, delayed N170 responses to faces, decreased brain activation during face processing, and difficulties in emotion recognition for meta-analyses see [58–61]. While research on behavioral responses to non-social emotional stimuli in autism is limited, our findings are also compatible with those of existing studies that have reported normative behavior or physiological responses (e.g., skin conductance) to non-social emotionally arousing stimuli in autistic individuals [26, 62].
The interaction was further nuanced by a significant three-way interaction between group, shift, and the social nature of the stimuli, showing that the switch cost reflected by the RTs was particularly pronounced for social stimuli in autistic individuals. These findings are consistent with the everyday difficulties experienced by autistic individuals in adjusting to social contexts, which are often marked by unpredictability. The results also echo previous conclusions suggesting that autistic adults may rely on learned patterns for social evaluation [63]. This would reduce their ability to adjust to social situations if they are not congruent with the learned pattern. Shifting trials would be one example of such a situation [63].
These results could also be interpreted in the context of the enhanced perceptual functioning theory of autism [64], which suggests that autistic individuals are more inclined to process local rather than global features. Since the processing of the scenes in our task required a more global approach than the processing of the cropped image, this might explain the increased switch cost in autism. However, the similar switch cost observed for non-social stimuli in both autistic and non-autistic participants suggests that autistic individuals can process the global scene and can recognize and adapt to unpredictable emotional changes in non-social contexts. This finding reduces the likelihood that enhanced perceptual functioning alone explains the results. Alternatively, the higher level of ambiguity associated with social cues might contribute to the observed difficulties [14, 65].
Overall, these results replicate, and more importantly extend the findings of Lacroix et al. [23], who used a similar task with socio-emotional stimuli and showed a larger increase in RT in the shift as measured against the non-shift condition in autistic versus non-autistic participants. Our study further shows that this pattern is specifically associated with social stimuli.
While emotion recognition is typically faster in females than in males, including in the field of autism research [23, 66, but 67], this effect was not replicated in our study. Instead, males responded faster than females. We also observed a two-way interaction between shift and sex on RT, as well as three-way interactions between group, shift, and sex on RT, indicating a reduced difference in RT between the shift and non-shift conditions in males, and particularly in non-autistic individuals. This reduced switch cost in males suggests higher emotional flexibility abilities, especially in non-autistic individuals. Since this effect was not found by Lacroix et al. [23], it might have been driven by the inclusion of non-social stimuli in our study. However, this is unlikely given the absence of an interaction between sex, shift, and the social nature of the stimuli. Additional research is needed to better understand this effect and examine whether it may be consistent with the literature suggesting sex differences in the way non-autistic individuals process emotional signals, due to socio-cultural and biological differences [31].
Furthermore, the results revealed an effect of the three-way interaction between group, sex and the social nature of the stimuli on accuracy. Like Lacroix et al. [23], our results did not indicate sex differences in accuracy for social stimuli. However, the inclusion of emotional non-social stimuli in this new task revealed higher accuracy for autistic females than for autistic males, a difference not observed in non-autistic individuals. Additionally, autistic females were less accurate than non-autistic females in the social condition, an effect not seen in males or in the non-social condition. These findings differ from Lacroix et al. [23], who observed lower accuracy only in autistic compared to non-autistic males. Therefore, our results should be interpreted with caution. Nevertheless, they suggest an intermediate profile for autistic females: while they struggle more than non-autistic females with social stimuli, they have fewer difficulties with non-social stimuli than autistic males. This pattern, showing characteristics of both autistic and non-autistic individuals, has been observed in other studies [33, 34, 68]. This finding could indicate that autistic females adapt better to real-life situations, although they still face challenges in social contexts.
Finally, our analyses revealed longer RT when the emotional context was negative, particularly among non-autistic individuals and especially in males. This finding contradicts our preregistered complementary hypothesis that this effect would be more pronounced in autism. However, no interaction with the shift condition was observed, an observation which differs from previous findings [23, 24]. Despite this, our results are consistent with the existing literature indicating shorter reaction times for positive stimuli in both non-autistic [35] and autistic individuals [for a review, see 18].
Limitations
The online nature of the study constitutes a limitation, as it reduces control over diagnosis and experimental conditions. However, participants were carefully selected to mitigate this issue (see Method). Despite this limitation, online research remains an appropriate method for studying autistic individuals, and particularly those without intellectual disabilities, as it mitigates the anxiety related to unfamiliar situations and social interactions [69].
Due to the requirements of the ethical committee and the General Data Protection Regulation, we were unable to ask specifically about other diagnoses. We asked general questions (e.g., “Have you been diagnosed with another neurodevelopmental disorder such as ADHD, dyslexia, dyspraxia, etc.?”) to minimize the online collection of sensitive information. This approach, while necessary, prevented a more precise understanding of which comorbidities might influence the results. Conditions such as ADHD [70], depression [71], and anxiety [72] are known, in particular, to affect task switching and processing speed. Nevertheless, comorbidities were included as covariates in our analyses, ensuring that our results account for the variance explained by other diagnoses. Thus, even though autistic and non-autistic individuals differed in the number of comorbidities, a finding consistent with the existing literature [73], this was taken into account in our analyses.
Certain socio-demographic differences may also be perceived as weaknesses. Specifically, autistic males exhibited lower educational attainment than the other groups. However, educational achievement in autism often falls below the levels expected based on IQ [74], and the mean educational attainment of autistic males in our study corresponds to the second year of a bachelor’s degree program. Although our sample appears homogeneous in terms of intellectual abilities, it does not fully represent the entire autism spectrum, a common limitation in studies involving autistic individuals.
Conclusions
Our study demonstrates that autistic individuals exhibit a larger switch cost than non-autistic individuals when evaluating the emotional content of an image in an unpredictable context, thus replicating previous findings. Importantly, this discrepancy is only evident for social stimuli. In non-social contexts, autistic individuals show no difference in switch cost compared to non-autistic individuals. These results suggest that the cognitive flexibility of autistic individuals is not inherently impaired; instead, their difficulty is modulated by the nature of the stimuli. This likely contributes to the mixed results observed when investigating flexibility abilities in autistic individuals and points to possible ways to better determine which other factors, beyond the social nature of the stimuli, contribute to their difficulties.
Additionally, our findings highlight the fact that autistic females differ both from autistic males (in the processing of non-social stimuli) and non-autistic females (in the processing of social stimuli). This result underscores the unique profile of autistic females, emphasizing the need for a better understanding and recognition of their experiences and the link between this profile and their under-recognition and under-diagnosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants for their participation in this study. We also thank the GNCRA and all CRA, expertise centers, and clinicians who helped with the recruitment.
Author contributions
Funding: MB, AL, MM. Conceptualization and implementation: AL, YBD, and MM. Data collection: AL, YBD Data curation and analysis: AL and YBD. Data interpretation: AL, YBD, MM, MB, MG, VM, FD, BM. Writing and revising the paper: AL, YBD, MM, VM, MB, MG, FD, BM. Supervision: MM, MB, MG, and FD.
Funding
This study was supported by a grant by the French Ministry of Higher Education, Research and Innovation (France) to Adeline Lacroix. This work was also supported by MIAI@Grenoble Alpes (ANR-19-P3IA-0003) and by the CerCoG@UGA IDEX UGA (ANR-15-IDEX-02).
Data availability
This study was preregistered on OSF. The datasets generated and analyzed during the current study, as well as the images used as stimuli, are available in the OSF repository: https://osf.io/7n8gm/?view_only=395f108fb0c74d8f96a0f86179f73ef5.
Declarations
Ethics approval and consent to participate
At the beginning of the study, participants were presented with an informed consent form, which they had to approve before proceeding. All procedures performed in this study involving human participants were conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the study was approved by the local ethics committee (CER-Grenoble Alpes, COMUE University Grenoble Alpes, IRB00010290).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mostert-Kerckhoffs MAL, Staal WG, Houben RH, de Jonge MV. Stop and Change: Inhibition and Flexibility Skills Are Related to Repetitive Behavior in Children and Young Adults with Autism Spectrum Disorders. J Autism Dev Disord. 2015;45:3148–58. 10.1007/s10803-015-2473-y [DOI] [PMC free article] [PubMed]
- 2.Lei J, Charman T, Leigh E, Russell A, Mohamed Z, Hollocks MJ. Examining the relationship between cognitive inflexibility and internalizing and externalizing symptoms in autistic children and adolescents: A systematic review and meta-analysis. Autism Res. 2022;15:2265–95. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10092776/ [DOI] [PMC free article] [PubMed]
- 3.Ionescu T. Exploring the nature of cognitive flexibility. New Ideas in Psychology. 2012;30:190–200. https://www.sciencedirect.com/science/article/pii/S0732118X11000705
- 4.Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–40. https://www.cell.com/trends/cognitive-sciences/abstract/S1364-6613(03)00028-7 [DOI] [PubMed]
- 5.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Child Neuropsychology (Neuropsychology, Development and Cognition: Section C). 2000;6:235–8. 10.1076/chin.6.3.235.3152 [DOI] [PubMed]
- 6.Roth RM, Isquith PK, Gioia GA. BRIEF-A: behavior rating inventory of executive function–adult version: Professional manual. Psychological Assessment Resources; 2005.
- 7.Wallace GL, Kenworthy L, Pugliese CE, Popal HS, White EI, Brodsky E et al. Real-World Executive Functions in Adults with Autism Spectrum Disorder: Profiles of Impairment and Associations with Adaptive Functioning and Co-morbid Anxiety and Depression. J Autism Dev Disord. 2016;46:1071–83. 10.1007/s10803-015-2655-7 [DOI] [PMC free article] [PubMed]
- 8.Granader Y, Wallace GL, Hardy KK, Yerys BE, Lawson RA, Rosenthal M et al. Characterizing the Factor Structure of Parent Reported Executive Function in Autism Spectrum Disorders: The Impact of Cognitive Inflexibility. J Autism Dev Disord. 2014;44:3056–62. 10.1007/s10803-014-2169-8 [DOI] [PMC free article] [PubMed]
- 9.Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences. 2009;13:74–82. http://www.sciencedirect.com/science/article/pii/S136466130800260X [DOI] [PMC free article] [PubMed]
- 10.Leung RC, Zakzanis KK. Brief Report: Cognitive Flexibility in Autism Spectrum Disorders: A Quantitative Review. J Autism Dev Disord. 2014;44:2628–45. 10.1007/s10803-014-2136-4 [DOI] [PubMed]
- 11.de Vries M, Geurts HM. Cognitive Flexibility in ASD; Task Switching with Emotional Faces. J Autism Dev Disord. 2012;42:2558–68. 10.1007/s10803-012-1512-1 [DOI] [PMC free article] [PubMed]
- 12.Van Eylen L, Boets B, Steyaert J, Wagemans J, Noens I. Executive functioning in autism spectrum disorders: Influence of task and sample characteristics and relation to symptom severity. Eur Child Adolesc Psychiatry. 2015;24:1399–417. https://link.springer.com/article/10.1007/s00787-015-0689-1 [DOI] [PubMed]
- 13.Landry O, Al-Taie S. A Meta-analysis of the Wisconsin Card Sort Task in Autism. J Autism Dev Disord. 2016;46:1220–35. 10.1007/s10803-015-2659-3 [DOI] [PubMed]
- 14.Latinus M, Cléry H, Andersson F, Bonnet-Brilhault F, Fonlupt P, Gomot M. Inflexibility in Autism Spectrum Disorder: Need for certainty and atypical emotion processing share the blame. Brain and Cognition. 2019;136:103599. http://www.sciencedirect.com/science/article/pii/S0278262618303294 [DOI] [PubMed]
- 15.Van Eylen L, Boets B, Steyaert J, Evers K, Wagemans J, Noens I. Cognitive flexibility in autism spectrum disorder: Explaining the inconsistencies? Research in Autism Spectrum Disorders. 2011;5:1390–401. https://www.sciencedirect.com/science/article/pii/S1750946711000316
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
- 17.Yeung MK. A systematic review and meta-analysis of facial emotion recognition in autism spectrum disorder: The specificity of deficits and the role of task characteristics. Neuroscience & Biobehavioral Reviews. 2022;133:104518. https://www.sciencedirect.com/science/article/pii/S0149763421005893 [DOI] [PubMed]
- 18.Harms MB, Martin A, Wallace GL. Facial Emotion Recognition in Autism Spectrum Disorders: A Review of Behavioral and Neuroimaging Studies. Neuropsychol Rev. 2010;20:290–322. 10.1007/s11065-010-9138-6 [DOI] [PubMed]
- 19.Uljarevic M, Hamilton A. Recognition of Emotions in Autism: A Formal Meta-Analysis. J Autism Dev Disord. 2013;43:1517–26. https://link.springer.com/article/10.1007/s10803-012-1695-5 [DOI] [PubMed]
- 20.Fabio RA, Esposito S, Carrozza C, Pino G, Caprì T. Correlations between facial emotion recognition and cognitive flexibility in autism spectrum disorder. Advances in Autism. 2020;6:195–204. 10.1108/AIA-02-2019-0005
- 21.Conill É, Stilgenbauer J-L, Mouren M-C, Goussé V. Rôle de la flexibilité cognitive dans la reconnaissance d’expressions émotionnelles chez les personnes atteintes de Troubles du Spectre Autistique. Annales Médico-psychologiques, revue psychiatrique. 2014;172:392–5. http://linkinghub.elsevier.com/retrieve/pii/S0003448714001255
- 22.Ciairano S, Bonino S, Miceli R. Cognitive flexibility and social competence from childhood to early adolescence. Cogniţie Creier Comportament. 2006;10:343–66. [Google Scholar]
- 23.Lacroix A, Dutheil F, Logemann A, Cserjesi R, Peyrin C, Biro B et al. Flexibility in autism during unpredictable shifts of socio-emotional stimuli: Investigation of group and sex differences. Autism. 2022;26:1681–97. 10.1177/13623613211062776 [DOI] [PubMed]
- 24.Biro B, Kôkônyei G, De Oliveira Negrao R, Dancsik A, Karsai S, Logemann A et al. Interaction between emotional context-guided shifting and cognitive shifting: Introduction of a novel task. Neuropsychopharmacologia Hungarica. 2021;23:319–30. https://mppt.hu/magazin/pdf/vol23issue3/v23i3p319.pdf [PubMed]
- 25.Rogers RD, Monsell S. Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–31. http://doi.apa.org/getdoi.cfm?doi=10.1037/0096-3445.124.2.207
- 26.South M, Ozonoff S, Suchy Y, Kesner RP, McMAHON WM, Lainhart JE. Intact emotion facilitation for nonsocial stimuli in autism: Is amygdala impairment in autism specific for social information? Journal of the International Neuropsychological Society. 2008;14:42–54. https://www.cambridge.org/core/journals/journal-of-the-international-neuropsychological-society/article/intact-emotion-facilitation-for-nonsocial-stimuli-in-autism-is-amygdala-impairment-in-autism-specific-for-social-information/97BCE98037D69445C4E0D4EEA467FCDA [DOI] [PubMed]
- 27.Lehnhardt F-G, Falter CM, Gawronski A, Pfeiffer K, Tepest R, Franklin J et al. Sex-Related Cognitive Profile in Autism Spectrum Disorders Diagnosed Late in Life: Implications for the Female Autistic Phenotype. J Autism Dev Disord. 2016;46:139–54. 10.1007/s10803-015-2558-7 [DOI] [PubMed]
- 28.White EI, Wallace GL, Bascom J, Armour AC, Register-Brown K, Popal HS et al. Sex differences in parent-reported executive functioning and adaptive behavior in children and young adults with autism spectrum disorder. Autism Research. 2017;n/a–. 10.1002/aur.1811/abstract [DOI] [PMC free article] [PubMed]
- 29.Demetriou EA, Pepper KL, Park SH, Pellicano L, Song YJC, Naismith SL et al. Autism spectrum disorder: An examination of sex differences in neuropsychological and self-report measures of executive and non-executive cognitive function. Autism. 2021;136236132110149. 10.1177/13623613211014991 [DOI] [PubMed]
- 30.Wood-Downie H, Wong B, Kovshoff H, Cortese S, Hadwin JA, Research Review. A systematic review and meta-analysis of sex/gender differences in social interaction and communication in autistic and nonautistic children and adolescents. J Child Psychol Psychiatry. 2021;62:922–36. [DOI] [PubMed] [Google Scholar]
- 31.Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012;50:1211–21. http://www.sciencedirect.com/science/article/pii/S0028393212000024 [DOI] [PubMed]
- 32.Wingenbach TSH, Ashwin C, Brosnan M. Sex differences in facial emotion recognition across varying expression intensity levels from videos. PLoS ONE. 2018;13:e0190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacroix A, Harquel S, Mermillod M, Garrido M, Barbosa L, Vercueil L et al. Sex modulation of faces prediction error in the autistic brain. Commun Biol. 2024;7:1–12. https://www.nature.com/articles/s42003-024-05807-4 [DOI] [PMC free article] [PubMed]
- 34.Lacroix A, Harquel S, Barbosa LS, Kovarski K, Garrido MI, Vercueil L et al. Reduced spatial frequency differentiation and sex-related specificities in fearful face detection in autism: Insights from EEG and the predictive brain model. Autism Research. 2024;n/a. 10.1002/aur.3209 [DOI] [PubMed]
- 35.Leppänen JM, Tenhunen M, Hietanen JK. Faster choice-reaction times to positive than to negative facial expressions: the role of cognitive and motor processes. J Psychophysiol. 2003;17:113–23. [Google Scholar]
- 36.Yang D, Tao H, Ge H, Li Z, Hu Y, Meng J. Altered Processing of Social Emotions in Individuals With Autistic Traits. Frontiers in Psychology. 2022;13. 10.3389/fpsyg.2022.746192 [DOI] [PMC free article] [PubMed]
- 37.Van der Donck S, Dzhelyova M, Vettori S, Mahdi SS, Claes P, Steyaert J, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61:1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoet G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach Psychol. 2017;44(1):24–31. 10.1177/0098628316677643.
- 39.Stoet G. PsyToolkit: A software package for programming psychological experiments using Linux. Behav Res Methods. 2010;42:1096–104. 10.3758/BRM.42.4.1096 [DOI] [PubMed]
- 40.Merlhiot G, Mermillod M, Pennec J-LL, Mondillon L. Introduction and validation of the Natural Disasters Picture System (NDPS). PLOS ONE. 2018;13:e0201942. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0201942 [DOI] [PMC free article] [PubMed]
- 41.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. 1997;1:39–58.
- 42.Dan-Glauser ES, Scherer KR. The Geneva affective picture database (GAPED): A new 730-picture database focusing on valence and normative significance. Behav Res. 2011;43:468. 10.3758/s13428-011-0064-1 [DOI] [PubMed]
- 43.Marchewka A, Żurawski Ł, Jednoróg K, Grabowska A. The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behav Res. 2014;46:596–610. 10.3758/s13428-013-0379-1 [DOI] [PMC free article] [PubMed]
- 44.Kurdi B, Lozano S, Banaji MR. Introducing the Open Affective Standardized Image Set (OASIS). Behav Res. 2017;49:457–70. 10.3758/s13428-016-0715-3 [DOI] [PubMed]
- 45.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Malesand Females, Scientists and Mathematicians. J Autism Dev Disord. 2001;31:5–17. 10.1023/A:1005653411471 [DOI] [PubMed]
- 46.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2020. https://www.R-project.org/
- 47.RStudio Team. RStudio: Integrated Development Environment for R. Boston, MA: RStudio, Inc. 2019. http://www.rstudio.com/
- 48.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using Lme4. J Stat Soft. 2015;67:1–48. http://www.jstatsoft.org/v67/i01/
- 49.Lo S, Andrews S. To transform or not to transform: Using generalized linear mixed models to analyse reaction time data. Front Psychol. 2015;6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4528092/ [DOI] [PMC free article] [PubMed]
- 50.Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang. 2013;68:255–78. http://www.sciencedirect.com/science/article/pii/S0749596X12001180 [DOI] [PMC free article] [PubMed]
- 51.Rights JD, Sterba SK. Quantifying explained variance in multilevel models: an integrative framework for defining R-squared measures. Psychol Methods. 2019;24:309–38. [DOI] [PubMed] [Google Scholar]
- 52.Lenth RV, Emmeans. Estimated Marginal Means, aka Least-Squares Means. 2021. https://CRAN.R-project.org/package=emmeans
- 53.Cumming G. Inference by eye: Reading the overlap of independent confidence intervals. Statistics in Medicine. 2009;28:205–20. https://onlinelibrary.wiley.com/doi/abs/10.1002/sim.3471 [DOI] [PubMed]
- 54.Garofalo S, Giovagnoli S, Orsoni M, Starita F, Benassi M. Interaction effect: Are you doing the right thing? PLOS ONE. 2022;17:e0271668. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0271668 [DOI] [PMC free article] [PubMed]
- 55.Biró B, Cserjesi R, Kocsel N, Galambos A, Gecse K, Kovács LN, et al. The neural correlates of context driven changes in the emotional response: an fMRI study. PLoS ONE. 2022;17:e0279823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weierich MR, Kleshchova O, Rieder JK, Reilly DM. The Complex Affective Scene Set (COMPASS): Solving the Social Content Problem in Affective Visual Stimulus Sets. Vazire S, Vazire S, editors. Collabra: Psychology. 2019;5:53. 10.1525/collabra.256
- 57.Hassin RR, Aviezer H, Bentin S. Inherently Ambiguous: Facial Expressions of Emotions, in Context. Emotion Review. 2013;5:60–5. http://journals.sagepub.com/doi/10.1177/1754073912451331
- 58.Costa C, Cristea IA, Dal Bò E, Melloni C, Gentili C. Brain activity during facial processing in autism spectrum disorder: An activation likelihood estimation (ALE) meta-analysis of neuroimaging studies. J Child Psychol Psychiatry. 2021;62:1412–24. 10.1111/jcpp.13412 [DOI] [PubMed]
- 59.Frazier TW, Strauss M, Klingemier EW, Zetzer EE, Hardan AY, Eng C et al. A Meta-Analysis of Gaze Differences to Social and Nonsocial Information Between Individuals With and Without Autism. J Am Acad Child Adolesc Psychiatry. 2017;56:546–55. http://www.sciencedirect.com/science/article/pii/S0890856717302071 [DOI] [PMC free article] [PubMed]
- 60.Kang E, Keifer CM, Levy EJ, Foss-Feig JH, McPartland JC, Lerner MD. Atypicality of the N170 Event-Related Potential in Autism Spectrum Disorder: A Meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2018;3:657–66. https://www.sciencedirect.com/science/article/pii/S2451902217302021 [DOI] [PMC free article] [PubMed]
- 61.Lozier LM, Vanmeter JW, Marsh AA. Impairments in facial affect recognition associated with autism spectrum disorders: a meta-analysis. Dev Psychopathol. 2014;26:933–45. [DOI] [PubMed] [Google Scholar]
- 62.Nuske HJ, Vivanti G, Dissanayake C. Are emotion impairments unique to, universal, or specific in autism spectrum disorder? A comprehensive review. Cognition and Emotion. 2013;27:1042–61. 10.1080/02699931.2012.762900 [DOI] [PubMed]
- 63.Palumbo L, Burnett HG, Jellema T. Atypical emotional anticipation in high-functioning autism. Molecular Autism. 2015;6:47. 10.1186/s13229-015-0039-7 [DOI] [PMC free article] [PubMed]
- 64.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced Perceptual Functioning in Autism: An Update, and Eight Principles of Autistic Perception. J Autism Dev Disord. 2006;36:27–43. 10.1007/s10803-005-0040-7 [DOI] [PubMed]
- 65.Fujino J, Tei S, Itahashi T, Aoki Y, Ohta H, Kubota M et al. Need for closure and cognitive flexibility in individuals with autism spectrum disorder: A preliminary study. Psychiatry Research. 2019;271:247–52. https://www.sciencedirect.com/science/article/pii/S0165178118306747 [DOI] [PubMed]
- 66.Sucksmith E, Allison C, Baron-Cohen S, Chakrabarti B, Hoekstra RA. Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia. 2013;51:98–105. https://www.sciencedirect.com/science/article/pii/S0028393212004800 [DOI] [PMC free article] [PubMed]
- 67.Baron-Cohen S, Bowen DC, Holt RJ, Allison C, Auyeung B, Lombardo MV et al. The Reading the Mind in the Eyes Test: Complete Absence of Typical Sex Difference in ~ 400 Men and Women with Autism. Yamasue H, editor. PLOS ONE. 2015;10:e0136521. 10.1371/journal.pone.0136521 [DOI] [PMC free article] [PubMed]
- 68.Lai M-C, Lombardo MV, Ruigrok ANV, Chakrabarti B, Wheelwright SJ, Auyeung B et al. Cognition in Males and Females with Autism: Similarities and Differences. PLoS One. 2012;7. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3474800/ [DOI] [PMC free article] [PubMed]
- 69.Gillespie-Lynch K, Kapp SK, Shane-Simpson C, Smith DS, Hutman T. Intersections Between the Autism Spectrum and the Internet: Perceived Benefits and Preferred Functions of Computer-Mediated Communication. Intellectual and Developmental Disabilities. 2014;52:456–69. 10.1352/1934-9556-52.6.456 [DOI] [PubMed]
- 70.Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: A meta-analytic review. Psychological Medicine. 2005;35:1097–108. https://www.cambridge.org/core/journals/psychological-medicine/article/abs/executive-functioning-in-adult-adhd-a-metaanalytic-review/858D3E13C533DA10FF6AD8E241794AF3 [DOI] [PubMed]
- 71.Nuño L, Gómez-Benito J, Carmona VR, Pino O. A Systematic Review of Executive Function and Information Processing Speed in Major Depression Disorder. Brain Sciences. 2021;11:147. https://www.mdpi.com/2076-3425/11/2/147 [DOI] [PMC free article] [PubMed]
- 72.Hartanto A, Yang H. Testing theoretical assumptions underlying the relation between anxiety, mind wandering, and task-switching: a diffusion model analysis. Emotion. 2022;22:493–510. [DOI] [PubMed] [Google Scholar]
- 73.Lugo-Marín J, Magán-Maganto M, Rivero-Santana A, Cuellar-Pompa L, Alviani M, Jenaro-Rio C et al. Prevalence of psychiatric disorders in adults with autism spectrum disorder: A systematic review and meta-analysis. Research in Autism Spectrum Disorders. 2019;59:22–33. https://www.sciencedirect.com/science/article/pii/S1750946718301879
- 74.Estes A, Rivera V, Bryan M, Cali P, Dawson G. Discrepancies Between Academic Achievement and Intellectual Ability in Higher-Functioning School-Aged Children with Autism Spectrum Disorder. J Autism Dev Disord. 2011;41:1044–52. 10.1007/s10803-010-1127-3 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was preregistered on OSF. The datasets generated and analyzed during the current study, as well as the images used as stimuli, are available in the OSF repository: https://osf.io/7n8gm/?view_only=395f108fb0c74d8f96a0f86179f73ef5.