Abstract
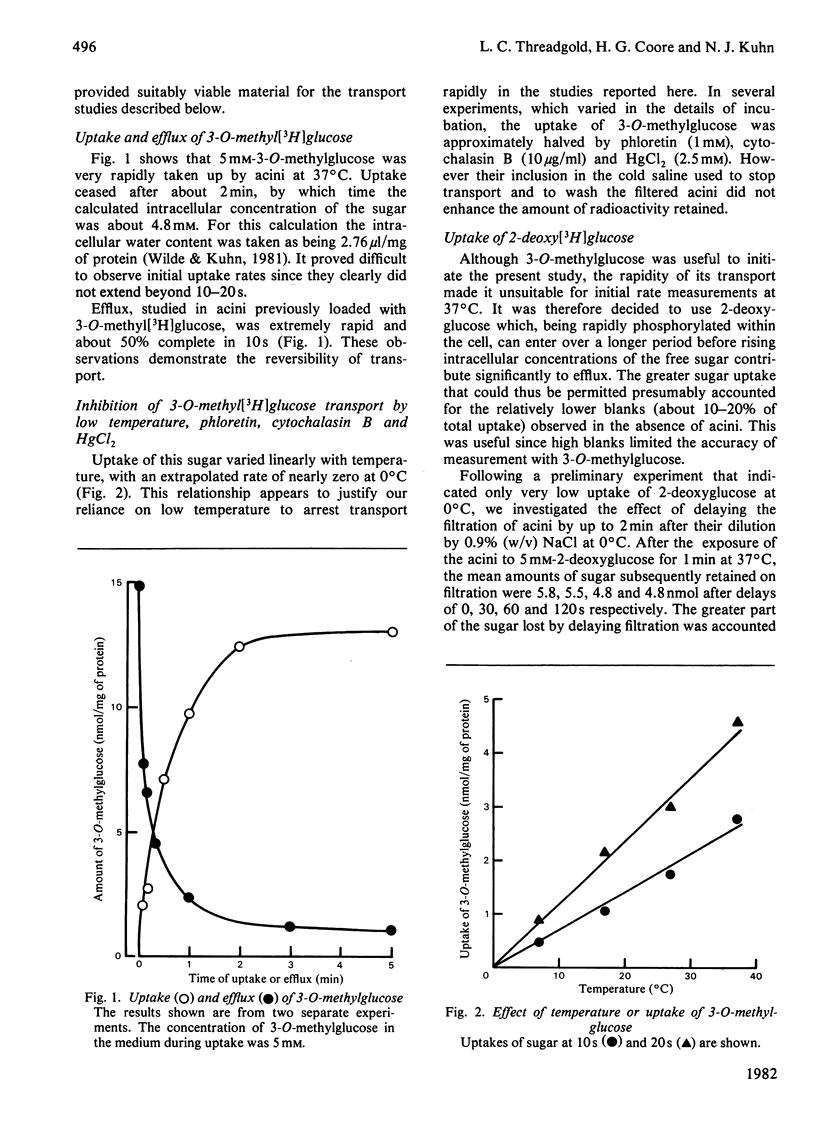
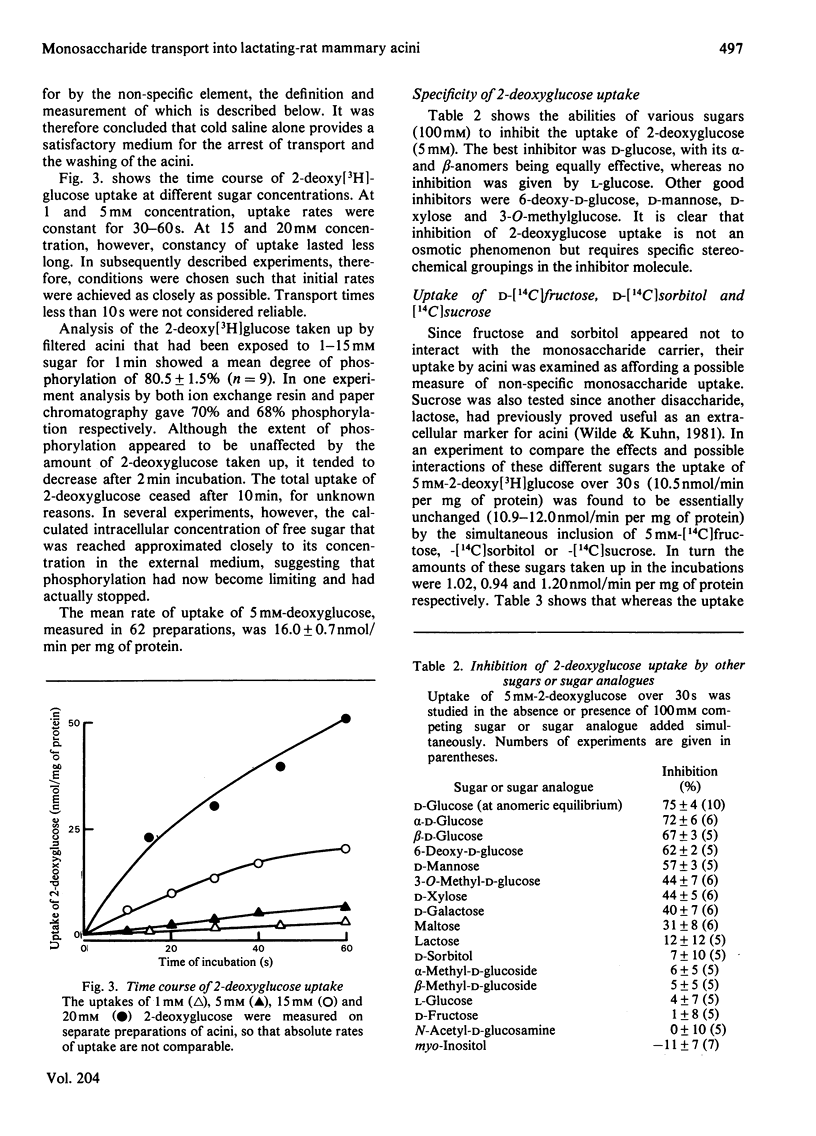
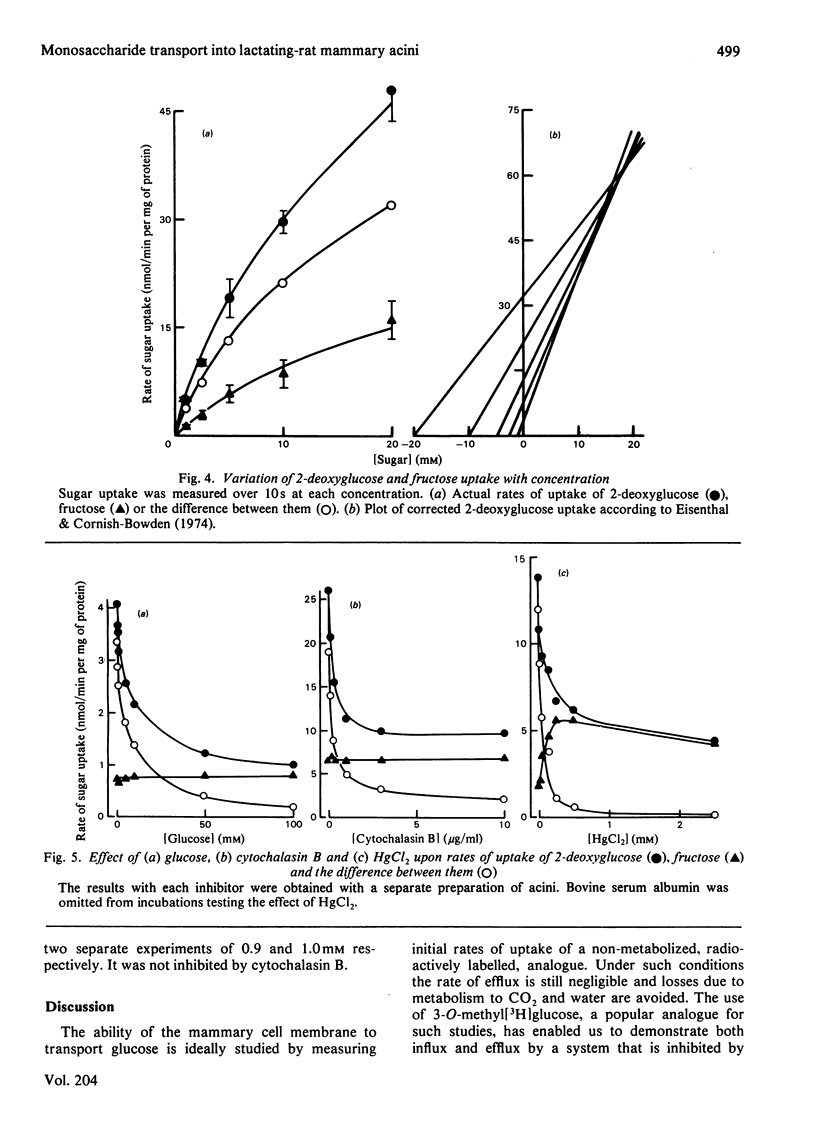
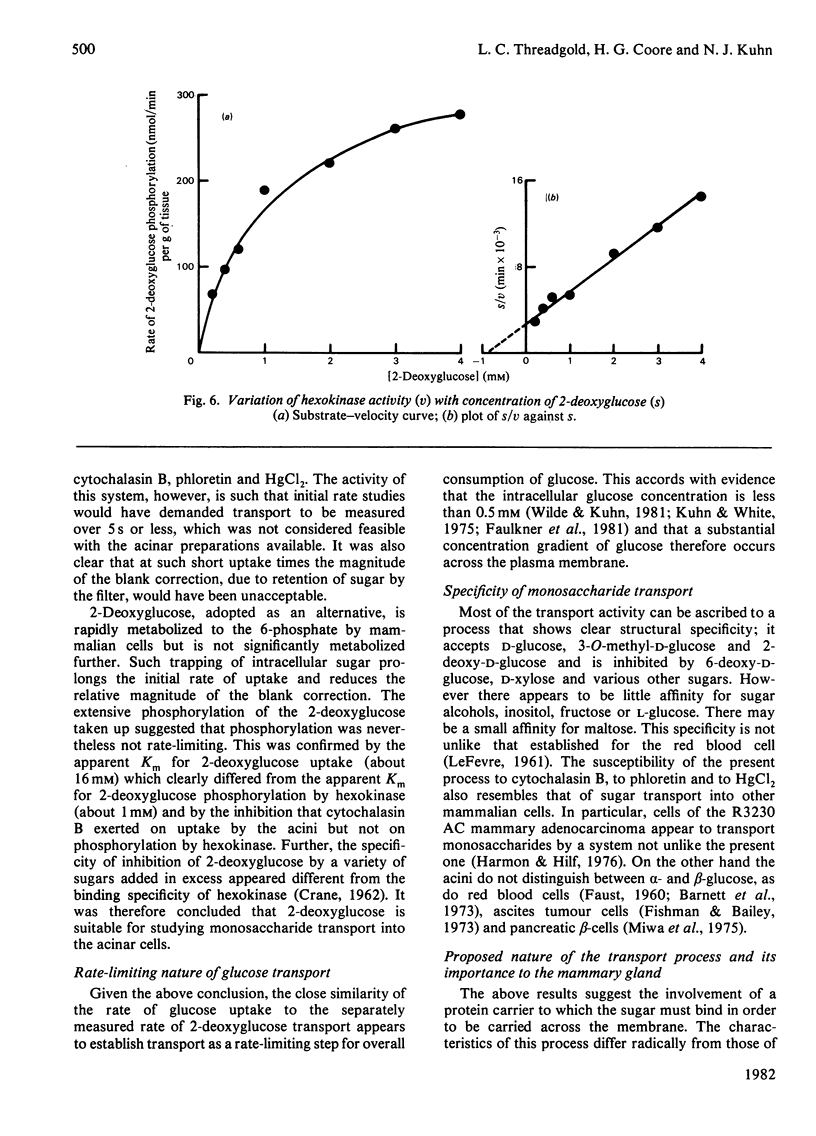
The uptake and release of 3-O-methyl-D-[3H]glucose at 37 degrees C by acini, prepared from lactating-rat mammary gland with collagenase, was inhibited by glucose, phloretin, cytochalasin B, HgCl2 and low temperature. Uptake and phosphorylation of 2-deoxy-D-[3H]glucose, studied in greater detail, could be ascribed to a specific, saturable, inhibitable, process of apparent Km 16 mM and Vmax. approx. 56 nmol/min per mg of protein, plus a non-specific, non-inhibitable process that was monitored with [14C]fructose. The mean rate of uptake of 5 mM-2-deoxyglucose (16 nmol/min per mg of protein) was similar to the rate of consumption of 5 mM-glucose, suggesting that transport was a rate-limiting step in the overall metabolism of glucose. This accords with evidence for a glucose gradient across the plasma membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUST R. G. Monosaccharide penetration into human red blood cells by an altered diffusion mechanism. J Cell Comp Physiol. 1960 Oct;56:103–121. doi: 10.1002/jcp.1030560205. [DOI] [PubMed] [Google Scholar]
- Faulkner A., Chaiyabutr N., Peaker M., Carrick D. T., Kuhn N. J. Metabolic significance of milk glucose. J Dairy Res. 1981 Feb;48(1):51–56. doi: 10.1017/s0022029900021440. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Bailey J. M. Active transport of glucose anomers by ascites tumour cells. Nat New Biol. 1973 May 9;243(123):59–60. [PubMed] [Google Scholar]
- Greenbaum A. L., Salam A. Regulation of mammary gland metabolism: pathways of glucose utilization, metabolite profile and hormone response of a modified mammary gland cell preparation. Eur J Biochem. 1978 Jul 3;87(3):505–516. doi: 10.1111/j.1432-1033.1978.tb12401.x. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon J. T., Hilf R. Effect of insulin to decrease glucose transport in dissociated cells from the R3230AC mammary adenocarcinoma of diabetic rats. Biochim Biophys Acta. 1976 Aug 4;443(1):114–125. doi: 10.1016/0005-2736(76)90495-8. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Van de Velde R. L. Lipogenesis by acini from mammary gland of lactating rats. J Biol Chem. 1974 Nov 25;249(22):7348–7357. [PubMed] [Google Scholar]
- Kuhn N. J. Glucose as a fuel for the mammary gland. Biochem Soc Trans. 1978;6(3):539–543. doi: 10.1042/bst0060539. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Milk glucose as an index of the intracellular glucose concentration of rat mammary gland. Biochem J. 1975 Oct;152(1):153–155. doi: 10.1042/bj1520153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- Miwa I., Okuda J., Niki H., Niki A. Uptake of radioactive D-glucose anomers by pancreatic islets. J Biochem. 1975 Nov;78(5):1109–1111. doi: 10.1093/oxfordjournals.jbchem.a130990. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Comparison of glucose metabolism in the lactating mammary gland of the rat in vivo and in vitro. Effects of starvation, prolactin or insulin deficiency. Biochem J. 1977 Apr 15;164(1):153–159. doi: 10.1042/bj1640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgold L. C., Coore H. G., Kuhn N. J. Monosaccharide transport into secretory cells of lactating-rat mammary gland. Biochem Soc Trans. 1981 Feb;9(1):66–66. doi: 10.1042/bst0090066. [DOI] [PubMed] [Google Scholar]
- White M. D., Kuhn N. J., Ward S. Mannitol and glucose movement across the Golgi membrane of lactating-rat mammary gland. Biochem J. 1981 Jan 15;194(1):173–177. doi: 10.1042/bj1940173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D., Kuhn N. J., Ward S. Permeability of lactating-rat mammary gland Golgi membranes to monosaccharides. Biochem J. 1980 Sep 15;190(3):621–624. doi: 10.1042/bj1900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis and the utilisation of glucose by rat mammary acini. Int J Biochem. 1981;13(3):311–316. doi: 10.1016/0020-711x(81)90083-5. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis in the rat, and the effects of litter size and malnutrition. Biochem J. 1979 Aug 15;182(2):287–294. doi: 10.1042/bj1820287. [DOI] [PMC free article] [PubMed] [Google Scholar]


