Abstract
Background
Hepatocellular carcinoma (HCC) is prevalent in Taiwan, primarily due to the high incidence of hepatitis B and C infections, with high recurrence rates of 50–70% within five years after initial treatment. Treatment options for recurrent HCC include salvage liver transplantation, trans-arterial chemoembolization, re-hepatectomy, and radiofrequency ablation. Repeat hepatectomy exhibits superior oncological outcomes compared with alternative approaches. Although laparoscopic liver resection has demonstrated safety and feasibility for primary HCC resection, the persistence of intrahepatic recurrence necessitates effective intervention. However, repeat liver resection poses several challenges including adhesions from previous surgeries, limited access to recurrent tumors, altered liver structure post-regeneration, difficulties in obtaining hilar control, and compromised liver reserves. Suggesting a laparoscopic approach for recurrent HCC is typically based on the surgeons’ experience and confidence. In this study, we reconfirmed the safety, feasibility and oncological outcome of laparoscopic repeat liver resection and investigated the optimal timing for initiation of this procedure by a pioneering team in minimally invasive liver resection.
Methods
We retrospectively reviewed our collective experience of 57 patients with recurrent HCC between January 2009 and December 2021.The patients were followed until June 30, 2024. Among them, 37 underwent laparoscopic approaches and 20 opted for open procedures.
Results
Both groups exhibited similar operative times and perioperative outcomes, with significantly reduced hospital stays in the laparoscopic cohort (median: 5 vs. 7, p < 0.001). The median follow-up duration was 41.5 months (range, 2.8 to 112.6 months). Mortality occurred in 22 patients (38.6%) and recurrence occurred in 26 patients (45.6%) The overall survival and disease-free survival after the operation were similar in both groups and comparative to the literatures.
Conclusion
Using a stepwise approach, laparoscopic repeat liver resection can be performed safely and effectively with a low incidence of conversion by an experienced surgical team with similar oncological outcomes. The introduction of laparoscopic techniques has also sparked a strategic shift in the surgical approach for recurrent HCC. This treatment option should be offered to patients by an experienced surgical team for minimally invasive liver resections.
Keywords: Hepatocellular carcinoma, Laparoscopic repeat liver resection, Minimally invasive hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality globally, with a noticeable increase in mortality over recent decades [1]. Taiwan, characterized by a high prevalence of Hepatitis B and C virus (HBV/HCV) infections, experiences an elevated incidence of HCC, standing as the second leading cause of cancer-related deaths in the country [2, 3]. Employing a multidisciplinary approach aligned with the Barcelona Clinic Liver Cancer (BCLC) classification is widely acknowledged and seeks to enhance treatment outcomes for HCC [4].
While liver resection can yield 60–80% 5-year overall survival rates in early-stage HCC, recurrence rates surge in patients’ post-primary treatment, reaching up to 80% according to various reports [5]. Among the treatment options for recurrent HCC, repeat resection has emerged as a promising avenue for improving survival [6].
The safety and feasibility of laparoscopic liver resection (LLR) for the treatment of HCC have been established in international consensus meetings [7]. However, laparoscopic repeat liver resection (LRLR) is perceived to be a more challenging procedure because of adhesions from prior operations, altered normal structural orientation, and the need for highly selective patients and experienced surgeons [8]. These complexities often hinder the surgeon’s decision to opt for a repeat laparoscopic approach. Notably, recent advancements in technologies such as the indocyanine green (ICG) scope, robotic surgery, intraoperative image guidance, and enhanced bleeding control, as discussed in the latest international laparoscopic liver surgery consensus, signify the evolving landscape of LLR [9]. Moreover, the most comprehensive multicenter propensity score-matched observational study reaffirmed the feasibility and safety of LRLR [10].
The decision to adopt a laparoscopic approach for recurrent intrahepatic malignancies is significantly influenced by surgeons’ experience and familiarity with the procedure. Furthermore, most studies on LRLR originate from high-volume centers [10]. Previously considered a relative contraindication, especially for novice laparoscopic liver surgeons, LRLR challenges traditional concepts. This retrospective study aimed to explore the optimal timing for the surgical team to initiate the first LRLR, as defined by the accumulated experience of performing laparoscopic hepatectomies. We conducted a retrospective comparison of the short-term perioperative outcomes and long-term oncological outcome between LRLR performed by our pioneering team and open repeat hepatectomy performed by experienced surgeons. This study provides evidence for the surgical team’s transition from traditional surgery to a minimally invasive approach in patients with recurrent HCC.
Methods
Patient selection
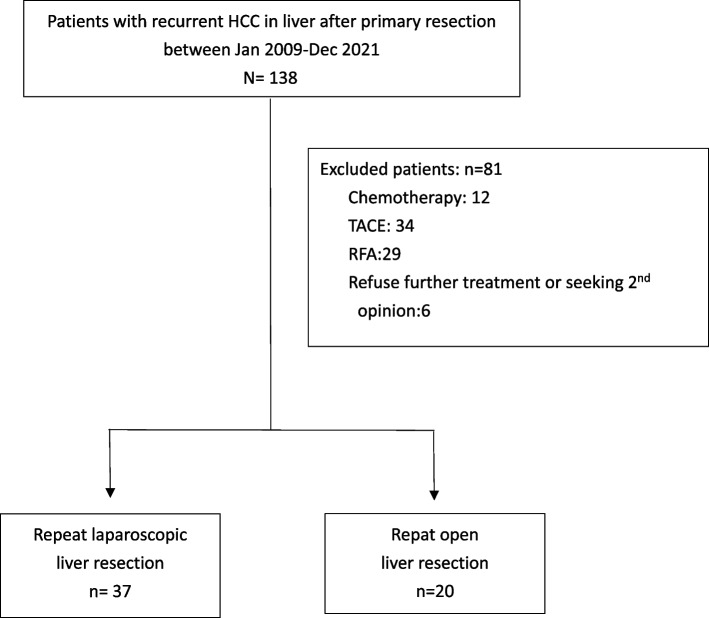
A retrospective study was conducted at the Chang Gung Memorial Hospital, Keelung between January 2009 and December 2021. The post-operative following time of survival ended in June 30, 2024. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB No. 02300984B0). This study is registered in Research Registry and the Identity number is researchregistry 10,487. The study enrolled patients who underwent surgical management for recurrent HCC. The selection of patients is illustrated in Figs. 1 and 2. The open-repeat liver resection was performed by experienced liver surgeons at the time of diagnosis. In contrast, LRLR was performed by a dedicated team comprising two fixed surgeons who are recognized as the pioneers of LLR in our institution. Recurrence was diagnosed using preoperative dynamic abdominal computed tomography (CT) or magnetic resonance imaging (MRI). Preoperative liver function was assessed using basic liver function tests, Child-Pugh Score, and ICG test. A multidisciplinary team consisting of doctors specializing in radiofrequency ablation (RFA), hepatobiliary surgery, radiology, radiation oncology, and medical oncology was involved in the pretreatment evaluation of all patients. The choice of the operative procedure was determined based on preserved liver function, tumor characteristics (location and size), and the patient’s previous surgical history. Open repeat liver resection (ORLR) would be suggested initially for patients with multiple histories of laparotomy (more than twice), or if the recurrent tumor would be at risk for major vessel injury (hepatic vein) during adhesiolysis or liver mobilization under our previous surgical experience.
Fig. 1.
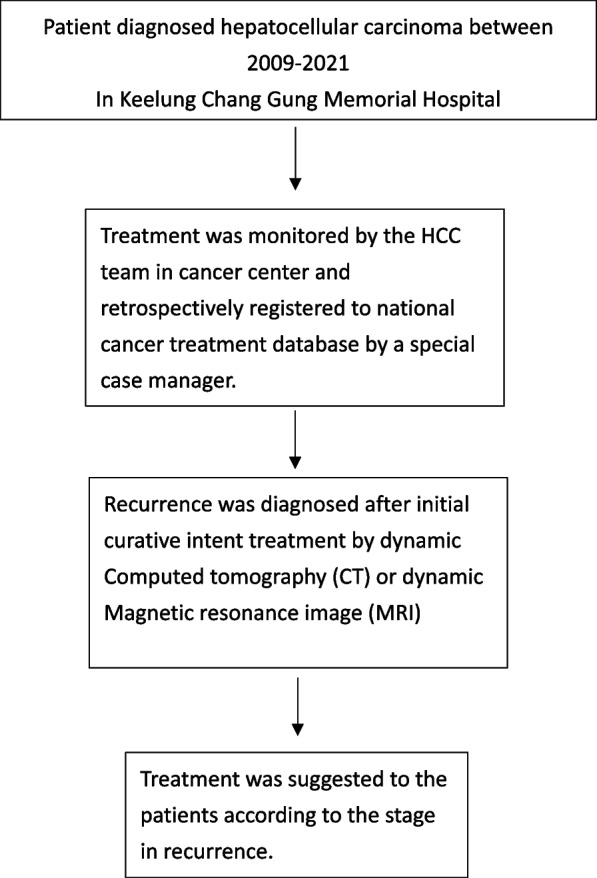
Follow up strategy for the patients diagnosed Hepatocellular carcinoma (HCC) underwent curative intent treatment
Fig. 2.
(a) Flow chart of patient selection process for repeat hepatectomy in recurrent HCC: hepatocellular carcinoma, TACE: trans-arterial chemo-embolization, RFA: radiofrequency ablation
Surgical procedure
Patients were positioned on a rotating table in the supine position with their legs suspended, transitioning to a reversed Trendelenburg position during LLR. For tumors located in the left lobe or anterior segment, upper arm extensions were utilized, whereas patients with tumors in the right posterior segment were placed in the left hemilateral decubitus position. The Hasson method [11]was employed to insert a camera port in the supraumbilical position, and extended adhesiolysis was performed around the incision to create adequate operational space. Subsequent trocars were inserted stepwise following the initial port using electrical cutting, monopolar coagulation, or energy devices for further adhesiolysis and trocar insertion. Trocar placement was optimized based on tumor location to facilitate liver rotation and adhesion dissection from previous surgeries. Intraoperative navigation using an ICG scope (Stryker, Kalamazoo, MI, USA) and intraoperative ultrasonography-guided parenchymal transection ensured adequate margins, especially for lesions that were not readily visible. Successful procedures were performed using pure LLR.
Hepatic parenchymal resection involved the crush-clamp method using a Harmonic Hi10000 (Ethicon Endo-Surgery, Cincinnati, OH, USA), a Cavitron ultrasonic surgical aspirator, and a bipolar clamp coagulation system (Karl Storz Endoscope, Tuttlingen, Germany). The Pringle maneuver by Huang’s loop [12] was applied as much as possible if the adhesion between the hepatoduodenal ligaments could be dissected; otherwise, partial vascular control using a Debakey Bulldog clamp (GerMed USA, New York, USA) was used in cases of severe adhesions around the hepatoduodenal ligament. A Seprafilm membrane (Baxter, Deerfield, Illinois, USA) was used to prevent postoperative adhesions around the resection margin, inferior vena cava, hepatoduodenal ligament, and under the midline mini-laparotomy wound in all patients [13]. Closed system drains employing the Jackson-Pratt draining system were inserted around the liver parenchymal. Prophylactic antibiotics with cefazolin 1 g were administered preoperatively, repeated every 4 h during the surgery, and continued for one postoperative day [14].
Postoperative outcome
The recorded parameters, including intraoperative blood loss, the need for blood transfusion, and surgical duration, were subsequently compared. Complications were graded according to the Clavien-Dindo classification [15]. Post-hepatectomy liver failure was defined as the combination of prothrombin time index < 50% and serum bilirubin > 50 µmol/L (i.e., 2.9 mg/dL) on postoperative day 5 according to the International Study Group of Liver Surgery [16], and postoperative intensive care unit (ICU) stay duration, postoperative hospital stay duration, 30-days readmission, and 90-days readmission were documented. Tumor resection margins were defined as R0 resection for no residual tumor detected microscopically and R1 resection for microscopically residual tumor [17]. Surgical morbidity encompassed readmission within 30 days, whereas surgical mortality constituted mortality within 30 days of the operation, and mortality within 90 days were also recorded. Survival was tracked until June 30, 2024, and all-cause mortality was included. Recurrence was documented based on imaging evidence, such as CT or MRI, showing tumor recurrence. The period until next recurrence is what we refer to as disease-free survival.
Statistical analysis
All statistical analyses were conducted using the SPSS software (version 23; IBM Corp., Armonk, NY, USA). Continuous variables that were not normally distributed, such as operative time and post-operative ICU stay, are recorded as medians with interquartile ranges, and differences were assessed using the Mann-Whitney U test. Categorical variables are presented as numbers and frequencies (%) and were compared using the chi-square test or Fisher’s exact test. If ≤ 20% of expected cell counts were < 5, we used the chi-square test; if > 20% of expected cell counts were < 5, we used Fisher’s exact test. Statistical significance was set at P < 0.05 (two-tailed).
The Kaplan-Meier method was used to estimate disease-free survival and overall survival, and the log-rank test was employed to compare the two groups (LRLR vs. ORLR).
Results
A total of 57 patients were included in the study: 37 in the laparoscopic group and 20 in the open hepatectomy group. Patient characteristics for both LRLR and ORLR are summarized in Table 1. The majority of patients were male, 86.5% in the laparoscopic group and 65% in the open hepatectomy group. The body mass index was comparable between groups. Most patients with recurrent HCC exhibited either HBV or HCV infection and the proportion of virus distribution is shown in Table 1. All patients were classified as Child-Pugh class A at the time of hepatectomy, the patients with pathological confirmation of cirrhosis were 18(48.6%) and 7(35%) in the laparoscopic and open group respectively and displayed equal Eastern Cooperative Oncology Group (ECOG) and American Society of Anesthesiologists (ASA) status. Most patients in the LRLR group underwent laparoscopic hepatectomy as their initial operation, whereas 11 patients (29.7%) had previously undergone open hepatectomy. There were no significant differences in the distribution of BCLC stages when encountering recurrence.
Table 1.
Demographic data
| Laparoscopic N = 37 |
Open N = 20 |
p | |
|---|---|---|---|
| Age, years | 63[61–70] | 67.5[58–73] | 0.508 |
| Sex, male | 32(86.5%) | 13(65%) | 0.088 |
| BMI | 25.35[23.06–27.1] | 23.83[22.18–24.83] | 0.21 |
| Hepatitis | |||
| HBV | 18(48.6%) | 6(30%) | 0.174 |
| HCV | 11(29.7%) | 12(60%) | 0.026 |
| Non B and non C | 9(24.3%) | 3(15%) | 0.51 |
|
Liver function Child-Pugh Score* |
NA | ||
| A | 37(100%) | 20(100%) | |
| B | 0 | 0 | |
| C | 0 | 0 | |
| Liver cirrhosis | 18(48.6%) | 7(35%) | 0.322 |
| Previous operation | < 0.001 | ||
| Laparoscopic hepatectomy | 26(70.3%) | 0 | |
| Open hepatectomy | 11(29.7%) | 20(100%) | |
| ECOG | 0[0–1] | 0[0–0] | 0.304 |
| ASA | 3[3–3] | 3[3–3] | 0.294 |
| Tumor size ( cm) | 2.4[1.5–3] | 2[2–3] | 0.624 |
| BCLC stage | 0.523 | ||
| 0 | 13(35.1%) | 4(20%) | |
| A | 20(54.1%) | 13(65%) | |
| B | 3(8.1%) | 3(15%) | |
| C | 1(2.7%) | 0 |
BMI body mass index, HBV hepatitis B virus, HCV hepatitis C virus, ECOG Eastern Cooperative Oncology Group, ASA American society of anesthesiologist, BCLC stage Barcelona Clinic Liver Cancer stage
Table 2 illustrates that most patients underwent minor hepatectomy and lateral segmentectomy during repeat hepatectomy, with both groups utilizing the intermittent Pringle’s maneuver intraoperatively. All the patients in the laparoscopic group underwent a second surgery. The rate of major hepatectomy was slightly higher in the laparoscopic group than in the open group (16.2% vs. 10%).
Table 2.
Surgical characteristic
| Laparoscopic N = 37 |
Open N = 20 |
p | |
|---|---|---|---|
| Extension of resection | 0.127 | ||
| Lateral segmentectomy | 2(5.4%) | 5(25%) | |
| Minor hepatectomy | 29(78.4%) | 13(65%) | |
|
Major hepatectomy (≥ 2 segments) |
6(16.2%) | 2(10%) |
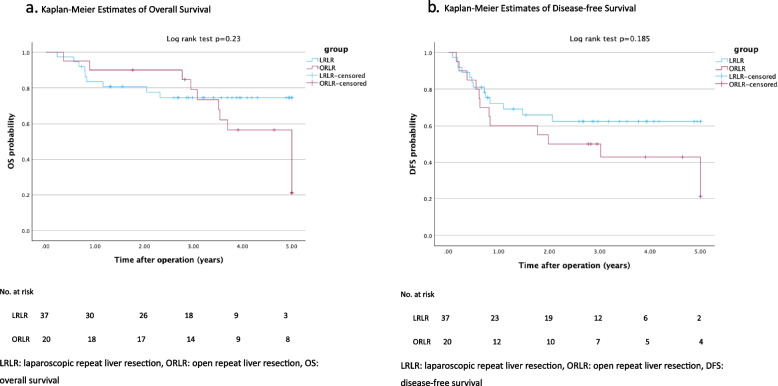
The perioperative outcomes outlined in Table 3 indicate comparable operative times and blood loss between the two groups, without statistical significance. Although the requirements for red blood cell transfusion were similar, the laparoscopic group demonstrated a significantly higher incidence of plasma transfusion (median: 2 vs. 0 unit, p = 0.014). The total time required for inflow control was significantly longer in the laparoscopic group than in the open group (median: 45 vs. 30 min; p = 0.038). No conversions were observed in the laparoscopic group, and the complication rates, post-hepatectomy liver failure, 30-day readmission, surgical mortality rates, 90-day readmission, 90-day mortality, and resectability were similar between the two groups. The complications greater than CDC IIIa included residual hematoma and pleural effusion, which were managed with drainage procedure under local anesthesia in the laparoscopic group, and bile leak requiring biliary stent insertion in the open group. One patient from each group was admitted in the ICU postoperatively for close monitoring. The laparoscopic group had a significantly shorter postoperative hospital stay (median: 5 vs. 7 days, p < 0.001). We made further subgroup analysis for peri-operative outcomes for cirrhotic patients with non-cirrhotic patients in the laparoscopic group in Table 4. which showed no significant difference for them underwent laparoscopic repeat liver resection. For the survival analysis (data cutoff, June 30, 2024; median duration of follow-up, 41.5 months [range, 2.8 to 112.6]). Mortality occurred in 22 patients (38.6%) while recurrence occurred in 26 patients (45.6%), and the overall survival and disease-free survival in both groups shown in Fig. 3a and b revealed no significant difference.
Table 3.
Perioperative outcome
| Laparoscopic N = 37 |
Open N = 20 |
p | |
|---|---|---|---|
| Operative time, min | 230[184–319] | 205.5[176–294] | 0.553 |
| Estimated blood loss, mL | 200[50–400] | 200[125–450] | 0.468 |
| Perioperative blood transfusion, u | |||
| PRBC | 0[0–0] | 0[0–0] | 0.681 |
| FFP | 2[0–2] | 0[0–1] | 0.014 |
| Platelet | 0[0–0] | 0[0–0] | 0.656 |
| Conversion | 0 | NA | NA |
| Pringle maneuver duration, min | 45[30–90] | 30[22.5–45] | 0.038 |
| Complications | 1 | ||
| CDC < IIIa | 35(94.6%) | 19(95%) | |
| CDC ≥ IIIa | 2(5.4%) | 1(5%) | |
| Post-hepatectomy liver failure | 0 | 0 | NA |
| Post-operative ICU stay, days | 0[0–0] | 0[0–0] | 0.675 |
| Hospital length of stays, days | 5[5–7] | 7[6.5–10] | < 0.001 |
| R1 rerection, n(%) | 6(16.2%) | 3(15%) | 1 |
| 30 days Readmission | 1(2.7%) | 0 | 1 |
| 90 days Readmission | 1(2.7%) | 0 | 1 |
| Mortality (90 days) | 1(2.7%) | 1(5%) | 1 |
PRBC packed red blood cell, FFP fresh frozen plasma, ICU intensive care unit, u unit of PRBC and FFP, 1 unit of PRBC is 140 mL and 1 unit FFP is 135 mL, CDC Clavien-Dindo Classification
Table 4.
Perioperative outcome of cirrhotic and non-cirrhotic patients in laparoscopic group
| Cirrhosis N = 18 |
Non-cirrhosis N = 19 |
p | |
|---|---|---|---|
| Operative time, min | 227.5[159–290] | 245[192–340] | 0.233 |
| Estimated blood loss, mL | 225[50–350] | 200[75–500] | 0.685 |
| Perioperative blood transfusion, u | |||
| PRBC | 0[0–0] | 0[0–0] | 0.578 |
| FFP | 2[0–2] | 0[2–3] | 0.538 |
| Platelet | 0[0–0] | 0[0–0] | 0.799 |
| Conversion | 0 | 0 | NA |
| Pringle’s maneuver duration, min | 37.5[30–75] | 50[30–105] | 0.245 |
| Complications | 0.23 | ||
| CDC < IIIa | 16(88.9%) | 19(100%) | |
| CDC ≥ IIIa | 2(11.1%) | 0 | |
| Post-hepatectomy liver failure | 0 | 0 | NA |
| Post-operative ICU stay, days | 0[0–0] | 0[0–0] | 0.775 |
| Hospital length of stay, days | 5[5–7] | 6[5–6.5] | 0.599 |
| Surgical margin < 1 mm, n (%) | 2(11.1%) | 4(21.1%) | 0.66 |
| 30 days Readmission | 0 | 1(5.3%) | 1 |
| 90 days Readmission | 0 | 1(5.3%) | 1 |
| Mortality (90 days) | 0 | 1(5.3%) | 1 |
PRBC packed red blood cell, FFP fresh frozen plasma, ICU intensive care unit, u unit of PRBC and FFP, 1 unit of PRBC is 140 mL and 1 unit FFP is 135 mL, CDC Clavien-Dindo Classification
Fig. 3.
Kaplan-Meier Estimates of Overall Survival. Kaplan-Meier Estimates of Disease-free Survival
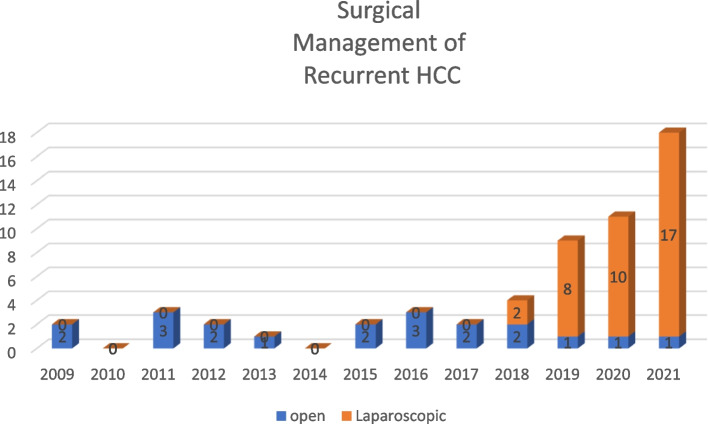
The distribution of procedures based on the year of operation is summarized in Fig. 4. This graphical representation indicates a gradual increase in the number and proportion of LRLR for recurrent HCC, accompanied by a decline in the number of ORLR. The first case of laparoscopic repeat liver resection was recorded in 2018, followed by a substantial increase in the subsequent years.
Fig. 4.
Trend of surgical management for resectable recurrent HCC in our institution HCC: Hepatocellular carcinoma
Discussion
HCC is the third leading cause of cancer-related deaths globally and the second in Taiwan [1, 2]. The increased prevalence of HBV and HCV infections significantly contributes to the incidence of HCC, reaching 47.05 per 100,000 person-years [3]. Recurrence rates for HCC range between 50% and 80%, making repeat resection the preferred choice over other strategies such as RFA, trans-arterial chemoembolization, chemotherapy, or targeted therapy, all of which offer limited treatment efficacy in cases of recurrence [6, 18]. Repeat hepatectomy is regarded as a potentially curative approach for recurrent HCC despite its inherent challenges. These challenges include repeated incisions through scar tissue, adhesions formed within the liver and hepatoduodenal ligament due to prior surgeries and altered anatomical structures post-reoperation [19, 20]. Insufficient adhesiolysis poses the risk of inadvertent organ injury and may compromise the effectiveness of the Pringle maneuver, potentially leading to uncontrollable bleeding during hepatectomy procedure [21].
Our primary findings indicate that repeat resection using a laparoscopic approach in patients with prior hepatectomy, regardless of whether it was a open or laparoscopic approach, was both safe and effective in short term and long-term outcome. Short-term outcomes appear to be comparable, if not superior, to those of patients undergoing open hepatectomy, with a noteworthy reduction in hospital stay attributed to the less invasive nature of the procedure. Our comprehensive timing methodology, starting at the incision and concluding at skin closure, revealed that the operative time spent on adhesiolysis was compensated for by the closure duration in the era of repeat open hepatectomy and not increased the duration of anesthesia.
Our data showed that the safety in LRLR was similar to those in traditional approaches while the initial LRLR was performed in our team after 60 initial procedure which was defined as experienced in the literature. According to previous literatures, learning curve analyses for laparoscopic hepatectomy suggest that approximately 60 cases can be considered experienced phase [22, 23]. LRLR should be considered for selected patients with recurrent HCC when the surgical team attains maturity in laparoscopic hepatectomy. There has been a noticeable paradigm shift in treatment preference for recurrent HCC among both patients and surgeons shown in Fig. 4. Although our follow-up period was relatively short, existing studies support the superior outcomes of repeat hepatectomies for recurrent HCC. Achieving a comparable ratio of R0 resections in LRLR suggests a recommendation for patients seeking curative treatment from an experienced surgical team. The overall survival and disease-free survival of both groups are similar without significant difference also reembraced this surgical outcome of R0 resection in laparoscopic group. According to previous literature, the disease-free survival in three years ranged from 30 to 50% [24, 25] and our results also revealed a similar pattern. The post operative recurrence and overall survival for patients with HCC are mainly affected by the environment- the cirrhotic liver. Risk factors for recurrence after resection HCC include large tumor size [26], positive margin [27] and requirement of blood transfusion [28], but not the procedure itself. In an experienced team, the perioperative quality for laparoscopic repeat liver resection is comparative to the open group and provide a safe choice of treatment to the patients.
Laparoscopic hepatectomy for primary HCC has demonstrated safety and efficacy, facilitating quicker post-operative recovery and shorter hospital stays owing to reduced blood loss and fewer complications [29], but repeat laparoscopic hepatectomy presents heightened complexities. Our experience suggests that in situ incisions with meticulous dissection or combined mini-laparotomies are viable approaches for individuals with a history of major abdominal surgery [30]. Intraoperative ultrasound has demonstrated efficacy as a simple yet effective technique for tumor localization and defining the precise surgical plane for hepatectomy [31]. Advancements in preoperative navigation methods have proven to be invaluable in surgical planning. Three-dimensional reconstruction of vessels and the biliary tree aids in preventing incidental iatrogenic injuries [32]. Additionally, the use of repeated intraoperative ultrasonography and ICG scope navigation assists in correlating preoperative imaging with intraoperative structural changes, ensuring greater surgical precision [33]. Most of our procedures were lateral sectionectomy and minor hepatectomy, laparoscopic repeat hepatectomy was most suitable for the patient with recurrence in the anterior-lateral segment. For patients with recurrence in the posterior segment, further investigation is required.
Liver hypertrophy following regeneration, which has been well-documented in previous studies [34], renders the liver more susceptible to bleeding during surgical intervention. A longer time for parenchymal transection and inflow control is required in the laparoscopic group However, the period of vascular control did not increase the incidence of post-hepatectomy liver failure, which has been mentioned for repeat ischemic-reperfusion injury to the liver [35, 36]. There was no increase in red blood cell transfusions in the laparoscopic group, indicating that blood loss remained controlled, which can be attributed to the meticulous parenchymal dissection and effective bleeding control. Despite equivalent blood loss, the laparoscopic group exhibited incidences of plasma infusion, was managed at our institution through goal-directed fluid management. Recent studies have advocated a protocolized plasma infusion based on SVV to prevent acute kidney injury in laparoscopic hepatectomy, a protocol that we adhered to for optimal patient care [37].Our experience, reflected in the laparoscopic group’s morbidity being greater than grade III at 5.4%, and 5% in the open group, showed no statistical significance. Based on updated literature, the complexity of laparoscopic liver resection significantly affects the incidence of post-operative complications, whereas repeat hepatectomy is not considered a risk factor [38]. In the laparoscopic group, most resections were minor hepatectomies or lateral segmentectomies, classified as Grade I or II complexity according to the 3-level complexity classification system [39]. which is suitable for the pioneer team in laparoscopic hepatectomy based on the learning curves of teams. The higher proportion of cirrhotic patients in the laparoscopic group with similar perioperative outcome also revealed the safety of this procedure in cirrhotic patients. The subgroup analysis for cirrhotic and noncirrhotic patients in the laparoscopic group also confirms this finding. The perioperative mortality rate was 0%. With accumulated experience and a stepwise approach to laparoscopic repeat hepatectomy, we can offer safer treatment options for patients with recurrent liver cancer.
This study has some limitations. Although our study aimed to be comprehensive, its retrospective nature might harbor selection bias; however, the preference for laparoscopic procedures might counteract this. The small sample size and short follow-up duration of this study could limit the accurate depiction of the oncological outcomes. Most patients received minor resection and the effectiveness of recurrent tumors in the posterior segments need further investigation. Randomized studies with extended follow-up periods are needed to substantiate our findings.
Conclusion
Repeat laparoscopic liver resection is feasible and safe for patients with recurrent intrahepatic HCC with shorter post-operative hospital stays with equivalent oncological outcome as compared with open group. It does not prolong the operative time for experienced surgical teams and could potentially shift the treatment trends for recurrent HCC. Successful outcomes depend on adequate adhesiolysis, intraoperative ultrasound guidance, use of ICG scope for tumor localization, and effective Pringle maneuver for hemorrhage control during parenchymal transection. These findings suggest that surgical teams with sufficient experience in laparoscopic hepatectomies can safely perform LRLRs in selected patients, offering patients a minimally invasive option with favorable short-term perioperative outcomes.
Acknowledgements
The author acknowledges all the patients participate in this study and the team participating in the perioperative care.
Abbreviations
- HCC
Hepatocellular carcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- BCLC
Barcelona Clinic Liver Cancer
- LLR
Laparoscopic liver resection
- LRLR
Laparoscopic repeat liver resection
- ICG
Indocyanine green
- RFA
Radiofrequency ablation
- ORLR
Open repeat liver resection
- ICU
Intensive care unit
- SVV
Stroke volume variation
Authors’ contributions
Y.C.C wrote the manuscript; Y.C.C and R.S.S designed the research; P.H.C and S.W.C collected the data;C.Y.C analyzed the data.
Funding
The study was supported by Keelung Chang Gung Memorial Hospital and National Yang Ming Chiao Tung University Joint Research Program [CGMH- NYCU − 2024- CORPG2P0031]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All data is contained within the manuscript. The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki because of the retrospective nature of this study, need for approval of the study was waived by ethics committee of Chang Gung Memorial Hospital. All methods were carried out in accordance with relevant guidelines and regulations in the ethics and consent to participate under declaration section. Due to the retrospective nature of this study, the informed consent was agreed to be waived by the Ethics Committee/Institutional Review Board of Chang Gung Memorial hospital.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, Negri E. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–9. [DOI] [PubMed] [Google Scholar]
- 2.111年國人死因統計結果. (n.d.). Ministry of Health and Welfare. https://www.mohw.gov.tw/cp-16-74869-1.html.
- 3.Shao Y-Y, Wang S-Y, Lin S-M, Chen K-Y, Tseng J-H, Ho M-C, Lee R-C, Liang P-C, Liao L-Y, Huang K-W, et al. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021;120(4):1051–60. [DOI] [PubMed] [Google Scholar]
- 4.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238(5):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francesco P, Matteo S, Alessandro C, Giorgio E. Treatment options for recurrence of hepatocellular carcinoma after surgical resection: review of the literature and current recommendations for management. Hepatoma Res. 2020;6:26. [Google Scholar]
- 7.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Annals of surgery. 2015;261(4):619–29. [DOI] [PubMed] [Google Scholar]
- 8.Goh BK, Teo JY, Chan CY, Lee SY, Cheow PC, Chung AY. Review of 103 cases of laparoscopic repeat liver resection for recurrent Hepatocellular Carcinoma. J Laparoendosc Adv Surg Tech A. 2016;26(11):876–81. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi G, Tanabe M. ILLS 2019 and the development of laparoscopic liver resection in Japan. J Hepatobiliary Pancreat Sci. 2020;27(1):1–2. [DOI] [PubMed] [Google Scholar]
- 10.Morise Z, Aldrighetti L, Belli G, Ratti F, Belli A, Cherqui D, Tanabe M, Wakabayashi G. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score-based study. Br J Surg. 2020;107(7):889–95. [DOI] [PubMed] [Google Scholar]
- 11.Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–7. [DOI] [PubMed] [Google Scholar]
- 12.Huang JW, Su WL, Wang SN. Alternative laparoscopic Intracorporeal Pringle Maneuver by Huang’s Loop. World J Surg. 2018;42(10):3312–5. [DOI] [PubMed] [Google Scholar]
- 13.Hong MK, Ding DC. Seprafilm® Application Method in Laparoscopic Surgery. JSLS: J Soc Laparoendoscopic Surg 2017, 21(1). [DOI] [PMC free article] [PubMed]
- 14.Guidelines for the use of prophylactic antibiotics in surgery in Taiwan. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2004, 37(1):71–74. [PubMed]
- 15.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 16.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver surgery (ISGLS). Surgery. 2011;149(5):713–24. [DOI] [PubMed] [Google Scholar]
- 17.Hermanek P, Wittekind C. Residual tumor (R) classification and prognosis. Semin Surg Oncol. 1994;10(1):12–20. [DOI] [PubMed] [Google Scholar]
- 18.Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14(8):2319–29. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Lin C, Zhang B, Cao J, Chen M, Shen J, Feng X, Xiao G, Pan L, Chen K, et al. Perioperative outcomes comparing laparoscopic with open repeat liver resection for post-hepatectomy recurrent liver cancer: a systematic review and meta-analysis. Int J Surg. 2020;79:17–28. [DOI] [PubMed] [Google Scholar]
- 20.Mohan R, Kabir T, Wu AGR, Lim KI, Goh BKP. Analysis of perioperative outcomes following laparoscopic repeat liver resection compared to laparoscopic primary liver resection based on a single surgeon’s experience: a 1:2 propensity score-matched study. Surg Oncol. 2020;35:382–7. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Tanaka H, Kubo S, Shuto T, Takemura S, Yamamoto T, Uenishi T, Hai S, Osugi H, Hirohashi K. Bowel injury associated with liver surgery for hepatocellular carcinoma. Hepatogastroenterology. 2006;53(70):571–5. [PubMed] [Google Scholar]
- 22.Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250(5):772–82. [DOI] [PubMed] [Google Scholar]
- 23.Sultana A, Nightingale P, Marudanayagam R, Sutcliffe RP. Evaluating the learning curve for laparoscopic liver resection: a comparative study between standard and learning curve CUSUM. HPB: Official J Int Hepato Pancreato Biliary Association. 2019;21(11):1505–12. [DOI] [PubMed] [Google Scholar]
- 24.Chan AC, Poon RT, Chok KS, Cheung TT, Chan SC, Lo CM. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg. 2014;38(5):1141–6. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, Tan W, Zhang L. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc. 2017;31(11):4790–8. [DOI] [PubMed] [Google Scholar]
- 26.Tzu-Hsuan Cheng Y-MC, Chao-Chuan Wu I-S, Tzeng C-C, Wang. Predictors of recurrence in early hepatocellular carcinoma patients treated with surgical resection. Adv Dig Med. October 2021;11(1):21–7. [Google Scholar]
- 27.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231(4):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai YH, Wu HL, Mandell MS, Tsou MY, Chang KY. The association of allogeneic blood transfusion and the recurrence of hepatic cancer after surgical resection. Anaesthesia. 2020;75(4):464–71. [DOI] [PubMed] [Google Scholar]
- 29.Jin B, Chen MT, Fei YT, Du SD, Mao YL. Safety and efficacy for laparoscopic versus open hepatectomy: a meta-analysis. Surg Oncol. 2018;27(2):A26–34. [DOI] [PubMed] [Google Scholar]
- 30.Rajih E, Alhathal N, Alenizi AM, El-Hakim A. Feasibility of planned mini-laparotomy and adhesiolysis at the time of robotic-assisted radical prostatectomy in patients with prior major abdominal surgery. Can Urol Assoc J. 2016;10(3–4):E125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garancini M, Gianotti L, Delitala A, Romano F, Degrate L, Giardini V. Intraoperative ultrasound: a review on its role in liver surgery for primitive and metastatic tumors. Minerva Chir. 2016;71(3):201–13. [PubMed] [Google Scholar]
- 32.Saito Y, Sugimoto M, Imura S, Morine Y, Ikemoto T, Iwahashi S, Yamada S, Shimada M. Intraoperative 3D Hologram support with mixed reality techniques in liver surgery. Ann Surg. 2020;271(1):e4–7. [DOI] [PubMed] [Google Scholar]
- 33.Jianxi W, Xiongfeng Z, Zehao Z, Zhen Z, Tianyi P, Ye L, Haosheng J, Zhixiang J, Huiling W. Indocyanine green fluorescence-guided laparoscopic hepatectomy versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: A single-center propensity score matching study. Front Oncol 2022;12:930065. [DOI] [PMC free article] [PubMed]
- 34.Gilgenkrantz H, Collin de l’Hortet A. Understanding liver regeneration: from mechanisms to Regenerative Medicine. Am J Pathol. 2018;188(6):1316–27. [DOI] [PubMed] [Google Scholar]
- 35.Zdujic P, Bogdanovic A, Djindjic U, Kovac JD, Basaric D, Zdujic N, Dugalic V. Impact of prolonged liver ischemia during intermittent Pringle maneuver on postoperative outcomes following liver resection. Asian J Surg. 2024;47(8):3485-91. [DOI] [PubMed]
- 36.Wei X, Zheng W, Yang Z, Liu H, Tang T, Li X, Liu X. Effect of the intermittent Pringle maneuver on liver damage after hepatectomy: a retrospective cohort study. World J Surg Oncol. 2019;17(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai E, Morohashi Y, Mishima K, Ozaki T, Igarashi K, Wakabayashi G. A goal-directed therapy protocol for preventing acute kidney injury after laparoscopic liver resection: a retrospective observational cohort study. Surg Today. 2022;52(9):1262–74. [DOI] [PubMed] [Google Scholar]
- 38.Mazzotta AD, Kawaguchi Y, Pantel L, Tribillon E, Bonnet S, Gayet B, Soubrane O. Conditional cumulative incidence of postoperative complications stratified by complexity classification for laparoscopic liver resection: optimization of in-hospital observation. Surgery. 2023;173(2):422–7. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi Y, Hasegawa K, Tzeng C-WD, Mizuno T, Arita J, Sakamoto Y, Chun YS, Aloia TA, Kokudo N, Vauthey J-N. Performance of a modified three-level classification in stratifying open liver resection procedures in terms of complexity and postoperative morbidity. Br J Surg. 2019;107(3):258–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is contained within the manuscript. The datasets used and analyzed during the current study available from the corresponding author on reasonable request.