Abstract
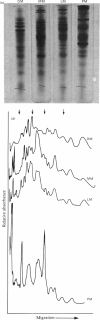
Lysosomes prepared from the livers of untreated rats and from the livers of rats injected with either Triton WR-1339 or dextran yielded membranes that were similar in both polypeptide composition and activities of ATPase and acid 5'-nucleotidase. The administration of Triton WR-1339 (and dextran) resulted in an increase in ATPase activity of liver homogenates that was associated with a parallel increase in the ATPase activity of the lysosomal membrane. On the other hand, plasma membranes appear to be different from lysosomal membranes with respect to polypeptide composition and enzyme activities. The ATPase activity of lysosomal membranes is not affected by ouabain and suramin, inhibitors of the plasma-membrane ATPase. The plasma-membrane alkaline 5'-nucleotidase has little activity at acid pH. Pulse-labelling of lysosomal membranes with [3H]fucose and with [3H]- and [14C]-leucine occurred rapidly, faster than labelling of plasma membranes. The labelling kinetics indicate that lysosomal membranes may be assembled independently of plasma membranes. These data suggest that, in liver, little bulk transport of plasma membrane to lysosomes takes place, and lysosomal-membrane proteins may not be derived from those of plasma membranes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Lysosomes and membrane recycling. A hypothesis. Biochem J. 1977 Dec 15;168(3):603–605. doi: 10.1042/bj1680603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Turnover of lysosomal proteins and induction and distribution of rat liver proteinases, after treatment with Triton WR-1339. Biochem Soc Trans. 1975;3(2):250–252. doi: 10.1042/bst0030250. [DOI] [PubMed] [Google Scholar]
- Doyle D., Baumann H., England B., Friedman E., Hou E., Tweto J. Biogenesis of plasma membrane glycoproteins in hepatoma tissue culture cells. J Biol Chem. 1978 Feb 10;253(3):965–973. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Evans W. H. The liver plasma membrane as a functional mosaic. Biochem Soc Trans. 1976;4(6):1007–1011. doi: 10.1042/bst0041007. [DOI] [PubMed] [Google Scholar]
- FINDLAY J., LEVVY G. A., MARSH C. A. Inhibition of glycosidases by aldonolactones of corresponding configuration. 2. Inhibitors of beta-N-acetylglucosaminidase. Biochem J. 1958 Jul;69(3):467–476. doi: 10.1042/bj0690467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Organization of glycoprotein and glycolipid in the plasma membrane of normal and transformed cells as revealed by galactose oxidase. Biomembranes. 1976;8:131–165. doi: 10.1007/978-1-4684-9087-9_4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Synthesis and turnover of lysosomal glycoproteins. Relation to the molecular heterogeneity of the lysosomal enzymes. FEBS Lett. 1974 Feb 15;39(2):176–181. doi: 10.1016/0014-5793(74)80045-1. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. II. Metabolic fate of iodinated polypeptides of mouse L cells. J Cell Biol. 1975 Feb;64(2):461–479. doi: 10.1083/jcb.64.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system I. Analysis of a novel method of intralysosomal iodination. J Cell Biol. 1980 Jul;86(1):292–303. doi: 10.1083/jcb.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system. II. Bidirectional flow between secondary lysosomes and plasma membrane. J Cell Biol. 1980 Jul;86(1):304–314. doi: 10.1083/jcb.86.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Schneider D. L., Burnside J., Gorga F. R., Nettleton C. J. Properties of the membrane proteins of rat liver lysosomes. The majority of lysosomal membrane proteins are exposed to the cytoplasm. Biochem J. 1978 Oct 15;176(1):75–82. doi: 10.1042/bj1760075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. L. Membranous localization and properties of ATPase of rat liver lysosomes. J Membr Biol. 1977 Jun 6;34(2-3):247–261. doi: 10.1007/BF01870302. [DOI] [PubMed] [Google Scholar]
- Schneider D. L. The acidification of rat liver lysosomes in vitro: a role for the membranous ATPase as a proton pump. Biochem Biophys Res Commun. 1979 Mar 30;87(2):559–565. doi: 10.1016/0006-291x(79)91831-x. [DOI] [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., de Duve C., Trouet A. Fate of plasma membrane during endocytosis. II. Evidence for recycling (shuttle) of plasma membrane constituents. J Cell Biol. 1979 Aug;82(2):466–474. doi: 10.1083/jcb.82.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Ashwell G. Studies on a mammalian hepatic binding protein specific for asialoglycoproteins. Evidence for receptor recycling in isolated rat hepatocytes. J Biol Chem. 1980 Apr 10;255(7):3008–3013. [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Howard D. J., Morell A. G., Scheinberg I. H. Functional segregation of hepatic receptors for asialoglycoproteins during endocytosis. J Biol Chem. 1980 Oct 10;255(19):9028–9029. [PubMed] [Google Scholar]
- Trouet A. Immunisation de lapins par des lysosomes hépatiques de rats traités au Triton WR 1339. Arch Int Physiol Biochim. 1964 Sep;72(4):698–700. [PubMed] [Google Scholar]
- Wang C-C, Touster O. Turnover studies on proteins of rat liver lysosomes. J Biol Chem. 1975 Jul 10;250(13):4896–4902. [PubMed] [Google Scholar]
- Wattiaux R., Wattiaux-De Coninck S., Ronveaux-dupal M. F., Dubois F. Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient. J Cell Biol. 1978 Aug;78(2):349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Widnell C. C. Cytochemical localization of 5'-nucleotidase in subcellular fractions isolated from rat liver. I. The origin of 5'-nucleotidase activity in microsomes. J Cell Biol. 1972 Mar;52(3):542–558. doi: 10.1083/jcb.52.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Ikehara Y., Kawamoto S., Kato K. Characterization of enzymes and glycoproteins in rat liver lysosomal membranes. J Biochem. 1980 Jan;87(1):237–248. doi: 10.1093/oxfordjournals.jbchem.a132731. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]