Right ventricular pacing percentage (%RVp) and paced QRS interval duration (pQRSd) are recognized as being potent independent predictors for the occurrence of pacing-induced cardiomyopathy (PICM). 1 Although the %RVp measurement is part of contemporary cardiac implantable electronic device (CIED) displays, measurement of the pQRSd is not included on trend display s , despite its prognostic significance. Time constraints in device clinics and during remote follow-up do not always allow for serial 12-lead electrocardiogram (ECG) monitoring. Through this case series, we highlight the need for CIED-based pQRSd monitoring and propose a display tool for incorporation into CIED data-capture that has potential to be of value in early intervention against progression of heart failure (HF).
Case Presentation
Patient 1
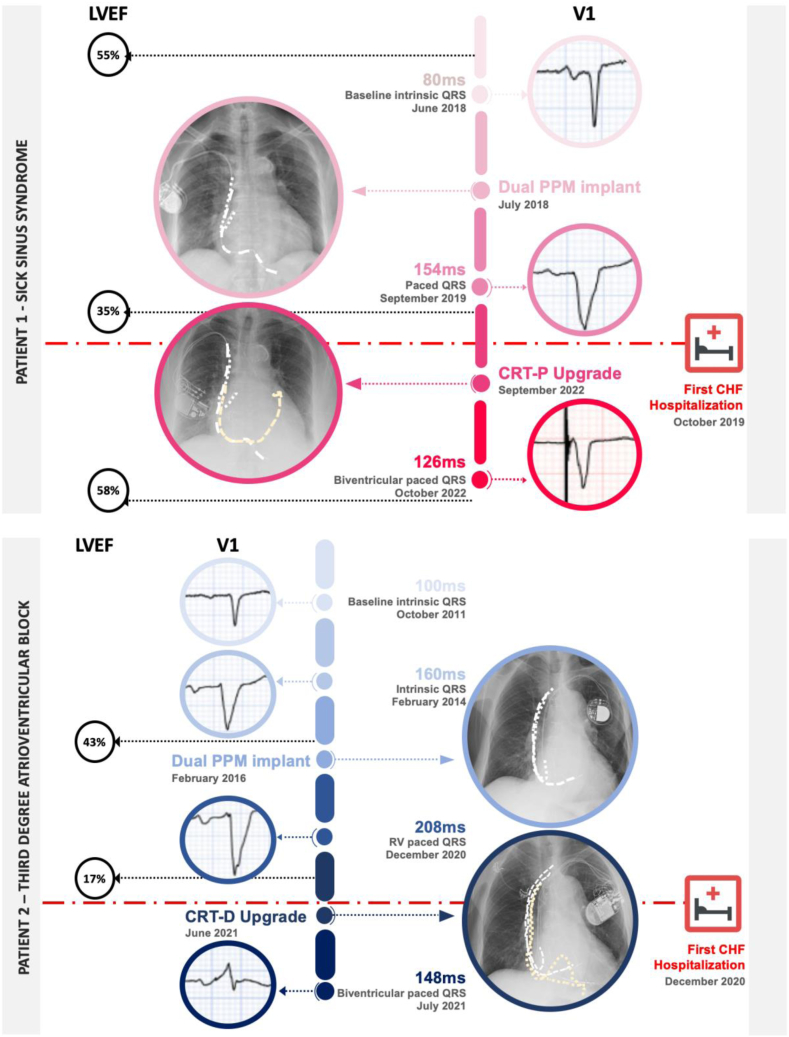
A 74-year-old female with diabetes mellitus type 2, hypertension, chronic kidney disease, and non-occlusive coronary artery disease presented with symptomatic sinus node dysfunction. She received a dual-chamber permanent pacemaker (PPM), programmed to the setting AAIR<>DDDR 60 beats per minute. Prior to implantation, her ECG-recorded intrinsic QRSd was 80 ms, and her left ventricular ejection fraction (LVEF) was 55% on echocardiography. Subsequently, she had an observed RV (right ventricular) pacing burden of 28%. A progressive increase in her pQRSd was observed via ECG assessment, as well as a decline in her LVEF. At 20 months, her pQRSd was 186 ms (with a concomittant increase in intrinsic QRSd to 156 ms), and her LVEF had dropped to less than 35%. Potential causes for deterioration in left ventricular (LV) function, including de novo coronary disease and cardiomyopathy, were excluded. She had recurrent hospitalizations with decompensated HF, despite receiving optimal guideline-directed medical therapy. She underwent an upgrade to a cardiac resynchronization therapy (CRT) pacemaker - after which her pQRSd narrowed to 126 ms, and her LVEF improved to 58%, with improvement in her HF status (biventricular pacing >95%). This clinical improvement can be attributed to the concomitant reduction in her pQRSd, as well as the enhanced ventricular synchrony from the upgrade to the CRT device. The lag time between the PPM implantation and her first HF hospitalization was 15 months (Fig. 1).
Figure 1.
Clinical timeline for cases 1 and 2, illustrating the clinical course of the 2 patients discussed. Both patients received a dual-chamber PPM and subsequently developed heart failure concurrently with LVEF reduction and increase in pQRSd before they were hospitalized at 15 months and 58 months, respectively. Both patients were upgraded to cardiac resynchronization therapy, which led to pQRSd narrowing and significant improvement in LVEF and heart failure status. CHF, congestive heart failure; CRT-D cardiac resynchronisation therapy with defibrillator; CRT-P, cardiac resynchronisation therapy pacemaker; LVEF, left ventircular ejection fraction; PPM, permanent pacemaker; pQRDs, paced QRS duration; RV, right ventricular.
Patient 2
A 77-year-old male with a history of mitral valve repair with coronary artery bypass grafting presented with asymptomatic third-degree atrioventricular block with an intrinsic QRSd of 160 ms, and an LVEF of 40%. He was offered a CRT pacemaker device, per guideline recommendations, but he declined it. Conduction system pacing was unavailable at the time, and he received a dual chamber PPM programmed to the setting DDD 60 beats per minute with 100% RV pacing dependence thereafter. His pQRSd immediately following PPM implantation was 186 ms. A progressive increase in pQRSd was observed, corresponding to a steep decline in LVEF. At the 36-month follow-up, an ECG showed that his pQRSd was 194 ms, and his LVEF was 27%. At 58 months post PPM implantation, he had his first hospitalization with decompensated HF, with an echocardiogram revealing severe cardiomyopathy, with an LVEF of 17% and a pQRSd of 208 ms, despite optimal guideline-directed medical therapy for HF. At this point, he accepted an upgrade to a CRT defibrillator, following which his pQRSd narrowed to 148 ms, and his LVEF improved to 40% after 1 year, and to 49% after 2 years. The lag time between the PPM implantation and his first HF hospitalization was 58 months (Fig. 1).
Discussion
With this case report, we aim to highlight a gap in modern device data display. We propose that pQRSd could be an important datastream collected by modern devices that is currently not utilized appropriately for patient benefit. In the current era of artificial intelligence and advanced technology, we hypothesize that the display of pQRSd in an easily accessible format could support practical decision-making by clinicians in a pacemaker clinic regarding whether to pursue early upgrade to CRT or conduction system pacing.
Briefly, PICM is defined as new-onset LV systolic dysfunction (LVEF ≤ 50%), with either a reduction in LVEF of ≥ 10%, or new regional wall motion abnormality that is unrelated to coronary artery disease, with no other identifiable cause for LV dysfunction.1 Based on these criteria, 10%-20% of patients who have a normal LVEF pre-implantation, and are subjected to frequent RV pacing, will develop PICM.1
A high degree of RV pacing burden has been associated with an increased risk of HF. In the Mode Selection Trial in Sinus-Node Dysfunction (MOST), an RV pacing burden of > 40% was associated with an increased incidence of HF hospitalization and atrial fibrillation.2 In a single-centre study, > 20% RV pacing was associated with negative LV remodelling.3 A prolonged pQRSd has also been associated independently with HF morbidity among patients with chronic RV pacing,4 with a pQRSd of ≥ 150 ms recognized as a threshold for HF development.5
This case series features the clinical course of 2 patients who received a dual chamber PPM for sick sinus syndrome and complete heart block, with 28% and 100% RV pacing, respectively. Both patients developed HF, with a significant reduction in LVEF that could not be attributed to other causes, culminating in HF hospitalization at 15 months and 58 months, respectively. In both cases, a progressive prolongation in pQRSd was observed, but it was not identified until after hospitalization, as 12-lead ECGs were not obtained routinely in the device clinic post implantation.
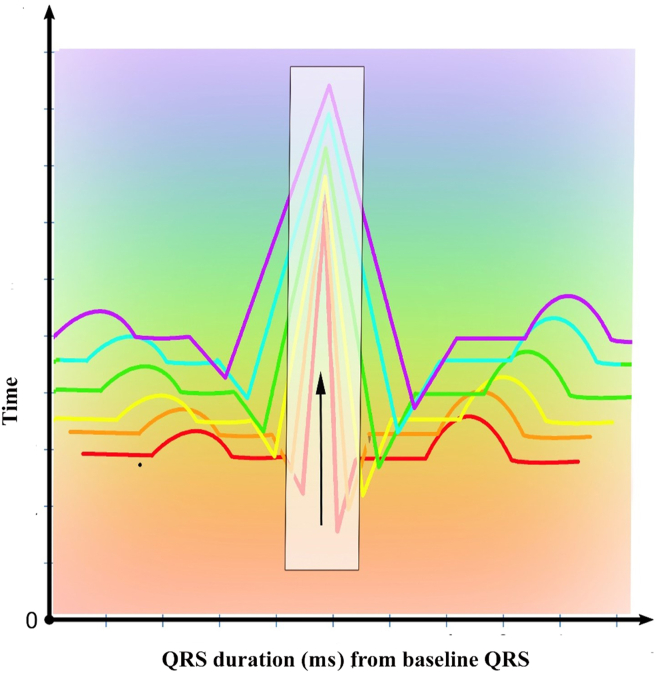
From this observation, we hypothesize that pQRSD displayed as a serial trend may be an important tool to predict progression to PICM, in addition to percentage RV pacing. Modern intracardiac defibrillators are capable of collecting and storing QRS morphology templates for the purposes of tachycardia discrimination. Similarly, if all CIEDs could collect the pQRSd as a practical trend display, this could be useful in screening for PICM. These data could provide a timely alert to device clinic staff that an upgrade to CRT or conduction system pacing is needed to prevent progression to HF morbidity and mortality, even when follow-up is remote. We propose here a hypothetical display tool (Fig. 2), which we call the temporal delta-paced QRS-interval duration (ΔpQRSd), that could be useful. If such a tool could be developed and validated in clinical trials, it may prove to be a useful CIED automatic capture-and-display tool to prevent PICM early, before significant HF morbidity and mortality occur.
Figure 2.
Proposed hypothetical display tool of temporal change in paced QRS-interval duration (temporal ΔpQRSd). This display tool could be implemented into CIEDs for serial monitoring and screening for PICM. The detection of a widening of the pQRSD could serve as an alert to electrophysiologists and device clinics, allowing for timely programming adjustment, medical optimization, and upgrade to cardiac resynchronization or conduction system pacing, before advanced HF develops. CIED, cardiac implantable electronic device; HF, heart failure; PICM, pacing-induced cardiomyopathy; pQRSd, paced QRS-interval duration.
Conclusion
We outline 2 distinct cases that emphasize the potential practical value of using the pQRSd, in addition to the %RVp, in predicting PICM. Although the %RVp is easily monitored via CIED data surveillance, currently, no method exists to monitor serial pQRSd changes via CIED data. We propose that the pQRSd may be a useful parameter in CIED data-capture and display. We hope that our observation could help inform future development of CIED treatment strategies and clinical trials to validate this hypothesis.
Novel Teaching Points.
-
•
Chronic percentage RV pacing (%RVp) and pQRSd are both strong independent predictors for pacing-induced cardiomyopathy. The %RVp can be monitored easily via device data-capture, but the same is not true for the pQRSd.
-
•
The pQRSd may be a useful prediction tool that could be incorporated into CIED algorithms for the screening, early detection, and decision to upgrade to CRT or conduction system pacing.
-
•
The use of device data to prevent HF morbidity and mortality is potentially an area for future research; this possibility will require randomized testing for further evaluation.
Acknowledgments
Ethics Statement
Institutional ethical approval was not required for this case report. Patient consent was obtained directly.
Patient Consent
In accordance with the Committee on Publication Ethics (COPE) guidelines, both patients gave their informed consent for the data related to their medical history to appear in a completely anonymous manner.
Funding Sources
The authors have no funding sources to declare.
Disclosures
K.N. is a consultant for Biosense Webster, Abbott, and BlueRock Therapeutics. M.R.B. is a recipient of the Miriam L Burnett Electrophysiology Fellowship Scholarship. The other authors have no conflicts of interest to disclose.
Footnotes
See page 1323 for disclosure information.
References
- 1.Merchant F.M., Mittal S. Pacing-induced cardiomyopathy. Card Electrophysiol Clin. 2018;10:437–445. doi: 10.1016/j.ccep.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney M.O., Hellkamp A.S., Ellenbogen K.A., et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 3.Kiehl E.L., Makki T., Kumar R., et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi F., Kobayashi Y., Itou H., et al. Prolonged paced QRS duration as a predictor for congestive heart failure in patients with right ventricular apical pacing. Pacing Clin Electrophysiol. 2005;28:1182–1188. doi: 10.1111/j.1540-8159.2005.50181.x. [DOI] [PubMed] [Google Scholar]
- 5.Khurshid S., Liang J.J., Owens A., et al. Longer paced QRS duration is associated with increased prevalence of right ventricular pacing-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:1174–1179. doi: 10.1111/jce.13045. [DOI] [PubMed] [Google Scholar]