Abstract
Background
Cardiac rehabilitation programs provide a valuable opportunity to promote the adoption of healthy lifestyle behaviors in patients with atherosclerotic cardiovascular diseases (ASCVDs) and metabolic comorbidities, including metabolic syndrome and prediabetes. However, strategies to reverse these conditions remain to be explored. The DIABEPIC-1 study aimed to assess the feasibility of an enhanced 6-month cardiac rehabilitation program for patients with ASCVD while investigating prediabetes and metabolic syndrome remission.
Methods
The study combined exercise training with a comprehensive nutritional intervention, emphasizing reduction in intake of ultraprocessed foods, adoption of a Mediterranean diet, and implementation of time-restricted eating. Baseline, 3-month, and 6-month assessments included segmental body-composition measurements, blood analysis, maximal exercise testing, nutritional diaries recorded with the Keenoa AI app, and lifestyle questionnaires. Remission criteria included a return to an HbA1c level of < 5.7%, and < 3 National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP-III) criteria for prediabetes and metabolic syndrome, respectively.
Results
A total of 36 participants were recruited. The study demonstrated completion rates of 94.4% at 3 months, and 88.9% at 6 months, and a mean compliance rate of 92.5% for planned clinical appointments. Significant reductions in waist circumference (–9.2 cm, P < 0.001) and weight (–8.0 kg, P < 0.001) were observed. Improvement in glycemic and lipid profiles, insulin-resistance marker levels, and liver health were noted. Participants enhanced their cardiorespiratory fitness, reduced their consumption of ultraprocessed food, and increased their adherence to the Mediterranean diet and time-restricted eating. Notably, 50% achieved prediabetes remission, and 70% with metabolic syndrome at baseline achieved remission.
Conclusions
The study demonstrates the possibility of enhancing cardiac rehabilitation with an intensive nutritional intervention, yielding clinically significant outcomes, including remission of key risk factors in a substantial number of ASCVD patients.
Clinical Trial Registration
ClinicalTrials.gov, NCT05459987.
Graphical abstract
Résumé
Contexte
Les programmes de réadaptation cardiaque offrent une occasion précieuse de promouvoir l'adoption d'un mode de vie sain chez les patients atteints de maladies cardiovasculaires athéroscléreuses (MCVA) et de comorbidités métaboliques, notamment le syndrome métabolique et le prédiabète. Cependant, les stratégies visant à inverser ces conditions restent à explorer. L'étude DIABEPIC-1 visait à évaluer la faisabilité d'un programme de réadaptation cardiaque renforcé de 6 mois pour les patients atteints de MCVA tout en examinant la rémission du prédiabète et du syndrome métabolique.
Méthodes
L'étude a combiné un entraînement physique avec une intervention nutritionnelle complète, mettant l'accent sur la réduction de la consommation d'aliments ultra-transformés, l'adoption d'un régime méditerranéen et la mise en œuvre d'une alimentation restreinte dans le temps. Les évaluations de base, à 3 mois et à 6 mois, comprenaient des mesures de la composition corporelle par segments, des analyses sanguines, des tests d'exercice maximal, la tenue de journaux nutritionnels enregistrés avec l'application Keenoa AI et des questionnaires sur le mode de vie. Les critères de rémission comprenaient un retour à un niveau d'hémoglobine glyquée HbA1c < 5,7 %, et < 3 critères de la table du « National Cholesterol Education Program Adult Treatment Panel III » (NCEP/ATP-III) pour le prédiabète et le syndrome métabolique, respectivement.
Résultats
Au total, 36 participants ont été recrutés. L'étude a montré des taux d'achèvement de 94,4 % à 3 mois et de 88,9 % à 6 mois, ainsi qu'un taux de conformité moyen de 92,5 % pour les rendez-vous cliniques planifiés. Des réductions significatives du tour de taille (-9,2 cm, p < 0,001) et du poids (-8,0 kg, p < 0,001) ont été observées. Une amélioration des profils glycémiques et lipidiques, des niveaux de marqueurs de résistance à l'insuline et de la santé hépatique a été constatée. Les participants ont amélioré leur condition cardiorespiratoire, réduit leur consommation d'aliments ultra-transformés et augmenté leur adhésion au régime méditerranéen et à l'alimentation restreinte dans le temps. Notamment, 50 % des participants ont obtenu une rémission du prédiabète et 70 % des participants atteints du syndrome métabolique au début de l'étude ont atteint la rémission.
Conclusions
L'étude démontre la possibilité d'améliorer la réadaptation cardiaque par une intervention nutritionnelle intensive, produisant des résultats cliniquement significatifs, y compris la rémission des principaux facteurs de risque chez un nombre substantiel de patients atteints de MCVA.
Enregistrement de l'essai clinique
ClinicalTrials.gov, NCT05459987.
Metabolic comorbidities are prevalent among patients with atherosclerotic cardiovascular diseases (ASCVDs), and they adversely affect the disease prognosis.1 Early insulin resistance, metabolic syndrome, and prediabetes are important underlying factors in the pathophysiology of ASCVD, with a substantial gradient of cardiovascular (CV) risk observed across glycated hemoglobin (HbA1c) levels, starting at those as low as HbA1c ≥ 5.4% (36 mmol/mol), well below the threshold for diabetes.2,3 Although current strategies aim primarily to manage cardiometabolic diseases through lifestyle modifications, recurrent medical checkups, or pharmacotherapy, only a few studies have explored the possibility of achieving remission of the root causes of ASCVD.
In 2021, the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus statement on the definition of type 2 diabetes (T2DM) remission was published, providing meaningful guidance in this area.4 This statement clearly defined remission, which entails maintaining a stable HbA1c level below the diagnostic threshold for at least 3 months without pharmacologic therapy. More recently, the Diabetes Canada guideline on remission of prediabetes and T2DM, and the associated user's guide, have also been released.5,6 underscoring the significant role of weight loss, particularly of excess intra-organ fat, in attaining this objective.7 In pursuit of this goal, individuals have various nonpharmacologic options, including following calorie-restricted diets, reducing consumption of ultraprocessed foods (UPFs), fasting, undergoing metabolic surgery, or receiving pharmacotherapy.8 Alternatively, one can increase energy expenditure through aerobic and resistance training.
One potential solution for patients with established ASCVD is to use cardiac rehabilitation programs, after an acute CV event, as an opportunity to resolve the underlying causes of ASCVD.9,10 Although these programs typically enhance cardiopulmonary fitness, their impact on body mass and fat mass is relatively modest,11 and very few studies have explored the potential to achieve remission through a cardiac rehabilitation program.
Therefore, the primary objective of the DIABEPIC-1 single-arm lifestyle clinical trial was to assess the feasibility of a cardiac rehabilitation program for patients with established ASCVD and new onset of prediabetes, combining exercise rehabilitation with an enhanced nutritional and educational program. Additionally, the study aimed to evaluate its potential effect on the following 2 outcomes: (i) the remission of prediabetes to normal glucose concentrations; and (ii) the remission of metabolic syndrome to an absence of it. Finally, the study sought to characterize baseline and intervention-related changes in body composition and blood analysis.
The study protocol was approved by the Research Ethics and New Technology Development Committee of the Montreal Heart Institute (#2022-3005), and it is registered in ClinicalTrials.gov (NCT05459987). The protocol has been published previously.12 All eligible participants provided written informed consent before they participated in the study.
Materials and Methods
Study population
Participants were recruited for cardiac rehabilitation from the Montreal Heart Institute if they had stable angina, had experienced an acute coronary event, had undergone coronary revascularization, or had undergone bypass surgery. Participants were included at the beginning of the program (at intake) if they were aged ≥ 40 years with a diagnosis of prediabetes within the past 6 months, as determined by their having HbA1c levels between 5.7% (39 mmol/mol) and 6.4% (46 mmol/mol), per the ADA guidelines.13 Exclusion criteria included the following: contraindications to exercise testing and/or physical training14; having T2DM (HbA1c level of ≥ 6.5% [48 mmol/mol], or HbA1c level ≥ 5.7%-6.4% with the use of oral hypoglycemic agents); taking medications known to cause weight gain, such as a psychotropic or cortisone; and taking recently introduced weight-loss medications, such as glucagon-like peptide-1 (GLP-1) agonists.
Study overview
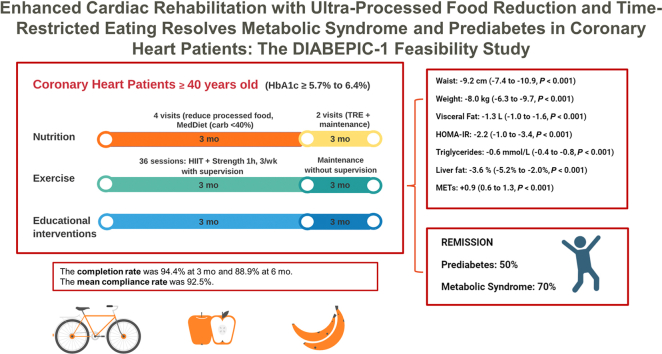
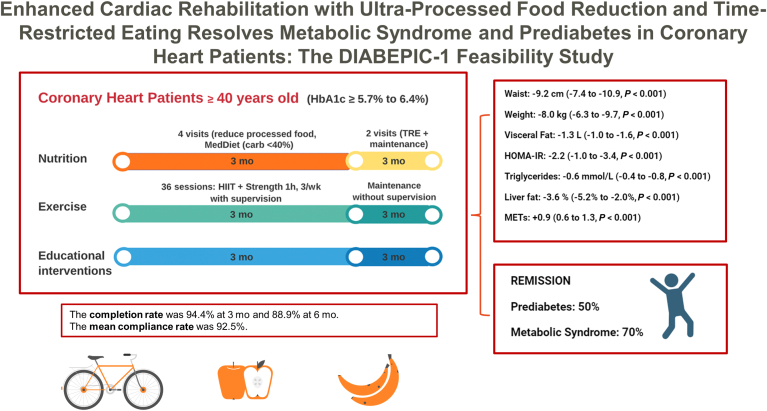
The DIABEPIC-1 feasibility study was conducted at the ÉPIC Centre, for preventive medicine and physical activity, of the Montreal Heart Institute. A visual illustration of the DIABEPIC-1 study intervention is provided in Figure 1.
Figure 1.
Following the inclusion process and the initial baseline assessment, patients with coronary heart disease who had recently been diagnosed with prediabetes (defined by a glycated hemoglobin (HbA1c) level between 5.7% and 6.4%) participated in a 3-pronged intervention. This intervention encompassed nutritional guidance, exercise training, and educational support, all delivered simultaneously. These patients were evaluated after 3 months of this intervention, and once more after an additional 3 months, during which they transitioned to training independently and adopted time-restricted eating practices. carb, carbohydrate; HIIT: high-intensity interval training; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; MedDiet, Mediterranean diet; MET, metabolic equivalent; TRE, time-restricted eating.
The study’s cardiac-rehabilitation program spanned 24 weeks and was comprised of 2 consecutive 3-month intervention phases. Phase 1 included onsite supervised aerobic and resistance training, with an enhanced nutritional intervention, involving counseling on reducing UPF consumption and adopting an ad libitum Mediterranean diet. Phase 2 involved participant continuation of the exercise training program independently, and participant counseling to follow a time-restricted eating (TRE) schedule, by which food consumption is restricted to an 8-hour window during the day, followed by a 16-hour fasting period. Nurses delivered educational interventions, following the collection of participant anthropometric measurements and the return of participant blood test results. These interventions covered topics such as insulin resistance and prediabetes, and concepts related to T2DM, body composition, factors contributing to disease development, and evidence-based methods for remission. A detailed description of all training and nutritional interventions can be found in the Supplemental Methods.
At the 3 time points (baseline, 3 months, and 6 months), the following measurements were conducted: segmental body composition measurements using a bioelectrical impedance balance (Seca medical Body Composition Analyzer (mBCA) 515 [Seca, Hamburg, Germany]); complete blood analysis; a maximal exercise stress test on a treadmill with electrocardiogram-monitoring, a medical visit, and examination; maximal–muscle strength measurements of upper (Matrix Versa Diverging Seated Row, Matrix Fitness, Cottage Grove, WI) and lower muscle groups (Matrix Versa Leg Press, Matrix Fitness); a 3-day food diary record using the photo-based, artificial intelligence–enhanced mobile application Keenoa15Cliquez ou appuyez ici pour entrer du texte.; and the following 4 questionnaires: the International Physical Activity Questionnaire (IPAQ)16; the Depression Anxiety Stress Scale-21 (DASS-21)17; the 14-item Mediterranean Diet Adherence Screener (MEDAS)18; and the Food Cravings Questionnaire score.19 A detailed definition of all variables collected can be found in the Supplemental Methods. Example copies of the questionnaires and information sheets provided to patients are included in the Supplemental Methods.
Study outcomes
The primary outcome of the DIABEPIC-1 study was to assess the feasibility of the interventions. The full-scale study was deemed feasible if it fulfilled the following predefined criteria: ≥ 50% of patients with new onset of prediabetes referred to the ÉPIC Centre for the cardiac rehabilitation program agreed to participate; the mean recruitment rate was ≥ 8 participants per month; the participant completion rate at 3 and 6 months was ≥ 70% (i.e., dropout rate ≤ 30%); and compliance with all protocol interventions reached ≥ 80%. The secondary outcome was to evaluate the proportions of participants with complete or partial remission of prediabetes and metabolic syndrome. The tertiary outcome was to characterize baseline and intervention-related changes in distinct anthropometric, physical, blood analysis, and questionnaire measures.
Definition of prediabetes and metabolic syndrome remission
Complete remission of prediabetes was defined as meeting all 3 of the following criteria: a return of HbA1c level to < 5.7% (39 mmol/mol) at 3 months of intervention (metabolic criterion) that was maintained at 6 months (duration criterion), without the use of glucose-lowering agents (pharmacologic criterion). Partial remission of prediabetes was defined as reaching an HbA1c level of < 5.7% at the end of the 6-month study intervention.
Complete remission of metabolic syndrome was defined as meeting < 3 National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP-III) criteria at 3 months of intervention (metabolic criterion), and maintenance of this status to the 6-month visit (duration criterion). Partial remission of metabolic syndrome was defined as meeting < 3 NCEP/ATP-III criteria at the end of the 6-month study intervention.
Statistical analysis
The statistical analysis is primarily descriptive, and 95% confidence intervals (Cis) are presented when applicable. Summary statistics describing baseline characteristics are presented as a mean ± standard deviation (SD), or as a median (interquartile range), as appropriate, for continuous variables, and as a count and frequency for categorical variables. A 1-way, repeated-measures analysis of variance model was used to compare differences among intervention periods (baseline, 3 months, and 6 months) to assess changes in tertiary outcomes, such as anthropometric measures, exercise-derived measurements, blood-analysis measures, and questionnaire scores. Effect sizes (Cohen's d), with their corresponding 95% CIs were calculated to measure the magnitude of the observed differences among groups. A Bonferroni correction was applied to account for multiple comparisons in the statistical analysis. The assumptions underlying the planned models were checked, and when they were not tenable, the Kruskal-Wallis nonparametric statistical test was employed. Statistical significance was defined as a P-value < 0.05. Statistical analyses were performed using Stata statistical software, release 15 (StataCorp, College Station, TX).
Results
Primary outcome: feasibility of the study
Recruitment
Over a period of 6 months, 57 patients with ASCVD who were referred to the ÉPIC Centre for cardiac rehabilitation, and who had been recently diagnosed with prediabetes per the ADA criteria, were screened. Among these patients, 36 provided consent and participated in the study. The screened participants' final enrollment rate was 63.2% (95% CI: 50.0%-74.8%). A consistent recruitment rate of 6 new participants per month was maintained throughout the study. In the context of the COVID-19 pandemic, the recruitment took 6 months to complete, instead of the anticipated 5 months.
Completion rate at 3 and 6 months
Of the initial 36 patients who began the study, 32 completed the entire protocol. At 3 months, the completion rate was 94.4% (95% CI: 80.0%-98.7%). Similarly, at 6 months, the completion rate was 88.9% (95% CI: 73.2%-95.9%).
Compliance with the clinical assessments
The mean compliance rate, for the 54 clinical appointments or interventions planned per the protocol, was 92.5% (95% CI: 83.3%-100.0%). Supplemental Tables S1 and S2 show a detailed breakdown of the compliance data; this includes the number of moderate-intensity training sessions and of high-intensity interval training sessions, as well as meetings with registered dietitians.
Clinical characteristics
The final analysis includes 32 participants who completed the protocol, a number sufficient to ensure data availability for analysis. The mean participant age was 61 ± 10 years, and 28% were women. The mean baseline body mass index was 30.3 ± 5.4 kg/m2. A total of 27 participants (84.4%) had experienced a recent acute coronary syndrome, and 5 were referred due to stable angina. At the start of the study, all participants had been recently diagnosed with prediabetes, with a median HbA1c level of 5.8%, with a range of 5.7%-6.4%. Additionally, 20 participants (62.5%) met the NCEP/ATP-III criteria for metabolic syndrome. A complete summary of the baseline characteristics of the study participants is provided in Table 1.
Table 1.
Baseline study population clinical characteristics
| Main baseline clinical characteristics | Participants (n = 32) |
| Age, y | 61 ± 10 |
| Sex, male | 23 (72) |
| HbA1c, % | 5.8 (5.7–5.8) |
| BMI, Kg/m2 | 30.3 ± 5.4 |
| Metabolic syndrome, NCEP-ATP- III criteria | 20 (62.5) |
| Risk factors | |
| Hypertension | 30 (92) |
| Dyslipidemia | 32 (100) |
| Smoking | 5 (15.6) |
| Overweight or obesity | 26 (81.3) |
| Sedentary lifestyle | 19 (59.4) |
| Sleep apnea | 7 (21.9) |
| Liver steatosis (estimated ≥ 5% liver fat) | 19 (67.9) |
| Coronary heart disease history | |
| Recent acute coronary syndrome (< 6 mo) | 27 (84.4) |
| Myocardial infarction | 16 (50) |
| Unstable angina | 11 (34.4) |
| Stable angina | 5 (15.6) |
| Percutaneous coronary intervention | 18 (56.3) |
| Bypass surgery | 9 (28.1) |
| Heart failure with reduced ejection fraction | 2 (6.2) |
| Treatment | |
| ACE inhibitors, ARB, or calcium-channel blockers | 28 (87.5) |
| Beta-blockers | 22 (68.8) |
| Statins | 31 (96.9) |
| Ezetimibe | 6 (18.8) |
Characteristics are presented as mean ± standard deviation, or median (25th percentile-75th percentile) for continuous variables, and as count and frequency (%) for categorical variables.
BMI, body mass index; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; HbA1c, glycated hemoglobin; NCEP-ATP-III, National Cholesterol Education Program Adult Treatment Panel III.
Secondary outcome: prediabetes and metabolic syndrome remission
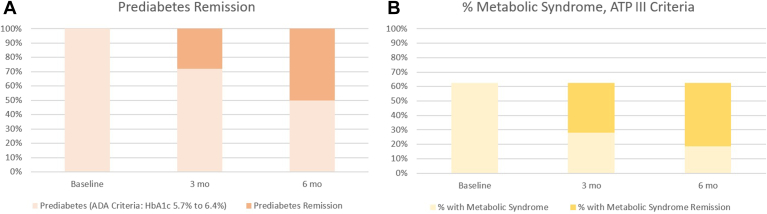
Of the 32 participants who completed the intervention, 9 (28.1%, 95% CI: 14.9%-46.6%) achieved an HbA1c level of < 5.7% at 3 months and maintained it until the 6-month visit, thus fulfilling the criteria for complete remission of prediabetes. Additionally, 7 participants (21.9%, 95% CI: 10.5%-40.1%) reached an HbA1c level of < 5.7% at 6 months, meeting the criteria for partial remission of prediabetes. Overall, these values represent a total of 16 participants (50.0%, 95% CI: 32.7%-67.3%; Fig. 2A who met either of the remission criteria after the 6 months of the intervention. None of the participants progressed to T2DM.
Figure 2.
Prediabetes and metabolic syndrome remission rates after 6 months of intervention. (A) All study participants met the American Diabetes Association (ADA) criteria for prediabetes, at the beginning of the study. Among these individuals, 16 participants (50.0%, 95% confidence interval: 32.7%-67.3%) met either of the remission criteria definitions for prediabetes after 6 months of the intervention. (B) Of the 32 participants, 20 (62.5%) initially met the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP-III) criteria for metabolic syndrome. Among these individuals, 14 (70%, 95% confidence interval: 45.7%-86.6%) met at least one of the remission criteria definitions for metabolic syndrome by the end of the study. HbA1c, glycated hemoglobin.
Among the 7 participants who initially had the highest HbA1c levels, ranging from 6.0% to 6.4% (the Diabetes Canada criteria for prediabetes), 5 ([71.4%, 95% CI: 24.4%-95.1%) achieved an HbA1c level of < 6.0% at 3 months and maintained it at 6 months. The remaining 2 participants reached an HbA1c level of < 6.0% at 6 months. Therefore, all participants with initial HbA1c levels of ≥ 6.0% met the criteria set by Diabetes Canada for returning to normal glucose concentrations by the study's conclusion.
Of the 32 participants, 20 (62.5%) met the NCEP/ATP-III criteria for metabolic syndrome at the start of the study. Among these 20 participants, 11 (55%, 95% CI: 32.3%-75.8%) achieved complete remission of metabolic syndrome. Additionally, 3 participants (15%, 95% CI: 4.5%-40.0%) reached the criteria for partial remission of metabolic syndrome at 6 months. In total, 14 participants (70%, 95% CI: 45.7%-86.6%) met the criteria for the definition of metabolic syndrome remission (Fig. 2B). Finally, none of the participants who did not have metabolic syndrome at the beginning of the study developed the criteria for a new diagnosis during the study period.
Tertiary outcomes: body composition, fasting blood test, cardiorespiratory fitness, and changes over time
A complete report of summary statistics, of baseline body-composition characteristics, fasting blood-test parameters, cardiorespiratory fitness, and changes over time, can be found in Table 2. After the 6-month follow-up period, all anthropometric, glycemic, and insulin-resistance measures showed clinically significant improvement. Blood pressure, cardiorespiratory fitness, and maximal muscle strength also improved. Most of these benefits were observed during phase 1 of the study, with the improvements persisting into the second follow-up phase, and occasionally showing further improvement. At 6 months, the mean reduction in waist circumference was –9.2 cm (95% CI: –7.4 to –10.9; P <0.001), and the mean body-mass loss was –8.0 kg (95% CI: –6.3 to –9.7; P <0.001), with 84% of participants losing ≥ 5% of weight. The participants also reported undergoing a significant reduction in self-reported time spent in a sedentary lifestyle, an increase in physical activity , and an improvement in their self-reported emotional state (Supplemental Tables S3 and S4).
Table 2.
Summary statistics of baseline body composition, fasting blood-test results, treadmill stress-test results, muscular strength characteristics, and changes over time
| Measure | T0 | T3 | T6 | F(2,61), P | Δ 0–3 mo | P | Δ 3–6 mo | P | Δ 0–6 mo | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Waist, cm | 105.0 ± 12.6 | 98.4 ± 12.6 | 95.9 ± 13.0 | 86.4, < 0.001 | –6.3 (–4.6 to –8.1) | < 0.001 | –2.8 (–1.0 to –4.6) | 0.001 | –9.2 (–7.4 to –10.9) | < 0.001 |
| Weight, kg | 87.4 ± 17.8 | 81.4 ± 16.8 | 79.4 ± 16.4 | 70.5, < 0.001 | –5.3 (–3.6 to –7.0) | < 0.001 | –2.7 (–1.0 to –4.4) | 0.001 | –8.0 (–6.3 to –9.7) | < 0.001 |
| BMI, kg/m2 | 30.3 ± 5.4 | 28.3 ± 5.2 | 27.5 ± 4.9 | 72.3, < 0.001 | –1.8 (–1.3 to –2.7) | < 0.001 | –1.0 (–0.4 to –1.6) | < 0.001 | –2.8 (–2.3 to –3.3) | < 0.001 |
| Adipose tissue | ||||||||||
| Fat mass, Kg | 29.9 ± 9.7 | 25.3 ± 9.3 | 23.7 ± 8.6 | 49.1, < 0.001 | –4.4 (–3.1 to –5.6) | < 0.001 | –1.7 (–0.2 to –3.4) | 0.023 | –6.1 (–4.6 to –7.7) | < 0.001 |
| Fat mass, % | 34.6 ± 7.9 | 31.3 ± 8.4 | 30.0 ± 7.8 | 37.6, < 0.001 | –3.3 (–2.2 to –4.4) | < 0.001 | –1.2 (–2.5 to 0.2) | 0.111 | –4.5 (–3.2 to –5.8) | < 0.001 |
| Visceral fat, L | 4.2 ± 2.2 | 3.2 ± 1.8 | 2.9 ± 1.9 | 41.5, < 0.001 | –1.0 (–0.7 to –1.3) | < 0.001 | –0.3 (–0.7 to 0.1) | 0.103 | –1.3 (–1.0 to –1.6) | < 0.001 |
| Lean tissue | ||||||||||
| Lean mass, % | 65.4 ± 7.8 | 68.7 ± 8.4 | 70.0 ± 8.4 | 37.6, < 0.001 | +3.2 (1.8–4.5) | < 0.001 | +1.3 (–0.1 to 2.6) | 0.071 | +4.4 (3.1–5.8) | < 0.001 |
| Skeletal mass, % | 30.8 ± 4.6 | 31.8 ± 4.8 | 32.3 ± 4.3 | 22.8, < 0.001 | +1.0 (0.5–1.6) | < 0.001 | +0.4 (–0.1 to 0.9) | 0.202 | +1.4 (0.9–2.0) | < 0.001 |
| Glycemic parameters | ||||||||||
| Glucose, mmol/L | 5.7 ± 0.7 | 5.3 ± 0.6 | 5.1 ± 0.6 | 19.5, < 0.001 | –0.4 (–0.2 to –0.6) | < 0.001 | –0.2 (–0.4 to 0.1) | 0.244 | –0.6 (–0.4 to –0.8) | < 0.001 |
| Insulin, pmol/L | 125.8 ± 88.4 | 78.5 ± 52.7 | 81.2 ± 53.2 | 11.1, < 0.001 | –40.0 (–14.0 to –65.9) | 0.001 | –4.7 (–30.6 to 21.3) | 1.000 | –44.6 (–19.0 to –70.3) | < 0.001 |
| HbA1c, % | 5.9 ± 0.2 | 5.7 ± 0.2 | 5.6 ± 0.2 | 19.4, < 0.001 | –0.2 (–0.1 to –0.3) | < 0.001 | –0.1 (–0.2 to 0.1) | 1.000 | –0.3 (–0.2 to –0.4) | < 0.001 |
| HOMA-IR | 5.3 ± 3.7 | 3.2 ± 2.3 | 3.1 ± 2.0 | 12.7, < 0.001 | –1.9 (–0.7 to –3.1) | 0.001 | –0.3 (–1.5 to 0.9) | 1.000 | –2.2 (–1.0 to –3.4) | < 0.001 |
| Lipid parameters | ||||||||||
| TC, mmol/L | 3.6 ± 0.8 | 3.3 ± 0.7 | 3.4 ± 0.7 | 3.40, 0.040 | –0.3 (–0.0 to –0.6) | 0.044 | –0.1 (–0.2 to 0.3) | 1.000 | –0.2 (–0.4 to 0.0) | 0.203 |
| LDL-C, mmol/L | 1.7 ± 0.7 | 1.5 ± 0.6 | 1.5 ± 0.6 | 3.13, 0.049 | –0.2 (–0.4 to 0.0) | 0.081 | 0.0 (–0.2 to 0.2) | 1.000 | –0.2 (–0.4 to 0.0) | 0.045 |
| HDL-C, mmol/L | 1.2 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | 6.13, 0.004 | 0.1 (–0.0 to 0.1) | 0.640 | 0.1 (–0.0 to 0.2) | 0.103 | 0.2 (0.1–0.2) | 0.003 |
| TGS, mmol/L | 1.7 ± 0.6 | 1.3 ± 0.5 | 1.1 ± 0.6 | 36.5, < 0.001 | –0.4 (–0.2 to –0.6) | < 0.001 | –0.2 (–0.3 to 0.0) | 0.146 | –0.6 (–0.4 to –0.8) | < 0.001 |
| ApoB, g/L | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 3.62, 0.033 | –0.1 (–0.1 to 0.0) | 0.173 | –0.0 (–0.1 to 0.1) | 1.000 | –0.1 (–0.1 to –0.0) | 0.037 |
| Inflammatory markers | ||||||||||
| Leukocytes, 109/L | 6.6 ± 1.7 | 6.0 ± 1.3 | 6.0 ± 1.4 | 4.41, 0.017 | –0.5 (–0.9 to 0.0) | 0.144 | –0.1 (–0.7 to 0.4) | 1.000 | –0.6 (–1.1 to –0.1) | 0.017 |
| hs-CRP, mg/L | 3.0 ± 4.1 | 2.7 ± 4.3 | 2.4 ± 3.5 | 1.35, 0.269 | –— | — | –— | — | –— | — |
| Liver measurements | ||||||||||
| ALT, U/L | 39.8 ± 14.9 | 35.2 ± 11.2 | 33.9 ± 9.5 | 3.39, 0.041 | –3.8 (–9.6 to 1.9) | 0.320 | –2.1 (–7.9 to 3.7) | 1.000 | –5.9 (–11.6 to –0.2) | 0.038 |
| AST, U/L | 23.5 ± 6.7 | 23.2 ± 6.6 | 22.6 ± 4.8 | 0.42, 0.657 | –— | — | –— | — | –— | — |
| Liver fat, % | 7.4 ± 4.5 | 4.4 ± 2.9 | 3.8 ± 2.0 | 17.4, < 0.001 | –3.0 (–4.6 to –1.4) | < 0.001 | –0.6 (–2.2 to 1.0) | 1.000 | –3.6 (–5.2 to –2.0) | <0.001 |
| Blood pressure | ||||||||||
| SBP, mm Hg | 124 ± 11 | 117 ± 12 | 118 ± 10 | 6.4, 0.003 | –6.4 (–1.7 to –11.2) | 0.004 | 1.1 (–3.6 to 5.8) | 1.000 | –5.3 (–0.6 to –10) | 0.021 |
| DBP, mm Hg | 73.8 ± 6.1 | 71.5 ± 8.9 | 70.9 ± 8.2 | 2.9, 0.062 | — | — | — | — | –— | — |
| Cardiorespiratory fitness | ||||||||||
| METs | 7.0 ± 1.5 | 7.7 ± 1.6 | 8.0 ± 1.7 | 18.3, < 0.001 | 0.6 (0.2–1.0) | 0.001 | 0.3 (–0.1 to 0.7) | 0.145 | 0.9 (0.6–1.3) | < 0.001 |
| Upper body, kg | 35 ± 14 | 50 ± 21 | 55 ± 19 | 18.91, < 0.001 | 15.1 (5.1–25.2) | 0.002 | 4.2 (–5.9 to 14.2) | 0.895 | 19.3 (11.0–27.6) | < 0.001 |
| Lower body, kg | 116 ± 49 | 168 ± 70 | 191 ± 82 | 22.08, < 0.001 | 61.3 (25.1–97.4) | 0.001 | 13.1 (–23.0 to 49.3) | 1.000 | 74.4 (44.6–104.2) | < 0.001 |
Summary statistics are presented as mean ± standard deviation for continuous variables. A 1-way analysis of variance was used to examine variations in means across the different time points. The F value in the analysis of variance helps determine whether the differences observed between intervention periods are likely true differences in the population means or rather are due to random variability. A significant F value suggests that true differences exist, warranting further investigation into their nature. Body composition was measured using the bioelectrical impedance analysis across the intervention’s time course; data were available for 29 participants. Liver fat content was calculated for each participant based on routinely available clinical data and 3 serological markers—fasting insulin, alanine transaminase (ALT), and aspartate transaminase (AST). For maximal muscle strength, data were available for 18 participants. The upper-body strength was measured with seated rows; the lower-body strength was measured with leg press. 1RM values were predicted using the Berger equation from submaximal repetitions (10 to 4 RM), performed to the point of muscle fatigue. Peak cardiorespiratory fitness was measured in metabolic equivalents of task (METs).
ApoB, apoprotein B; BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TGS, triglycerides; T0, at baseline; T3, at 3 months; T6, at 6 months.
Nutritional intervention data
During the study, a significant increase (P < 0.001) in adherence to the Mediterranean diet was assessed using the 14-item Mediterranean Diet Adherence Screener (MEDAS) (Supplemental Table S5). Total energy intake decreased by a mean of –203 ± 81 Kcal (P = 0.048). Carbohydrate intake (–53 ± 10 g, P < 0.001), especially of simple sugars and alcohol, decreased, and protein and fat intake, particularly of polyunsaturated fat, increased. Total energy intake from UPFs (NOVA group 4) decreased by –10% ± 3% (P = 0.008). Complete data and changes over time in nutritional scores and nutrient intakes can be found in Table 3.
Table 3.
Reported nutrient intakes collected by the Keenoa artificial intelligence app, and changes over time
| Intake | T0 | T3 | T6 | F(2,44), P | Δ 0–3 mo | P | Δ 3–6 mo | P | Δ 0–6 mo | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Caloric intake | ||||||||||
| Energy, Kcal | 1891 ± 323 | 1573 ± 381 | 1688 ± 351 | 7.9, 0.001 | –318 ± 81 (–154 to –481) | < 0.001 | 114 ± 81 (–87 to –317) | 0.493 | –203 ± 81 (–2 to –405) | 0.048 |
| Macronutrient sources, % | ||||||||||
| Carbohydrate | 46 ± 7 | 42 ± 8 | 39 ± 7 | 8.4, 0.001 | –4 ± 2 (–8 to 0) | 0.080 | –3 ± 2 (–7 to 2) | 0.235 | –7 ± 1 (–3 to –11) | < 0.001 |
| Protein | 19 ± 3 | 21 ± 3 | 21 ± 5 | 6.3, 0.004 | 2 ± 1 (1–4) | 0.008 | 0 ± 1 (–2 to 2) | 1.000 | 2 ± 1 (0 to 4) | 0.016 |
| Fat | 33 ± 6 | 37 ± 8 | 40 ± 7 | 11.5, < 0.001 | 4 ± 1 (–0 to 7) | 0.052 | 3 ± 1 (–0 to 7) | 0.074 | 7 ± 1 (3 to 11) | < 0.001 |
| Dietary components | ||||||||||
| Carbohydrate | 217 ± 43 | 162 ± 33 | 164 ± 39 | 20.6, < 0.001 | –55 ± 10 (–30 to –79) | < 0.001 | 1 ± 10 (–23 to 25) | 1.000 | –53 ± 10 (–29 to –77) | < 0.001 |
| Sugar | 77 ± 23 | 52± 13 | 56 ± 18 | 20.7, < 0.001 | –25 ± 4 (–15 to –35) | < 0.001 | 4 ± 4 (–7 to 14) | 1.000 | –21 ± 4 (–11 to –32) | < 0.001 |
| Fiber | 26 ± 9 | 26 ± 9 | 27 ± 11 | 0.1, 0.897 | — | — | — | — | — | — |
| Saturated fat | 19 ± 5 | 15 ± 6 | 18 ± 5 | 3.5, 0.040 | –4 ± 1 (–7 to 0) | 0.058 | 3 ± 1 (–0 to 6) | 0.125 | –1 (–4 to 3) | 1.000 |
| Monosat. fat | 27 ± 8 | 24 ± 12 | 29 ± 11 | 2.7, 0.079 | — | — | — | — | — | — |
| Polyunsat. fat | 17 ± 6 | 18 ± 10 | 20 ± 9 | 2.3, 0.116 | — | — | — | — | — | — |
| Trans | 0.3 [0.1–0.6] | 0.3 [0.2–0.6] | 0.4 [0.2–0.6] | χ 2 = 1,0, 0.608 | — | — | — | — | — | — |
| Omega 6 | 6 [5–10] | 7 [4–11] | 7 [5–13] | χ 2 = 0.6, 0.729 | — | — | — | — | — | — |
| Omega 3 | 2 [1–4] | 4 [2–5] | 4 ± [2–6] | χ 2 = 6.0, 0.048 | 2 (0–3) | 0.128 | 0 ± 1 (–1 to 2) | 0.561 | 2 ± 1 (1 to 3) | 0.035 |
| Ratio Omega 6/3 | 3 [2–4] | 2 [1–3] | 2 [1–3] | χ 2 = 6.1, 0.047 | –1 (–3 to 1) | 0.058 | 0 (–2 to 2) | 0.815 | –1 (–3 to 0) | 0.034 |
| Sodium | 2 [2–3] | 1 [1–2] | 1 [1–2] | χ 2 = 10.9, 0.004 | –1 (–2 to 0) | 0.024 | 0 (–1 to 1) | 0.877 | –1 (–2 to 0) | 0.017 |
| BCAA (L, I, V), n | 11 [8–16] | 11 [8–13] | 12 [10–14] | χ 2 = 0.6, 0.732 | — | — | — | — | — | — |
| Alcohol | 10 ± 15 | 4 ± 7 | 4 ± 6 | 3.7, 0.034 | –6 ± 2 (–11 to 0) | 0.079 | –0 ± 2 (–6 to 6) | 1.000 | –6 ± 2 (–11 to 0) | — |
| NOVA classification, % energy | ||||||||||
| 1 | 42 ± 16 | 50 ± 13 | 50 ± 12 | 2.8, 0.076 | — | — | — | — | — | — |
| 2 | 7 ± 4 | 8 ± 4 | 9 ± 5 | 2.8, 0.070 | — | — | — | — | — | — |
| 3 | 26 ± 10 | 25 ± 10 | 25 ± 9 | 0.2, 0.977 | — | — | — | — | — | — |
| 4 | 25 ± 13 | 16 ± 10 | 15 ± 13 | 6.5, 0.003 | –9 ± 3 (–3 to –14) | 0.013 | –1 ± 3 (–8 to –7) | 1.000 | –10 ± 3 (–2 to –17) | 0.008 |
Dietary components are given in g, unless otherwise indicated. Data related to completing a 3-day journal involving 23 participants. This 3-day journal required participants to record data for 2 weekdays and 1 weekend day, during each of the 3 evaluation time frames. A research dietician verified and summarized the recorded values by calculating the mean ± standard deviation for each evaluation period (baseline [T0], 3 months [T3], and 6 months [T6]), or medians (25th percentile–75th percentile), as appropriate. An analysis of variance was used to examine variations in means across the different time points. When data did not meet the assumptions required for parametric tests, the Kruskal-Wallis nonparametric statistical test was used. BCAA, branched-chain amino acids, including leucine, isoleucine, and valine.
Monosat., monosaturated; Polyunsat., polyunsaturated.
TRE
Finally, in Phase 2, TRE was introduced. A total of 22 participants (68.8%, 95% CI: 50.3%- 82.7%) followed the TRE protocol (16:8) for > 5 days a week, and 6 participants maintained the TRE pattern (16:8) for at least 2-4 days a week, bringing the total proportion of participants adhering to TRE to 87.6% (95% CI: 70.1%-95.4%; Supplemental Fig. S1). Although this study was not designed originally to test this hypothesis, Fisher’s exact test was employed to investigate any significant association between adherence to TRE and the remission of prediabetes or metabolic syndrome. No statistically significant association was found between the 2 variables, with 1-tailed P-values of 0.126 and 0.336 for prediabetes and metabolic syndrome remission, respectively.
Discussion
The DIABEPIC-1 lifestyle intervention study, offered to patients with a history of ASCVD and newly diagnosed prediabetes, demonstrated the positive impact of an upgraded exercise-based cardiac rehabilitation program that integrates an intensive nutritional intervention targeting UPFs, promotion of a Mediterranean diet, and incorporation of TRE in a stepwise manner. As a result, this comprehensive approach yielded beneficial results in 4 key areas: (i) improved body composition, and clinical improvements in blood pressure, glycemic and lipid profile, insulin-resistance markers, and liver health; (ii) enhanced cardiorespiratory fitness and muscle strength, increased physical activity, and reduced sedentary behavior; (iii) heightened adherence to the Mediterranean diet, improved nutritional quality, lower levels of UPF consumption, lower caloric intake, and high TRE compliance; and (iv) attainment of prediabetes remission to normal glucose levels in 50% of participants, with those with the highest initial HbA1c levels experiencing a remarkable 100% remission rate. Additionally, 70% of participants with metabolic syndrome achieved remission.
The study was performed with high completion rates (94.4% at 3 months, and 88.9% at 6 months) and high compliance rates (92.5% for clinical appointments), although recruitment was delayed by the COVID-19 pandemic, extending it to a 6-month, instead of a 5-month, period. This study demonstrates the possibility of reinforcing a cardiac rehabilitation program with an enhanced stepwise nutritional intervention that yields clinical improvements of key causal risk factors for ASCVD patients, notably prediabetes and metabolic syndrome, thereby addressing the intricate interrelationship between metabolic and CV health.
Cardiac rehabilitation and remission of CV risk factors
Standard cardiac rehabilitation programs provide a valuable opportunity to promote the adoption of healthy lifestyle behaviors for patients with ASCVD, typically focusing on exercise training, and blood pressure, lipid, and glycemic management.20 However, the role of nutrition in cardiac rehabilitation success faces structural constraints from both patient and program administrative perspectives. Notably, a gap is present in access to nutritional interventions or research that tests high-quality nutrition interventions within cardiac rehabilitation settings.21 This hinders progress in improving body composition and potentially achieving remission of CV risk factors. For instance, in the absence of a dedicated weight-loss program, conventional cardiac rehabilitation usually results in minor weight reduction (∼2 %) and modest changes in body composition.22,23 In our study, the mean percentage reduction in body weight was −6.0% (95% CI: −4.5% to −7.5%) at 3 months, and −9.1% (95% CI: −7.2% to −11.0%) at the end of the study, with 84% of participants lost at least >5% of initial body weight.
Numerous studies in the past 2 decades have demonstrated that significant weight loss can prevent progression from prediabetes to T2DM, particularly when exercise is combined with nutrition intervention.24 A Cochrane systematic review of 8 trials investigating the effect of exercise, or exercise and diet, for the prevention of T2DM (2241 participants) and standard care (2509 participants) revealed that, overall, interventions combining exercise and diet reduced the risk of progression to T2DM, compared to standard recommendations, by 37% (relative ratio 0.63, 95% CI: 0.49-0.79).25 A point worth noting is that none of these studies incorporated supervised high-intensity exercise training, and none combined intermittent fasting as a dietary strategy with other lifestyle interventions that have been demonstrated to have benefits for metabolic biomarkers.26 A point worth mentioning is that none of these studies evaluated the criteria for the remission of prediabetes to normal glucose concentrations, most likely because agreed-upon criteria were lacking at the time.
In the different contexts of primary-care and community settings, the Diabetes Remission Clinical Trial (DiRECT) study and the Diabetes Intervention Accentuating Diet and Enhancing Metabolism-I (DIADEM-I) trial have shown that T2DM remission was achievable in 46% of participants (HbA1c level of < 6.5% at 12 months, without hypoglycemic agents) in the DiRECT study intervention group,27 and in 61% of participants in the DIADEM-I intervention group (800 kcal/d hypocaloric diet for 3-5 months, followed by a structured long-term weight-maintenance program).28 These studies have fundamentally altered the landscape by revealing that a significant loss of weight (> 10%-15%) induced by the diet could lead to T2DM remission, mainly when a substantial reduction in visceral adiposity was observed, including liver-fat content.29
Our study used a multisegmental bioimpedance scale to assess various aspects of body composition. Beyond quantifying changes in body composition, the scale facilitated the explanation to patients of the different body components in the pathophysiology of prediabetes and metabolic syndrome remission. The scale also allowed for tracking of patients' progress, which clinicians and patients appreciated. This approach emphasized the importance of preserving muscle and lean body mass in relation to total body mass and muscle function while reducing fat mass, especially visceral fat. We observed a substantial mean reduction of –31.9% (95% CI: –25.3% to –38.6%) in visceral fat at the 6-month mark. We also noted a highly significant decrease in liver enzymes and computed hepatic steatosis, with 67.9% of participants having a predicted intrahepatic fat level of ≥ 5% at the beginning of the study, which was reduced to 10.7% of participants by the study's end (Supplemental Fig. S2).
Caloric reduction and impact of reducing intake of UPFs on weight reduction and satiety signals
In the field of nutrition, we have gained insights into the health advantages of calorie restriction. For instance, in the Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) study, the intervention group aimed at a 25% reduction in calories, resulting in a 10% loss in body mass, a 71% decrease in fat mass, and improved metabolic parameters.30 Furthermore, the substantial health benefits of the Mediterranean diet are well established, as demonstrated in trials such as Prevención con Dieta Mediterránea (PREDIMED) and Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV), in which intervention groups adhering to the Mediterranean diet exhibited notable health improvements without significant changes in body mass.31,32 One surprising result of our study was the reduced calorie intake at 3 months, maintained at 6 months, with the concomitant improvement of diet quality as evidenced by the increased adherence to the Mediterranean diet and without changes in the Food Cravings Questionnaire score. We found this result to be remarkable because no indications were present of specific caloric-restriction targets or established weight-loss goals. Yet, a significant weight reduction was observed among the participants, without an increase in hunger signals, which is often seen with reduction of calories. Recently, a study involving 20 weight-stable adults sought to examine the impact of UPFs on energy intake.33 The results revealed that consuming UPFs significantly increased energy intake, primarily owing to a higher consumption of carbohydrates and fats, leading to weight gain. These findings underscore the potential efficacy of reducing the consumption of UPFs as a strategy for addressing obesity. The observed outcomes in our study may be attributed to the emphasis on reducing intake of the NOVA-4 food group as the initial focus of our study, complementing the recommendation to adopt a quality Mediterranean diet. Consequently, a notable decrease occurred in total energy intake, as did reductions in intake of simple sugars, saturated fat, sodium, grams of alcohol, and the percentage of calories derived from NOVA-4 group products for our study participants (refer to Table 3). These changes may have implications for rebalancing satiety signals and fostering adherence to subsequent nutritional recommendations. Having observed the potential impact of this intervention, we believe that the effect of this strategy on its own should be studied meticulously in future randomized research.
Exploring the benefits of fasting in cardiac rehabilitation
Numerous experimental intermittent fasting trials, including TRE, have been conducted in populations without ASCVD.34 These studies have demonstrated potential for promoting weight loss and improving metabolic health among individuals with metabolic syndrome, prediabetes, and T2DM. However, the DIABEPIC-1 trial, to our knowledge, represents the first endeavor to provide insights into the feasibility and health benefits of implementing this approach within this patient group and clinical framework. A noteworthy point is that several ongoing randomized controlled trials are currently investigating this topic (ClinicalTrials.gov: NCT05075317, NCT05014880).
In developing the DIABEPIC-1 study, we aimed to assess the feasibility and impact of combining various nutritional interventions in a cardiac rehabilitation program that yield favourable metabolic results individually. Concurrently, we sought to explore the independent effects of incorporating TRE in a cardiac rehabilitation program. Consequently, we decided to introduce this strategy within the follow-up period during months 3 to 6 of the intervention, and it was met with a remarkably high level of adherence. Based on participant feedback, most opted for the 12 PM-8 PM window, due to its convenience in social, work, and family life. When considering the effects of introducing TRE during phase 2 of the study, the anthropometric parameters noted significant enhancements, including improved waist circumference, weight, body mass index, and fat- mass reductions. A further modest improvement in glycemic, lipid, inflammatory, and liver parameters also was observed, although this change was not statistically significant. An important result was that none of the assessed parameters deteriorated (see Table 2).
Of particular significance was the observation that stepwise incorporation of TRE helped maintain a stable HbA1c level of < 5.7% for 9 participants, which fulfilled the criteria for complete prediabetes remission at the 6-month mark. In addition, 3 additional participants achieved this status during phase 2. Similarly, participants with metabolic syndrome at baseline showed promising results, with 11 participants achieving the durability criterion of remission within phase 2, and 3 more meeting the metabolic criteria following the TRE phase.
Considering the results and the adherence rates observed, a compelling need is to investigate further the introduction of the 16:8 TRE pattern, among other possibilities of TRE, within cardiac rehabilitation. Such research holds promise for elucidating the distinct effects of this strategy, with the potential to enhance metabolic health and achieve the remission of CV risk factors. Additionally, given the adherence levels and findings from prior literature,35 an earlier implementation of this approach also could have been feasible.
Limitations
This study has limitations that need to be addressed. First, the study's recruitment of participants from a specific cardiac rehabilitation program and centre may limit the comparability or generalizability of its findings, including its small-scale nature. Second, whether the effects observed are better than those that would be observed in response to a standard cardiac rehabilitation program is not known. Further investigation, such as a randomized controlled trial, could help to clarify these points. Third, the 6-month study may only partially capture long-term results and the sustainability of observed improvements, particularly regarding remission rates. To address this issue, a 12-month follow-up evaluation will be conducted to assess the continuation of remission. Finally, reduction of UPF intake, and introduction of TRE, were integrated into a combined strategy that yielded positive results. However, these changes limit the ability to draw independent conclusions about the effects of each approach, and we cannot dissociate the potential effect from the different dietary interventions and training. Future randomized controlled trials are crucial, to investigate further the individual impacts of these interventions in these populations and specific settings.
Conclusions
The study underscores the possibility of implementing an intensive lifestyle intervention utilizing cardiac rehabilitation, including high rates of completion and adherence, suggesting that a combination of exercise and enhanced dietary interventions can be implemented effectively in this clinical setting. The intervention also yielded clinically meaningful improvements in CV risk factors, body composition, cardiorespiratory fitness, and diet quality. Notably, half of the participants achieved remission from prediabetes, and 70% achieved remission from metabolic syndrome, emphasizing the potential of lifestyle interventions in mitigating these conditions in individuals with ASCVD. Given the absence of a control group, the study results warrant a randomized controlled trial to investigate this question further.
Acknowledgements
The authors thank the other investigators, the staff, and particularly the participants of the DIABEPIC-1 study for their valuable contributions.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. L.B. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics Statement
The study protocol was approved by the Research Ethics and New Technology Development Committee of the Montreal Heart Institute (#2022-3005).
Patient Consent
The authors confirm that patient consent forms have been obtained for this article.
Funding Sources
This work was funded by the Mirella and Lino Saputo Research Chair in Cardiovascular Health and the Prevention of Cognitive Decline from Université de Montréal at the Montreal Heart Institute.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1420 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.07.018.
Supplementary Material
References
- 1.Cosentino F., Verma S., Ambery P., et al. Cardiometabolic risk management: insights from a European Society of Cardiology Cardiovascular Round Table. Eur Heart J. 2023;44:4141–4156. doi: 10.1093/eurheartj/ehad445. [DOI] [PubMed] [Google Scholar]
- 2.Honigberg M.C., Zekavat S.M., Pirruccello J.P., Natarajan P., Vaduganathan M. Cardiovascular and kidney outcomes across the glycemic spectrum: insights from the UK Biobank. J Am Coll Cardiol. 2021;78:453–464. doi: 10.1016/j.jacc.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossello X., Raposeiras-Roubin S., Oliva B., et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J Am Coll Cardiol. 2021;77:2777–2791. doi: 10.1016/j.jacc.2021.03.335. [DOI] [PubMed] [Google Scholar]
- 4.Riddle M.C., Cefalu W.T., Evans P.H., et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44:2438–2444. doi: 10.2337/dci21-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKay D., Chan C., Dasgupta K., et al. Remission of type 2 diabetes: Diabetes Canada Clinical Practice Guidelines Expert Working Group. Can J Diabetes. 2022;46:753–761.e8. doi: 10.1016/j.jcjd.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Jin S., Bajaj H.S., Brazeau A.S., et al. Remission of type 2 diabetes: user’s guide: Diabetes Canada Clinical Practice Guidelines Expert Working Group. Can J Diabetes. 2022;46:762–774. doi: 10.1016/j.jcjd.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Taylor R., Ramachandran A., Yancy W.S., Forouhi N.G. Nutritional basis of type 2 diabetes remission. BMJ. 2021;374 doi: 10.1136/bmj.n1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallberg S.J., Gershuni V.M., Hazbun T.L., Athinarayanan S.J. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:1–16. doi: 10.3390/nu11040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milani R.V., Lavie C.J. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92:50–54. doi: 10.1016/s0002-9149(03)00464-8. [DOI] [PubMed] [Google Scholar]
- 10.Lavie C.J., Milani R.V. Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes. J Cardiopulm Rehabil. 2005;25:59–66. doi: 10.1097/00008483-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ades P.A., Savage P.D. The treatment of obesity in cardiac rehabilitation: a review and practical recommendations. J Cardiopulm Rehabil Prev. 2021;41:295. doi: 10.1097/HCR.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesies-Grau J., Dionne V., Latour É., et al. Mediterranean diet and time-restricted eating as a cardiac rehabilitation approach for patients with coronary heart disease and pre-diabetes: the DIABEPIC-1 protocol of a feasibility trial. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2023-073763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 14.Pelliccia A., Sharma S., Gati S., et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa735. [DOI] [PubMed] [Google Scholar]
- 15.Ji Y., Plourde H., Bouzo V., Kilgour R.D., Cohen T.R. Validity and usability of a smartphone image-based dietary assessment app compared to 3-day food diaries in assessing dietary intake among Canadian adults: randomized controlled trial. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/16953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig C.L., Marshall A.L., Sjöström M., et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 17.Henry J.D., Crawford J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-González M.A., García-Arellano A., Toledo E., et al. A 14-Item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meule A., Hermann T., Kübler A. A short version of the Food Cravings Questionnaire—Trait: the FCQ-T-reduced. Front Psychol. 2014;5:190. doi: 10.3389/fpsyg.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor R.S., Dalal H.M., McDonagh S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol. 2022;19:180. doi: 10.1038/s41569-021-00611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lara-Breitinger K., Lynch M., Kopecky S. Nutrition intervention in cardiac rehabilitation: a review of the literature and strategies for the future. J Cardiopulm Rehabil Prev. 2021;41:383–388. doi: 10.1097/HCR.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 22.Gomadam P.S., Douglas C.J., Sacrinty M.T., et al. Degree and direction of change of body weight in cardiac rehabilitation and impact on exercise capacity and cardiac risk factors. Am J Cardiol. 2016;117:580–584. doi: 10.1016/j.amjcard.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 23.Prescott E., Eser P., Mikkelsen N., et al. Cardiac rehabilitation of elderly patients in eight rehabilitation units in western Europe: outcome data from the EU-CaRE multi-centre observational study. Eur J Prev Cardiol. 2020;27:1716–1729. doi: 10.1177/2047487320903869. [DOI] [PubMed] [Google Scholar]
- 24.Beals J.W., Kayser B.D., Smith G.I., et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat Metab. 2023;5:1221–1235. doi: 10.1038/s42255-023-00829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orozco L.J., Buchleitner A.M., Gimenez-Perez G., et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD003054.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z., Cai J., Wu Z., Gong W. Effect of high-intensity interval training combined with fasting in the treatment of overweight and obese adults: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:4638. doi: 10.3390/ijerph19084638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lean M.E., Leslie W.S., Barnes A.C., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 28.Taheri S., Zaghloul H., Chagoury O., et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:477–489. doi: 10.1016/S2213-8587(20)30117-0. [DOI] [PubMed] [Google Scholar]
- 29.Taylor R., Al-Mrabeh A., Zhyzhneuskaya S., et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab. 2018;28:547–556.e3. doi: 10.1016/j.cmet.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Kraus W.E., Bhapkar M., Huffman K.M., et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:673–683. doi: 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estruch R., Ros E., Salas-Salvadó J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378 doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 32.Delgado-Lista J., Alcala-Diaz J.F., Torres-Peña J.D., et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399:1876–1885. doi: 10.1016/S0140-6736(22)00122-2. [DOI] [PubMed] [Google Scholar]
- 33.Hall K.D., Ayuketah A., Brychta R., et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patikorn C., Roubal K., Veettil S.K., et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.39558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Small S., Iglesies-Grau J., Gariepy C., et al. Time-restricted eating: a novel dietary strategy for cardiac rehabilitation. Can J Cardiol. 2023;39(11S):S384–S394. doi: 10.1016/j.cjca.2023.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. L.B. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.