Abstract
Spontaneous coronary artery dissection (SCAD) is an increasingly recognized cause of acute coronary syndromes. Fibromuscular dysplasia (FMD) is an idiopathic, nonatherosclerotic, and noninflammatory arterial disease that affects small- to medium-sized arteries that can result in multifocal aneurysms, stenosis, tortuosity, and dissections. Extracoronary FMD has been identified in approximately 70% of SCAD patients and it is recommended that all SCAD patients undergo screening for FMD once in their lifetime using computed tomography angiography from head to pelvis. This focused review for cardiologists outlines current approaches to diagnosis and management of patients with FMD.
Résumé
La dissection spontanée de l’artère coronaire (DSAC) est une cause de plus en plus reconnue du syndrome coronarien aigu. La dysplasie fibromusculaire (DFM) est une maladie idiopathique, non athéroscléreuse et non inflammatoire qui affecte les petites et moyennes artères et peut entraîner des anévrismes multifocaux, une sténose, une tortuosité et des dissections. Une DFM extracoronarienne a été observée chez environ 70 % des patients présentant une DSAC. Il est recommandé que tous les patients présentant une DSAC devraient une fois dans leur vie subir un test de dépistage de la DFM au moyen d’une angiographie par tomodensitométrie de la tête au bassin. Cette analyse destinée aux cardiologues décrit les approches actuelles dans le diagnostic et la prise en charge des patients atteints de DFM.
During medical school, we learned about “beads on a string,” the classic imagery to describe fibromuscular dysplasia (FMD) of the renal arteries. We knew this was a potential etiology for early onset hypertension in younger women, but most cardiologists never felt the need to dive deeper into this diagnosis. The emergence of spontaneous coronary artery dissection (SCAD) as an alternate pathophysiology for acute myocardial infarction (MI), especially among women younger than 55 years of age, and its association with FMD has changed the relevance of this condition for the cardiology community. SCAD is caused by a spontaneous intimal tear or spontaneous vasa vasorum bleeding resulting in intramural hematoma formation within the tunica media with separation of the intima from the underlying vessel.1 The intramural hematoma compresses the true lumen causing myocardial ischemia. When carefully screened, more than 70% of SCAD patients have extracardiac vascular findings of FMD, and most have moderate to severe coronary tortuosity.2 Therefore, cardiologists should be familiar with FMD to better inform discussions with patients who have experienced SCAD. Herein we review the practical aspects of FMD for clinical cardiologists.
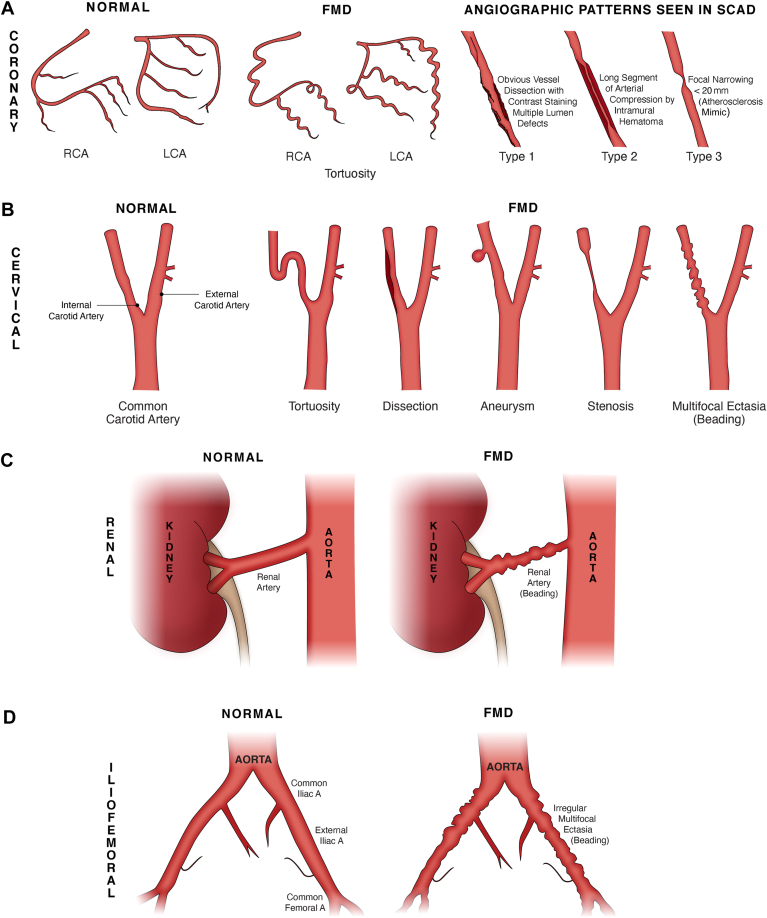
FMD is an idiopathic, nonatherosclerotic and noninflammatory arterial disease resulting in abnormal arterial musculature and distorted arterial architecture from abnormal cellular proliferation and connective tissue matrix deposition.3 It typically affects small- to medium-sized arteries with multifocal aneurysms or ectasia (beading), stenosis, tortuosity, and dissection (Fig. 1).3 In SCAD patients, the most commonly affected extracardiac territories are the renal, carotid, vertebral, and iliac arteries. Most patients with FMD have multifocal FMD.3 Because of the strong association of FMD with SCAD, it is recommended that all SCAD patients undergo screening for FMD from head to pelvis at least once in their lifetime.1
Figure 1.
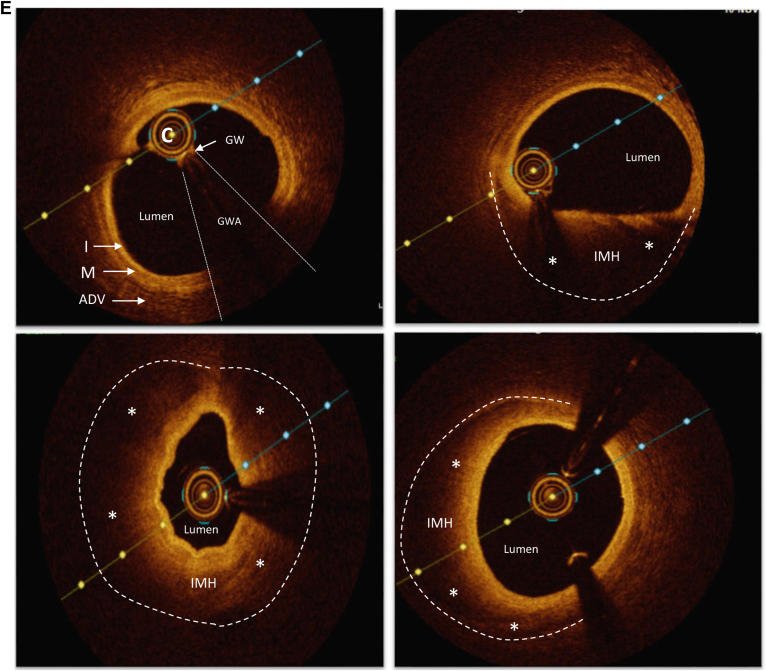
FMD patterns of disease. (A) Coronary FMD and angiographic patterns observed among patients with SCAD. (B) Manifestations of cervical FMD observed in the carotid arteries. (C) Renal FMD. (D) Iliofemoral FMD. (E) Optical coherence tomography examination of the left anterior descending coronary artery in a patient with SCAD-related myocardial infarction. Top left, normal vessel architecture distal to the SCAD segment. The other panels show varying degrees of intramural hematoma (IMH and asterisks) compressing the arterial lumen from the distal (top right), mid (bottom left), and proximal (bottom right) aspects of the SCAD segment. The dotted line in each of these panels illustrates the outer vessel contour. A, artery; ADV, adventitia; C, catheter; FMD, fibromuscular dysplasia; GW, guide wire; GWA, normal comet-like artifact caused by presence of a GW in the coronary artery; I, intima; LCA, left coronary artery; M, media; RCA, right coronary artery; SCAD, spontaneous coronary artery dissection.
Epidemiology
FMD most commonly affects women with an average age of 56 years at the time of diagnosis (range, 5-83 years).4, 5, 6 Only 10%-20% of cases occur in men; typically with a more aggressive natural history that includes a greater frequency of aneurysms and dissections.3 Although FMD most commonly affects White individuals (95%),7 there is no evidence to support an association with race, and this finding is confounded by the racial demographic characteristics of the countries from which registry data were collected (United States or France), and possible referral bias.8
Classification
The international consensus on FMD described 2 angiographic appearances for classification of FMD: focal and multifocal (Fig. 1).3 Focal FMD is defined as a solitary lesion (< 1 cm in length) or tubular stenosis (> 1 cm in length) due to fibroplasia of the intima or adventitia.9 Focal FMD can occur in any part of the artery along its length. Multifocal FMD is described as alternating areas of stenosis (at least 2) and dilation, classically described as a “string of beads” appearance.3 Multifocal FMD (beading) affects the media of the vessel wall and involves mid to distal portions of the artery.9
Risk Factors and Genetics
Risk factors include smoking, mechanical stress, and exposure to endogenous or exogenous female hormones. Although the exact role of female hormones in the pathogenesis of FMD remains unclear, one study showed elevated progesterone receptor expression in smooth muscle cell nuclei among patients with renal FMD.10 Elevated plasma levels of circulating transforming growth factor β1 and β2 and lysophosphatidylcholine were reported in multifocal FMD.3,11 Finally, FMD has been identified in > 70% of patients with SCAD-related MI.2,12
Further research is required to understand the genetic determinants of FMD because very few familial and sporadic gene mutations have been identified. Recent imaging studies have suggested that 11% of FMD is familial, however, other registry data suggest that only 2%-7% of patients have an affected family member.6,7,13 In a retrospective study of 104 patients with imaging-confirmed FMD, all family members with FMD were siblings and there were no cases of vertical transmission.6
A PHACTR1 (phosphatase and actin regulator 1) gene mutation on chromosome 6 was identified as a risk factor for FMD (odds ratio 1.4).6,14 This gene is involved in angiogenesis and cell migration. It was also identified as a risk factor for cervical artery dissection (CeAD), hypertension, and migraines.6 Other studies that have assesses gene associations with known arteriopathies have not shown a clear association. Because of the limited evidence for genetic determinants of FMD, genetic testing for asymptomatic relatives of patients with FMD is not recommended at this time.
Clinical Presentation and Diagnosis
The clinical presentation of FMD is heterogeneous on the basis of the vascular beds affected and severity of disease (Table 1). Many patients with FMD remain asymptomatic over their lifetime, with the diagnosis being uncovered as an incidental finding in the context of imaging performed for another indication. In the Assessment of Renal and Cervical Artery DysplasIA (ARCADIA) Registry, 48% of patients with renal or cerebrovascular FMD had lesions in another vascular bed and 18% of patients had aneurysms or dissections.9 Overall, the rate of multisystem FMD involvement is approximately 66%.9 Regardless of the initial site of FMD involvement, all patients with FMD should undergo screening for multisystem involvement with computed tomography angiography (CTA) from head to pelvis3; contrast-enhanced magnetic resonance angiography (MRA) could also be considered if CTA is contraindicated. Although CTA examinations involve exposure to radiation and contrast, CTA is prioritized over MRA and duplex ultrasound because of better spatial resolution, ability to identify vessel calcification, and widespread availability and convenience (Table 2).
Table 1.
FMD summary
| Fibromuscular dysplasia | Findings |
|---|---|
| Main characteristics | |
| Sex |
|
| Classification |
|
| Vascular territory |
|
| Environmental factors |
|
| Clinical scenarios |
|
| Diagnosis |
|
| Genetic screening |
|
| Medical therapy | |
| Antiplatelet therapy |
|
| β-Blocker therapy |
|
| Other antihypertensive therapies |
|
| Statins |
|
| Heart failure therapies |
|
| Migraine therapy |
|
| Lifestyle | |
| Diet |
|
| Physical activity |
|
| Stress management |
|
| Smoking Cessation |
|
CTA, computed tomography angiography; DAPT, dual antiplatelet therapy; FMD, fibromuscular dysplasia; MI, myocardial infarction; MRA, magnetic resonance angiography; NSTEMI, non–ST-elevation myocardial infarction; SCAD, spontaneous coronary artery dissection; STEMI, ST-elevation myocardial infarction; TIA, transient ischemic attack.
Table 2.
Summary of clinical presentation, diagnosis, and management of fibromuscular dysplasia according to vascular territory involvement
| Vascular bed | Clinical presentation | Diagnosis | Management |
|---|---|---|---|
| Coronary artery |
|
|
|
| Renal artery |
|
|
|
| Cerebrovascular |
|
|
|
| Visceral artery |
|
|
|
| Extremity |
|
|
|
CABG, coronary artery bypass graft surgery; CeAD, cervical artery dissection; CTA, computed tomography angiography; DAPT, dual antiplatelet therapy; IVUS, intravascular ultrasound; MRA, magnetic resonance angiography; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; TIA, transient ischemic attack.
Coronary artery FMD
Coronary artery FMD might be an incidental finding on angiography or identified after presentation with SCAD-related MI. Although extracardiac FMD is common (> 70%) among patients with SCAD, the prevalence of SCAD among FMD patients is low (2.7%).15 Patients who experience SCAD typically present with chest pain, that is indistinguishable from a patient experiencing a traditional atherosclerosis-related MI; however, some patients can have more severe presentations such as ventricular arrhythmias (4%-14%), cardiogenic shock (2%), or sudden cardiac death.16 SCAD patients are usually middle-aged women (mean age 52 years; nearly 90% female) with few or no traditional cardiac risk factors.1,16 Although previously believed to be an uncommon condition, SCAD is increasingly recognized as an etiology for acute coronary syndromes (ACS). Causing nearly a third of ACS cases among women aged 50 years or younger, SCAD is also a common cause of pregnancy-associated MI.1
Coronary artery FMD has been associated most commonly with the angiographic finding of severe coronary artery tortuosity (Fig. 1A). Severe coronary tortuosity is characterized by pronounced 360° loops, sinusoidal twisting or bending of the arteries, and is indicative of potential structural weaknesses, possibly predisposing to dissection or intramural hematoma formation (Fig. 2). Among SCAD patients with extracoronary FMD, the prevalence of severe coronary artery tortuosity on coronary angiography is notably greater compared with those without extracoronary FMD (58.4% vs 36.5%; P < 0.001).17 Additional angiographic findings include irregular stenosis patterns, marked by irregular borders in focal or diffuse patterns (with or without systolic accentuation), and smooth stenoses have also been observed.17 Coronary ectasia, indicating positive remodelling of arterial segments, might also be associated with FMD among SCAD patients.2,17 Notably, arterial beading is not as prevalent in coronary arteries compared with other arterial beds.17
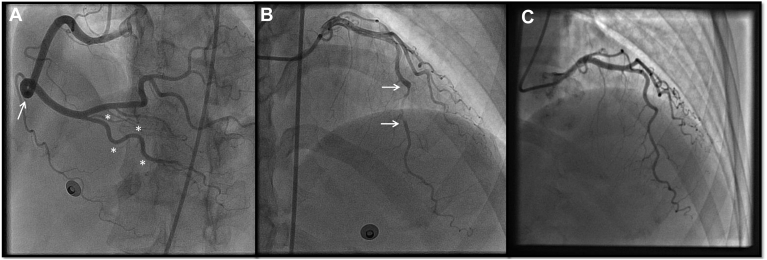
Figure 2.
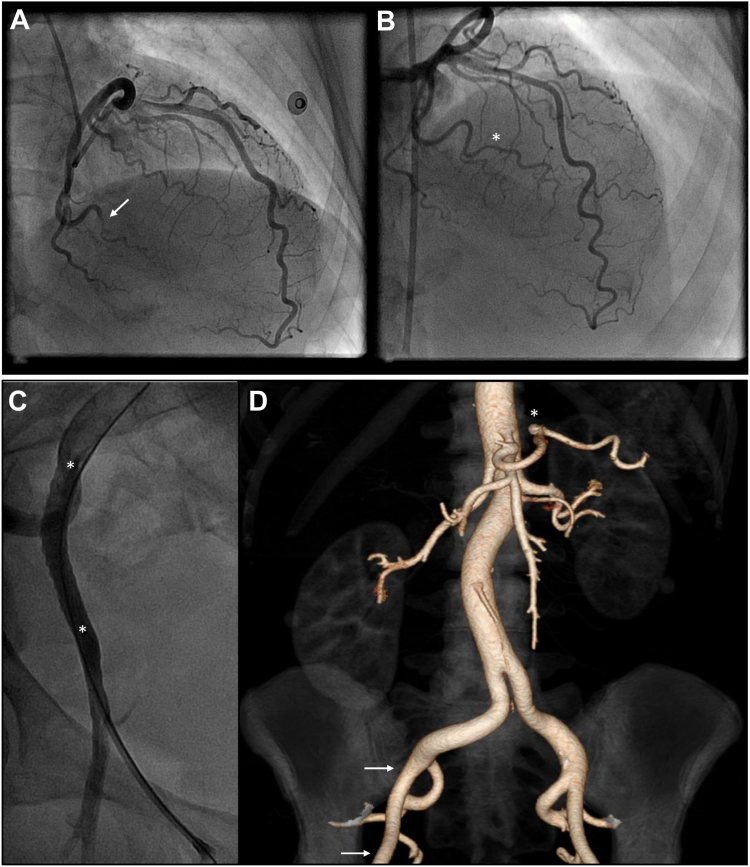
Coronary tortuosity in a patient with SCAD-related MI, who was managed conservatively. (A) Right coronary artery, left anterior oblique projection. Note 360° loop (arrow) and undulating course (asterisks). (B) Left coronary artery, cranial projection. Note the SCAD segment in the mid left anterior descending artery (arrows). (C) Left coronary artery, cranial projection. Complete healing of the mid left anterior descending artery 8 weeks after initial angiography. MI, myocardial infarction; SCAD, spontaneous coronary artery dissection.
Although these observations suggest a potential correlation of coronary FMD with SCAD, confirming a direct causal relationship requires histopathological validation, which is challenging because of limited access to autopsy data. It remains controversial whether SCAD is a distinct entity or a consequence of coronary artery FMD. Some postmortem reports describe histological findings consistent with FMD in SCAD patients, whereas others have not shown this correlation.3 In a retrospective analysis of 346 SCAD patients, the presence of extracardiac FMD was not associated with an increase in adverse clinical events compared with SCAD patients without extracardiac FMD.2
Routine coronary artery imaging in patients with FMD to screen for potential coronary artery involvement is not recommended in the absence of an ACS, to avoid iatrogenic coronary artery dissection associated with invasive coronary angiography. For patients with ACS, SCAD might be suspected when the patient is of younger age, peripartum, or with few preexisting cardiac risk factors, and invasive coronary angiography is considered the gold standard for diagnosis. Although coronary CTA has adequate spatial resolution in the proximal and middle portions of most coronary arteries, the ability to exclude dissection in small tapering distal vessels is limited. To solely rely on coronary CTA risks leaving the patient without a definite diagnosis of SCAD, which has implications for management, lifestyle, and prognosis.
Three distinct angiographic patterns of SCAD have been previously described (Fig. 1A).18 Angiographically, type 3 SCAD (focal) can be mistaken for atherosclerotic coronary artery disease. In cases of diagnostic uncertainty, for very tight lesions, relook angiography performed 2-4 weeks after initial presentation, with or without intracoronary imaging might be helpful to determine etiology of the ACS presentation. Lesion resolution would favour SCAD, and lesion persistence should prompt a careful image-guided approach to confirm traditional atherosclerosis.19 Optical coherence tomography images of SCAD are characteristic (Fig. 1E) with evidence of intimal disruption and intramural hematoma, whereas intravascular ultrasound images require closer scrutiny to discriminate between traditional atherosclerosis and SCAD because of the lower spatial resolution.16
Renal FMD
Renal FMD is often clinically silent or diagnosed incidentally and thus its true prevalence is likely underestimated.3 In renal transplant studies, the prevalence of renal FMD in the transplanted kidney was 3%-4%.3 Up to 90% of cases are multifocal and typically affect White, middle-aged women with a personal or family history of hypertension (Figs. 1C and Figure 3, Figure 4, Figure 5).7,13 Focal renal FMD is less common and associated with hypertension at younger than 30 years of age, with a more balanced sex distribution. Occasionally, renal FMD patients might present with distal thromboembolism and renal infarction in the setting of renal artery dissection. Sudden onset or worsening of hypertension and age older than 55 years or younger than 30 years should prompt consideration of renovascular hypertension, and if younger, renal FMD.
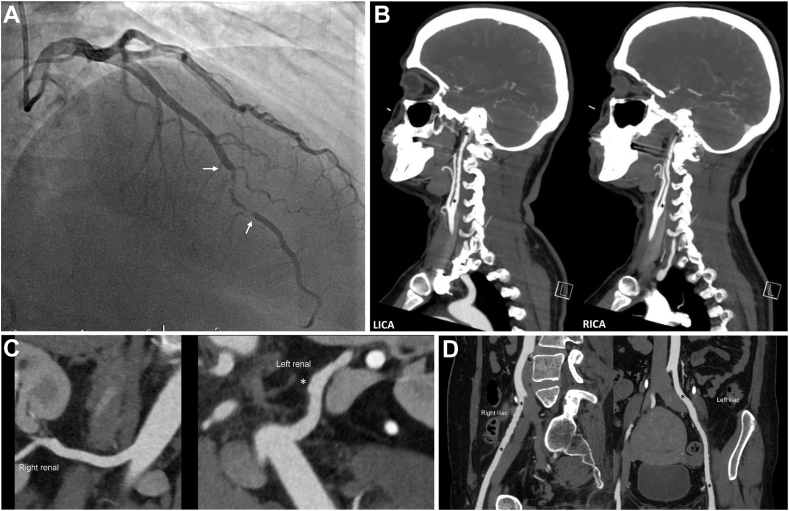
Figure 3.
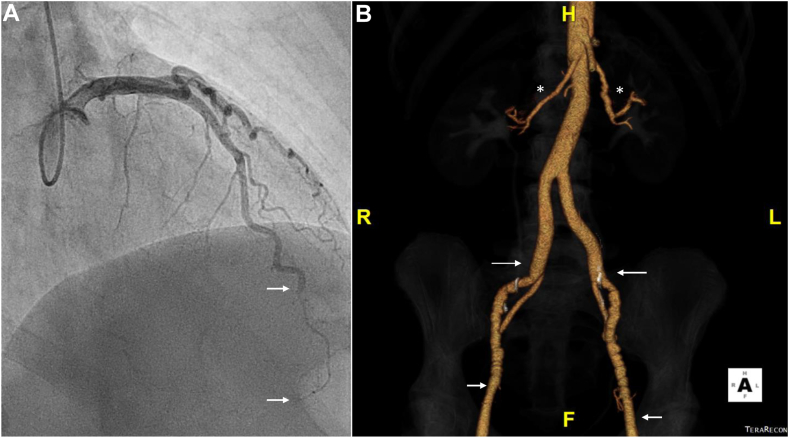
A 49-year-old woman with NSTEMI due to SCAD and results of subsequent CTA screening for FMD from head to pelvis. (A) Left coronary artery angiography in the right anterior oblique-cranial projection shows a 30-mm segment of type 2 SCAD (between white arrows) in the distal left anterior descending artery. (B) FMD of both internal carotid arteries. Note the luminal irregularities between the black asterisks. (C) FMD (luminal irregularity white asterisk) of left renal artery. (D) FMD of right and left iliac arteries (note luminal irregularities between black asterisks). CTA, computed tomography angiography; FMD, fibromuscular dysplasia; LICA, left internal carotid artery; NSTEMI, non–ST-elevation myocardial infarction; RICA, right internal carotid artery; SCAD, spontaneous coronary artery dissection.
Figure 4.
Coronary angiography and subsequent 3-D reconstruction of an abdominal-pelvic CTA performed as part of screening for FMD in a 52-year-old woman with anterior STEMI due to SCAD. (A) Left coronary angiography in a cranial projection with a long segment (between white arrows) of abrupt luminal compression by intramural hematoma affecting the distal left anterior descending artery (type 2 SCAD). (B) FMD (beading) of both main renal arteries (white asterisks) and more distally, FMD (beading) involving both external iliac arteries (between white arrows) was observed. CTA, computed tomography angiography; FMD, fibromuscular dysplasia; SCAD, spontaneous coronary artery dissection; STEMI, ST-elevation myocardial infarction.
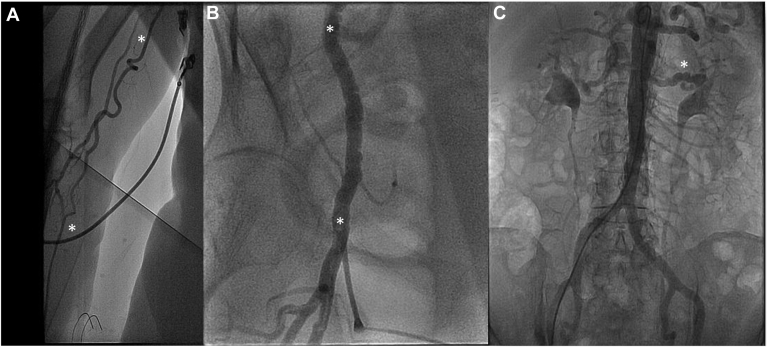
Figure 5.
A 72-year-old woman had inferior STEMI due to distal occlusion of the distal posterior descending branch of the right coronary artery, for which conservative therapy was pursued. Her coronary angiogram could not be completed from the right radial artery because of excessive right brachial artery tortuosity (A, between the white asterisks). Coronary angiography was undertaken via the right femoral artery, and angiography showed right iliofemoral FMD (B, note luminal irregularity in between the white asterisks). Aortic run-off angiography showed left renal artery FMD (C, white asterisk). The patient was subsequently diagnosed with paroxysmal atrial fibrillation during prolonged Holter monitoring, and an embolic event was the suspected etiology for her myocardial infarction. In this case, FMD was an incidental finding. FMD, fibromuscular dysplasia; STEMI, ST-elevation myocardial infarction.
The primary modality to diagnose renal FMD is invasive catheter-based angiography; however, current guidelines recommend initial noninvasive imaging with CTA, or MRA if CTA is contraindicated.3 CTA provides better spatial resolution to visualize small calcifications to differentiate FMD from atherosclerotic renal artery stenosis. In specialized centres, duplex ultrasound can be used if expertise is available.3 Compared with CTA, ultrasound has lower spatial resolution and is highly operator-dependent. During percutaneous intervention, translesional pressure gradient measurement is helpful to assess the hemodynamic significance of stenoses in patients with multifocal FMD. This may be combined with intravascular ultrasound or optical coherence tomography to further assess anatomy, and accurately size equipment.3 A pressure gradient of 10% of the mean aortic pressure (ratio of distal coronary artery pressure to aortic pressure < 0.90) is considered significant in patients with atherosclerotic renal artery stenosis; however, this threshold has not been validated in patients with renal FMD.20
Cerebrovascular FMD
Extracranial (carotid and vertebral artery) cerebrovascular FMD has been reported in approximately 65% of FMD patients, often coexisting with renal FMD (Fig. 1B).13 Extracranial carotid dissections typically occur ≥ 2 cm beyond the carotid bifurcation or at the level of the skull base and vertebral artery dissections usually occur at the level of C2-C6 or in the extracranial segment between C2 and the skull base.21,22 Carotid webs (carotid bulb diaphragms) are considered a focal variant of cerebrovascular FMD, and are increasingly recognized as a cause of ischemic stroke in younger patients.3,23 Compared with the general population, the prevalence of intracranial aneurysms is significantly elevated in FMD patients (13%-22%), and the aneurysms are often larger in size and involve high-risk locations such as the posterior circulation.5,15
Patients with cerebrovascular FMD frequently report nonspecific symptoms of pulsatile tinnitus, headaches, neck pain, and dizziness. A cervical bruit might be present. Acute onset of focal neurological symptoms, cranial or cervical neuropathies, Horner syndrome, or transient ischemic attacks suggest cerebral ischemia from thromboembolism or cerebral hypoperfusion. There might be associated severe pain involving the head, neck, or face, depending on the location of FMD and suggests the presence of a severe stenosis, acute CeAD involving the carotid or vertebral arteries, cerebral thromboembolism, or aneurysm rupture (Figs. 1B and 3).15,24
In most centres, CTA or contrast-enhanced MRA has replaced invasive angiography to diagnose cerebrovascular FMD, with catheter-based angiography being reserved for interventions on aneurysms, dissections, or severely stenotic lesions. There are currently no diagnostic criteria for FMD on carotid duplex and transcranial Doppler ultrasound, but it may be considered for screening in specialized centres with expertise in ultrasound. Carotid duplex might detect 68%-95% of cases, but it is operator-dependent.25,26 It is suboptimal for detecting dissection in patients with isolated Horner syndrome and for dissections near the skull base.25,26
Visceral artery FMD
Visceral involvement occurs in approximately 20% of patients with FMD and is associated with an increased risk for aneurysms and dissections (Fig. 6D).7 The celiac arteries, hepatic arteries, splenic arteries, and the superior and inferior mesenteric arteries might be involved.3,27 Clinical presentation can range from postprandial abdominal pain or abdominal bruit to mesenteric ischemia, splenic infarct, aneurysms, and dissections. CTA or MRA are typically used for the diagnosis of visceral artery FMD. Duplex ultrasound has not been validated for diagnosis.
Figure 6.
A 54-year-old woman had inferolateral STEMI due to SCAD involving the OM3 of the left circumflex artery. (A) Left coronary artery angiography in right anterior oblique-cranial projection showing (white arrow) subtotal occlusion of the upper subbranch of the OM3 due to intramural hematoma. Note other coronary vessels are smooth and normal-appearing with severe tortuosity (sinusoidal waves). (B) Repeat left coronary angiogram in cranial projection, performed 8 weeks after SCAD, shows vessel healing with OM3 recanalization (white asterisk) after resorption of intramural hematoma. (C) During repeat coronary angiography (B) via the right common femoral artery, femoral angiography was performed by injecting a 10-cc contrast bolus through the vascular access sheath. Standing waves are shown in the right external iliac artery (regular corrugated luminal contour, between the white asterisks). Standing waves represent vascular artifact, and are possibly due to transient vasospasm induced by the arterial injection of a rapid bolus of contrast. (D) CTA of the abdomen and pelvis with 3-D reconstruction performed 7 years after myocardial infarction to investigate abdominal discomfort. The previously noted luminal irregularity in the right external iliac artery is resolved (arterial segment between white arrows) and a small known saccular splenic artery aneurysm (white asterisk) has a stable appearance. CTA, computed tomography angiography; OM3, third obtuse marginal branch; SCAD, spontaneous coronary artery dissection; STEMI, ST-elevation myocardial infarction.
Extremity FMD
Extremity FMD is most commonly multifocal and bilateral. In lower extremity FMD, the iliac arteries are most commonly affected (7%-14% of patients; Figs. 1D and Figure 3, Figure 4, Figure 5).7 Upper extremity FMD has been reported in 16% of patients.24 It can involve the brachial, subclavian, axillary, radial, or ulnar arteries. Patients with lower extremity FMD might present with claudication, foot ischemia, weakness, femoral bruits, or aneurysms/dissections.28 In contrast, patients with upper extremity FMD might present with hand ischemia, claudication, dissections, aneurysms, paresthesia, or Raynaud phenomenon. Diminished pulses, brachial bruits, and discrepant arm blood pressure (BP) might be present.28
Duplex ultrasound can be used to screen for extremity FMD. CTA, MRA, or catheter-based angiography are usually used to confirm the diagnosis. Misdiagnosis can occur in lower extremity FMD in the presence of benign transient standing arterial waves on catheter-based angiography (Fig. 6C and D),29 which are regular areas of corrugation, or sinusoidal undulating contour of medium- and small-sized arteries such as iliofemoral or renal arteries. Standing waves are usually transient, and might not be present on repeat contrast injections.29 FMD usually imparts a fixed, irregular ectatic contour on angiography. The mechanism of standing arterial waves is unclear, however, it has been proposed that it is related to vasospasm, or other arterial reaction stemming from rapid contrast administration.
Pharmacologic Management
Although there is no cure for FMD, it can be managed with medications, and lifestyle recommendations that will help maintain arterial wall integrity throughout the body. FMD management is guided by the site(s) of involvement and associated complications.
Antiplatelet therapy
There are no randomized placebo-controlled trials to guide antiplatelet therapy for FMD patients. It has been suggested that routine antiplatelet therapy for patients with symptomatic and asymptomatic FMD is reasonable because patients can present with multiple FMD-associated complications such as thrombosis or thromboembolic events.3 In our clinical experience, we have observed a greater tendency to prescribe antiplatelet therapy for patients with symptomatic FMD. There are currently no clear guidelines on which antiplatelet agent would be preferred, however, most centres use acetylsalicylic acid (ASA) 75-100 mg daily in the absence of contraindications.
In some subgroups, there are clear indications for use of antiplatelet therapy. For example, in patients with SCAD-related MI who have undergone coronary stenting, dual antiplatelet therapy (DAPT) is recommended for 1 year. In patients with SCAD who have not undergone percutaneous coronary intervention (PCI), there is a lack of consensus data on the use of single antiplatelet therapy with aspirin or DAPT. Many Canadian centres currently use single antiplatelet therapy with ASA, so not to delay resorption of coronary intramural hematoma. An international randomized controlled trial Anti-Platelet Therapy in Spontaneous Coronary Artery Dissection (APT-SCAD) trial to establish optimal antiplatelet therapy after SCAD is planned. For patients with renal FMD who have undergone angioplasty, lifelong ASA is recommended, with some operators recommending a short course of DAPT for 4-6 weeks.3
Antihypertensive therapy
There are no FMD-specific guidelines for BP management and patients would fall into the category of increased cardiovascular risk, but not high risk, therefore targeting BP < 140/90 mm Hg for office BP measurement and < 135/85 mm Hg for standardized automated office BP, or home BP monitoring, or daytime ambulatory BP monitoring according to the 2020 Hypertension Canada Clinical Practice Guideline.30
In patients with renal FMD, the need for antihypertensive therapy is common and referral to a hypertension specialist should be considered. Angiotensin converting enzyme inhibitors or angiotensin receptor blockers are favoured because the renin-angiotensin-aldosterone system is activated in patients with renal FMD. In severe bilateral stenotic lesions, a 10%-30% increase in plasma creatinine might occur with treatment, underscoring the need to monitor kidney function after initiation of therapy. Patients with FMD often require additional therapies, including long-acting dihydropyridine calcium channel blockers, and thiazide-like diuretics.
There have been no randomized controlled trials to guide SCAD therapy, however, lower recurrence rates with the use of β-blockers and antihypertensive therapy to maintain normal BP were reported in a single retrospective review.12 In the Canadian SCAD registry, 75% of patients continued single antiplatelet therapy and β-blockade, and the risk of SCAD recurrence was much lower than previously reported at 2.4% by 3 years.31 As a result, β-blockers are recommended in patients with SCAD. There is no evidence that angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, or nitroglycerin have direct benefit in patients with SCAD; however, they can be used as antihypertensive medications. Overall, further research is required to guide medical therapy.
Statin therapy
Because FMD is a nonatherosclerotic, and noninflammatory connective tissue disease, statin therapy is not routinely recommended. This includes patients who have had SCAD or CeAD. Most experts reserve statin therapy for cases of severe dyslipidemia (low-density lipoprotein cholesterol > 5 mmol/L). In our experience, this is a difficult concept for most cardiovascular specialists to grasp, because of the fairly ubiquitous use of statin therapy for patients with most types of vascular disease.
Migraine therapy
More than two-thirds of patients with FMD report migraine with at least 50% of them experiencing symptoms weekly.32 Treatment for migraine in FMD patients is dependent on the severity of migraine and might involve appropriate preventive and acute therapies with the appropriate safety profile. Currently, there have been no trials to guide migraine therapy in FMD patients. If medical therapy is required, it is important to be cautious with the use of triptans, ergots, or vasoconstrictive agents. In patients with a history of SCAD or CeAD, these agents should be avoided.1 β-Blocker therapy is often used to reduce shear stress, heart rate, and BP among patients with SCAD-related MI. β-Blockers also have evidence in migraine prevention, and in our experience, many SCAD patients not infrequently report some amelioration of migraine headaches after taking daily β-blocker therapy after SCAD. For FMD patients who require β-blockade and having migraine, it might be worth selecting an agent that has evidence in migraine prevention such as propranolol, metoprolol, or nadolol rather than newer agents that have less evidence for this indication.33 Acute therapy for migraine can be more challenging and although there are no direct studies, direct oral calcitonin gene-related peptide antagonists (rimegepant, ubrogepant) might be better options for acute migraine treatment because of their absence of vasoconstrictive properties compared with triptans.
Pulsatile tinnitus therapy
Pulsatile tinnitus is present in 32% of patients with cerebrovascular FMD and is caused by turbulent blood flow patterns near the ear, resulting in a rhythmic noise (whooshing sound) at the same frequency as the heart rate.34 Patient reassurance is recommended. In the presence of severe symptoms, referral to an ENT specialist should be considered because medical therapy is limited.
Management of Clinical Scenarios Observed in Patients With FMD
Although FMD might remain clinically silent over a patient’s lifetime, the disease might manifest clinically in certain well recognized scenarios. The management of FMD depends on the location of vascular involvement and extent of complications.
SCAD
In patients with SCAD, most cases can be safely managed conservatively (> 80%), without coronary revascularization. For severe cases with active or ongoing coronary ischemia, or hemodynamic instability, or SCAD involving the left main artery or proximal vessel(s), a revascularization attempt might be necessary. The goal of intervention is to completely or even partially restore myocardial perfusion, as feasible. For those managed with an initial conservative approach, spontaneous healing with resorption of intramural hematoma occurs in 95% of cases by 30 days after a SCAD event, however, in 2%-8% of cases conservative therapy fails.35 Options for revascularization (acutely or deferred) include PCI or coronary artery bypass graft surgery (CABG). The results of previous studies reveal that only 30% of PCI attempts have successful and durable results, and another 40% are partially successful.16,36,37 PCI in patients with SCAD is associated with an increased risk of complications and suboptimal outcomes including iatrogenic dissection, hematoma propagation, residual stenosis, and repeat target lesion revascularization because of stent thrombosis, in-stent restenosis, and stent malapposition.18,36 In select cases, CABG might be life-saving, and considered if a significant myocardial territory is at risk and the lesion is not amenable to PCI. Patients who undergo CABG have increased early mortality rates (5%) and more than two-thirds of grafts occlude in the long term because of completive flow as the SCAD lesion heals.37
CeAD
In patients with acute ischemic stroke, thrombolysis and endovascular thrombectomy should be considered; however, there is limited evidence to guide this approach specifically in the FMD population. CeAD is not a contraindication to intravenous thrombolysis, despite the theoretical risk of intramural hematoma enlargement. Current American Heart Association/American Stroke Association guidelines recommend treatment with 3-6 months of anticoagulation or antiplatelet therapy in CeAD patients with ischemic stroke or transient ischemic attack. The Cervical Artery Dissection in Stroke study failed to show superiority for either anticoagulation or antiplatelet therapy. In the STrOke Prevention in Cervical Artery Dissection (STOP-CAD) study it was shown that the subgroup of patients with occlusive CeAD might derive benefit from anticoagulation over antiplatelet therapy in the short term (first 180 days).38 Endovascular therapy is typically restricted to patients with persistent cerebrovascular ischemia despite medical therapy and for cases of expanding pseudoaneurysms and worsening stenosis with cerebral hypoperfusion.39,40
Intracranial aneurysms
The management of intracranial aneurysms in patients with FMD is controversial. The mean annual risk of rupture for an intracranial aneurysm in the general population is < 1% per year; however, it is unknown whether the risk is increased in FMD patients. With a 13% prevalence of unruptured intracranial aneurysms among patients with FMD, screening using brain CTA or MRA is recommended at least once regardless of the initial vascular site involved. Yet, it remains unclear if subsequent vascular imaging is warranted after initial negative screening.4,41 For small unruptured aneurysms with diameter < 5 mm, serial noninvasive imaging is suggested, using MRA without contrast to limit patient’s exposure to radiation, and contrast agents. Risk of rupture depends on aneurysm-related factors including aneurysm diameter > 5 mm, posterior circulation location, multilobulated aneurysm morphology, and patient characteristics. Patient factors include history of rupture, family history, hypertension, smoking, and alcohol use disorder.4,41 For aneurysms without high-risk characteristics or symptoms, conservative management involves close observation without immediate intervention.
Options for endovascular management include surgical clipping and endovascular coiling. Current American Heart Association/American Stroke Association guidelines recommend that patients with no history of subarachnoid hemorrhage with aneurysms ≤ 7 mm may be followed conservatively with routine imaging surveillance to assess changes in aneurysm size and/or morphology.42 The frequency of imaging remains unclear, however, it is advisable to consider conducting MRA time of flight (noncontrast exam) every 6-12 months after initial detection of the aneurysm.43 Subsequently, yearly imaging for 3 years is recommended, followed by biennial scans if the aneurysm size remains stable thereafter.43
Renal and visceral artery dissection
Patients with renal and visceral artery dissection might be asymptomatic or present with distal thromboembolic events such as renal or splenic infarction. This is more common in men than in women. Most patients can be managed with medical therapy and surveillance imaging; however, current guidelines on medical therapy after renal or visceral artery dissection remains controversial. Some centres recommend 3-6 months of anticoagulation followed by long-term antiplatelet therapy,44 especially in the setting of thromboembolism. In contrast, other centres prefer antiplatelet therapy only; ether ASA alone or DAPT with clopidogrel.44 In severe cases with progressive end organ hypoperfusion, dissection, or secondary aneurysms, endovascular intervention might be required. This might include covered stents, coil embolization, or surgical repair.3
Renal and visceral artery aneurysms
Renal and visceral artery aneurysms are typically managed with surveillance, antihypertensive therapy, and smoking cessation. Aneurysms might rupture or result in renal infarction from embolization of thrombus within the aneurysm. Large aneurysms can be treated with coiling, covered stents, or surgery. Success rates are similar for percutaneous and surgical options, however, surgery is associated with higher morbidity rates. There is currently no evidence to guide the timing of intervention in patients with renal FMD; therefore, intervention is typically offered if the aneurysm exceeds 2 cm, which is in line with current guidelines for non–FMD-associated aneurysms. When these measure > 2 cm the risk of rupture ranges from 25% to 40%, with an associated mortality rate up to 76%.45 The 2-cm cutoff for intervention in patients with visceral artery aneurysms is a general guideline because most reports of these rare aneurysms include cases with heterogeneous etiology. The decision for intervention should include consideration of location, symptoms, the risk of rupture, and/or other predisposing factors rather than a simple size cutoff.45 For example, earlier intervention may be considered in patients who are at high risk for rupture such as before pregnancy.45 In our centre’s experience, rupture of aneurysms attributable to FMD alone are extremely rare.
Renal FMD
Treatment options for renal FMD include medical therapy alone, percutaneous intervention, and surgery. Renal artery revascularization for stenotic lesions can be considered for hemodynamically significant stenosis; however, there have been no randomized controlled trials to compare medical therapy with revascularization to guide this. At our centre, the proportion of lesions that are hemodynamically significant is small.
Revascularization is typically achieved using angioplasty alone for focal and multifocal FMD because stenting might be complicated by stent kinking and fracture. Stenting is typically only considered in the setting of procedural complications such as flow-limiting dissection or renal artery rupture. After angioplasty, patients require lifelong antiplatelet therapy. In patients with complex FMD lesions involving bifurcations, branches, complex stenosis with aneurysms, or failed angioplasty, surgery is the preferred management strategy. This is typically an aortorenal bypass using a saphenous vein graft. After intervention for renal FMD, the rate of hypertension cure has been reported at 36%, with a greater likelihood of success in patients with focal FMD.3 This is likely a result of the later age at diagnosis in patients with multifocal FMD and longer duration of hypertension with nephrosclerosis. After intervention, surveillance duplex ultrasound for restenosis is recommended every 6 months for 2 years, then annually.3 The rate of restenosis after percutaneous angioplasty ranges from 12% to 34% over 6-24 months of follow-up.46,47
Extremity
Most patients remain asymptomatic (> 70%) and are usually managed conservatively (> 90%). If revascularization is required, percutaneous angioplasty may be performed. Surgical intervention might include bypass surgery, or aneurysm resection.48,49 Treatment of limb-threatening extremity FMD typically involves anticoagulation.
Lifestyle Modifications
Smoking
Smoking cessation is recommended for general cardiovascular protection. In patients with FMD, smoking is associated with increased risk of aneurysms, dissection, and need for intervention.
Physical activity
Arterial dissections have been associated with physical activity in FMD patients; however, individualized, regular, moderate-intensity exercise likely outweighs the theoretical risks of dissection with exercise. Patients with previous dissections such as SCAD or CeAD should avoid high-risk activities. SCAD has been associated with physical activity in 32% of patients, therefore it is recommended that patients avoid lifting, pushing, or pulling heavy objects that require prolonged straining, high-intensity exercise, extreme endurance training, and elite competitive sports.1 Patients with CeAD should avoid activities that require prolonged neck extension or severe neck tractions such as chiropractic neck manipulation. In addition, they are advised to refrain from exercises like push-ups and sit-ups for 8-12 weeks. Afterward, their activity recommendations align with those for SCAD (moderate-intensity exercise, and lifting of light to moderate loads). Although there are currently no data to indicate restriction of activities like riding roller coasters, skydiving, scuba diving, or chiropractic manipulation,50 it is not unreasonable to exercise caution with these activities to avoid excess torsion on the cervical arteries. Among patients with SCAD, the safety of participation in cardiac rehabilitation has been reported,51 although it might be prudent to wait 6-8 weeks before initiating an exercise program to permit vessel healing.
Mental health considerations
FMD is often under-recognized because of the broad number of symptoms possible, with patients often experiencing a delay in diagnosis. A quality of life survey study revealed that 22% of FMD patients experience moderate depression, 10% had moderate general anxiety, and 41% experienced significant physical symptoms.52 Compared with the general population, the rates of anxiety and depression were nearly double, and rates of somatoform symptoms were also increased.52 A retrospective review identified that 30%-40% of patients experience post-traumatic stress disorder symptoms, anxiety, and depression after their SCAD diagnosis.53 Anxiety diminished over time, but post-traumatic stress disorder and depression were time-independent. In our experience, patients struggling with a new diagnosis of SCAD and/or FMD might benefit greatly from a psychiatry or psychology referral to assist with restoring emotional well-being.
Hormone and reproductive concerns
Because SCAD and FMD occur predominantly among women, there is a presumed pathophysiologic role for endogenous and exogenous forms of female sex hormones; however, previous studies have not identified a causal relationship. At present, there is no definitive evidence that the use of hormonal contraceptives or hormone replacement therapy for menopause increases the risk of SCAD recurrence.54 Moreover, the fact that SCAD occurs across a broad age range, representing a wide variety of estrogen and progesterone levels, and also among men, leads one to believe that the role of female sex hormones in the pathogenesis of SCAD and FMD might be less prominent than previously assumed.
Recognizing the absence of direct evidence linking estrogen and progesterone to the pathophysiology of SCAD, consensus statements have recommended avoidance of exposure to exogenous hormones for contraception and postmenopausal therapy, if possible, and only support use if nonhormonal strategies have been explored. For contraception, preferred methods include vasectomy of male partners of SCAD patients, tubal ligation, long-acting progesterone-only therapies (intrauterine devices with local delivery of levonorgestrel, or levonorgestrel subdermal implants), and the avoidance of estrogen-containing contraceptives.1,54 To guide the use of postmenopausal hormone therapy, it might be necessary to individualize recommendations on a case-by-case basis, taking into account patient preferences, shared decision-making about risks and benefits, and giving preference to topical agents over oral hormone replacement therapy. Every effort should be made to use the lowest effective dose possible, if oral agents cannot be avoided.54 These difficult cases might be best supported through collaboration with menopausal experts.
Preconception counselling with a maternal-fetal medicine specialist is recommended for all patients with FMD. An increased risk of preeclampsia is reported among patients with renal FMD.55 Patients with a history of CeAD, SCAD, or poorly controlled hypertension are at significantly increased peripartum risk. Pregnancy-associated SCAD-related MI accounts for 1.81 per 100,000 pregnancies.56 Approximately 20% of patients with SCAD have recurrence with pregnancy, with > 70% of cases occurring within the first week postpartum.1,57 Patients who develop SCAD during pregnancy tend to have more extensive infarction and often multivessel SCAD.57 It is recommended that SCAD patients should avoid subsequent pregnancy because of the high risk of recurrent SCAD.
Follow-up
After initial screening with head-to-pelvis CTA, it is recommended that patients with FMD have annual clinical follow-up to review symptoms, vascular events, and monitor for hypertension. More frequent follow-up might be required depending on the site of vascular involvement and complications. Annual electrolyte and creatinine levels should be monitored in patients with renal FMD with periodic urinalysis to assess for albuminuria.3 Counselling on clinical symptoms, when to seek medical attention, and lifestyle modifications should be reviewed at follow-up visits. There are currently no consensus guidelines on the frequency and modality of imaging surveillance. This is typically individualized on the basis of severity of disease including the site of involvement, size of lesions, and previous interventions. There is presently no established role for routine surveillance of vascular territories for patients diagnosed with FMD, other than a reactive approach when new symptoms arise to guide further imaging in these patients.
Conclusions
Systematic screening for FMD should be performed in all patients with SCAD-related MI. Pharmacological management and intervention depend on the site of involvement and associated complications. The long-term management of patients should include routine symptom surveillance, hypertension management, lifestyle modifications, education, and counselling. In this review the importance of a comprehensive multidisciplinary care model is emphasized because of the complexity of FMD and its multisystem involvement. Further research is required to define optimal medical therapy in these patients.
Acknowledgments
Ethics Statement
The research summarized in this review article adhered to relevant ethical guidelines.
Patient Consent
The authors confirm that patient consent forms have been obtained for this article.
Funding Sources
Dr Madan is supported by the Heart and Stroke Foundation Polo Chair in Cardiology at the University of Toronto. Additional funding for this work was provided by the Sunnybrook Foundation SCAD Research Fund.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1287 for disclosure information.
References
- 1.Hayes S.N., Tweet M.S., Adlam D., et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:961–984. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- 2.Inohara T., Alfadhel M., Choi D., Starovoytov A., Saw J. Coronary angiographic manifestations and outcomes in spontaneous coronary artery dissection patients with and without fibromuscular dysplasia. Can J Cardiol. 2021;37:1725–1732. doi: 10.1016/j.cjca.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Gornik H.L., Persu A., Adlam D., et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med. 2019;24:164–189. doi: 10.1177/1358863X18821816. [DOI] [PubMed] [Google Scholar]
- 4.Paré G., Bhatt D.L. Vol 73. American College of Cardiology Foundation; Washington, DC: 2019. Linking Spontaneous Coronary Artery Dissection, Cervical Artery Dissection, and Fibromuscular Dysplasia: Heart, Brain, and Kidneys; pp. 67–69. [DOI] [PubMed] [Google Scholar]
- 5.Lather H.D., Gornik H.L., Olin J.W., et al. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: a report from the US registry for fibromuscular dysplasia. JAMA Neurol. 2017;74:1081–1087. doi: 10.1001/jamaneurol.2017.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivapour D.M., Erwin P., Kim E.S. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med. 2016;21:376–381. doi: 10.1177/1358863X16637913. [DOI] [PubMed] [Google Scholar]
- 7.Plouin P.F., Baguet J.P., Thony F., et al. High prevalence of multiple arterial bed lesions in patients with fibromuscular dysplasia: the ARCADIA Registry (Assessment of Renal and Cervical Artery Dysplasia) Hypertension. 2017;70:652–658. doi: 10.1161/HYPERTENSIONAHA.117.09539. [DOI] [PubMed] [Google Scholar]
- 8.Shivapour D.M., Erwin P., Gornik H.L., Kim E.S. Spontaneous coronary artery dissection: characterizing presentation, management, and outcomes at a large referral cardiovascular center. Circulation. 2015;132 [Google Scholar]
- 9.Van Twist D.J., Houben A.J., de Haan M.W., de Leeuw P.W., Kroon A.A. Pathophysiological differences between multifocal fibromuscular dysplasia and atherosclerotic renal artery stenosis. J Hypertens. 2017;35:845–852. doi: 10.1097/HJH.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 10.Silhol F., Sarlon-Bartoli G., Daniel L., et al. Intranuclear expression of progesterone receptors in smooth muscle cells of renovascular fibromuscular dysplasia: a pilot study. Ann Vasc Surg. 2015;29:830–835. doi: 10.1016/j.avsg.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Shimabukuro M. A new plausible link between lysophosphatidylcholine, TGFβ, and fibromuscular dysplasia. J Atheroscler Thromb. 2016;23:665–667. doi: 10.5551/jat.ED040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saw J., Humphries K., Aymong E., et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Olin J.W., Froehlich J., Gu X., et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125:3182–3190. doi: 10.1161/CIRCULATIONAHA.112.091223. [DOI] [PubMed] [Google Scholar]
- 14.Kiando S.R., Tucker N.R., Castro-Vega L.J., et al. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadian-Dodov D., Gornik H.L., Gu X., et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the US Registry for FMD. J Am Coll Cardiol. 2016;68:176–185. doi: 10.1016/j.jacc.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Saw J., Starovoytov A., Humphries K., et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188–1197. doi: 10.1093/eurheartj/ehz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faiella W., Bishop H., Mulvagh S.L. Spontaneous coronary artery dissection and fibromuscular dysplasia: chicken or egg? Which comes first? Can J Cardiol. 2021;37:1695–1698. doi: 10.1016/j.cjca.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Saw J., Aymong E., Sedlak T., et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 19.Jubran A., Hassan R., Siu V., et al. Etiology of acute coronary syndrome in a young woman: can intracoronary imaging help? CJC Open. 2023;5:345–347. doi: 10.1016/j.cjco.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bruyne B., Manoharan G., Pijls N.H., et al. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48:1851–1855. doi: 10.1016/j.jacc.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 21.Downer J., Nadarajah M., Briggs E., Wrigley P., McAuliffe W. The location of origin of spontaneous extracranial internal carotid artery dissection is adjacent to the skull base. J Med Imaging Radiat Oncol. 2014;58:408–414. doi: 10.1111/1754-9485.12170. [DOI] [PubMed] [Google Scholar]
- 22.Chaves C., Estol C., Esnaola M.M., et al. Spontaneous intracranial internal carotid artery dissection: report of 10 patients. Arch Neurol. 2002;59:977–981. doi: 10.1001/archneur.59.6.977. [DOI] [PubMed] [Google Scholar]
- 23.Sharashidze V., Nogueira R.G., Al-Bayati A.R., et al. Carotid web phenotype is uncommonly associated with classic fibromuscular dysplasia: a retrospective observational study. Stroke. 2022;53:e33–e36. doi: 10.1161/STROKEAHA.121.036188. [DOI] [PubMed] [Google Scholar]
- 24.Olin J.W., Gornik H.L., Bacharach J.M., et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129:1048–1078. doi: 10.1161/01.cir.0000442577.96802.8c. [DOI] [PubMed] [Google Scholar]
- 25.Nebelsieck J., Sengelhoff C., Nassenstein I., et al. Sensitivity of neurovascular ultrasound for the detection of spontaneous cervical artery dissection. J Clin Neurosci. 2009;16:79–82. doi: 10.1016/j.jocn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Traenka C., Streifler J., Lyrer P., Engelter S.T. Clinical usefulness of serial duplex ultrasound in cervical artery dissection patients. Cerebrovasc Dis. 2020;49:206–215. doi: 10.1159/000507485. [DOI] [PubMed] [Google Scholar]
- 27.Moore E.K., Gu X., Olin J.W., et al. Registry findings of fibromuscular dysplasia of the mesenteric arteries. Vasc Med. 2014;19:224–225. [Google Scholar]
- 28.Nguyen N., Sharma A., West J.K., et al. Presentation, clinical features, and results of intervention in upper extremity fibromuscular dysplasia. J Vasc Surg. 2017;66:554–563. doi: 10.1016/j.jvs.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A.M., Gornik H.L. Standing arterial waves is NOT fibromuscular dysplasia. Circ Cardiovasc Interv. 2012;5:e9–e11. doi: 10.1161/CIRCINTERVENTIONS.111.967828. [DOI] [PubMed] [Google Scholar]
- 30.Rabi D.M., McBrien K.A., Sapir-Pichhadze R., et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36:596–624. doi: 10.1016/j.cjca.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 31.Saw J., Starovoytov A., Aymong E., et al. Canadian spontaneous coronary artery dissection cohort study: 3-year outcomes. J Am Coll Cardiol. 2022;80:1585–1597. doi: 10.1016/j.jacc.2022.08.759. [DOI] [PubMed] [Google Scholar]
- 32.Swan K., Gu X., Kline-Rogers E., et al. Prevalence of headaches in patients with fibromuscular dysplasia: a report from the US Registry for FMD. Circ Cardiovasc Qual Outcomes. 2017;10:A099. [Google Scholar]
- 33.Pringsheim T., Davenport W., Mackie G., et al. Canadian Headache Society guideline for migraine prophylaxis. Can J Neurol Sci. 2012;39:S1–S59. [PubMed] [Google Scholar]
- 34.Mahmood R.Z., Olin J., Gu X., et al. Unraveling pulsatile tinnitus in FMD: a report of the United States Registry for Fibromuscular Dysplasia. J Am Coll Cardiol. 2014;63:A2060. [Google Scholar]
- 35.Hassan S., Prakash R., Starovoytov A., Saw J. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. JACC Cardiovasc Interv. 2019;12:518–527. doi: 10.1016/j.jcin.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Prakash R., Starovoytov A., Heydari M., Mancini G.J., Saw J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9:1851–1853. doi: 10.1016/j.jcin.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Tweet M.S., Eleid M.F., Best P.J., et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 38.Yaghi S., Shu L., Mandel D., et al. Antithrombotic treatment for stroke prevention in cervical artery dissection: the STOP-CAD study. Stroke. 2024;55:908–918. doi: 10.1161/STROKEAHA.123.045731. [DOI] [PubMed] [Google Scholar]
- 39.Rahme R.J., Aoun S.G., McClendon J., El Ahmadieh T.Y., Bendok B.R. Spontaneous cervical and cerebral arterial dissections: diagnosis and management. Neuroimaging Clin N Am. 2013;23:661–671. doi: 10.1016/j.nic.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Kleindorfer D.O., Towfighi A., Chaturvedi S., et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 41.Kesav P., Manesh Raj D., John S. Cerebrovascular fibromuscular dysplasia–a practical review. Vasc Health Risk Manag. 2023;19:543–556. doi: 10.2147/VHRM.S388257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson B.G., Brown RD Jr, Amin-Hanjani S., et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2368–2400. doi: 10.1161/STR.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 43.Etminan N., Rinkel G.J. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713. doi: 10.1038/nrneurol.2016.150. [DOI] [PubMed] [Google Scholar]
- 44.Cavalcante R.N., Motta-Leal-Filho J.M., De Fina B., et al. Systematic literature review on evaluation and management of isolated spontaneous celiac trunk dissection. Ann Vasc Surg. 2016;34:274–279. doi: 10.1016/j.avsg.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Juntermanns B., Bernheim J., Karaindros K., Walensi M., Hoffmann J. Visceral artery aneurysms. Gefasschirurgie. 2018;23:19–22. doi: 10.1007/s00772-018-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alhadad A., Mattiasson I., Ivancev K., Gottsäter A., Lindblad B. Revascularisation of renal artery stenosis caused by fibromuscular dysplasia: effects on blood pressure during 7-year follow-up are influenced by duration of hypertension and branch artery stenosis. J Hum Hypertens. 2005;19:761–767. doi: 10.1038/sj.jhh.1001893. [DOI] [PubMed] [Google Scholar]
- 47.Carmo M., Bower T.C., Mozes G., et al. Surgical management of renal fibromuscular dysplasia: challenges in the endovascular era. Ann Vasc Surg. 2005;19:208–217. doi: 10.1007/s10016-004-0164-9. [DOI] [PubMed] [Google Scholar]
- 48.Okazaki J., Guntani A., Homma K., et al. Fibromuscular dysplasia of the lower extremities. Ann Vasc Dis. 2011;4:143–149. doi: 10.3400/avd.cr.10.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg I., Jaff M.R. Nonatherosclerotic arterial disorders of the lower extremities. Circulation. 2012;126:213–222. doi: 10.1161/CIRCULATIONAHA.111.060335. [DOI] [PubMed] [Google Scholar]
- 50.Tweet M.S., Olin J.W., Bonikowske A.R., Adlam D., Hayes S.N. Physical activity and exercise in patients with spontaneous coronary artery dissection and fibromuscular dysplasia. Eur Heart J. 2021;42:3825. doi: 10.1093/eurheartj/ehab307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou A.Y., Prakash R., Rajala J., et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32:554–560. doi: 10.1016/j.cjca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Bumpus S.M., Heidt S.T., Krallman R., Kline-Rogers E. Single site lifestyle survey in FMD patients suggests improvement over time. Circ Cardiovasc Qual Outcomes. 2017;10 [Google Scholar]
- 53.Johnson A.K., Hayes S.N., Sawchuk C., et al. Analysis of posttraumatic stress disorder, depression, anxiety, and resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes S.N., Kim E.S.H., Saw J., et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berra E., Dominiczak A.F., Touyz R.M., et al. Management of a pregnant woman with fibromuscular dysplasia. Hypertension. 2018;71:540–547. doi: 10.1161/HYPERTENSIONAHA.118.10819. [DOI] [PubMed] [Google Scholar]
- 56.Faden M.S., Bottega N., Benjamin A., Brown R.N. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart. 2016;102:1974–1979. doi: 10.1136/heartjnl-2016-309403. [DOI] [PubMed] [Google Scholar]
- 57.Tweet M.S., Hayes S.N., Codsi E., et al. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. doi: 10.1016/j.jacc.2017.05.055. [DOI] [PubMed] [Google Scholar]