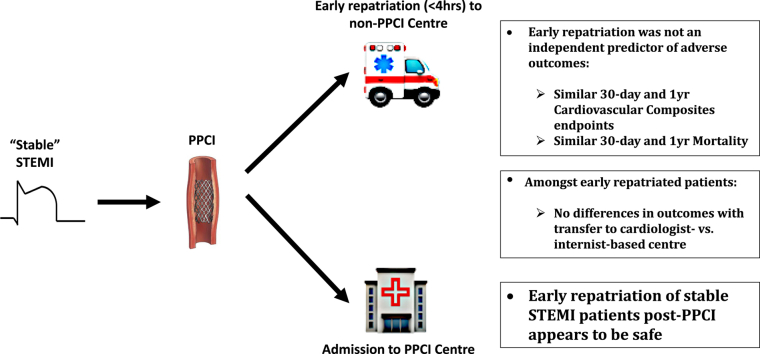
Abstract
Background
Repatriation of ST-segment elevation myocardial infarction (STEMI) patients after primary percutaneous coronary intervention (PPCI) is common in regional health care programs. We examined the short- and long-term safety of early repatriation after PPCI in stable STEMI patients.
Methods
Consecutive stable STEMI patients undergoing PPCI between 2016 to 2018 in the Fraser Health Authority were included. Outcomes were compared between early and nonrepatriated cohorts. Co-primary outcomes were a composite of death, myocardial infarction, congestive heart failure, and stroke at 30 days and 1 year. Logistic regression analyses were performed to determine association between early repatriation and outcomes, and to assess impact of transfer to cardiologist- vs internist-based care centres.
Results
A total of 788 patients were included, with 62% being repatriated early. Primary composite and individual outcomes rates were similar between both cohorts. Early repatriation was not an independent predictor of 30-day (odds ratio [OR] 0.93, 95% confidence interval [CI] 0.50-1.72; P = 0.82) or 1-year (OR 1.05, 95% CI 0.67-1.65; P = 0.8) primary outcome, or of 30-day (OR 1.35, 95% CI 0.41-4.47, P = 0.63) or 1-year (OR 1.03, 95% CI 0.44-2.40; P = 0.95) mortality. Among early repatriated patients, transfer to cardiologist- vs internist-based care centres was not an independent factor for 30-day (OR 1.07, 95% CI 0.45-2.54; P = 0.87) or 1-year (OR 1.17, 95% 0.55-2.50, P = 0.69) primary outcome.
Conclusions
Early repatriation of stable STEMI patients after PPCI appears to be safe based on short- and long-term outcomes, and transfer to internist- vs cardiology-based centres did not affect outcomes. After PPCI, early repatriation allows for redistribution of stable STEMI patients to lower-acuity settings across regional hospitals.
Graphical abstract
RéSUMé
Contexte
Le rapatriement des patients ayant eu un infarctus du myocarde avec élévation du segment ST (STEMI) après une intervention coronarienne percutanée primaire (ICPP) est une pratique courante des programmes régionaux de soins de santé. Nous avons examiné l’innocuité à court et à long terme du rapatriement précoce de patients avec STEMI stables après une ICPP.
Méthodologie
Des patients avec STEMI stables ayant subi une ICPP entre 2016 et 2018 dans un centre de la Fraser Health Authority ont été inclus dans l’étude. Les résultats des cohortes rapatriées de façon précoce et non rapatriées ont été comparés. Les paramètres d’évaluation principaux représentaient un critère composite comprenant le décès, un infarctus du myocarde, une insuffisance cardiaque congestive et un AVC après 30 jours et après 1 an. Des analyses de régression logistique ont été réalisées pour déterminer le lien entre le rapatriement précoce des patients et les résultats ainsi que pour évaluer l’incidence du transfert de centres de cardiologie vers des centres de médecine interne.
Résultats
L’étude a porté sur 788 patients au total, dont 62 % avaient été rapatriés de façon précoce. La fréquence des paramètres d’évaluation principaux pour le critère composite et les critères individuels était comparable entre les deux cohortes. Le rapatriement précoce n’était pas un facteur prédictif indépendant du paramètre d’évaluation principal composite à 30 jours (rapport de cotes [RC] 0,93, intervalle de confiance [IC] à 95 % 0,50-1,72; p = 0,82) ou à 1 an (RC 1,05, IC à 95 % 0,67-1,65; p = 0,8), ni de la mortalité à 30 jours (RC 1,35, IC à 95 % 0,41-4,47, p = 0,63) ou à 1 an (RC 1,03, IC à 95 % 0,44-2,40; p = 0,95). Dans le cas des patients rapatriés de façon précoce, le transfert d’un centre de cardiologie à un centre de médecine interne n’était pas un facteur prédictif indépendant du paramètre d’évaluation principal composé à 30 jours (RC 1,07, IC à 95 % 0,45-2,54; p = 0,87) ou à 1 an (RC 1,17, IC à 95 % 0,55-2,50, p = 0,69).
Conclusions
Le rapatriement précoce des patients avec STEMI stables après une ICPP semble sécuritaire d’après les résultats obtenus à court et à long terme, et le transfert vers un centre de médecine interne par rapport à un centre de cardiologie n’a pas eu d’incidence sur les résultats. Après une ICPP, le rapatriement précoce permet une redistribution des patients avec STEMI stables vers des hôpitaux de soins actifs régionaux.
Primary percutaneous coronary intervention (PPCI) after ST-segment elevation myocardial infarction (STEMI) has been shown to reduce short- and long-term morbidity and mortality compared with thrombolysis.1, 2, 3 However, most hospitals in Canada do not offer PPCI. Through a regional hub and spoke model, patients within health authorities are transferred to centres with cardiac catheterisation laboratories for PPCI followed by eventual repatriation back to the hospital site of original care. In our health authority, STEMI patients after PPCI are deemed to be suitable for early repatriation unless they present with cardiogenic shock, have persistent dysrhythmias, require mechanical ventilation, or have a major intraprocedural complication. Early repatriation after cardiac catheterisation allows for reduced congestion at PPCI centres where bed capacity may be limited. Approximately, 80% of patients transferred for PPCI were repatriated in our regional STEMI program according to a previous study.4
Several Canadian registries have offered disparate results on the safety of early repatriation. In registries from British Columbia and Ontario, similar rates of in-hospital or index-admission mortality were noted between repatriated and nonrepatriated PPCI patients.4,5 In contrast, Abuzeid et al. demonstrated an increased risk of recurrent myocardial infarction (MI) at 1 year in repatriated patients, despite observing no differences in 1-year mortality between cohorts.6 However, those studies predominantly included a broad spectrum of STEMI patients with varying definitions of early repatriation.
In the present study, we sought to assess the safety of early repatriation, defined as transfer to the hospital of original care within 4 hours of PPCI completion, in a population of stable STEMI patients. We examined differences in 30-day and 1-year outcomes between repatriated and nonrepatriated patients. And in substudy analysis, we sought to assess the association between outcomes and transfer to centres with cardiologist- vs internist-based care.
Methods
Study population
This study assessed stable STEMI patients who underwent PPCI at the Royal Columbian Hospital in the Fraser Health Authority. Patients included those presenting either directly to a PPCI centre or as transferred from non-PPCI centres with subsequent early repatriation. Patients were repatriated back to the referring centre where the initial referral for PPCI was made. STEMI was defined by the standard guideline-based criteria according to 12-lead electrocardiography.7 Stable STEMI patients were those not having evidence of cardiogenic shock or persistent dysrhythmias or requiring mechanical ventilation before or during PPCI. Persistent dysrhythmias were defined as ventricular arrhythmias that failed initial synchronised or chemical cardioversion or shock, atrial fibrillation with rapid ventricular response, or nontransient second degree type II or complete heart block. Typically, patients with these exclusionary conditions were not deemed to be suitable for early repatriation, because ancillary services such as cardiac intensivists and mechanical circulatory support were not readily available at non-PPCI centres. Also, the inclusion of such patients in the analysis was thought to potentially lead to selection bias by disproportionately increasing the number of more complex, sicker patients within the nonrepatriated group, as was evidenced by markedly disparate unadjusted outcomes noted between cohorts in previous analysis.4 Patients presenting with cardiac arrest or congestive heart failure not requiring mechanical ventilation or necessitating critical care admission were included in the analysis.
Early repatriation was defined as patient arrival to the hospital of original care within 4 hours after PPCI completion. Among transfer patients, those who were repatriated after 4 hours or those remaining at PPCI centre were excluded from analysis, as were individuals referred from centres without adequate resources to care for those after PPCI. Verification of PPCI completion time and arrival to hospital of original care was checked manually with the use of electronic medical records, focusing specifically on PPCI procedural notes, nursing flow sheets, and ambulance records. Early-repatriation patients were transferred directly from the cardiac catheterisation laboratory or neighbouring hold bay without admission to the PPCI centre. In addition, there was no assessment or further treatment by cardiologists other than the interventionalist at the PPCI centre. All transfers for early repatriation patients required an ambulance and included 2 paramedics and a transfer nurse. Ambulances transferring STEMI patients to the PPCI centre for the procedure were usually able to stay for the duration of PPCI, allowing for the same car and emergency medical services crew to be involved in early repatriation. A demarcation of 4 hours to arrival at hospital of original care after PPCI was used as a cutoff for defining early repatriation because it allowed for long transport times resulting from the large dispersion of distances to non-PPCI hospitals in the health authority (9.5-133.4 km), as well as short delays in ambulance pick-up from the PPCI centre. Early repatriation was determined based on time for transfer rather than distance to non-PPCI centre because there were considerable variations in the duration of transfer regardless of distance during daytime hours, particularly at rush hours. Patients presenting to the PPCI centre came via its emergency room or from the field directly through ambulance pick-up in the catchment area of the health authority. Patients were preloaded with P2Y12 inhibitors on arrival to the PPCI centre.
The PPCI centre was the only hospital within the health authority capable of providing mechanical circulatory support in the form of intra-aortic balloon pump or extracorporeal membranous oxygenation, as well as being the sole regional site with an in-house cardiac surgery department. All non-PPCI centres receiving early repatriated patients had to have the capacity to treat individuals needing mechanical ventilation or vasopressors, and had staffing by internists or cardiologists. In addition to providing cardiologist-based consultations, non-PPCI centres with cardiology departments had greater resources to obtain expedited cardiac testing, such as transthoracic and transesophageal echocardiography.
Patients referred for PPCI from 2 smaller hospitals in the Fraser Health Authority that did not have the capacity to offer care for those requiring mechanical ventilation or vasopressors were excluded from analysis. Distance from the PPCI centre was not used as criteria to exclude hospitals accepting early repatriated patients.
Patient characteristics and clinical outcomes
Data relating to baseline demographics, clinical presentation features, PPCI procedural details, discharge medications and both 30-day and 1-year outcomes were collected retrospectively from the health authority electronic medical records. Baseline demographics included age, sex, cardiovascular risk factors, previous MI, and the presence of anemia (hemoglobin ≤ 110 g/L) or chronic kidney disease (estimated glomerular filtration rate ≤ 60 mL/min/m2). Clinical presentation features noted were congestive heart failure (CHF) and cardiac arrest before or at the time of PPCI. Procedural details collected were the presence of multivessel disease, left ventricular end-diastolic pressure (LVEDP) ≥ 20 mm Hg, culprit left anterior descending coronary artery disease on angiography, Thrombolysis in Myocardial Infarction (TIMI) 3 flow after PPCI, and use of glycoprotein IIb/IIIa inhibitors. Discharge with evidence-based medications (EBM) was also examined. EBMs at discharge required a combined prescription for guideline-recommended medications after PPCI including aspirin or oral anticoagulant, P2Y12 inhibitor, beta-blocker, statin, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker. Dosing of these medications at the time of discharge was not recorded or analysed.
Short- and long-term clinical outcomes were assessed. The primary outcomes were a composite of death, recurrent MI, stroke, or CHF exacerbation requiring emergency room presentation at the time points of 30 days and 1 year. Secondary outcomes included mortality, recurrent MI, CHF exacerbation, and stroke at 30 days and 1 year. Length of stay during PPCI admission also was assessed for each cohort. MI was defined as presentation with chest pain or chest pain–equivalent symptoms and associated troponin elevation, or a syndrome consistent with unstable angina. CHF was defined as presentation of shortness of breath and evidence of pulmonary edema on roentrographic study or clinical responsiveness to furosemide. Finally, stroke was defined as presentation with new focal neurologic deficits and associated imaging findings consistent with cerebral infarction.
Statistical methods
A comparison of patient characteristics and outcomes was made between stable STEMI patients who presented directly to the PPCI centre and those who were transferred for PPCI with subsequent early repatriation. Continuous variables were expressed as mean ± SD and compared with the use of a Wilcoxon sum test, and categoric variables were expressed as n (%) and analysed with the use of a Fisher exact test. Multivariate analysis was performed with the use of logistic regression modelling to determine whether early repatriation or other patient characteristics were independent predictors for 30-day primary composite outcomes, 30-day mortality, 1-year primary composite outcomes ,and 1-year mortality. Multivariate analysis was also performed in the subgroup of patients who were repatriated to determine whether transfer to a cardiology- vs internist-based centre was an independent predictor of 30-day and 1-year primary composite outcomes. Variables for all multivariate analysis models involved baseline clinical, presenting, and procedural characteristics, including age, sex, diabetes, hypertension, dyslipidemia, anemia, chronic kidney disease, previous MI, multivessel disease on PPCI angiography, increased LVEDP or congestive heart failure requiring diuretics, and use of combined EBMs. Results were presented as odds ratios (ORs) with 95% confidence intervals (CIs). A P value < 0.05 was considered to be significant for analysis.
Results
Study population
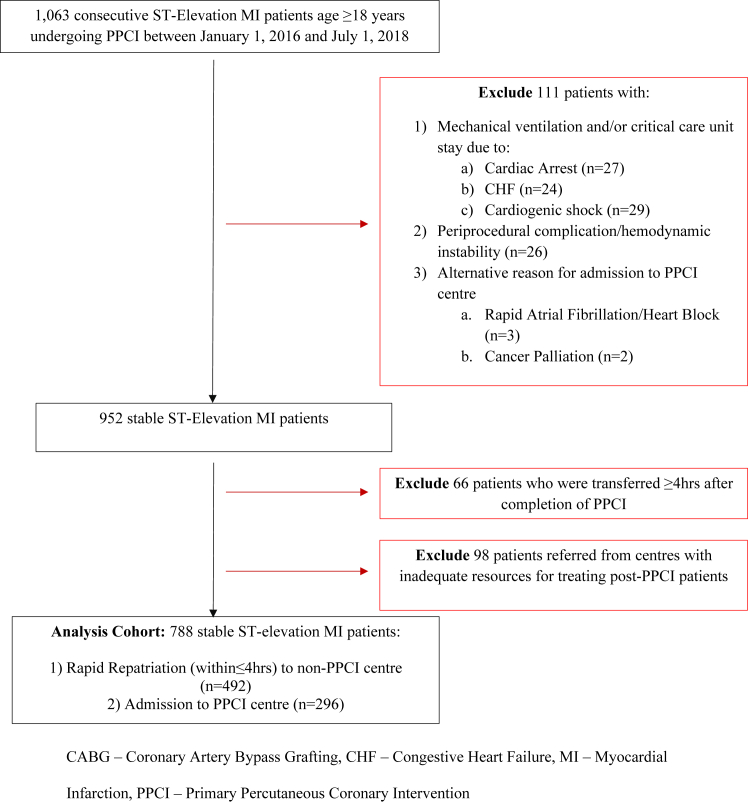
From January 1, 2016, to July 1, 2018, there were a total of 1063 patients who presented with STEMI and underwent PPCI at the Royal Columbian Hospital. Of these, 80 were classified as unstable and excluded from analysis (Fig. 1). Unstable patients were those requiring mechanical ventilation, mechanical circulatory support, or any vasopressor agent necessitating admission to the PPCI centre’s critical care unit (CCU). Also excluded were 26 patients with periprocedural complications or intraprocedural hemodynamic instability resulting in prolonged stay at the PPCI centre. Periprocedural complications (n = 10; 1% of stable STEMI patients) included femoral access site bleeding (n = 3), forearm hematoma (n = 1), distal wire perforation (n = 2), large vessel perforation after balloon dilation (n = 2), and intraprocedural stent thrombosis (n = 2). Hemodynamic instability in initially stable STEMI patient was due to the intraprocedural development of congestive heart failure (n = 8), recurrent ventricular arrhythmias (n = 5) or cardiogenic shock (n = 3) requiring mechanical ventilation, and initiation of vasopressor agents.
Figure 1.
Flow chart of study cohort selection. CHF, congestive heart failure; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention.
There were 66 patients who required more than 4 hours for repatriation from PPCI centre to the hospital of original care, owing to greater need for observation at the PPCI centre or delay in ambulance transfer, that were also excluded from analysis. Of these, 21 patients were not included in the early repatriation cohort owing to suspected post-PPCI delay in ambulance transfer. There were 4 cases of delays in transfer that could be attributed to the access site used for PPCI (transfemoral = 3; transradial = 1). Finally, 98 patients were excluded because they were referred from centres that did not have the capacity to care for individuals after PPCI.
After exclusions, there were a total of 788 stable STEMI patients included for study (Fig. 1). In this cohort, there were 492 patients (62%) repatriated to the hospital of original care within 4 hours of PPCI completion, whereas 296 patients (38%) were admitted to the PPCI centre from the centre’s emergency room or through direct transfer from the field via emergency medical services. The mean age for this combined population was 63.5 ± 12.5 years, and 24.7% of patients were female. Median time for repatriation was 124 min (interquartile range 72-176 min).
Patient characteristics
Baseline and procedural characteristics were similar between repatriated and nonrepatriated cohorts (Table 1). Early repatriated patients were more likely to have culprit left anterior descending coronary artery diagnosis (46% early repatriated vs 39% nonrepatriated; P = 0.03). There were no differences in mean age (63.7 years early repatriated vs 63.3 years nonrepatriated; P = 0.98), frequency of diabetes (25% early repatriated vs 28% nonrepatriated; P = 0.40), and presence of multivessel disease (46% early repatriated vs 46% nonrepatriated; P = 1.0) between patient cohorts. There were similar rates of transradial approach for PPCI among both cohorts (80% early repatriated vs 82% nonrepatriated; P = 0.40)
Table 1.
Baseline characteristics of stable STEMI patients in early and nonrepatriated cohorts
| Early repatriated (n = 492) | Nonrepatriated (n = 296) | P value | |
|---|---|---|---|
| Age, y | 63.7 ± 12.4 | 63.3 ± 12.3 | 0.98 |
| Male | 359 (73) | 214 (72) | 0.74 |
| Hypertension | 261 (57) | 162 (55) | 0.77 |
| Dyslipidemia | 215 (44) | 131 (44) | 1.0 |
| Diabetes | 123 (25) | 83 (28) | 0.40 |
| Smoking | 120 (24) | 72 (24) | 1.0 |
| Prior MI | 41 (8) | 26 (9) | 0.9 |
| Anemia (Hg < 110) | 66 (13) | 43 (15) | 0.75 |
| CKD (eGFR < 60) | 97 (19) | 49 (17) | 0.26 |
| Presenting CHF | 36 (7) | 24 (8) | 0.68 |
| Presenting cardiac arrest | 23 (5) | 7 (2) | 0.12 |
| Post-PPCI TIMI 3 flow | 484 (99) | 282 (95) | 0.08 |
| Multivessel disease | 227 (46) | 136 (46) | 0.94 |
| LVEDP ≥ 20 mm Hg | 112 (23) | 80 (27) | 0.20 |
| LAD | 227 (46) | 114 (39) | 0.03 |
| GIIB/IIIA bolus/infusion | 30 (6) | 24 (8) | 0.31 |
| GIIB/IIIA bolus only | 4 (1) | 2 (1) | 1.0 |
| EBM | 431 (88) | 246 (83) | 0.06 |
Values are mean ± SD or n (%).
CHF, congestive heart failure; CKD, chronic kidney disease; EBM, evidence-based medication; eGFR, estimated glomerular filtration rate; GIIB/IIIA, glycoprotein IIb/IIIa inhibitor; Hg, hemoglobin; LAD, left anterior descending coronary artery; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention.
In subgroup analysis, patients repatriated to cardiologist-based centres had a higher rate of hypertension (58% cardiologist-based vs 47% internist-based; P = 0.01) and anemia (17% cardiologist-based vs 10% internist-based; P = 0.03) compared with those transferred to internist-based centres (Supplemental Table S1). In contrast, patients at internist-based centres had a greater frequency of smoking (19% cardiologist-based vs 31% internist-based; P = 0.0022) and previous MI (8% cardiologist-based vs 24% internist-based; P < 0.001).
Clinical outcomes
The rate of the primary composite outcome was not significantly different between repatriated and nonrepatriated patients at both 30 days (early repatriated 7% vs nonrepatriated 7%; P = 0.88) and 1 year (early repatriated 9% vs nonrepatriated 13%; P = 0.09). Rates of individualised outcomes, including mortality, MI, CHF, and stroke, also were similar between both cohorts at both specified time periods (Table 2). There was no difference noted in length of stay for repatriated and nonrepatriated patients (early repatriated 5.0 ± 5.9 days vs nonrepatriated 5.1 ± 5.0 days; P = 0.99). Of the 492 patients who had early repatriation, 2 were transferred back to the PPCI centre during the index admission owing to stent thrombosis. There were no episodes of post-PPCI chest pain warranting return of ambulances to the PPCI centre during repatriation. Among those repatriated, there were no differences in the unadjusted outcomes between patients repatriated to centres with and without cardiologists (Supplemental Table S2).
Table 2.
Unadjusted outcomes of stable STEMI patients in early and nonrepatriated cohorts
| Early repatriated (n = 492) | Nonrepatriated (n = 296) | P value | |
|---|---|---|---|
| 30-d composite | 35 (7) | 22 (7) | 0.88 |
| 30-d mortality | 10 (2) | 5 (2) | 1.0 |
| 30-d MI | 3 (1) | 6 (2) | 0.09 |
| 30-d CHF | 30 (6) | 18 (6) | 1.0 |
| 30-d stroke | 4 (1) | 3 (1) | 1.0 |
| 1-y composite | 43 (9) | 38 (13) | 0.09 |
| 1-y mortality | 18 (4) | 10 (3) | 1.0 |
| 1-y MI | 12 (2) | 15 (5) | 0.07 |
| 1-y CHF | 33 (7) | 20 (7) | 1.0 |
| 1-y stroke | 7 (1) | 7 (2) | 0.41 |
| LOS, d | 5.0 ± 5.9 | 5.1 ± 5.0 | 0.99 |
CHF, congestive heart failure; MI, myocardial infarction; LOS, length of stay.
There were 15 patients who had died at 30 days. Cardiogenic shock secondary to pump failure was the cause of death in 7 patients. Two patients had angiographically confirmed stent thrombosis, both of which were in the early repatriated group. One patient had a cardiac arrest in hospital. Three patients developed sepsis and had in-hospital deaths. One death was attributed to upper gastrointestinal bleeding and 1 to a cerebrovascular accident. Two patients had an unknown cause of death at home. There were an additional 13 deaths between 30 days and 1 year. Causes of death in that period included pump failure in 2 patients, cerebrovascular accident in 3, cancer in 2, intracranial hemorrhage in 1, sepsis in 1, upper gastrointestinal bleeding in 1, cirrhosis in 1, and end-stage renal disease in 1. One patient had an unknown cause of death.
There were 66 STEMI patients deemed to be stable that were repatriated after 4 hours. These patients had a 30-day primary composite outcome rate of 13.6% and 30-day MI rate of 9.1%. At 1 year, the rate of the primary outcome was 19.6% and MI occurred in 12.1%. In patients with delayed repatriation, 30-day and 1-year mortalities were 4.5% and 7.5%, respectively. Outcome rates were numerically higher than those noted in the early-repatriation cohort. The cause for ambulance delay for most patients could not be determined from the electronic medical records.
Using multivariable modelling for short-term outcomes, early repatriation was not associated with the 30-day primary composite outcome (OR 0.93, 95% CI 0.50-1.72; P = 0.82) or mortality (OR 1.35, 95% CI 0.41-4.47; P = 0.63) (Tables 3 and 4). Independent predictors of the 30-day primary composite outcome included discharge with EBMs (OR 0.44, CI 0.21-0.92; P = 0.028), multivessel disease (OR 1.96, CI 1.04-3.70; P = 0.037), anemia (OR 3.39, CI 1.67-6.89; P = 0.001), and increased LVEDP/CHF (OR 4.55 CI 2.43-8.53; P < 0.001) (Table 3). Characteristics independently associated with 30-day mortality were discharge with EBMs (OR 0.17 CI 0.05-0.55; P = 0.003), anemia (OR 3.75, CI 1.08-13.07; P = 0.003), and multivessel disease (OR 12.37, CI 1.56-98.05; P = 0.017) (Table 4).
Table 3.
Independent predictors for 30-day composite outcomes in stable STEMI patients
| OR | 95% CI | P value | |
|---|---|---|---|
| Increased LVEDP/CHF | 4.55 | 2.43-8.53 | < 0.001 |
| Anemia (Hg < 110 g/L) | 3.39 | 1.67-6.89 | 0.001 |
| Multivessel disease | 1.96 | 1.04-3.70 | 0.037 |
| Age ≥ 70 y | 1.98 | 0.99-3.98 | 0.05 |
| Diabetes | 1.59 | 0.81-3.10 | 0.18 |
| Hypertension | 1.54 | 0.77-3.10 | 0.23 |
| CKD (eGFR < 60 mL/min/m2) | 1.08 | 0.51-2.31 | 0.84 |
| Male sex | 1.06 | 0.54-2.05 | 0.87 |
| Early repatriation | 0.93 | 0.50-1.72 | 0.82 |
| Dyslipidemia | 0.58 | 0.30-1.13 | 0.11 |
| EBM | 0.44 | 0.21-0.92 | 0.028 |
| Previous MI | 0.42 | 0.18-1.49 | 0.18 |
CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; EBM, evidence-based medication; eGFR, estimated glomerular filtration rate; Hg, hemoglobin; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction; OR, odds ratio.
Table 4.
Independent predictors for 30-day mortality in stable STEMI patients
| OR | 95% CI | P value | |
|---|---|---|---|
| Multivessel disease | 12.37 | 1.56-98.05 | 0.017 |
| Anemia (Hg < 110 g/L) | 3.75 | 1.08-13.07 | 0.003 |
| CKD (eGFR < 60 mL/min/m2) | 2.52 | 0.66-9.60 | 0.18 |
| Increased LVEDP/CHF | 2.10 | 0.67-6.57 | 0.20 |
| Early repatriation | 1.35 | 0.41-4.47 | 0.63 |
| Diabetes | 1.35 | 0.38-4.76 | 0.64 |
| Hypertension | 1.03 | 0.29-3.63 | 0.97 |
| Age ≥ 70 y | 0.78 | 0.20-3.10 | 0.73 |
| Male sex | 0.74 | 0.22-2.45 | 0.62 |
| Dyslipidemia | 0.60 | 0.17-2.17 | 0.44 |
| Previous MI | 0.55 | 0.07-4.65 | 0.58 |
| EBM | 0.17 | 0.05-0.55 | 0.003 |
CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; EBM, evidence-based medication; eGFR, estimated glomerular filtration rate; Hg, hemoglobin; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction; OR, odds ratio.
Similarly, in multivariable analysis examining 1-year end points, early repatriation was not associated with the primary composite outcome (OR 0.84, 95% CI 0.49-1.44; P = 0.53) or mortality (OR 1.03, 95% CI 0.44-2.40; P = 0.95) (Tables 5 and 6). Factors independently associated with 1-year primary composite outcome included diabetes (OR 1.91, CI 1.07-3.42; P = 0.029), age ≥ 70 years (OR 2.13, CI 1.16-3.90; P = 0.014), anemia (OR 2.20, 95% CI 1.14-4.27; P = 0.019), multivessel disease (OR 2.36, CI 1.34-4.13; P = 0.003), and increased LVEDP/CHF (OR 4.53, CI 2.62-7.82; P < 0.0001) (Table 5). Independent predictors of 1-year mortality were discharge with EBMs (OR 0.37, CI 0.15-0.89; P = 0.027), increased LVEDP/CHF (OR 2.38, CI 1.05-5.44; P = 0.039), and anemia (OR 3.32, CI 1.35-8.21; P = 0.009) (Table 6).
Table 5.
Independent predictors for 1-year composite outcomes in stable STEMI patients
| OR | 95% CI | P value | |
|---|---|---|---|
| Increased LVEDP/CHF | 4.53 | 2.62-7.82 | < 0.001 |
| Multivessel disease | 2.36 | 1.34-4.13 | 0.003 |
| Age ≥ 70 y | 2.13 | 1.16-3.90 | 0.014 |
| Anemia (Hg < 110 g/L) | 2.20 | 1.14-4.27 | 0.019 |
| Diabetes | 1.91 | 1.07-3.42 | 0.029 |
| Hypertension | 1.24 | 0.68-2.25 | 0.49 |
| Male sex | 0.96 | 0.54-1.72 | 0.90 |
| Early repatriation | 0.84 | 0.49-1.44 | 0.53 |
| Previous MI | 0.82 | 0.32-2.13 | 0.69 |
| CKD (eGFR < 60 mL/min/m2) | 0.77 | 0.38-1.55 | 0.46 |
| Dyslipidemia | 0.58 | 0.33-1.05 | 0.07 |
| EBM | 0.52 | 0.27-1.02 | 0.06 |
CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; EBM, evidence-based medication; eGFR, estimated glomerular filtration rate; Hg, hemoglobin; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction; OR, odds ratio.
Table 6.
Independent predictors for 1-year mortality in stable STEMI patients
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Anemia (Hg < 110 g/L) | 3.32 | 1.35-8.21 | 0.009 |
| Increased LVEDP/CHF | 2.38 | 1.05-5.44 | 0.039 |
| Age ≥ 70 y | 2.31 | 0.86-6.19 | 0.10 |
| CKD (eGFR < 60 mL/min/m2) | 2.06 | 0.80-5.31 | 0.13 |
| Multivessel disease | 1.83 | 0.77-4.33 | 0.17 |
| Hypertension | 1.48 | 0.56-3.95 | 0.43 |
| Male sex | 1.07 | 0.44-2.58 | 0.89 |
| Early repatriation | 1.03 | 0.44-2.40 | 0.95 |
| Diabetes | 0.86 | 0.33-2.27 | 0.77 |
| Previous MI | 0.81 | 0.22-3.04 | 0.76 |
| Dyslipidemia | 0.79 | 0.33-1.90 | 0.59 |
| EBM | 0.37 | 0.15-0.89 | 0.027 |
CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; EBM, evidence-based medications; eGFR, estimated glomerular filtration rate; Hg, hemoglobin; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction; OR, odds ratio.
In the subcohort of repatriated patients, multivariate analysis suggested that transfer to cardiologist-based vs internist-based centres was not associated with 30-day (OR 1.07, 95% CI 0.45-2.54; P = 0.87) or 1-year (OR 1.17, 95% 0.55-2.50; P = 0.69) primary composite outcomes (Supplemental Tables S3 and S4). Independent predictors of 30-day primary composite outcome in this subcohort of patients included dyslipidemia (OR 0.35, CI 0.13-0.94; P = 0.038), increased LVEDP/CHF (OR 3.74, CI 1.64-8.51; P = 0.002), and anemia (OR 5.40, CI 2.12-13.78; P < 0.001) (Supplemental Table S3). Similarly, characteristics independently associated with 1-year primary composite outcomes included dyslipidemia (OR 0.36, 95% CI 0.15-0.87; P = 0.022), age ≥ 70 years (CI 2.45, CI 1.05-5.71; P = 0.038), multivessel disease (OR 2.46, CI 1.14-5.31; P = 0.022), anemia (OR 3.63, CI 1.52-8.63; P = 0.004), and increased LVEDP/CHF (OR 4.08, CI 1.95-8.52; P < 0.001) (Supplemental Table S4).
Discussion
In this study, early repatriation was found to be safe regarding short-and long-term outcomes in a population of stable STEMI patients. Furthermore, in the subset of patients repatriated, transfers to hospitals with cardiologist- vs internist-based care were associated with similar outcomes. These findings suggest that stable STEMI patients after PPCI may be safely managed in lower-acuity settings.
When assessing short-term outcomes, these results are in concordance with previous studies, including an earlier cohort of STEMI patients from within the Fraser Health Authority, which showed repatriation to be safe based on similar in-hospital and 30-day mortality rates between repatriated and nonrepatriated patients (Table 7). The present study extends that analysis to suggest the safety of early repatriation regarding longer-term outcomes. Similar short- and long-term outcomes observed between repatriated and nonrepatriated STEMI patients may stem from the fact that most patients in both groups of our study had successful revascularisation and received EBMs at discharge. Successful revascularisation, defined by TIMI 3 flow in the culprit artery, was documented in more than 90% of patients in both repatriated and nonrepatriated cohorts. Evidence from both clinical trial substudies and STEMI registries have noted that restoration of TIMI 3 flow in PPCI portends to good short- and long-term prognosis.8, 9, 10, 11 In the present study, both repatriated and nonrepatriated groups had a similarly high (> 80%) rate of discharge with EBMs, which also has been associated with reduced short- and long-term mortality after STEMI.12,13 Combined, the high frequency of successful revascularisation and EBMs at discharge likely resulted in 2 cohorts with similarly low event rates.
Table 7.
Summary of studies examining early repatriation
| Authors | Definition of early repatriation | Median time of repatriation | Frequency of early repatriation | STEMI period | Total population | Exclusion | Outcomes |
|---|---|---|---|---|---|---|---|
| Chan et al.4 | “Immediate” (time not specified) | Not specified | 81.2% (among transfer-centre presentation subcohort) | June 1, 2003–June 30, 2007 | 1479 | Prolonged cardiac arrest without neurologic recovery | In-hospital mortality: PPCI-centre presentation 4.0% vs transfer-centre presentation 3.7% (P = 0.87). Transfer subcohort in-hospital mortality: repatriated 11.5% vs nonrepatriated 1.9% (P < 0.001) |
| Ting et al.5 | ≤ 24 h | 21.8 h | Early repatriation 65.2% (total repatriation 83.2) | January 1, 2008–June 2014 | 979 | Death ≤ 72 h | Repatriation index-admission mortality (OR 0.46; P = 0.15) |
| Abuzeid et al.6 | Transfer at any point during PPCI hospitalisation | ∼1.5 d | 55% | January 1, 2010–December 31, 2012 | 860 | Shock/death ≤ 24 h after hospitalization | 30-day mortality: repatriated 2.8% vs nonrepatriated 5.1%, (P = 0.08). 1-year mortality: repatriated 6.7% vs nonrepatriated 5.6% (P = 0.545). 30-day MI readmission: repatriated 5.8% vs nonrepatriated 2.0% (P = 0.003). 1-year MI readmission: repatriated 12.1% vs nonrepatriated 5.8% (P = 0.02) |
OR, odds ratio; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
The long-term safety of early repatriation in STEMI patients demonstrated in our study diverges from one earlier analysis. Abuzeid et al. noted higher rates of readmission for MI at 1 year among repatriated vs nonrepatriated STEMI patients in a propensity-matched cohort study6 (Table 7). Those authors observed that MI-related readmission curves appeared to diverge between the 2 groups over the 1-year follow-up period. Although our study did not examine MI specifically as a primary end point or in multivariate analysis, the rates of composite outcomes, including ischemic events, were similar between repatriated and nonrepatriated STEMI patients at both 30 days and 1 year. In fact, there was a numerically higher rate of unadjusted MI among nonrepatriated patients at 1 year (Table 2). Differences between our analysis and the previous study may be attributed in part to the populations examined. Our patient population consisted solely of stable STEMI patients who were repatriated within 4 hours after undergoing PPCI. These restrictions may have led to the exclusion of those with 1) more complex coronary anatomies or unsuccessful PCIs where target lesion revascularisation rates may be higher,11,14 and 2) acute complications of higher-risk STEMI (eg, bleeding, cerebrovascular accidents, hypotension, etc), which may have independent prognostic effects as well as affecting the initiation of EBMs, resulting in worsened long-term ischemic outcomes.15, 16, 17, 18 The authors from the previous study had postulated incomplete revascularisation as a possible reason for higher rates of MI among the nonrepatriated. Although completeness of revascularisation was not assessed in our study, a policy of PCI for treatment of residual coronary artery disease had already been adopted in the Fraser Health Authority during this time period, based on the results of landmark randomised trials published before this study’s onset.19, 20, 21 Finally, our analysis was not propensity matched and included fewer patients than the above study, and therefore it may have had inherent biases not accounted for or inadequate power to detect differences in specific ischemic outcomes such as MI.
Repatriation of stable STEMI patients to centres with cardiologist- vs internist-based care was associated with similar outcomes in this study. These results contrast with previous studies where treatment of MI directed by cardiologists compared with noncardiologists demonstrated reduced in-hospital and 1-year standardised mortality rates, as well as shorter lengths of stay.22, 23, 24, 25 In a contemporary Canadian study, admission of MI patients to a cardiology service vs noncardiology service was associated with reductions in both adjusted 30-day and 4-year mortality, partly driven by underutilisation of cardiac catheterisation, reduced electrocardiographic surveillance, and underuse of EBMs among noncardiologists.25 Similarly, when exclusively examining STEMI patients studied before the routine use of PPCI, treatment in hospitals with cardiology compared with general internist departments was associated with reduced adjusted in-hospital mortality. These results were again attributed to the increased use of cardiac catheterisation and EBMs in hospitals with cardiology departments.26 However, those studies suggesting outcome benefit with cardiologist-based care involved MI patients being treated from the time of initial hospital admission rather than in the post-PPCI setting. In this patient population, the impact of subspecialty care may have been reduced because revascularisation after STEMI diagnosis had taken place in all of the included patients. In addition, an assessment and therapeutic plan, including introduction of EBMs, had been initiated by the interventional cardiologist at the PPCI centre. Finally, because our study involved a health authority with a high annual STEMI burden and an associated repatriation program more than a decade old, general internist experience with STEMI care had increased greatly with time, likely contributing to similarities in outcomes among differing specialties.
Historically, STEMI patients were routinely admitted to specialised CCUs for intensive monitoring and treatment of potential ventricular arrhythmias, which were more common before the era of rapid reperfusion.27 Access to higher-level CCUs, capable of providing care for patients requiring mechanical ventilation and mechanical circulatory support, have traditionally been restricted to PPCI or tertiary care centres. Despite the notable reductions in STEMI-related complications with PPCI, there remains discordance in current guidelines regarding the need for CCU admission for lower risk STEMI populations.28,29 In Canada, contemporary interprovincial analysis suggests that more than two-thirds of all STEMI patients receive CCU care.30 However, owing to the significant critical care strain and health care cost demands of this approach, several studies have examined outcomes associated with lower-acuity care for stable STEMI patients in whom critical care therapies may not be required. In a retrospective case-control analysis, there were no differences in 1-year mortality among uncomplicated STEMI patients admitted to a step-down unit vs CCU after PPCI.31 In 2 distinct Canadian health authorities examining stable STEMI cohorts, 2% to 5% of patients had adverse events during or after PPCI that would mandate care in a CCU.32,33 Among a national registry of STEMI patients ≥ 65 years old in the United States, only ∼ 16% of hemodynamically stable individuals developed complications requiring higher-level care despite > 90% being admitted to a CCU.34 Our study further demonstrates the safety of rapid deescalation of care for stable STEMI patients after PPCI to lower-acuity and less resource-dependent settings, as well as extending previous findings by suggesting that this care can occur in non-PPCI centres.
Limitations
The patients included in this analysis were stable, and therefore our results should not be extended to a more complex, wider spectrum of MI population. The importance of having a cardiac catheterisation laboratory, cardiologists on service, and additional higher-level medical services may be greater for higher-risk unstable patients. The distribution of other in-hospital complications affecting long-term outcomes, such as bleeding or acute kidney injury, among repatriated and nonrepatriated patients was not tracked, possibly affecting analysis or interpretation of our results. In this study, it was not possible to determine whether patients discharged from internist-based centres received follow-up with cardiologists, thereby concealing potential differences in long-term outcomes attributed to medical speciality. It should be noted that both internist- and cardiologist-based hospitals have cardiac rehabilitation services, although the frequency of STEMI patients utilising these services was not tracked. In addition, postdischarge measures of medical adherence associated with long-term outcomes after MI, such as proportion of days covered, were not readily available for assessment. Finally, owing to the study’s smaller size, these results may be viewed more as qualitative or hypothesis generating. Larger and preferably randomised studies would be required for assessing the definitive safety of early repatriation after PPCI.
Conclusion
This study demonstrates the short- and long-term safety of early repatriation after PPCI for stable STEMI patients. Outcomes for repatriated patients did not appear to differ based on transfer to hospitals with cardiologist- vs internist-based care. Overall, these results suggest that stable STEMI patients may be managed in lower-acuity settings after PPCI across hospitals throughout a regional health authority. Analysis of only stable STEMI patients in this study prevents the generalisability of these results to broader MI populations.
Acknowledgements
The authors thank the Royal Columbian Hospital STEMI database department for their efforts.
Ethics Statement
This study has adhered to the relevant ethical guidelines.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This was a retrospective analysis using de-identified data and therefore patient consent was not required.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1297 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjco.2024.07.010.
Supplementary Material
References
- 1.Grines C.L., Browne K.F., Marco J., et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med. 1993;328:673–679. doi: 10.1056/NEJM199303113281001. [DOI] [PubMed] [Google Scholar]
- 2.Zijlstra F., de Boer M.J., Hoorntje J., et al. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 3.Huynh T., Perron S., O’Loughlin J., et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction: bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation. 2009;119:3101–3109. doi: 10.1161/CIRCULATIONAHA.108.793745. [DOI] [PubMed] [Google Scholar]
- 4.Chan A.W., Bakar S.N., Brown R.I., et al. In-hospital outcomes of a regional ST-segment elevation myocardial infarction acute transfer and repatriation program. Can J Cardiol. 2011;27:664.e1–664.e8. doi: 10.1016/j.cjca.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 5.Ting R., Tejpal A., Finken L., et al. Repatriation to referral hospital after reperfusion of STEMI patients transferred for primary percutaneous coronary intervention: insights of a Canadian regional STEMI care system. Am Heart J. 2016;177:145–152. doi: 10.1016/j.ahj.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Abuzeid W., Bennell M., Qiu F., et al. Clinical outcomes of early repatriation for patients with ST-elevationn myocardial infarction. Can J Cardiol. 2014;30:S66–S67. doi: 10.1016/j.cjca.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 7.O’Gara P.T., Kushner F.G., Ascheim D.D., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Brener S.J., Moliterno D.J., Aylward P.E., et al. Reperfusion after primary angioplasty for ST-elevation myocardial infarction: predictors of success and relationship to clinical outcomes in the APEX-AMI angiographic study. Eur Heart J. 2008;29:1127–1135. doi: 10.1093/eurheartj/ehn125. [DOI] [PubMed] [Google Scholar]
- 9.Brener S.J., Mehran R., Brodie B.R., et al. Predictors and implications of coronary infarct artery patency at initial angiography in patients with acute myocardial infarction (from the CADILLAC and HORIZONS-AMI trials) Am J Cardiol. 2011;108:918–923. doi: 10.1016/j.amjcard.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Cura F.A., l’Allier P.L., Kapadia S.R., et al. Predictors and prognosis of suboptimal coronary blood flow after primary coronary angioplasty in patients with acute myocardial infarction. Am J Cardiol. 2001;88:124–128. doi: 10.1016/s0002-9149(01)01605-8. [DOI] [PubMed] [Google Scholar]
- 11.Kammler J., Kypta A., Hofmann R., et al. TIMI 3 flow after primary angioplasty is an important predictor for outcome in patients with acute myocardial infarction. Clin Res Cardiol. 2009;98:165–170. doi: 10.1007/s00392-008-0735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K., Han S., Lee M., et al. Evidence-based optimal medical therapy and mortality in patients with acute myocardial infarction after percutaneous coronary intervention. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.121.024370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasevic D., El Khoury C., Subtil F., et al. Effect of optimal medical therapy at discharge in patients with reperfused ST-segment elevation myocardial infarction on 1-year mortality (from the Regional RESCUE Registry) Am J Cardiol. 2018;121:403–409. doi: 10.1016/j.amjcard.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Magro M., Nauta S., Simsek C., et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: the MI SYNTAXscore study. Am Heart J. 2011;161:771–781. doi: 10.1016/j.ahj.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Mehran R., Pocock S., Nikolsky E., et al. Impact of bleeding on mortality after percutaneous coronary intervention: results from a patient-level pooled analysis of the REPLACE-2 (Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trials. JACC Cardiovasc Interv. 2011;4:654–664. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Mouhat B., Putot A., Hanon O., et al. Low systolic blood pressure and mortality in elderly patients after acute myocardial infarction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwok C.S., Kontopantelis E., Myint P.K., et al. Stroke following percutaneous coronary intervention: type-specific incidence, outcomes and determinants seen by the British Cardiovascular Intervention Society 2007-12. Eur Heart J. 2015;36:1618–1628. doi: 10.1093/eurheartj/ehv113. [DOI] [PubMed] [Google Scholar]
- 18.Alkhouli M., Alqahtani F., Tarabishy A., Sandhu G., Rihal C.S. Incidence, predictors, and outcomes of acute ischemic stroke following percutaneous coronary intervention. JACC Cardiovas Interv. 2019;12:1497–1506. doi: 10.1016/j.jcin.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Gershlick A.H., Khan J.N., Kelly D.J., et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits P.C., Abdel-Wahab M., Neumann F.-J., et al. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- 21.Engstrøm T., Kelbæk H., Helqvist S., et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–671. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 22.Frances C.D., Go A.S., Dauterman K.W., et al. Outcome following acute myocardial infarction: are differences among physician specialties the result of quality of care or case mix? Arch Intern Med. 1999;159:1429–1436. doi: 10.1001/archinte.159.13.1429. [DOI] [PubMed] [Google Scholar]
- 23.Casale P.N., Jones J.L., Wolf F.E., Pei Y., Eby L.M. Patients treated by cardiologists have a lower in-hospital mortality for acute myocardial infarction. J Am Coll Cardiol. 1998;32:885–889. doi: 10.1016/s0735-1097(98)00325-8. [DOI] [PubMed] [Google Scholar]
- 24.Roe M.T., Chen A.Y., Mehta R.H., et al. Influence of inpatient service specialty on care processes and outcomes for patients with non–ST-segment elevation acute coronary syndromes. Circulation. 2007;116:1153–1161. doi: 10.1161/CIRCULATIONAHA.107.697003. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill D.E., Southern D.A., Norris C.M., et al. Acute coronary syndrome patients admitted to a cardiology vs noncardiology service: variations in treatment and outcome. BMC Health Serv Res. 2017;17:354. doi: 10.1186/s12913-017-2294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottwik M., Zahn R., Schiele R., et al. Differences in treatment and outcome of patients with acute myocardial infarction admitted to hospitals with compared to without departments of cardiology. Results from the pooled data of the Maximal Individual Therapy in Acute Myocardial Infarction (MITRA 1+2) registries and the Myocardial Infarction Registry (MIR) Eur Heart J. 2001;22:1794–1801. doi: 10.1053/euhj.2001.2630. [DOI] [PubMed] [Google Scholar]
- 27.Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet. 1998;352:1771–1774. doi: 10.1016/S0140-6736(98)03212-7. [DOI] [PubMed] [Google Scholar]
- 28.Ibanez B., James S., Agewall S., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 29.Antman E.M., Anbe D.T., Armstrong P.W., et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) J Am Coll Cardiol. 2004;44:e1–e211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 30.van Diepen S., Lin M., Ezekowitz J.A., et al. Interprovincial differences in Canadian coronary care unit resource use and outcomes. Can J Cardiol. 2017;33:166–169. doi: 10.1016/j.cjca.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Chou Y.-S., Lin H.-Y., Weng Y.-M., et al. Step-down units are cost-effective alternatives to coronary care units with noninferior outcomes in the management of ST-elevation myocardial infarction patients after successful primary percutaneous coronary intervention. Intern Emer Med. 2020;15:59–66. doi: 10.1007/s11739-019-02037-z. [DOI] [PubMed] [Google Scholar]
- 32.Amon J., Wong G.C., Lee T., et al. Incidence and predictors of adverse events among initially stable ST-elevation myocardial infarction patients following primary percutaneous coronary intervention. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.025572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caddell A., Belliveau D., Moeller A. Stable patients with STEMI rarely require intensive-care-level therapy after primary PCI. CJC Open. 2022;4:390–394. doi: 10.1016/j.cjco.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shavadia J.S., Chen A.Y., Fanaroff A.C., et al. Intensive care utilization in stable patients with ST-segment elevation myocardial infarction treated with rapid reperfusion. JACC Cardiovasc Interv. 2019;12:709–717. doi: 10.1016/j.jcin.2019.01.230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.