Abstract
Background
Clinically important perioperative atrial fibrillation (POAF) is a common cardiac complication after noncardiac surgery. Little is known about how patients with POAF are managed acutely and whether practices have changed over time.
Methods
We conducted an observational substudy of patients who had POAF, were at elevated cardiovascular risk, and were enrolled in the PeriOperative Ischemic Evaluation (POISE)-1, 2 and 3 trials between 2002 and 2021. POAF was defined as new, clinically important atrial fibrillation occurring within 30 days after surgery. We assessed the use of rhythm-control and anticoagulation treatment in response to POAF, at hospital discharge and at 30 days after surgery. We assessed for temporal trends using multivariable logistic regression.
Results
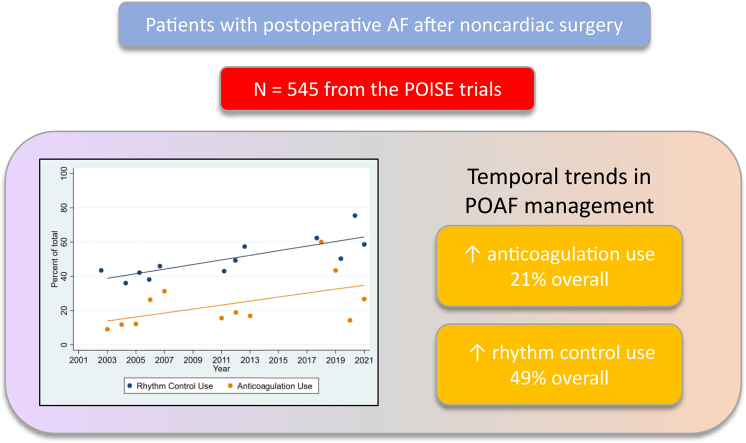
Of the 27,896 patients included, 545 (1.9%) developed clinically important POAF. Patients received rhythm-control treatment in 48.6% of cases. The level of use of rhythm-control treatment increased over the course of the trials (POISE-1 vs POISE-2 vs POISE-3; 40.9% vs 49.5% vs 59.1%). A later randomization date was associated independently with use of rhythm-control treatment (odds ratio, 1.05 per year; 95% confidence interval, 1.01-1.09). Anticoagulation treatment was prescribed in 21% of POAF cases. The level of anticoagulation treatement use was higher in POISE-3, compared to that in the 2 previous trials (POISE-1 vs POISE-2 vs POISE-3—16.4% vs 16.5% vs 33.6%). A later randomization date was associated independently with use of anticoagulation treatment (odds ratio, 1.06 per year; 95% confidence interval, 1.02-1.11).
Conclusions
Despite the absence of randomized controlled trials, the level of use of rhythm-control and anticoagulation treatment for POAF is rising. High-quality trials are needed urgently to determine whether these interventions are safe and effective in this population.
Graphical abstract
Résumé
Contexte
En chirurgie non cardiaque, la fibrillation auriculaire postopératoire (FAPO) d’importance clinique est une complication cardiaque fréquente. La prise en charge ponctuelle des patients présentant une FAPO est mal connue et on ne sait pas précisément si les pratiques ont évolué au fil du temps.
Méthodologie
Nous avons mené une sous-étude d’observation auprès de patients ayant présenté une FAPO et dont le risque cardiovasculaire était élevé, et qui avaient participé aux études 1, 2 ou 3 du programme d’essais cliniques POISE (PeriOperative Ischemic Evaluation) entre 2002 et 2021. La FAPO était définie comme un nouvel épisode de fibrillation auriculaire (FA) d’importance clinique au cours des 30 jours suivant une intervention chirurgicale. Nous avons évalué l’utilisation d’un traitement visant à régulariser le rythme cardiaque et de l’anticoagulothérapie dans la prise en charge d’une FAPO, au moment du congé de l’hôpital, puis 30 jours après l’intervention chirurgicale. Nous avons procédé à l’évaluation des tendances temporelles par régression logistique multivariée.
Résultats
Sur les 27 896 patients inclus dans l’analyse, 545 (1,9 %) ont présenté une FAPO d’importance clinique. Dans 48,6 % des cas, les patients ont reçu un traitement visant à régulariser le rythme cardiaque. Le taux d’utilisation d’un traitement visant à régulariser le rythme cardiaque a augmenté d’une étude à l’autre (étude POISE-1 vs étude POISE-2 vs étude POISE-3; 40,9 % vs 49,5 % vs 59,1 %, respectivement). Une date plus tardive de la répartition aléatoire était un facteur indépendant de l’utilisation d’un traitement visant à régulariser le rythme cardiaque (rapport de cotes = 1,05 par an; intervalle de confiance [IC] à 95 % : 1,01-1,09). Une anticoagulothérapie a été prescrite à 21 % des patients ayant présenté une FAPO. Le taux d’utilisation d’une anticoagulothérapie était plus élevé dans l’étude POISE-3, comparativement aux deux essais précédents (étude POISE-1 vs étude POISE-2 vs étude POISE-3; – 16,4 % vs 16,5 % vs 33,6 %, respectivement). Une date plus tardive de la répartition aléatoire était un facteur indépendant d’utilisation d’une anticoagulothérapie (rapport de cotes = 1,06 par an; IC à 95 % : 1,02-1,11).
Conclusions
Malgré l’absence d’essais comparatifs avec répartition aléatoire, le taux d’utilisation d’un traitement visant à régulariser le rythme cardiaque ou d’une anticoagulothérapie chez les patients présentant une FAPO est en augmentation. Il existe un besoin urgent de réaliser des études cliniques de grande qualité afin de déterminer si ces interventions sont sûres et efficaces dans cette population de patients.
Perioperative atrial fibrillation (POAF) is the most common arrhythmia after noncardiac surgery, with an incidence ranging between 2% and 3%.1 Patients with POAF have an elevated risk of postoperative stroke, myocardial infarction, and death.2,3 POAF also is associated with longer hospital stays and higher care costs.4 Although POAF is a serious complication after surgery, little is known about how clinicians choose to manage this condition.
No high-quality studies have assessed the effectiveness of different treatment strategies for managing new atrial fibrillation (AF) in the postoperative setting. Accordingly, many existing guidelines do not provide clear recommendations regarding the use of anticoagulation and the use of rate- and rhythm-control treatment in this population.5, 6, 7, 8 Exploring past and present practice patterns could inform the rationale and design of interventional trials in this population. We therefore conducted an observational analysis assessing temporal changes in the management practices of patients with clinically important POAF who were at elevated cardiovascular risk and had undergone noncardiac surgery over a period spanning 2 decades.
Materials and Methods
The Perioperative Ischemic Evaluation (POISE) trials
We undertook an observational substudy of patients with new POAF who participated in the POISE-1, POISE-2, and POISE-3 trials. The POISE-1 trial evaluated the effects of metoprolol vs placebo on perioperative cardiovascular complications; 8351 patients were recruited between October 2002 and July 2007.9 The POISE-2 trial evaluated the effects of aspirin vs placebo, and clonidine vs placebo, on perioperative cardiovascular complications; 10,010 patients were recruited between July 2010 and December 2013.10,11 The POISE-3 trial evaluated the effects of tranexamic acid vs placebo, and a hypertension-avoidance strategy vs a hypotension-avoidance strategy, on vascular events and bleeding; 9535 patients were recruited between June 2018 and July 2021.12,13 Patients enrolled in the trials were aged ≥ 45 years, either had or were at elevated risk of developing cardiovascular disease, and were planning to undergo noncardiac surgery requiring at least an overnight hospital stay. The complete list of eligibility criteria for each trial is provided in the Supplemental Methods.
Follow-up
All patients enrolled in the POISE trials were followed throughout their hospitalization and were contacted 30 days after randomization. The 30-day follow-up period was completed in 99.8%, 99.9%, and 99.9% of patients, in the POISE-1, POISE-2, and POISE-3 trials, respectively.
Eligibility criteria
Patients were eligible for inclusion in this substudy if they were enrolled in one of the POISE trials and had been diagnosed with new POAF during the follow-up period. All patients with POAF were included in the analyses pertaining to the use of rate- and rhythm-control treatment. For the analyses pertaining to anticoagulation treatment use, we excluded patients with POAF if they were on therapeutic anticoagulation treatment before surgery, had missing data on preoperative anticoagulation treatment use, or had no available data on postoperative anticoagulation treatment use.
POAF
New, clinically important POAF was an outcome in all 3 trials, and data were reported systematically on case-report forms specific to the diagnosis, throughout the 30-day follow-up period. In all 3 trials, new, clinically important POAF was defined as new-onset AF occurring within 30 days after surgery, and resulted in angina, symptomatic hypotension, and/or congestive heart failure, or required treatment with a rate-controlling drug, an antiarrhythmic drug, and/or electrical cardioversion. The same definition was used in previous analyses of the POISE trials that demonstrated the prognostic and predictive importance of POAF.3,14
Use of rhythm- and rate-control treatment
We defined the use of rhythm-control treatment as the initiation and/or dose increase of a rhythm-controlling drug and/or the use of electrical cardioversion. All other patients, including those receiving neither rate- nor rhythm-control drugs, were classified as having received rate-control treatment. The use of rate-controlling drugs, rhythm-controlling drugs, and electrical cardioversion in response to POAF were recorded on case-report forms. The eligible rate- and rhythm-controlling drugs were not defined explicitly on the forms. For participants enrolled in the POISE-1 trial, we did not consider the use of study metoprolol when classifying patients as having received either rate- or rhythm-control treatment.
Anticoagulation treatment use
In the POISE-1 trial, anticoagulation treatment use was recorded only on case-report forms at the time of hospital discharge. In the POISE-2 and POISE-3 trials, anticoagulation treatment use was recorded on case-report forms both at the time of hospital discharge and at the 30-day follow-up visit. We defined the initiation of anticoagulation treatment as the use of a therapeutically dosed anticoagulant after the date of the first occurrence of POAF, as recorded on case-report forms. Therapeutically dosed anticoagulation treatment was defined as the use of a vitamin-K antagonist, a non–vitamin K oral anticoagulant (NOAC), or a parenteral anticoagulant prescribed at therapeutic doses. All other patients were classified as having no anticoagulation treatment. For episodes of POAF occurring prior to hospital discharge, we used medication data at the time of discharge whenever possible. We used medication data collected at 30 days after randomization, if discharge medication data were unavailable or if the first POAF episode occurred after hospital discharge.
Statistical analyses
We compared the baseline characteristics between patients treated with rhythm- vs rate-control and anticoagulation vs no anticoagulation. We assessed the normality of the distribution of continuous variables using the Shapiro–Wilk test. We compared non-normally distributed continuous variables using the Wilcoxon rank-sum test. Categorical variables were compared using χ2 tests.
To visually assess for temporal trends, we created a scatterplot showing the proportion of patients receiving rhythm-control and anticoagulation treatment vs the year they were randomized into one of the POISE trials. We drew lines of best fit to illustrate the associations between use of each treatment and the randomization year. When fewer than 10 patients were enrolled in a given year, patients were aggregated into the adjacent year during which enrollment had taken place over the full year.
We used multivariable logistic regression models to assess for the presence of temporal trends. We conducted analyses comparing the levels of use of rhythm- vs rate-control treatment, and anticoagulation vs no anticoagulation treatment. For both analyses, the main independent variable of interest was the patient’s randomization date. We followed the rule of thumb of limiting the number of predictor variables to 1 per every 10 outcome events.15 Both models were adjusted for age, sex, congestive heart failure, diabetes, hypertension, stroke or transient ischemic attack, vascular disease, and preoperative antiplatelet use. The multivariable model comparing the levels of rhythm- vs rate-control treatment use was adjusted additionally for a history of current or recent smoking, a preoperative serum creatinine level > 175 μmol/L, a patient having undergone urgent or emergent surgery, type of surgery, and preoperative use of cardiovascular medications (ie, beta-blocker, statin, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker).
For the rhythm- and rate-control treatment use analysis, we conducted a sensitivity analysis excluding patients assigned to the beta-blocker arm of the POISE-1 trial. However, given that the POISE trials were conducted with a blinded design, the possibility that study allocation influenced acute treatment decisions is unlikely. For the anticoagulation treatment use vs no anticoagulation treatment use analysis, we conducted sensitivity analyses excluding patients with a Congestive Heart Failure, Hypertension, Age (65 to 74 years: single-weight; over 75 years: double-weight), Diabetes, Stroke or Transient Ischemic Attack (Double-Weight), Vascular Disease, Female Sex (CHA2DS2-VASc) score of 0 or 1, patients assigned to the aspirin arm of the POISE-2 trial, and patients who experienced bleeding during the index hospitalization.
We used a complete case–analysis approach, without imputation for missing data. All statistical analyses were performed using STATA version 16.1 (StataCorp, College Station, TX). We considered findings with a P-value of < 0.05 to be statistically significant.
Consent and registration
The trials were registered at ClinicalTrials.gov (POISE-1, NCT00182039; POISE-2, NCT01082874; POISE-3, NCT03505723). All participating sites obtained ethical approval from institutional ethics boards before patient recruitment. All patients, or their substitute decision-makers, provided written informed consent.
Results
Rhythm- and rate-control treatment use
Of the 27,896 patients included in the 3 trials, 545 (1.9%) had newly detected, clinically important POAF (POISE-1, 2.5%; POISE-2, 1.9%; POISE-3, 1.4%). Patients were treated with rhythm control in 48.6% of cases. Of the 265 patients treated with rhythm control, 232 (88%) received an antiarrhythmic drug alone, 9 (3%) received electrical cardioversion alone, and 24 (10%) received both an antiarrhythmic drug and electrical cardioversion.
Baseline characteristics for the rhythm- and rate-control treatment groups are reported in Table 1. The use of rhythm-control treatment was more common in those who were younger (aged 73 vs 75 years; P = 0.002), those with diabetes (32% vs 24%; P = 0.05), those who had undergone urgent or emergent surgery (12% vs 6%; P = 0.01), and those who were using beta-blockers preoperatively (30% vs 19%; P < 0.001). Rhythm-control treatment use was less common in female patients (31% vs 42%; P = 0.009) and in those who had undergone orthopedic surgery (13% vs 31%; P = 0.005). Patients receiving rhythm-control treatment had lower CHA2DS2-VASc scores (3.0 vs 4.0; P = 0.004).
Table 1.
Baseline characteristics, stratified by rhythm- vs rate-control use
| Variable | Overall N = 545 |
Rhythm-control use N = 265 |
Rate-control use N = 280 |
P |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 74 (9) | 73 (9) | 75 (9) | 0.002 |
| Sex, female | 199 (37) | 82 (31) | 117 (42) | 0.009 |
| Comorbidities | ||||
| History of congestive heart failure | 43 (8) | 23 (9) | 20 (7) | 0.51 |
| History of hypertension | 432 (79) | 210 (79) | 222 (79) | 0.99 |
| History of diabetes on treatment | 152 (28) | 84 (32) | 68 (24) | 0.05 |
| History of TIA or stroke | 84 (15) | 35 (13) | 49 (18) | 0.17 |
| History of peripheral arterial disease | 114 (21) | 53 (20) | 61 (22) | 0.61 |
| History of coronary artery disease | 173 (32) | 76 (29) | 97 (35) | 0.14 |
| Preoperative serum creatinine > 175 μmol/L | 30 (6) | 17 (6) | 13 (5) | 0.37 |
| History of current or recent smoking | 124 (23) | 66 (25) | 58 (21) | 0.25 |
| Surgical characteristics | ||||
| Having undergone urgent and/or emergent surgery | 49 (9) | 32 (12) | 17 (6) | 0.01 |
| Orthopedic | 93 (17) | 33 (13) | 60 (31) | 0.005 |
| Thoracic | 75 (14) | 42 (15) | 33 (12) | 0.17 |
| Vascular | 150 (28) | 71 (27) | 79 (28) | 0.71 |
| General | 168 (31) | 92 (35) | 76 (27) | 0.06 |
| Preoperative medications | ||||
| Beta-blockers | 132 (24) | 79 (30) | 53 (19) | 0.003 |
| Statin | 252 (46) | 119 (45) | 133 (48) | 0.52 |
| ACE inhibitor or ARB | 298 (55) | 136 (51) | 162 (58) | 0.11 |
| Therapeutic anticoagulation | 44 (8) | 27 (10) | 17 (6) | 0.08 |
| Antiplatelet (any) | 196 (36) | 89 (34) | 107 (38) | 0.25 |
| Stroke risk scores | ||||
| CHADS2 | 2 (1) | 2 (1) | 2 (1) | 0.68 |
| CHA2DS2-VASc | 4 (1) | 3 (1) | 4 (2) | 0.004 |
Data other than P values are presented as n (%). Age and stroke risk scores (CHADS and CHADSVASc) are median/IQR values (IQR is q3-q1).
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CHADS2, congestive heart failure, hypertension, age over 75 years, diabetes, stroke or transient ischemic attack (double-weight). CHA2DS2-VASc, congestive heart failure, hypertension, age (65 to 74 years: single-weight; over 75 years: double-weight), diabetes, stroke or transient ischemic attack (double-weight), vascular disease, female sex; TIA, transient ischemic attack.
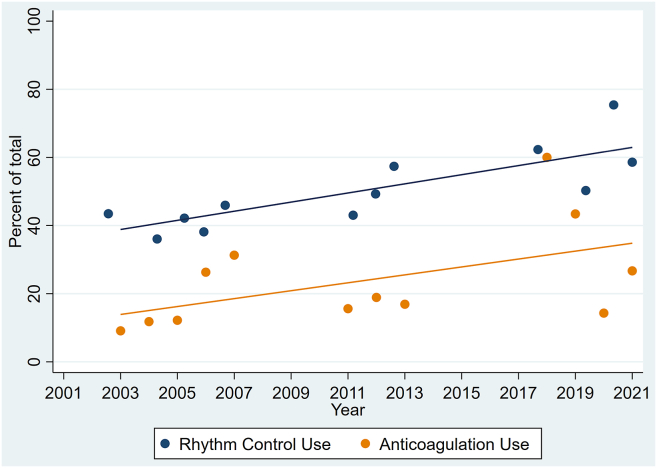
Figure 1 illustrates the temporal trend for rhythm-control treatment use. The use of rhythm-control treatment increased over the course of the 3 trials (POISE-1 vs POISE-2 vs POISE-3; 40.9% vs 49.5% vs 59.1%). The level of use of rhythm-control treatment was higher overall in South America (78%), Europe (63%), Asia (59%), and the US (59%), and was lower in Oceania (39%) and Canada (33%). Temporal trends generally remained consistent across geographic regions (Supplemental Fig. S1). An independent association occurred between having a later date of study randomization (ie, enrollment into 1 of the 3 trials) and the use of rhythm-control treatment (odds ratio [OR], 1.05 per year; 95% confidence interval [CI], 1.01-1.09; P = 0.02). The sensitivity analysis showed similar findings (Supplemental Table S3). A patient’s having undergone urgent or emergent surgery (OR, 3.03; 95% CI, 1.54-5.95; P = 0.001) and being of a younger age (OR, 0.98 per year; 95% CI, 0.95-1.00; P = 0.05) were also associated with their receipt of rhythm-control treatment (Supplemental Table S1).
Figure 1.
Temporal trends in the use of rhythm control and anticoagulation.
Anticoagulation treatment use
Of the patients with POAF, 473 were included in the analyses pertaining to anticoagulation treatment use. Patients with a history of preoperative anticoagulation treatment use (n = 44) or with missing medication data (n = 28) were excluded.
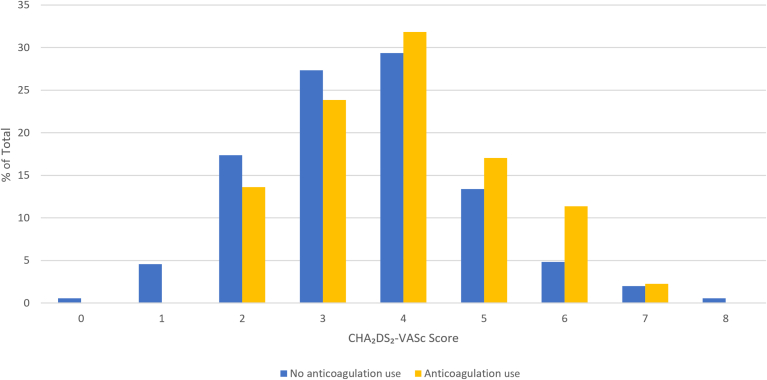
Overall, 21% of patients received therapeutic anticoagulation treatment. Table 2 presents the baseline characteristics for those patients who did and those who did not receive anticoagulation treatment. The level of anticoagulation treatment use was greater in those with a history of congestive heart failure (12% vs 6%; P = 0.03), a history of hypertension (88% vs 78%; P = 0.02), preoperative statin use (56% vs 44%; P = 0.04), and preoperative antiplatelet use (47% vs 35%; P = 0.02). The level of anticoagulation treatment use was lower in those who had undergone intrathoracic surgery (7% vs 17%; P = 0.03). The Congestive Heart Failure, Hypertension, Age over 75 years, Diabetes, Stroke or Transient Ischemic Attack (double-weight) (CHADS2 score; 2.0 vs 2.0; P = 0.03) and the CHA2DS2-VASc score (4.0 vs 3.5; P = 0.01) were higher in patients receiving anticoagulation, although a large overlap occurred in the distribution of CHA2DS2-VASc scores, as shown in Figure 2.
Table 2.
Baseline characteristics, stratified by anticoagulation use
| Characteristics | Overall N = 473 |
Anticoagulation use N = 99 |
No anticoagulation use N = 374 |
P |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 74 (9) | 75 (10) | 74 (10) | 0.96 |
| Sex, female | 172 (36) | 34 (37) | 138 (37) | 0.64 |
| Comorbidities | ||||
| History of congestive heart failure | 34 (7) | 12 (12) | 22 (6) | 0.03 |
| History of hypertension | 377 (80) | 87 (88) | 290 (78) | 0.02 |
| History of diabetes | 135 (29) | 35 (35) | 100 (27) | 0.09 |
| History of TIA or stroke | 70 (15) | 16 (16) | 54 (14) | 0.67 |
| History of peripheral arterial disease | 93 (20) | 24 (24) | 69 (18) | 0.20 |
| History of coronary artery disease | 152 (32) | 37 (37) | 115 (31) | 0.21 |
| Preoperative serum creatinine > 175 μmol/L | 23 (5) | 3 (3) | 20 (5) | 0.34 |
| History of current or recent smoking | 109 (23) | 18 (18) | 91 (24) | 0.20 |
| Surgical characteristics | ||||
| Having undergone urgent and/or emergent surgery | 41 (9) | 6 (6) | 35 (9) | 0.30 |
| Vascular | 127 (27) | 32 (32) | 95 (25) | 0.17 |
| Intra-abdominal | 141 (30) | 24 (24) | 117 (31) | 0.17 |
| Orthopedic | 85 (18) | 21 (21) | 64 (17) | 0.35 |
| Intrathoracic | 69 (15) | 7 (7) | 62 (17) | 0.02 |
| Preoperative medications | ||||
| Beta-blockers | 106 (22) | 26 (26) | 80 (21) | 0.30 |
| Statin | 220 (47) | 55 (56) | 165 (44) | 0.04 |
| ACE inhibitor or ARB | 259 (55) | 62 (63) | 197 (53) | 0.08 |
| Antiplatelet (any) | 177 (37) | 47 (48) | 130 (35) | 0.02 |
| Stroke risk scores | ||||
| CHADS2 score | 2 (1) | 2 (1) | 2 (1) | 0.03 |
| CHA2DS2-VASc score | 4 (1) | 4 (2) | 3.5 (1) | 0.01 |
Data other than P values are presented as n (%). Stroke risk scores (CHADS and CHADSVASc) are median/IQR values (IQR is q3-q1).
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CHADS2, congestive heart failure, hypertension, age over 75 years, diabetes, stroke or transient ischemic attack (double-weight); CHA2DS2-VASc, congestive heart failure, hypertension, age (65 to 74 years: single-weight; over 75 years: double-weight), diabetes, stroke or transient ischemic attack (double-weight), vascular disease, female sex; TIA, transient ischemic attack.
Figure 2.
Distribution of Congestive Heart Failure, Hypertension, Age (65 to 74 years: single-weight; over 75 years: double-weight), Diabetes, Stroke or Transient Ischemic Attack (double-weight), Vascular Disease, Female Sex (CHA2DS2-VASc) scores, stratified by anticoagulation use.
The proportion of patients treated with anticoagulation while concomitantly receiving one or more antiplatelet drugs (47%) was similar to the proportion who were not treated with anticoagulation (43%). Of the 49 patients who had POAF during their index hospitalization, who received anticoagulation treatment on discharge, and who had a 30-day follow-up visit, 30 (61%) remained on anticoagulation treatment at follow-up. Of the 161 patients who had POAF during their index hospitalization, received no anticoagulation treatment on discharge, and had a 30-day follow-up visit, 20 (12%) were receiving anticoagulation treatment at follow-up.
Temporal trends for anticoagulation treatment use are shown in Figure 1. The level of anticoagulation treatment use was higher in the POISE-3 trial, compared to that in the 2 previous trials (POISE-1 vs POISE-2 vs POISE-3; 16.4% vs 16.5% vs 33.6%). The level of anticoagulation use was higher overall in Europe (29%), Canada (27%), and the US (19%), and was lower in South America (12%), Oceania (11%), and Asia (9%). Temporal trends were generally consistent across geographic regions (Supplemental Fig. S2). A later date of study randomization (ie, enrollment into 1 of the 3 trials) was associated with receipt of anticoagulation treatment (OR, 1.06 per year; 95% CI, 1.02-1.11; P = 0.005; Supplemental Table S2). The sensitivity analyses showed similar findings (Supplemental Table S3).
Discussion
In this study of 545 patients with POAF after noncardiac surgery, we demonstrated that the level of use of rhythm-control and anticoagulation treatments has risen over the past 2 decades. Contemporary data from our analysis suggest that about 3 in 5 patients are now being treated with rhythm control, and about 1 in 3 patients are being treated with anticoagulation. These trends have appeared despite the absence of any direct data suggesting that the use of these strategies has a treatment benefit in the postoperative setting.
Unlike in nonoperative AF, no randomized trials have compared the efficacy and safety of rate- and rhythm-control treatment use in patients with POAF after noncardiac surgery. Despite the lack of available evidence, however, our findings suggest that the use of rhythm-control treatment is increasing steadily. Several reasons may account for these findings. First, as technological advances in surgical care progress rapidly, complex and urgent surgeries increasingly are being performed in those with multiple preexisting comorbidities.16 These patients are at higher risk of postoperative complications, and they therefore are more likely to be placed in a highly monitored postoperative bed, where intravenous antiarrhythmic drugs can be given readily, and electrical cardioversion can be performed. Second, one possibility is that more clinicians are choosing to use rhythm-control treatment to achieve faster conversion to sinus rhythm, as this may facilitate earlier discontinuation of continuous cardiac monitoring, and earlier hospital discharge. Finally, this trend may reflect an increasing interest in the use of rhythm-control treatment for nonoperative AF, which has followed in the wake of development of new antiarrhythmic drugs and more-effective ablation techniques.17
Currently, no randomized trials have evaluated whether anticoagulation treatment use is safe and effective in patients with POAF. Consequently, anticoagulation treatment use for stroke prevention in this population remains an unproven strategy. Nevertheless, we found that anticoagulation treatment use has become increasingly common in this population. The steady rise in anticoagulation treatment use for chronic nonoperative AF likely plays an important role in this observed trend.18,19 Another probable major contributor to the trend is the widespread proliferation of use of NOACs, which are safer and easier for patients to take vs vitamin K antagonists, and the fact that surgeons have become increasingly comfortable with the use of NOACs during the postoperative period. Additionally, as major studies demonstrating that POAF is associated with long-term stroke risk were published only within the past decade,20,21 a growing interest around the use of stroke-prevention strategies in this population may have developed. Consequently, an increase in physician knowledge regarding this issue may have contributed to the observed trends. Finally, guidelines from several international societies have been published regarding the level of anticoagulation treatment use in this population over the past decade,5,8,22, 23, 24 and these may have led to an increase in the level of use. However, whether our findings reflect a trend toward an increased level of use of anticoagulation treatment in the longer term remains unclear. Many patients in our study who received anticoagulation treatment at discharge did not continue taking the medication upon follow-up, and vice versa. These practice variabilities likely reflect the lack of high-quality evidence available regarding the long-term use of anticoagulation treatment in this population.
Given that anticoagulation treatment use is associated with higher risks of bleeding and is an unproven treatment, our findings raise concerns. Even if anticoagulation treatment can be effective in this population, observational data suggest that its level of efficacy may be lower than that observed with its application in chronic AF.6 Therefore, any potential benefits of anticoagulation treatment use could be outweighed by the negative impact of increased bleeding risks. Possibly, a higher-risk subset exists of patients with POAF who may derive greater benefit from receiving anticoagulation therapy. Observational data have suggested that POAF patients with higher CHADS2 and CHA2DS2VASc scores are at greater risk of stroke in the long-term.3,20 Despite these findings, however, we found only small differences in these traditional AF risk scores, for those who did vs did not receive anticoagulation treatment, suggesting that such scoring systems may not play a significant role in decision-making in this setting. Although no studies have explored the underlying factors influencing physicians' decisions to prescribe anticoagulation treatment in this population, our study did show that the level of use of anticoagulation treatment was much lower than that for rhythm-control treatment, even though guidelines have suggested that patients with > 48 hours of nonoperative AF require anticoagulation treatment prior to undergoing cardioversion.5,25,26 Possibly, our findings reflect the fact that many POAF episodes are brief in duration. Alternatively, physicians may choose not to use anticoagulation treatment, as its role for stroke prevention in this setting remains unproven. Whether additional factors, such as postoperative bleeding risk, AF burden, and postoperative timing of AF, should be considered when deciding whether anticoagulation treatment should be used remains unknown, and further high-quality evidence is needed to identify patients who will benefit from receiving anticoagulation therapy. Future studies also should record bleeding-risk scores, to elucidate their role in the decision-making process. The ongoing Anticoagulation for Stroke Prevention in Patients with Recent Episodes of Perioperative Atrial Fibrillation After Noncardiac Surgery (ASPIRE-AF) trial, which is randomizing patients with POAF to receive either an NOAC or no anticoagulation therapy, will provide high-quality data on whether anticoagulation treatment is safe and effective for patients who are at an elevated risk of stroke.27
A strength of our study is the use of a consistent and prognostically important definition of POAF across all 3 trials. POAF was a prespecified study outcome for all 3 trials and was recorded systematically across all 3 trials. Some limitations should be considered when interpreting the results of our study. First, only those patients who have or are at high risk of developing cardiovascular disease were included in the POISE trials. Whether the observed trends can be extrapolated appropriately to patients with fewer comorbidities is unknown, although anticoagulation treatment use in patients with AF usually is restricted to high-risk patients. Second, we did not collect detailed information on patient hemodynamics, which may have altered the decision to use rhythm-control treatment. Furthermore, changes may have occurred in the perioperative hemodynamic parameters over time, which could have led to changes in the incidence of POAF, but not in subsequent treatment decisions. Third, we did not collect data on AF duration or its recurrences. Possibly, the total burden of AF played a role in the use of different strategies, although no evidence indicates that such factors should be taken into consideration as part of decision-making. Fourth, AF-detection strategies likely differed across sites, which may have affected the incidence and characteristics of the POAF episodes detected. However, the main outcome for this analysis was clinically important POAF, which is mostly independent of POAF episodes that are detected by continuous monitoring but do not lead to symptoms or therapeutic interventions. Fifth, although categories of pharmacologic treatment for POAF were recorded systematically across the trials, data pertaining to individual medications were not collected. Sixth, medication data were missing for about 5% of patients in the anticoagulation analyses, which may have led to systematic biases. Seventh, for our temporal trend analyses, we were unable to adjust for additional perioperative complications (eg, sepsis) that could have affected the choice of treatment strategy for POAF. Finally, patients who experienced POAF that did not meet our criteria for clinical importance, including those with asymptomatic events detected on continuous cardiac monitoring, were not included in this analysis. Such patients plausibly may be managed differently than those who meet our study criteria for POAF. These patients should be investigated in future studies.
Conclusion
The level of use of rhythm-control and anticoagulation treatment for the early management of POAF is rising, despite the absence of high-quality studies to support the use of either modality. Additional data regarding the optimal management of POAF are needed urgently, and randomized controlled trials should be conducted to determine whether these treatment strategies are safe and effective in this population.
Acknowledgments
Ethics Statement
All participating sites obtained ethical approval from institutional ethics boards before patient recruitment. All patients or their substitute decision-makers provided written informed consent.
Patient Consent
The authors confirm that patient consent forms have been obtained for this article.
Funding Sources
POISE-1 received funding from the Canadian Institutes of Health Research, the Commonwealth Government of Australia's National Health and Medical Research Council, the Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo) in Spain, the British Heart Foundation, and AstraZeneca, who provided the study drug and funding for drug labelling, packaging, and shipping and helped support the cost of some national POISE investigator meetings. POISE-2 received funding from the Canadian Institutes of Health Research, the National Health and Medical Research Council of Australia, and the Spanish Ministry of Health and Social Policy. Bayer Pharma provided the aspirin used in the study, and Boehringer Ingelheim provided the clonidine and some funding. POISE-3 received funding from a Foundation Grant (FDN-143302) from the Canadian Institutes of Health Research, a Project Grant (1162362) from the Australian National Health and Medical Research Council, and a grant from General Research Fund 14104419, Research Grant Council, Hong Kong, and by the Population Health Research Institute.
Disclosures
M.K.W. is supported by the Physicians’ Services Incorporated (PSI) Foundation—Research Trainee Award. P.J.D. is a member of a research group that has a policy of not accepting honoraria or other payments from industry for its own personal financial gain. They do accept honoraria or payments from industry to support research endeavours and costs to participate in meetings. Based on study questions that P.J.D. originated, and grants he has written, he has received grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, BristolMyers Squibb, Cloud DX, Covidien, Octapharma Plasma, Philips Healthcare, Roche Diagnostics, Siemens, and Stryker. P.J.D. also has participated in an advisory board meeting for GlaxoSmithKline, and an expert panel meeting with AstraZeneca, Boehringer Ingelheim, and Roche Diagnostics. F.K.B. has received investigator-initiated research grants from Roche Diagnostics and Siemens. F.K.B. holds a Research Early Career Award from Hamilton Health Sciences. J.D.D. has received consulting fees from Servier, Leo Pharma, Fresenius Kabi, Pfizer, and CytoSorbents; speaking fees from Leo Pharma and Pfizer; research funding from the Canadian Institutes of Health Research; and royalties from UpToDate and Merck Manuals. W.F.M. has received consulting fees from TRIMEDX and AtriCure; and speaking fees from Bayer, Servier, and Eli Lilly and Company. E.P. is funded by a research contract (SLT017/20/000089) supported by the Department of Health of the Generalitat de Catalunya, Spain. A.B. has received lecture honoraria from Boehringer Ingelheim and Bristol-Myers Squibb; and research grants from Theravance Biopharma, Region Zealand (Denmark), the Canadian Institutes of Health Research, and the Danish Heart Foundation. J.S.H. has received research grants and speaking fees from Medtronic, Boston Scientific, and Bristol-Myers Squibb–Pfizer Alliance, with speaking and consulting fees from Bayer and Servier. D.C. has received research grants from the Canadian Institutes of Health Research; speaker fees from Servier; and advisory board fees from Roche Diagnostics and TRIMEDX. The other authors have no conflicts of interest to disclose.
Footnotes
See page 1370 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.08.003
Supplementary Material
References
- 1.McIntyre W.F., Vadakken M.E., Rai A.S., et al. Incidence and recurrence of new-onset atrial fibrillation detected during hospitalization for non-cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. 2021;68:1045–1056. doi: 10.1007/s12630-021-01944-0. [DOI] [PubMed] [Google Scholar]
- 2.Huynh J.T., Healey J.S., Um K.J., et al. Association between perioperative atrial fibrillation and long-term risks of stroke and death in noncardiac surgery: systematic review and meta-analysis. CJC Open. 2021;3:666–674. doi: 10.1016/j.cjco.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conen D., Alonso-Coello P., Douketis J., et al. Risk of stroke and other adverse outcomes in patients with perioperative atrial fibrillation 1 year after non-cardiac surgery. Eur Heart J. 2020;41:645–651. doi: 10.1093/eurheartj/ehz431. [DOI] [PubMed] [Google Scholar]
- 4.Bhave P.D., Goldman L.E., Vittinghoff E., Maselli J., Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164:918–924. doi: 10.1016/j.ahj.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade J.G., Aguilar M., Atzema C., et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang M.K., Heo R., Meyre P.B., et al. Anticoagulation use in perioperative atrial fibrillation after noncardiac surgery: a systematic review and meta-analysis. Swiss Med Wkly. 2023;153 doi: 10.57187/smw.2023.40056. [DOI] [PubMed] [Google Scholar]
- 7.Chyou J.Y., Barkoudah E., Dukes J.W., et al. Atrial fibrillation occurring during acute hospitalization: a scientific statement from the American Heart Association. Circulation. 2023;147:e676–e698. doi: 10.1161/CIR.0000000000001133. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 9.Devereaux P.J., Yang H., Yusuf S., et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 10.Devereaux P.J., Mrkobrada M., Sessler D.I., et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 11.Devereaux P.J., Sessler D.I., Leslie K., et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–1513. doi: 10.1056/NEJMoa1401106. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux P.J., Marcucci M., Painter T.W., et al. Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med. 2022;386:1986–1997. doi: 10.1056/NEJMoa2201171. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci M., Painter T.W., Conen D., et al. Hypotension-avoidance versus hypertension-avoidance strategies in noncardiac surgery. Ann Intern Med. 2023;176:605–614. doi: 10.7326/M22-3157. [DOI] [PubMed] [Google Scholar]
- 14.Alonso-Coello P., Cook D., Xu S.C., et al. Predictors, prognosis, and management of new clinically important atrial fibrillation after noncardiac surgery: a prospective cohort study. Anesth Analg. 2017;125:162–169. doi: 10.1213/ANE.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 15.Vittinghoff E., McCulloch C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 16.Moridzadeh R.S., Sanaiha Y., Madrigal J., et al. Nationwide comparison of the medical complexity of patients by surgical specialty. J Vasc Surg. 2021;73:683–688.e2. doi: 10.1016/j.jvs.2020.05.072. [DOI] [PubMed] [Google Scholar]
- 17.Camm A.J., Naccarelli Gerald V., Mittal S., et al. The increasing role of rhythm control in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79:1932–1948. doi: 10.1016/j.jacc.2022.03.337. [DOI] [PubMed] [Google Scholar]
- 18.Gorczyca-Głowacka I., Bielecka B., Wałek P., et al. Temporal trends in oral anticoagulant prescription in atrial fibrillation patients between 2004 and 2019. Int J Environ Res Public Health. 2022;19:5584. doi: 10.3390/ijerph19095584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko D., Lin K.J., Bessette L.G., et al. Trends in use of oral anticoagulants in older adults with newly diagnosed atrial fibrillation, 2010-2020. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.42964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gialdini G., Nearing K., Bhave P.D., et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312:616–622. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt J.H., Olesen J.B., Havers-Borgersen E., et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol. 2018;72:2027–2036. doi: 10.1016/j.jacc.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 23.Frendl G., Sodickson A.C., Chung M.K., et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148:e153–e193. doi: 10.1016/j.jtcvs.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Brieger D., Amerena J., Attia J., et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart, Lung Circ. 2018;27:1209–1266. doi: 10.1016/j.hlc.2018.06.1043. [DOI] [PubMed] [Google Scholar]
- 27.US National Library of Medicine Anticoagulation for Stroke Prevention In Patients With Recent Episodes of Perioperative Atrial Fibrillation After Noncardiac Surgery—The ASPIRE-AF Trial. https://clinicaltrials.gov/ct2/show/NCT03968393 Available at: Accessed xxx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.