Abstract
Background
Transitional care programs help improve continuity of care and post-discharge outcomes for frail older adults who are hospitalized. In this study, we examined the effectiveness of a transitional care model, based in a long-term care (LTC) home, on the functional independence of older hospitalized patients post-discharge.
Methods
We used a propensity-score matched cohort, whereby cases comprised patients who were admitted to a transitional care program—called the Sub-Acute Care for Frail Elderly (SAFE) Unit—following a hospitalization between March 1, 2018 and June 30, 2019. Controls were matched to Usual Care patients discharged from hospitals within the same health region and accrual period who did not receive transitional care in the SAFE Unit. Outcomes included acute care, LTC, and home care use within six-month post-discharge.
Results
Compared to Usual Care, SAFE Unit patients were less likely to be admitted into an LTC home (RR 0.44, 95% CI 0.23–0.86) within six months post-discharge. Additionally, on average, SAFE Unit patients spent 34 fewer days in LTC homes than controls. SAFE Unit patients also incurred significantly fewer home care service days (median: 52 days, IQR: 12–132 days) than Usual Care patients (median: 65.5 days, IQR: 19–158 days), particularly in terms of their reliance on general nursing and personal support. Both groups had similar risks of six-month hospital readmission and having an ED visit.
Conclusion
Rehabilitative and restorative-focused care provided through transitional programs, such as the SAFE Unit, have the potential to enable independent living for older hospitalized patients discharged to the community.
Keywords: transitional care, case-control studies, functional status, frailty, older adults
INTRODUCTION
In Canada, more than one-third of hospitalized seniors are considered frail, which leads them to be nearly three times more likely to be frequent users of hospital-based care and experience prolonged hospital stays of 30 days or longer.(1) They are also twice as likely to be readmitted to a hospital within 30 days of discharge.(1)
In Canada, patients who no longer require the intensity of services provided in hospital settings, but remain in a hospital awaiting discharge to a more appropriate care setting, are labelled as requiring alternate level of care or ALC.(2,3) With more than 35% of ALC patients comprising individuals who are 85 years of age or older, frail seniors with complex care needs are at the highest risk of being designated as ALC.(4) Prolonged hospital stays have been shown to be associated with poorer health outcomes in older patients(5) including functional decline,(6–8) increased rates of hospital-acquired infections,(9) increased stress and anxiety symptoms,(7,10) and feelings of social isolation.(11–13) Despite the deleterious effects of prolonged hospital stays, for many older adults their frailty presents a challenge to care continuity as there are often inadequate resources in the community to support patients who are medically complex.(14,15)
Transitional care—sometimes referred to as intermediate care, post-acute care, or sub-acute care—provides continuity between acute care settings and a patient’s primary residence in the community or in a congregate care setting.(16,17) These programs are often designed to help patients return to their homes by providing interdisciplinary care with a focus on functional ability and client-centred care planning.(18) Existing studies suggest transitional care with a focus on restorative and rehabilitative care has the potential to enable functional independence and avoid hospital readmissions and institutionalization in older adults.(16,18–21)
There have been increased investments in transitional care across Canada with the aim of supporting older adults’ return to home.(19,22,23) However, there have been few evaluations of transitional care programs in Canada. A recent scoping review found a limited number of studies conducted in Canada,(18) and the vast majority of studies focused on acute care-related outcomes (i.e., hospital readmission).(24) The effectiveness of transitional care on subsequent home care and long-term care (LTC) utilization, which are proxy measures for functional independence, is less known. This study aimed to examine the effectiveness of a Canadian-based restorative and integrated transitional care model on six-month use of acute care, home care, and admission to LTC (i.e., nursing) home following discharge from hospital.
METHODS
Setting
The Sub-Acute Care for the Frail Elderly (SAFE) Unit is a 0-bed transitional care unit located within Perley Health, a LTC home with 450 beds in Ontario, Canada. It is specifically designed for medically complex older adults, who are at a higher risk of deconditioning due to prolonged hospitalization(25) and require short-term (up to 30 days) restorative care.(26)
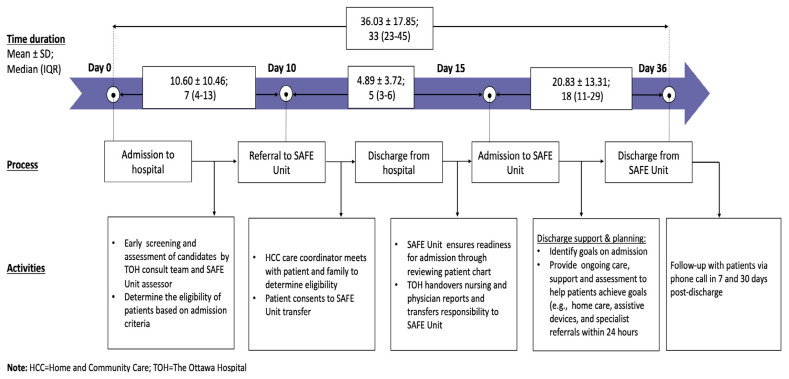
In partnership with The Ottawa Hospital (TOH), this unique model of care has three main features: proactive, restorative and rehabilitative, and collaborative and integrated approach to care. When older patients are admitted to TOH, there is proactive assessment and screening of their functional capacity and rehabilitation potential. The results are shared by the care team at TOH with the staff in the SAFE Unit to determine if patients meet the eligibility criteria and could benefit from the program.(25) Eligibility for admission to the SAFE Unit is determined by the interdisciplinary team at Perley Health. Criteria for admission include: (a) being at least 60 years old; (b) capable of bearing weight; (c) requiring and can benefit from interdisciplinary care;(27) and (d) having defined and achievable rehabilitative/restorative care goals within 30 days and a discharge plan. (28) Once patients are considered medically stable for discharge and transferred to the SAFE Unit, they will receive interdisciplinary care consisting of nursing care, geriatrics care, physiotherapy, and allied health care with a focus on function preservation and holistic care that may include, for example, therapeutic recreation and creative arts programs. (25) The collaboration between TOH and Perley Health is the final and core component enabling patients to access this continuum of medical care from an interdisciplinary clinical team, from hospital to LTC home. The typical trajectory of hospitalized older patients, from eligibility assessment while in the hospital to discharge from the SAFE Unit, is provided in Figure 1.
FIGURE 1.
The overall trajectory of patients who received care in the SAFE Unit
A previous study of the SAFE Unit by our research team focused on acute care and primary care use within 30 days post-discharge.(26) We demonstrated that frail older patients in the SAFE Unit had shorter length of stay in hospital and greater odds of being discharged to home. In this analysis, we extend the observation period to 180 days and include other important health-care outcomes, such as LTC home admission and the use of various types of home care services.
Study Design and Cohort
We designed a matched case-control study by linking patient-level data collected by the SAFE Unit team (for cases) to provincial-level health administrative data housed at ICES to identify controls. ICES is an independent and non-profit organization that houses population-level health and social data in Ontario. Cases included 154 patients admitted to the SAFE Unit post-discharge from a hospital stay between March 1, 2018 and June 30, 2019. Our control group comprised ALC (i.e., Usual Care) patients discharged from hospitals in the Champlain Local Health Integration Network (LHIN), the same health region where the SAFE Unit and Perley Health are situated. Usual Care patients were hospitalized within the same period as cases but did not receive transitional care in the SAFE Unit. Hospitalization records were derived from the Discharge Abstract Database (DAD).
To create the matched cohort, we first hard-matched the SAFE Unit and Usual Care patients based on their age and sex. Age and sex of Usual Care patients were derived from the Ontario Registered Persons Database (RPDB). Then, we generated propensity scores from a logistic regression model that included:
the Johns Hopkins Adjusted Clinical Group (ACG)(29) as an indicator of patients’ medical complexity. Data comprising ICD-10 codes in the DAD and diagnostic codes from the Ontario Health Insurance Plan (OHIP) physician billing database, based on services/visits provided in the two years prior to the index date, were pulled and collapsed into 32 Aggregated Diagnosis Groups (ADGs) using the Johns Hopkins ACG® software (Version 10.0; https://www.hopkinsacg.org/documents/version-10-0/), which was then used to create the ACGs;
geographic location of the patient’s primary residence, dichotomously defined as rural or urban, using postal code obtained from the RPDB;
the number of prior acute care admissions recorded in the DAD in the six months before index hospitalization; and
selected chronic conditions and diseases (congestive heart failure [CHF], chronic obstructive pulmonary disease [COPD], stroke, dementia, arrhythmia, lower respiratory tract infections [LRTIs]) known to be leading risk factors of hospital readmissions and LTC admission,(30,31) which were defined using validated algorithms and relevant diagnostic codes in ICES datasets (see Table 1).
TABLE 1.
Disease and other chronic conditions among SAFE Unit and Usual Care patients identified using algorithm developed in ICES databases
| Chronic Conditions | ICD-9 Codes a | ICD-10 Codes |
|---|---|---|
| Asthmae | 493 | J45 |
| Arrythmiaf | 427 (OHIP)b/427.3 (DAD)c | I48.0, I48.1 |
| Cancerf | 140–239 | C00–C26, C30–C44, C45–C97 |
| Congestive heart failuree | 428 | I500, I501, I509 |
| Chronic Obstructive Pulmonary Disorder (COPD)e | 491, 492, 496 | J41, J43, J44 |
| Coronary artery diseasef | 411–414 | I20, I22–I25 |
| Dementiad,e | 290, 331 (OHIP)/046.1, 290.0, 290.1, 290.2, 290.3, 290.4, 294, 331.0, 331.5, F331.82 (DAD) | F00–F03, G30 |
| Diabetese | 250 | E08–E13 |
| Hypertensione | 401–405 | I10–I13, I15 |
| Mental health disorderf | 291, 292, 295, 297, 298, 299, 301–307, 313, 314, 315, 319 | F04, F050, F058–F064, F07, F08, F10–F29, F340, F35–F37, F430, F439, F453, F454, F458, F46–F52, F531, F538, F539, F54–F67, F681, F688, F69–F92, F931, F932, F933, F938, F939, F94–F98 |
| Osteoarthritisf | 715 | M15–M19 |
| Osteoporosisf | 733 | M81, M82 |
| Renal failuref | 403, 404, 584, 585, 586, v451 | N17, N18, N19, T82.4, Z49.2, Z99.2 |
| Strokef | 430, 431, 432, 434, 436 | I60–I64 |
| Mood disorderf | 296, 300, 309, 311 | F30–F34, F38–F42, F43.1, F43.2, F43.8, F44, F45.0, F45.1, F45.2, F48, F53.0, F68.0, F93.0, F99 |
| Rheumatoid arthritisf | 714 | M05, M06 |
ICD = International Classification of Disease
OHIP = Ontario Health Insurance Plan; contains claims of Ontario’s residents under insurance coverage information from health care provider (e.g., physicians)
DAD = Discharge Abstract Database; contains demographic, clinical, and administrative information for inpatient hospital admissions
identified through prescription of Cholinesterase inhibitors in Ontario Drug Database (ODB), in addition to ICD codes; ODB contains prescribed drug claims for Ontario’s residents aged 65 years and older
We used nearest-neighbour matching to pair cases and controls using a caliper width of 0.2 standard deviations (SD).(47) We assessed the balance of characteristics between cases and controls by examining the distribution of measured baseline covariates (Table 2) and standardized differences. Standardized differences greater than 0.1 were interpreted as a potential imbalance in baseline characteristics between SAFE Unit and Usual Care patients.(47)
TABLE 2.
Baseline characteristics of SAFE Unit and Usual Care patients, following propensity score matching
| Characteristics | SAFE Unit, n(%) | Usual Care, n(%) | Standardized Differences | ||
|---|---|---|---|---|---|
|
| |||||
| N =154 | N = 154 | ||||
| Age (in years) | |||||
| Mean ± SD | 82.47 ± 9.03 | 82.47 ± 9.02 | 0.00 | ||
| 60–64 | 1–5a | (0.6–3.2) | 1–5a | (0.6–3.2) | 0.00 |
| 65–69 | 10–14a | (6.5–9.1) | 10–14a | (6.5–9.1) | 0.00 |
| 70–74 | 17 | (11.0) | 16 | (10.4) | 0.02 |
| 75–79 | 26 | (16.9) | 27 | (17.5) | 0.02 |
| 80–84 | 26 | (16.9) | 25 | (16.2) | 0.02 |
| 85–89 | 34 | (22.1) | 34 | (22.1) | 0.00 |
| 90+ | 36 | (23.4) | 37 | (24.0) | 0.02 |
|
| |||||
| Sex | |||||
| Female | 94 | (61.0) | 94 | (61.0) | 0.00 |
| Male | 60 | (39.0) | 60 | (39.0) | 0.00 |
|
| |||||
| Rural | |||||
| Yes | 1–5a | (0.6–3.2) | 1–5a | (0.6–3.2) | 0.04 |
| No | 149–153a | (96.8–99.4) | 149–153a | (96.8–99.4) | 0.04 |
|
| |||||
| Comorbidities | |||||
| Arrythmia | 37 | (24.0) | 37 | (24.0) | 0.00 |
| CHF | 86 | (55.8) | 84 | (54.5) | 0.03 |
| COPD | 51 | (33.1) | 47 | (30.5) | 0.06 |
| Dementia | 27 | (17.5) | 28 | (18.2) | 0.00 |
| Stroke | 15 | (9.7) | 8 | (5.2) | 0.08 |
|
| |||||
| ACG score | |||||
| Mean ± SD | 13.70 ± 3.24 | - | 13.78 ± 3.16 | - | 0.05 |
|
| |||||
| LRTI during index hospitalization | 49 | (31.8) | 47 | (30.5) | 0.03 |
|
| |||||
| Prior acute care admission (within 6 months prior to index) | |||||
| 0 | 100 | (64.9) | 102 | (66.2) | 0.03 |
| 1 | 36 | (23.4) | 34 | (22.1) | 0.03 |
| 2+ | 18 | (11.7) | 18 | (11.7) | 0.00 |
Results in these cells were suppressed due to the concern of re-identification.
CHF = Congestive Heart Failure; COPD = Chronic Obstructive Pulmonary Disease; ACG = Adjusted Clinical Group; LRTI = Lower Respiratory Tract Infection.
Baseline Characteristics
The RPDB was used to capture baseline sociodemographic information (i.e., sex and age, rurality of residence, and neighbourhood income quintile). In addition to the variables captured in the propensity score matching, we also reported the prevalence of other relevant conditions, and number of chronic conditions (i.e., 0–2, 4, 5, 5, 6, 7+) to reflect the level of multimorbidity in this population (see Table 3); these include asthma, cancer, coronary heart disease, diabetes, hypertension, mood disorder, osteoarthritis, osteoporosis, renal failure, rheumatoid arthritis. The prevalence of these chronic conditions was ascertained using validated algorithms and relevant diagnostic codes in ICES datasets (see Table 1).
TABLE 3.
Additional sociodemographic and health characteristics not used in propensity score matching
| Characteristics | SAFE Unit, n(%) | Usual Care, n(%) | Standardized Differences | ||
|---|---|---|---|---|---|
|
| |||||
| N=154 | N=154 | ||||
| Neighbourbood income quintile | |||||
| 1 | 34 | (22.1) | 29 | (18.8) | 0.08 |
| 2 | 42 | (27.3) | 31 | (20.1) | 0.17 |
| 3 | 33 | (21.4) | 37 | (24.0) | 0.06 |
| 4 | 16 | (10.4) | 23 | (14.9) | 0.14 |
| 5 | 29 | (18.8) | 34 | (22.1) | 0.08 |
|
| |||||
| Comorbidities | |||||
| Asthma | 39 | (27.4) | 37 | (33.2) | 0.03 |
| Cancer | 58 | (39.5) | 51 | (34.7) | 0.10 |
| Coronary heart disease | 68 | (46.3) | 67 | (42.1) | 0.01 |
| Diabetes | 65 | (42.1) | 61 | (44.2) | 0.05 |
| Hypertension | 138 | (89.5) | 140 | (88.4) | 0.04 |
| Mood disorder | 38 | (23.7) | 44 | (27.4) | 0.09 |
| Osteoarthritis | 123 | (80.0) | 123 | (82.6) | 0.00 |
| Osteoporosis | 23 | (15.8) | 20 | (21.1) | 0.06 |
| Renal failure | 83 | (50.5) | 62–66 | (40.3–42.9) | 0.25 |
| Rheumatoid arthritis | 11 | (7.4) | 1–5 | (0.6–3.2) | 0.25 |
|
| |||||
| Number of Prevalent Conditions | |||||
| 0–2 | 1–5 | (0.6–3.2) | 1–5 | (0.6–3.2) | 0.00 |
| 3 | 5–10 | (3.2–6.5) | 5–10 | (3.2–6.5) | 0.10 |
| 4 | 14 | (10.0) | 21 | (8.4) | 0.14 |
| 5 | 15 | (11.1) | 18 | (13.7) | 0.06 |
| 6 | 28 | (18.9) | 28 | (16.3) | 0.00 |
| 7+ | 89 | (54.7) | 76 | (56.3) | 0.17 |
|
| |||||
| Presence of Infections | 62 | (42.1) | 52 | (36.3) | 0.13 |
Outcomes
We captured health-care utilization of patients in the cohort who were alive for at least 180 days post-discharge, which included: emergency department (ED) visits using the National Ambulatory Care Reporting System; hospital readmissions using the DAD; follow-up consultations with family physicians using claims made to OHIP; LTC admission using records in the Continuing Care Reporting System; and home care visits using the Home Care Database, which were classified into all services, general or specialized nursing, personal support, and allied health care.
Statistical Analysis
We reported frequencies, proportions, means (SD), and medians (interquartile ranges [IQR]) to compare baseline characteristics and outcomes of interest between SAFE Unit and Usual Care patients. We calculated risk ratios (RRs) between SAFE Unit and Usual Care patients using McNemar paired Chi-square test; a p value of less than .05 was considered as statistically significant. For means and medians, a standardized difference of greater than 0.1 was considered as statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Ethics Approval
ICES is a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act. Section 45 authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to or planning for all or part of the health system. Projects conducted under section 45 and which have received approval by ICES’ Privacy and Legal Office, such as this one, do not require review by a Research Ethics Board.
RESULTS
Study Population Characteristics
Table 2 presents the sociodemographic and health profiles of patients who received care in the SAFE Unit and Usual Care patients after matching; baseline characteristics prior to matching are included in Table 4. The mean age of the matched cohorts was 82.5 ± 9.0 years at discharge. The majority of patients are female (61%) and more than 97% resided in an urban area. In terms of conditions that are associated with the highest risk of hospital readmission, CHF (55.8% for SAFE vs. 54.5% for Usual Care), COPD (33.1% vs. 30.5%), and LRTIs (31.8% vs. 30.5%) were the most prevalent conditions. Most SAFE Unit and Usual Care patients did not have an acute care admission within six months before the index hospitalization (64.9% vs. 66.2%). In terms of the discharge location, SAFE Unit patients were more likely to be discharged home without home support (56.8%), compared to the control group (7.9%; Table 5). All baseline covariates were well-balanced with standardized differences that were less than or equal to 0.1. Additional baseline characteristics which were not used in the propensity score matching are reported in Table 3.
TABLE 4.
Sociodemographic and health characteristics of patients who received care in the SAFE Unit and Usual Care, before propensity score matching
| SAFE Unit, n(%) | Usual Care, n(%) | Standardized Difference | |||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | N=155 | N=3,336 | |||
| Age | |||||
| Mean ± SD | 82.49 ± 9.00 | 79.95 ± 9.68 | |||
| 60–64 | 1–5 | (0.6–3.2) | 242 | (7.3) | 0.18 |
| 65–69 | 10–14 | (6.5–9.1) | 336 | (10.1) | 0.13 |
| 70–74 | 17 | (11.0) | 435 | (13.0) | 0.06 |
| 75–79 | 26 | (16.8) | 492 | (14.7) | 0.06 |
| 80–84 | 26 | (16.8) | 615 | (18.4) | 0.04 |
| 85–89 | 35 | (22.6) | 619 | (18.6) | 0.1 |
| 90+ | 36 | (23.1) | 597 | (17.9) | 0.13 |
|
| |||||
| Sex | |||||
| Female | 95 | (61.3) | 1,972 | (59.1) | 0.04 |
| Male | 60 | (38.7) | 1,364 | (40.9) | 0.04 |
|
| |||||
| Rural | |||||
| Yes | 1–5 | (0.6–3.2) | 325 | (9.7) | 0.34 |
| No | 150–154 | (96.8–99.4) | 2,981 | (89.4) | 0.36 |
|
| |||||
| Comorbidities | |||||
| Arrythmia | 38 | (24.5) | 386 | (11.6) | 0.34 |
| Congestive heart failure (CHF) | 87 | (56.1) | 936 | (28.1) | 0.59 |
| Chronic obstructive pulmonary disorder (COPD) | 52 | (33.5) | 678 | (20.3) | 0.3 |
| Dementia | 24 | (15.5) | 1,170 | (35.1) | 0.46 |
| Stroke | 12 | (7.7) | 497 | (14.9) | 0.23 |
| Asthma | 40 | (25.8) | 652 | (19.5) | 0.15 |
| Cancer | 59 | (38.1) | 888 | (26.6) | 0.25 |
| Coronary heart disease | 69 | (44.5) | 1,066 | (32.0) | 0.26 |
| Diabetes | 66 | (42.6) | 1,320 | (39.6) | 0.06 |
| Hypertension | 139 | (89.7) | 1,747 | (82.3) | 0.21 |
| Mood disorder | 39 | (25.2) | 930 | (27.9) | 0.06 |
| Osteoarthritis | 124 | (80.0) | 2,565 | (76.9) | 0.08 |
| Osteoporosis | 23 | (14.8) | 626 | (18.8) | 0.11 |
| Renal failure | 84 | (54.2) | 971 | (29.1) | 0.53 |
| Rheumatoid arthritis | 12 | (7.7) | 164 | (4.9) | 0.12 |
|
| |||||
| ACG score | |||||
| Mean ± SD | 13.74 ± 3.27 | 12.86 ± 3.47 | 0.26 | ||
|
| |||||
| Lower respiratory tract infections during index hospitalization | 50 | (32.3) | 467 | (14.0) | 0.44 |
|
| |||||
| Prior acute care admission (within 6 months prior to index) | |||||
| 0 | 101 | (65.2) | 2,517 | (75.4) | 0.23 |
| 1 | 36 | (23.2) | 605 | (18.1) | 0.13 |
| 2+ | 18 | (11.6) | 214 | (6.4) | 0.18 |
|
| |||||
| Number of prevalent conditions | |||||
| 0–2 | 1–5 | (0.6–3.2) | 233 | (7.0) | 0.25 |
| 3 | 5–10 | (3.2–6.5) | 266 | (8.0) | 0.21 |
| 4 | 14 | (9.0) | 440 | (13.2) | 0.13 |
| 5 | 15 | (9.7) | 525 | (15.7) | 0.18 |
| 6 | 28 | (18.1) | 512 | (15.3) | 0.07 |
| 7+ | 90 | (58.1) | 1,360 | (40.8) | 0.35 |
|
| |||||
| Presence of infections | 63 | (40.6) | 574 | (17.2) | 0.54 |
TABLE 5.
Discharge location of patients who received care in the SAFE Unit and Usual Care
| Characteristic | SAFE Unit Patient, N (%) | ALC Patients, N (%) | Standard Difference |
|---|---|---|---|
| N=190 | N=190 | ||
| Home with Support | 23 (12.1%) | 81 (42.6%) | 0.73 |
| Home without support | 108 (56.8%) | 15 (7.9%) | 1.23 |
| Other | 1 (0.5%) | 0 (0.0%) | 0.1 |
| Rehabilitation facility | 2 (1.1%) | 0 (0.0%) | 0.15 |
| Retirement home | 28 (14.7%) | 21 (11.1%) | 0.11 |
Post-Discharge Hospital Readmission, ED Visits, Primary Care Visits, & LTC Admission
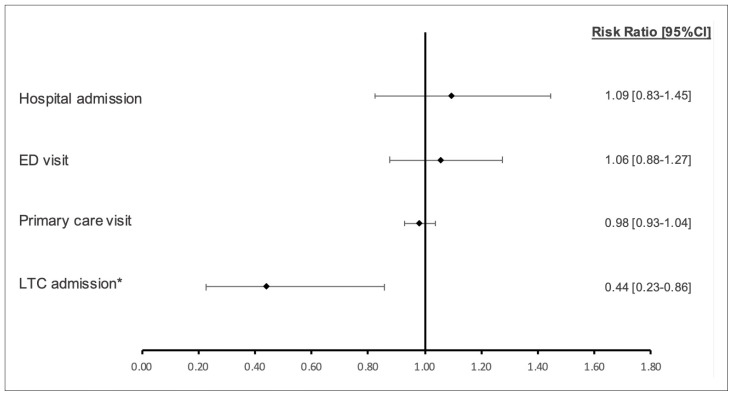
As shown in Figure 2, over the 180-day post-discharge period, we did not find statistically significant differences between SAFE Unit and Usual Care patients with respect to their risk of hospital readmissions (RR 1.09, 95% CI 0.83–1.45), ED visits (RR 1.06, 95% CI 0.88–1.27), or primary care visits (RR 0.98, 95% CI 0.93–1.04). We observed a significantly lower risk of entering an LTC home, by 55%, among SAFE Unit patients relative to those who received Usual Care (RR 0.44, 95% CI 0.23–0.86).
FIGURE 2.
Relative risk of a hospital readmission, emergency department (ED) visit, primary care visit, and long-term care (LTC) admission within 180 days post-discharge in SAFE Unit compared to Usual Care patients
aDenotes statistical significance at p<.05.
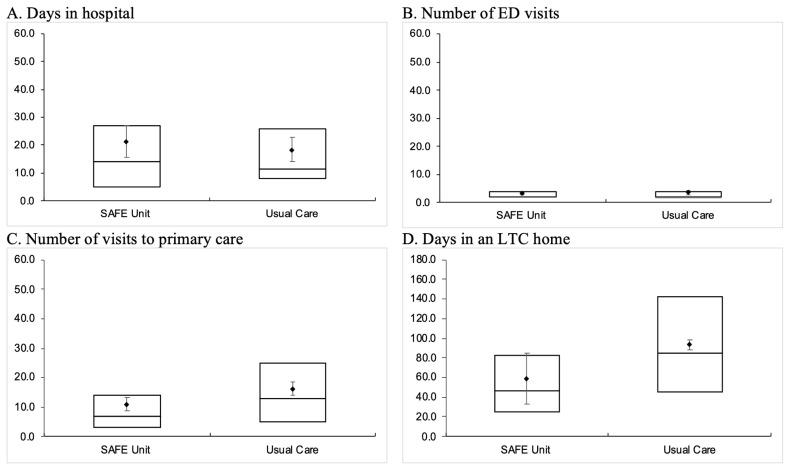
As shown in Figure 3, SAFE Unit patients had a similar median length of stay (LOS) in the hospital (SAFE: median 14 days, IQR 5–27 days; Usual Care: median 11.5 days, IQR 8–26 days) and a similar number of ED visits (SAFE: median 2 visits, IQR 2–4 visits; Usual Care: median 2 visits, IQR 1.5–4 visits) as Usual Care patients within 180 days post-discharge. However, SAFE Unit patients had significantly fewer primary care visits (SAFE: median 7 visits, IQR 3–14 visits; Usual Care: median 13 visits, IQR 5–25 visits) and spent fewer days in an LTC home (SAFE: median 46 days, IQR 24.5–82.5 days; Usual Care: median 85 days, IQR 45–142 days) than those who received Usual Care.
FIGURE 3.
Length of stay (in days) of hospitalization and in long-term care (LTC), and number of visits to the emergency department (ED) and primary care providers within 180 days post-discharge in SAFE Unit and Usual Care patients
ED = Emergency Department; LTC = Long-term Care.
Post-Discharge Home Care Use
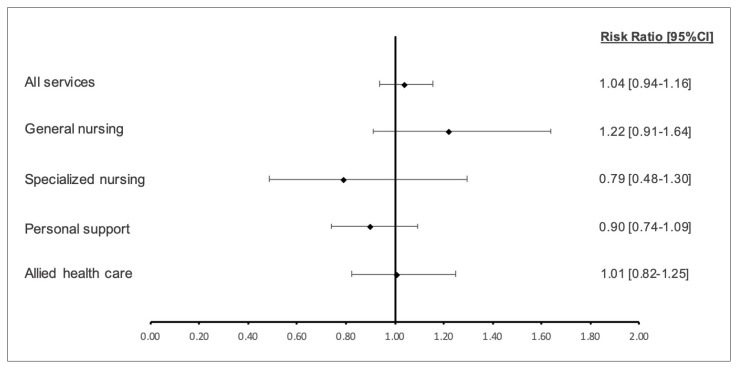
Overall, SAFE Unit and Usual Care group patients had a similar likelihood of using any home care within 180 days post-discharge (RR 1.04, 95% CI 0.94–1.16, Figure 4). SAFE Unit patients were slightly more likely to require general nursing care (RR 1.22, 95% CI 0.91–1.64) as well as allied health-care support (RR 1.01, 95% CI 0.82–1.25), though these were not statistically significant differences. Meanwhile, SAFE Unit patients were less likely to need specialized nursing care (RR 0.79, 95% CI 0.48–1.30) and personal support (RR 0.90, 95% CI 0.74–1.09). These differences were, again, not statistically significant.
FIGURE 4.
Relative risk of requiring various home-care services within 180 days post-discharge in SAFE Unit and Usual Care patients
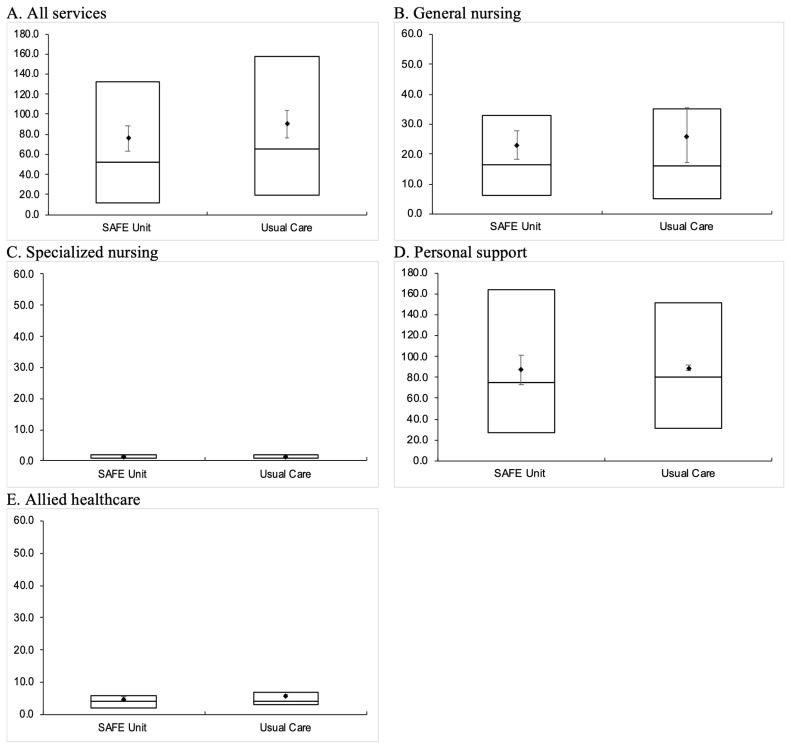
As shown in Figure 5, among those who used home care, on average, SAFE Unit patients had used significantly fewer days of service than those who received usual care; specifically, SAFE Unit patients had a median of 52 service days (IQR 12–132 days), compared to a median of 65.5 service days (IQR 19–158 days) among Usual Care patients. In terms of specific home-care services, we found significant differences in general nursing (SAFE: median 16.5 days, IQR 6–33 days; Usual Care: median 16 days, IQR 5–35 days) and personal support use (SAFE: median 75 days, IQR 27–164 days; Usual Care: median 80 days, IQR 31–151 days). The median number of service days did not differ in other categories of home-care services (i.e., specialized nursing and allied health care).
FIGURE 5.
Number of service days associated with various home-care services utilized by SAFE Unit and Usual Care patients within 180 days post-discharge
DISCUSSION
In this study, we found that patients who were admitted to the SAFE Unit were significantly less reliant on follow-up care in the community. This was demonstrated by having fewer primary care visits and fewer home-care service days, especially for home-based personal support, than Usual Care patients. Furthermore, patients who received care in the SAFE Unit had significantly lower odds of entering LTC than those who received usual care. Among patients who required care in LTC homes, SAFE Unit patients spent fewer days in LTC within the 180-day observation period, indicating a delayed admission compared to those who received usual care. Delayed admission to LTC, combined with fewer home care service days—particularly in terms of personal care and support—may indicate that care provided in the SAFE Unit optimized the functional independence of hospitalized older patients who were supported through this model.
These observations echo existing evidence on the effectiveness of transitional care units,(15,18,48,49) which tend to suggest a positive association between transitional care and improvements in functional status,(16,18,48) as well as reduced home-care use among older adults.(15,50–52) The unique features of the SAFE Unit—including cognitive screening, comprehensive frailty assessment, as well as rehabilitation- and restorative-focused treatment—are key contributors to the observed outcomes.(25) The initial design of the SAFE Unit was modelled after the Acute Care for Elders (ACE) Unit at a local hospital in Cleveland, Ohio. The ACE Unit, which adopted a patient-centred, multidimensional assessment and care planning approach, was set up to prevent functional deterioration and enable restoration of functional independence in frail older adults.(53,54) In a randomized controlled trial of ACE, the investigators found that functional decline from baseline and nursing home placement were less frequent in the intervention group at discharge and during the year following hospitalization.(55) In a similar evaluation of a national transitional care program in Australia—which provides short-term, interdisciplinary, rehabilitative- and restorative-focused care to older patients in residential settings (i.e., nursing home)—the investigators found improved functional independence upon discharge from the program.(19)
Functional decline is a common concern for older adults who experience extended stay and delayed discharge from the hospital.(56) Therefore, preventing further deterioration towards critical frailty and functional decline in this population is particularly important to mitigate their need for more institutional care.(57) Not only is maintaining older adults’ independence at a level where they are able to remain living at home aligned with most seniors’ preferences when considering their place of residence and care,(58) this could have important implications from a health system perspective as well, in terms of reducing the relatively higher cost associated with residential care while increasing capacity within hospitals. In a parallel study conducted by our research team, we examined the cost-effectiveness of the SAFE Unit in enabling institution-free days at home. We demonstrated that SAFE Unit patients had incurred a lower total cost of care (by approximately $1,100 in 2019 Canadian dollars), owing to their reduced hospital length of stay.(59) Patients who received care in the SAFE Unit also spent more institution-free days at home within six months post-discharge. Therefore, it was deemed to be a cost-effective model of care.
Limitations
We acknowledge that our study has limitations. First, we used the likelihood and days of LTC and home care service utilization as indications of functional independence, which is an inferred effect rather than an objective measure of functional independence using a standard measurement scale, such as the modified Barthel Index.(18) The lack of available services may contribute to a person not receiving care, and not because the patient is functionally independent. Second, our use of a quasi-experimental design also has limitations. While we were able to match cases and controls using a variety of factors (e.g., age, sex, morbidity, and ACG score) that are relevant to the outcomes of interest, these do not represent a comprehensive depiction or measurement of the patients’ frailty or overall health stability. As such, there may be unobserved and clinically meaningful differences between the cases and controls that we did not control for in our matching algorithm. For example, there may be individuals in the Usual Care group who are incapable of bearing weight and have limited rehabilitative/restorative potential, and they would not have qualified for the SAFE Unit even if this care option was available to them. Unfortunately, we are unable to assess the magnitude of potential bias using health administrative data sources due to the unavailability of information pertaining to general frailty and weight-bearing status. Despite this, the potential bias may be small, as studies comparing the ACG to other clinical frailty instruments suggest similar performance in predicting various health outcomes.(60–63)
CONCLUSION
Function-focused transitional care models, like the SAFE Unit, have the potential to enable older adults to live independently without relying on significant community-based resources and delays their entry into LTC homes. In order to holistically address an older patient’s needs, transitional care programs must include a comprehensive frailty assessment, care planning by a multidisciplinary team that involves patients and families, mobility and rehabilitation-treatments, discharge planning, and patient, family and staff education. Despite these promising findings, the continued investment in transitional care in Ontario and elsewhere within Canada should be supported by rigorous evaluations of their effectiveness. Future work would benefit from a formal evaluation framework to guide the assessment of similar transitional care models.
ACKNOWLEDGEMENTS
The authors acknowledge the contributions of Mary Boutette (Chief Operating Officer, Perley Health) and the entire SAFE Unit staff at Perley Health for their contribution to this program and evaluation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: We have read and understand the Canadian Geriatrics Journal’s policy on disclosing conflicts of interest and declare that we have none.
FUNDING: This research was funded by the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario and supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care.
REFERENCES
- 1.Canadian Institute for Health Information. A profile of hospitalized seniors at risk of frailty in Canada — Infographic. 2022. [cited 2023 January 18]. Available from: https://www.cihi.ca/en/a-profile-of-hospitalized-seniors-at-risk-of-frailty-in-canada-infographic.
- 2.Lim SC, Doshi V, Castasus B, Lim JK, Mamun K. Factors causing delay in discharge of elderly patients in an acute care hospital. Ann Acad Med Singap. 2006 Jan 1;35(1):27–32. doi: 10.47102/annals-acadmedsg.V35N1p27. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Institute for Health Information. Seniors in Transition: Exploring Pathways Across the Care Continuum. Ottawa, ON: 2017. Available from: https://www.cihi.ca/sites/default/files/document/seniors-in-transition-report-2017-en.pdf. [Google Scholar]
- 4.Canadian Institute for Health Information. Health Care in Canada 2011: a Focus on Seniors and Aging. Ottawa, ON: 2011. Available from: https://publications.gc.ca/collections/collection_2011/icis-cihi/H115-15-2011-eng.pdf. [Google Scholar]
- 5.Murtaugh CM, Litke A. Transitions through postacute and long-term care settings: patterns of use and outcomes for a national cohort of elders. Med Care. 2002 Mar 1;40(3):227–36. doi: 10.1097/00005650-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Barnable A, Welsh D, Lundrigan E, Davis C. Analysis of the influencing factors associated with being designated alternate level of care. Home Health Care Manage Pract. 2015 Feb;27(1):3–12. doi: 10.1177/1084822314539164. [DOI] [Google Scholar]
- 7.Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: a mixed-studies systematic review. Health Expect. 2018 Feb;21(1):41–56. doi: 10.1111/hex.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCloskey R, Jarrett P, Stewart C, Nicholson P. Alternate level of care patients in hospitals: what does dementia have to do with this? Can Geriatr J. 2014 Sep;17(3):88–94. doi: 10.5770/cgj.17.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuluski K, Ho JW, Cadel L, Shearkhani S, Levy C, Marcinow M, et al. An alternate level of care plan: Co-designing components of an intervention with patients, caregivers and providers to address delayed hospital discharge challenges. Health Expect. 2020 Oct;23(5):1155–65. doi: 10.1111/hex.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuluski K, Im J, McGeown M. “It’s a waiting game” a qualitative study of the experience of carers of patients who require an alternate level of care. BMC Health Serv Res. 2017 Dec;17(1):318. doi: 10.1186/s12913-017-2272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AdvantAge Ontario. Appropriate level of care: a patient flow, system integration and capacity solution. Dec, 2006. Available from: https://www.advantageontario.ca/oanhssdocs/Issue_Positions/External_Resources/ALC_Report_December_2006.pdf.
- 12.Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004 May 25;170(11):1678–86. doi: 10.1503/cmaj.1040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landeiro F, Roberts K, Gray AM, Leal J. Delayed hospital discharges of older patients: a systematic review on prevalence and costs. Gerontologist. 2019 Mar 14;59(2):e86–e97. doi: 10.1093/geront/gnx028. [DOI] [PubMed] [Google Scholar]
- 14.Sheets DJ, Gallagher EM. Aging in Canada: state of the art and science. Gerontologist. 2013 Feb 1;53(1):1–8. doi: 10.1093/geront/gns150. [DOI] [PubMed] [Google Scholar]
- 15.Weeks LE, Macdonald M, Martin-Misener R, Helwig M, Bishop A, Iduye DF, et al. The impact of transitional care programs on health services utilization in community-dwelling older adults: a systematic review. JBI Evid Synth. 2018 Feb 1;16(2):345–84. doi: 10.11124/JBISRIR-2017-003486. [DOI] [PubMed] [Google Scholar]
- 16.Manville M, Klein MC, Bainbridge L. Improved outcomes for elderly patients who received care on a transitional care unit. Can Fam Physician. 2014 May 1;60(5):e263–e271. [PMC free article] [PubMed] [Google Scholar]
- 17.Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations [Research Letter] JAMA Intern Med. 2015 Feb 1;175(2):295–96. doi: 10.1001/jamainternmed.2014.6383. [DOI] [PubMed] [Google Scholar]
- 18.McGilton KS, Vellani S, Krassikova A, Robertson S, Irwin C, Cumal A, et al. Understanding transitional care programs for older adults who experience delayed discharge: a scoping review. BMC Geriatr. 2021 Dec;21(1):210. doi: 10.1186/s12877-021-02099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cations M, Lang C, Crotty M, Wesselingh S, Whitehead C, Inacio MC. Factors associated with success in transition care services among older people in Australia. BMC Geriatr. 2020 Dec;20(1):496. doi: 10.1186/s12877-020-01914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah T, Churpek MM, Perraillon MC, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015 May 1;147(5):1219–26. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim WK, Lambert SF, Gray LC. Effectiveness of case management and post-acute services in older people after hospital discharge. Med J Aust. 2003 Mar;178(6):262–66. doi: 10.5694/j.1326-5377.2003.tb05191.x. [DOI] [PubMed] [Google Scholar]
- 22.Piraino E, Heckman G, Glenny C, Stolee P. Transitional care programs: who is left behind? A systematic review. Int J Integr Care. 2012 Jul;12:e132. doi: 10.5334/ijic.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray LC, Peel NM, Crotty M, Kurrle SE, Giles LC, Cameron ID. How effective are programs at managing transition from hospital to home? A case study of the Australian Transition Care Program. BMC Geriatr. 2012 Dec;12:6. doi: 10.1186/1471-2318-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fønss Rasmussen L, Grode LB, Lange J, Barat I, Gregersen M. Impact of transitional care interventions on hospital readmissions in older medical patients: a systematic review. BMJ Open. 2021 Jan 1;11(1):e040057. doi: 10.1136/bmjopen-2020-040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutette M, Hoffer A, Plant J, Robert B, Sinden D. Establishing an integrated model of subacute care for the frail elderly. Healthc Manage Forum. 2018 Jul;31(4):133–36. doi: 10.1177/0840470418774807. [DOI] [PubMed] [Google Scholar]
- 26.Robert B, Sun AH, Sinden D, Spruin S, Hsu AT. A case-control study of the Sub-Acute Care for Frail Elderly (SAFE) unit on hospital readmission, emergency department visits and continuity of post-discharge care. J Am Med Dir Assoc. 2021 Mar 1;22(3):544–50. doi: 10.1016/j.jamda.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Theou O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J. 2020 Sep;23(3):210–15. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colognesi S, Fagnani C, Panceri F, Ruggero M, Di Florio F, Passoni C, et al. Hospital discharge: testing the “Blaylock Risk Assessment Screening Score” in a surgical department. Acta Biomed. 2021;92(S2):e2021039. doi: 10.23750/abm.v92iS2.10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Johns Hopkins University. The Johns HopkinsACG® System Technical Reference Guide Version 10.0. Dec, 2011. https://www.hopkinsacg.org/document/acg-system-version-10-0-technical-reference-guide/
- 30.Canadian Institute for Health Information (CIHI) All-cause readmission to acute care and return to the emergency department. 2012. [Accessed June 1, 2023]. Available from: https://secure.cihi.ca/free_products/Readmission_to_acutecare_en.pdf.
- 31.Pedersen MK, Meyer G, Uhrenfeldt L. Risk factors for acute care hospital readmission in older persons in Western countries: a systematic review. JBI Database System Rev Implement Rep. 2017;15(2):454–85. doi: 10.11124/JBISRIR-2016-003267. [DOI] [PubMed] [Google Scholar]
- 32.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009 Nov;16(6):183–88. doi: 10.1155/2009/963098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Health Promo Chronic Dis Prevent Can. 2013 Jun 1;33(3):160–66. [PubMed] [Google Scholar]
- 34.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. J COPD. 2009 Jan 1;6(5):388–94. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 35.Jaakkimainen RL, Bronskill SE, Tierney MC, Hermann N, Green D, Young J, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016 Jan 1;54(1):337–49. doi: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 36.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002 Mar 1;25(3):512–16. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 37.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002 Aug 1;144(2):290–96. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 39.Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015 Dec;15:415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruneir A, Bronskill SE, Maxwell CJ, Bai YQ, Kone AJ, Thavorn K, et al. The association between multimorbidity and hospitalization is modified by individual demographics and physician continuity of care: a retrospective cohort study. BMC Health Serv Res. 2016 Dec;16:154. doi: 10.1186/s12913-016-1415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane NE, Maxwell CJ, Gruneir A, Bronskill SE, Wodchis WP. Absence of a socioeconomic gradient in older adults’ survival with multiple chronic conditions. EBioMedicine. 2015 Dec 1;2(12):2094–2100. doi: 10.1016/j.ebiom.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mondor L, Maxwell CJ, Bronskill SE, Gruneir A, Wodchis WP. The relative impact of chronic conditions and multimorbidity on health-related quality of life in Ontario long-stay home care clients. Qual Life Res. 2016 Oct;25(10):2619–32. doi: 10.1007/s11136-016-1281-y. [DOI] [PubMed] [Google Scholar]
- 43.Petrosyan Y, Bai YQ, Pefoyo AJ, Gruneir A, Thavorn K, Maxwell CJ, et al. The relationship between diabetes care quality and diabetes-related hospitalizations and the modifying role of comorbidity. Can J Diabetes. 2017 Feb 1;41(1):17–25. doi: 10.1016/j.jcjd.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Mondor L, Maxwell CJ, Hogan DB, Bronskill SE, Gruneir A, Lane NE, et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: a retrospective analysis of a population-based cohort. PLoS Med. 2017 Mar 7;14(3):e1002249. doi: 10.1371/journal.pmed.1002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thavorn K, Maxwell CJ, Gruneir A, Bronskill SE, Bai Y, Pefoyo AJ, et al. Effect of socio-demographic factors on the association between multimorbidity and healthcare costs: a population-based, retrospective cohort study. BMJ Open. 2017 Oct 1;7(10):e017264. doi: 10.1136/bmjopen-2017-017264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mondor L, Cohen D, Khan AI, Wodchis WP. Income inequalities in multimorbidity prevalence in Ontario, Canada: a decomposition analysis of linked survey and health administrative data. Int J Equity Health. 2018 Dec;17(1):90. doi: 10.1186/s12939-018-0800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical statistics. 2011;2011;10(2):150–61. doi: 10.1002/pst.433. Available from: https://doi.org/10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hang JA, Naseri C, Francis-Coad J, Jacques A, Waldron N, Knuckey R, et al. Effectiveness of facility-based transition care on health-related outcomes for older adults: A systematic review and meta-analysis. Int J Older People Nurs. 2021 Nov;16(6):e12408. doi: 10.1111/opn.12408. [DOI] [PubMed] [Google Scholar]
- 49.Cumal A, Colella TJF, Puts MT, Sehgal P, Robertson S, McGilton KS. The impact of facility-based transitional care programs on function and discharge destination for older adults with cognitive impairment: a systematic review. BMC Geriatr. 2022 Nov 14;22(1):854. doi: 10.1186/s12877-022-03537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999 Feb 17;281(7):613–20. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 51.Boult C, Reider L, Leff B, Frick KD, Boyd CM, Wolff JL, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med. 2011 Mar 14;171(5):460–66. doi: 10.1001/archinternmed.2010.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004 May;52(5):675–84. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 53.Palmer RM. The acute care for elders unit model of care. Geriatrics. 2018 Sep 11;3(3):59. doi: 10.3390/geriatrics3030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer RM, Landefeld CS, Kresevic D, Kowal J. A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994 May;42(5):545–52. doi: 10.1111/j.1532-5415.1994.tb04978.x. [DOI] [PubMed] [Google Scholar]
- 55.Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000 Dec;48(12):1572–81. doi: 10.1111/j.1532-5415.2000.tb03866.x. [DOI] [PubMed] [Google Scholar]
- 56.Peel NM, Hubbard RE, Gray LC. Impact of post-acute transition care for frail older people: a prospective study. J Frailty Aging. 2013 Jan 1;2(3):165–71. doi: 10.14283/jfa.2013.24. [DOI] [PubMed] [Google Scholar]
- 57.Loeffler K. Geriatric intermediate care and transitional care for frailty-related patients: Kerstin Loeffler. Eur J Public Health. 2016 Nov 1;26(suppl_1):ckw174.246. doi: 10.1093/eurpub/ckw174.246. [DOI] [Google Scholar]
- 58.Hestevik CH, Molin M, Debesay J, Bergland A, Bye A. Older persons’ experiences of adapting to daily life at home after hospital discharge: a qualitative metasummary. BMC Health Serv Res. 2019 Dec;19(1):224. doi: 10.1186/s12913-019-4035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murmann M, Sinden D, Hsu AT, Thavorn K, Eddeen AB, Sun AH, et al. The cost-effectiveness of a nursing home-based transitional care unit for increasing the potential for independent living in the community among hospitalized older adults. J Med Econ. 2023 Dec 31;26(1):61–69. doi: 10.1080/13696998.2022.2156152. [DOI] [PubMed] [Google Scholar]
- 60.Tran DTT, Tu JV, Dupuis JY, Bader Eddeen A, Sun LY. Association of frailty and long-term survival in patients undergoing coronary artery bypass grafting. J Am Heart Assoc. 2018 Aug 7;7(15):e009882. doi: 10.1161/JAHA.118.009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grudzinski AL, Aucoin S, Talarico R, Moloo H, Lalu MM, McIsaac DI. Measuring the predictive accuracy of preoperative clinical frailty instruments applied to electronic health data in older patients having emergency general surgery: a retrospective cohort study. Ann Surg. 2023 Aug 1;278(2):e341–e348. doi: 10.1097/SLA.0000000000005718. [DOI] [PubMed] [Google Scholar]
- 62.Sternberg SA, Bentur N, Abrams C, Spalter T, Karpati T, Lemberger J, et al. Identifying frail older people using predictive modeling. Am J Manag Care. 2012 Oct 1;18(10):e392–e397. [PubMed] [Google Scholar]
- 63.Sun LY, Jabagi H, Fang J, Lee DS. Comparison of multidimensional frailty instruments for estimation of long-term patient-centered outcomes after cardiac surgery. JAMA Netw Open. 2022 Sep 1;5(9):e2230959. doi: 10.1001/jamanetworkopen.2022.30959. [DOI] [PMC free article] [PubMed] [Google Scholar]