Abstract
Rice grown in Yunnan Province is known for its excellent taste and consumer preference. However, the metabolite composition of this unique rice remains unclear. In this study, the metabolic profile of different rice planted in various producing regions was evaluated. A total of 1,005 metabolites were identified, including nucleotides and their derivatives, amino acids and their derivatives, alkaloids, organic acids, phenolic acids, lignans and coumarins, lipids, terpenoids, quinones, flavones, tannins, and others. Procucing region and varieties can be clearly distinguished on the PCA diagram. Differential metabolites accumulated in the MSD502 vs. MSR88 (138)/LHHG (234)/LHR88 (188) comparison groups. The results in this study provide scientific information for the origin tracing and variety differentiation of raw rice materials.
Keywords: Rice, Producing region, Differential metabolites, Metabolic pathways
Introduction
Rice is the most important staple food crop in Yunnan Province, which produces 40% of the total grain output with 25% of the cultivated land area, and 67% of the population of the province take rice as the staple food (Deng et al., 2017, 2019). The annual planting area is about one million square hectares. Most of the rice farming areas in Yunnan Plateau range from 1,200 to 2,400 m above sea level, which is one of the special rice farming areas with the most biodiversity in China and even in the world. Yunnan Province has a variety of terrain and climate, which can adapt to different types of plateau characteristic rice varieties, but also has a unique rice planting area, which has an independent flavor grown in these areas. Due to the special natural ecological conditions, Yunnan has its own unique rice system. According to statistics, more than 30 quality rice varieties have formed a certain scale of planting and production in Yunnan, which is one of the quality rice farming areas with the most industrialization development prospects in China.
In recent years, quality grain project in China is closely related to the prosperity of food industry, the increase of farmers’ income and the improvement of enterprises’ efficiency. With the advantage of rich plateau characteristic food resources (such as, soft rice, red rice, purple rice), Yunnan accelerates the development of modern agriculture, meets the needs of consumers from the rigid demand type to the quality type, and starts to pay attention to the food quality. A variety of new analytical techniques are needed for grain quality and adulteration identification, and metabolomics may be an important technical means to solve this problem (Deng et al., 2015).
Starting from the overall level, metabolomics mainly studies the differences that the whole biological system responds to changes in certain conditions under different behavioral conditions, and then reflects the overall changes through the monitoring of biomarkers of differences (Tang & Wang, 2006). It is widely used in nutrition science (Tebani & Bekri, 2019), food adulteration quality identification, food origin traceability (Hao et al., 2017), disease diagnosis (Gowda et al., 2008), toxicology (Ramirez et al., 2013), plant metabolism and response mechanism (Li et al., 2014), safety of food (Organization for Economic Co-operation and Development, 1993) (such as rice (Chang et al., 2012), potato (Catchpole et al., 2005), tomato (Le Gall et al., 2003)) and so on. The metabolome can more accurately reflect the nutrient composition and content of plant cells, so it plays an important role in evaluating food quality (Fiehn, 2002; Scalbert et al., 2009). In this study, 15 rice samples (divided into five groups) were used as research materials, and the metabolites of rice were detected using an extensively employed method in the field of metabolomics, known as ultra high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), and their differences were compared and analyzed. By examining various metabolites, variations in phenolic acids, alkaloids, flavonoids, lipids, amino acids and their derivatives were identified.
The objective of this study was to provide possible solutions to the problems of identifying quality rice varieties and origin tracing in Yunnan. We hypothesized that different rice cultivars have various metabolic compositions, and colored rice has higher flavonoid content.
Materials and Methods
Genetic materials
Fifteen rice samples from Lianghe County and Mangshi City of Dehong in Yunnan Province were collected and divided into five groups for metabolic study, namely, Mangshi Diantun 502 (MSD502), Lianghe Soft Rice 88 (LHR88), Mangshi Soft 88 (MSR88), Lianghe Honggu (LHHG) and mixed samples, with three biological replicates in each group. These rice were harvested in September 2020 and stored at −20 °C.
Preparation and extraction of rice samples
The specimens were subject to freeze-drying under vacuum conditions using the lyophilizer (Scientz-100F), followed by pulverization into a fine powder using the grinder (MM 400; Retsch, Haan, Germany) at a frequency of 30 Hz for a duration of 1.5 min. A quantity of 100 mg powder was precisely weighed and dissolved in 1.2 mL of methanol solution containing 70%, with vortexing performed every half an hour for a period of 30 s each time, totaling six cycles. Subsequently, the sample was refrigerated overnight at a temperature of 4 °C. The centrifuge operated at a speed of 12,000 rpm for 10 min to separate the supernatant from the solid residue. After centrifugation, filtration through microporous membranes with pore size measuring at 0.22 μm was conducted on the obtained sample solution. Finally, storage in designated vials ensued prior to UPLC-MS/MS analysis.
Parameters for UPLC
The UPLC-ESI-MS/MS system utilized for the analysis of the sample extracts consisted of a SHIMADZU Nexera X2 UPLC and an Applied Biosystems 4500 Q TRAP mass spectrometer. The analytical conditions involved the use of an Agilent SB-C18 column (100 × 2.1 mm, 1.8 µm). A gradient program was employed for sample processing, starting with a composition of 95% A and 5% B. Over a period of 9 min, there was a gradual transition to a composition of 5% A and 95% B, which was maintained for another minute. Subsequently, within just over 1 min, the composition was adjusted to be predominantly composed of 95% A and only 5% B, which remained constant for approximately 3 min. The flow rate used during analysis was set at a value equivalent to injecting 0.35 ml per minute into the system. To maintain optimal temperature conditions, the column oven temperature was maintained at a steady state of 40 °C throughout the analysis process while ensuring that each injection contained precisely four microliters.
Parameters for MS/MS
The acquisition of LIT and triple quadrupole (QQQ) scans was performed using an AB4500 Q TRAP UPLC/MS/MS System, which is a triple quadrupole-linear ion trap mass spectrometer. The system was equipped with an ESI Turbo Ion-Spray interface and operated in both positive and negative ion mode. Control of the instrument was carried out using Analyst 1.6.3 software developed by AB Sciex. The operational parameters for the ESI source were as follows: the temperature of the ion source was set at 550 °C; positive ion mode, the ion spray voltage (IS) was 5,500 V, while in negative ion mode it was −4,500 V; gas I (GSI), gas II (GSII), and curtain gas (CUR) pressures were adjusted to 50, 60, and 25.0 psi respectively; collision-activated dissociation (CAD) was set to high. The instrument underwent tuning and mass calibration using polypropylene glycol solutions with concentrations of 10 and 100 μmol/L in QQQ and LIT modes respectively. MRM experiments were conducted for QQQ scans with medium collision gas (nitrogen). DP and CE optimization were performed for individual MRM transitions by adjusting DP and CE values accordingly. A specific set of MRM transitions were monitored during each period based on the eluted metabolites within that period.
Analysis of metabolite concentration with multiple variables
Utilizing a self-constructed metabolite database and relevant mass spectrometry database from Metware Biotechnology Co., Ltd (Wuhan, China), the detection of MRM metabolites was conducted through multi-peak mapping. The triple quadrupole technique was employed to screen characteristic ions for each substance, and subsequently, the detector measured the signal intensity of these ions.
This study employed multivariate statistical analysis, utilizing R (http://www.r-project.org/) for conducting principal component analysis (PCA) and cluster analysis (CA) on five distinct sample groups. Orthogonal partial least squares discriminant analysis (OPLS-DA) and OPLS-DA S-plot were utilized to identify orthogonal metabolites between the two samples. Variable importance in project (VIP) values, one-dimensional statistical P-values, and differential multiples were used to identify different metabolites. A cluster system was employed to screen the diverse metabolites in the samples, which were subsequently subjected to correlated pathway analysis using the KEGG (Kyoto Encyclopedia of Genes and Genomes) database website.
Analysis of KEGG annotations and enrichment
The KEGG database is utilized to annotate various metabolites and link them to the KEGG pathways database. Metabolite concentration analysis (MSEA) was conducted on pathways where significant regulation of metabolites occurred. The significance of these findings was assessed using the P value derived from a hypergeometric test.
Results
Metabolic composition profiling
Metabolome analysis was performed on 15 rice samples. According to the self-constructed metabolite database and relevant mass spectrometry database, a total of 1,005 metabolites belonging to 12 different classes were successfully identified (Table 1), The metabolites included 63 nucleotides and their derivatives, 110 amino acids and their derivatives, 87 alkaloids, 80 organic acids, 137 phenolic acids, 21 lignans and coumarins, 172 lipids, 23 terpenoids, five quinones, 184 flavonoids, six tannins, and 117 others metabolites.
Table 1. Overview of annotated metabolites.
| Class | Number | Percentage (%) |
|---|---|---|
| Nucleotides and their derivatives | 63 | 6.27 |
| Amino acids and their derivatives | 110 | 10.95 |
| Alkaloid | 87 | 8.66 |
| Organic acid | 80 | 7.96 |
| Phenolic acids | 137 | 13.63 |
| Lignans and coumarins | 21 | 2.09 |
| Lipid | 172 | 17.11 |
| Terpenoids | 23 | 2.29 |
| Quinones | 5 | 0.50 |
| Flavonoids | 184 | 18.31 |
| Tannins | 6 | 0.60 |
| Others | 117 | 11.64 |
| Total | 1,005 | 100 |
Multivariate analysis of metabolite profiles
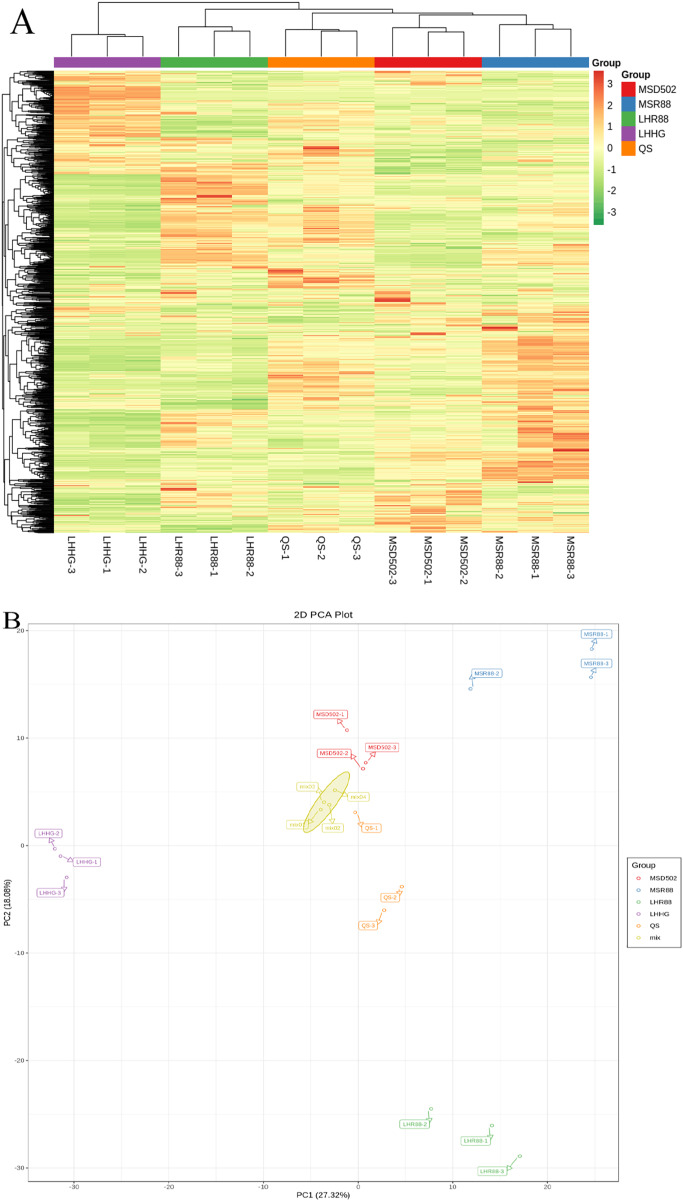
Multivariate statistical analysis was conducted to evaluate the variations in metabolic profiles across various rice samples. The rice samples exhibited distinct categorization into five groups (Fig. 1), with noticeable segregation between the groups, suggesting substantial variations in metabolite composition among these five groups of rice samples. Furthermore, the three biological replicates demonstrated close clustering together (Fig. 1A). Through conducting PCA analysis on a set of rice samples, we were able to assess the extent of variation both within and between the five groups of rice samples. The contribution rate of PC1 was 27.32%, and that of PC2 was 18.08%. The separation between the five groups of rice samples was obvious in the two-dimensional diagram, which can generally reflect the differences across the five distinct sample categories. The close clustering of the three biological replicates for each sample suggests that the test exhibited high repeatability and reliability, thereby ensuring reliable quality control analysis (Fig. 1B).
Figure 1. Heatmap (A) and PCA plot (B) illustrating the distribution of metabolites across different treatments in rice examples.
(A) Each sample is represented by a column, while each metabolite is represented by a row. The colors green and red are used to indicate low and high abundance, respectively. (B) PC1 and 2 demonstrate strong cohesion within groups and effective separation among the rice accessions.
Analysis of differential metabolites
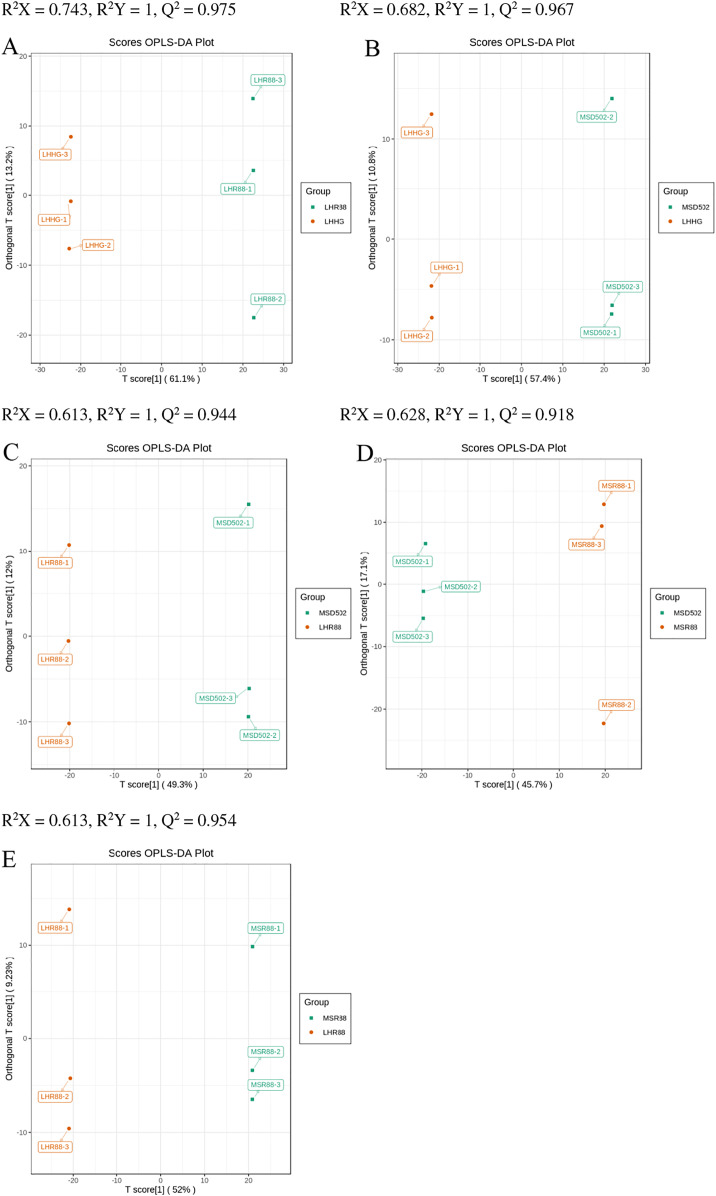
The OPLS-DA method was used to screen for variables that contributed to the differences between the five groups. In this research, the OPLS-DA model was utilized to analyze and compare metabolite composition in rice samples, aiming to identify variations between different groups, LHR88 and LHHG (R2X = 0.743, R2Y = 1, Q2 = 0.975), MSD502 and LHHG (R2X = 0.682, R2Y = 1, Q2 = 0.967), MSD502 and LHR88 (R2X = 0.613, R2Y = 1, Q2 = 0.944), MSD502 and MSR88 (R2X = 0.628, R2Y = 1, Q2 = 0.918), MSR88 and LHR88 (R2X = 0.613, R2Y = 1, Q2 = 0.954) (Figs. 2, 3). All comparison groups had Q2 values of more than 0.9, indicating that the model was stable. The OPLS-DA score map showed that the five groups of rice samples were well separated in pairs, suggesting that the metabolic phenotypes of the five groups of rice samples were significantly different.
Figure 2. (A–E) The OPLS-DA score chart displays the comparison of five groups of rice, namely LHR88 vs LHHG, MSD502 vs LHHG, MSD502 vs LHR88, MSD502 vs MSR88, and MSR88 vs LHR88.
Charts (A) and (E) represent the OPLS-DA model diagrams for these comparisons.
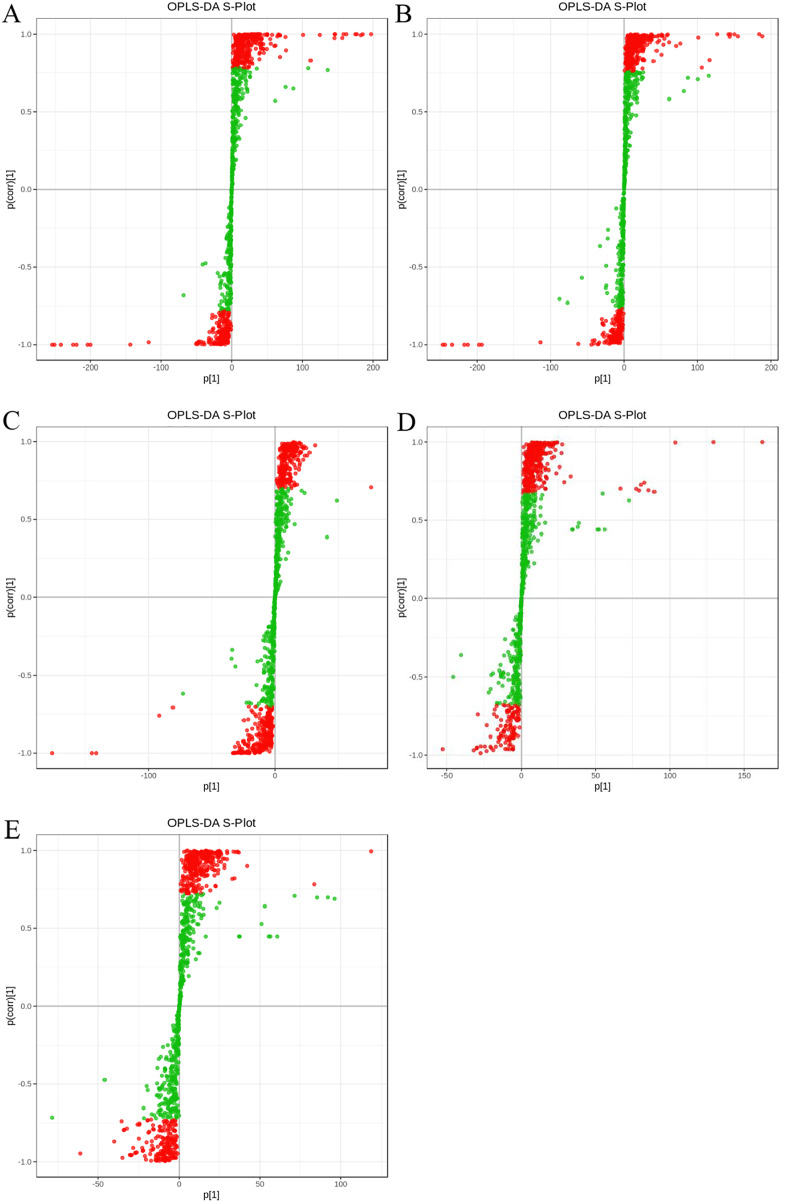
Figure 3. The S plot (A–E) displays the comparison of five groups of rice, namely LHR88 vs LHHG, MSD502 vs LHHG, MSD502 vs LHR88, MSD502 vs MSR88, and MSR88 vs LHR88.
According to the screening criteria, FC ≥ 2 to ≤ 0.5 and VIP ≥ 1, of differential metabolites, there was a significant difference in the expression levels of metabolites between LHR88 and LHHG, with 228 metabolites showing down-regulation and 99 metabolites showing up-regulation. The identified down-regulated and up-regulated metabolites mainly belonged to categories such as lipids, flavonoids, phenolic acids, amino acids, and their derivatives (Table 2). Terpenoids, lignans and coumarins were the least up-regulated, while quinones and blends were the least down-regulated. There was a significant decrease in the levels of 174 metabolites and an increase in the levels of 60 metabolites when comparing MSD502 to LHHG (Table 2). The most down-regulated and up-regulated metabolites were flavonoids, amino acids and their derivatives, phenolic acids, etc. The least down-regulated metabolites were quinones and tannins, while the least up-regulated metabolites were terpenoids, other metabolites, nucleotides and their derivatives, lipins and coumarins. There was a significant difference in the expression levels of 79 metabolites down-regulated and 109 metabolites up-regulated when comparing MSD502 to LHR88 (Table 2). The most down-regulated metabolites were flavonoids and phenolic acids, while the most up-regulated metabolites were lipids and alkaloids. Quinones, blends, lignans and coumarins were least down-regulated and up-regulated. Between MSD502 vs MSR88, there were 31 down-regulated metabolites and 107 up-regulated metabolites (Table 2). The most down-regulated metabolites were amino acids and their derivatives, while the most up-regulated metabolites were flavonoids and lipids. Quinones, blends, lignans and coumarins were least down-regulated and up-regulated. There was a significant decrease in the levels of 139 metabolites and an increase in the levels of 53 metabolites when comparing MSR88 to LHR88 (Table 2). The most down-regulated metabolites were flavonoids, phenolic acids, etc., and the most up-regulated metabolites were amino acids and their derivatives, alkaloids, lipids, etc. Terpenoids, quinones and blends were least down-regulated and up-regulated.
Table 2. Metabolites significantly changed in different treatments.
| Class | LHR88 vs LHHG |
MSD502 vs LHHG |
MSD502 vs LHR88 |
MSD502 vs MSR88 |
MSR88 vs LHR88 |
|
|---|---|---|---|---|---|---|
| Nucleotides and their derivatives | Down | 13 | 11 | 4 | 5 | 2 |
| Up | 8 | 2 | 0 | 1 | 3 | |
| Amino acids and their derivatives | Down | 30 | 24 | 2 | 11 | 2 |
| Up | 3 | 5 | 11 | 6 | 14 | |
| Alkaloid | Down | 23 | 13 | 4 | 4 | 1 |
| Up | 10 | 7 | 18 | 8 | 12 | |
| Organic acid | Down | 23 | 18 | 6 | 3 | 7 |
| Up | 8 | 3 | 5 | 6 | 2 | |
| Phenolic acids | Down | 16 | 19 | 21 | 2 | 28 |
| Up | 19 | 13 | 7 | 11 | 1 | |
| Lignans and coumarins | Down | 4 | 4 | 2 | 0 | 6 |
| Up | 2 | 2 | 0 | 1 | 1 | |
| Lipid | Down | 50 | 5 | 14 | 1 | 7 |
| Up | 20 | 6 | 49 | 32 | 10 | |
| Terpenoids | Down | 8 | 5 | 1 | 1 | 1 |
| Up | 0 | 0 | 5 | 2 | 0 | |
| Quinones | Down | 0 | 1 | 0 | 0 | 2 |
| Up | 2 | 2 | 0 | 2 | 0 | |
| Flavonoids | Down | 34 | 53 | 25 | 2 | 77 |
| Up | 21 | 16 | 2 | 33 | 4 | |
| Tannins | Down | 0 | 0 | 0 | 0 | 0 |
| Up | 3 | 3 | 0 | 0 | 0 | |
| Others | Down | 27 | 21 | 0 | 2 | 6 |
| Up | 3 | 1 | 12 | 5 | 6 | |
| Total | Down | 228 | 174 | 79 | 31 | 139 |
| Up | 99 | 60 | 109 | 107 | 53 | |
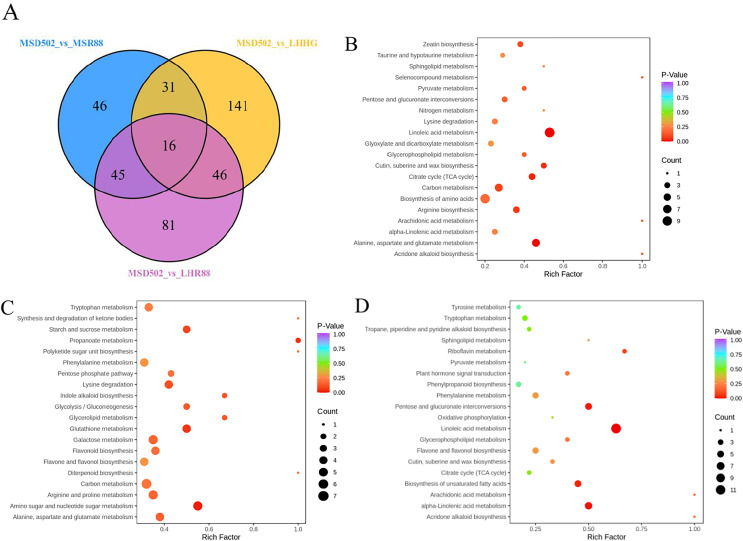
We analyzed the pathway enrichment of metabolites through KEGG database. The pathways differential metabolites remarkably enriched in the group of MSD502 vs. MSR88 were “linoleic acid metabolism”, “cutin, suberine and wax biosynthesis”, “citrate cycle (TCA cycle)”, “carbon metabolism”, “arginine biosynthesis” and “alanine, aspartate and glutamate metabolism” (P < 0.05) (Fig. 4B). The pathways differential metabolites remarkably enriched in the group of MSD502 vs. LHHG were “starch and sucrose metabolism”, “propanoate metabolism”, “lysine degradation”, “glutathione metabolism” and “amino sugar and nucleotide sugar metabolism” (P < 0.05) (Fig. 4C). The pathways differential metabolites remarkably enriched in the group of MSD502 vs. LHR88 were “riboflavin metabolism”, “pentose and glucuronate interconversions”, “linoleic acid metabolism”, “biosynthesis of unsaturated fatty acids” and “alpha-linolenic acid metabolism” (P < 0.05) (Fig. 4D).
Figure 4. Visual representations including Venn diagrams and bubble diagrams were utilized to illustrate comparisons among three distinct sets of differentially expressed metabolites (MSD502_vs_MSR88, MSD502_vs_LHHG, MSD502_vs_LHR88).
A Venn diagram (A) was employed to showcase both shared and unique metabolites across these comparison groups. Additionally, (B–D) present KEGG enrichment analyses for differentially expressed metabolites within each group (MSD502 vs. MSR88/LHHG/LHR88). Each individual bubble within these figures corresponds to a specific metabolic pathway; its position on an axis as well as its size collectively indicate its level of influence within that particular pathway. Larger bubbles signify greater influence factors. Furthermore, variations in bubble color reflect p values obtained from enrichment analysis with darker colors indicating higher degrees of enrichment.
In this research, a total of 406 distinct metabolites were identified in these groups. By comparing the different groups using a Venn diagram (Fig. 4A), only 16 unique metabolites (MSD502 vs. MSR88/LHHG/LHR88) were found to be common among them. These shared metabolites include flavonoids, organic acids, amino acids and their derivatives, alkaloids, lipids, phenolic acids, terpenoids, and others. The findings indicate significant variations in the metabolic profiles of four rice varieties obtained from diverse sources.
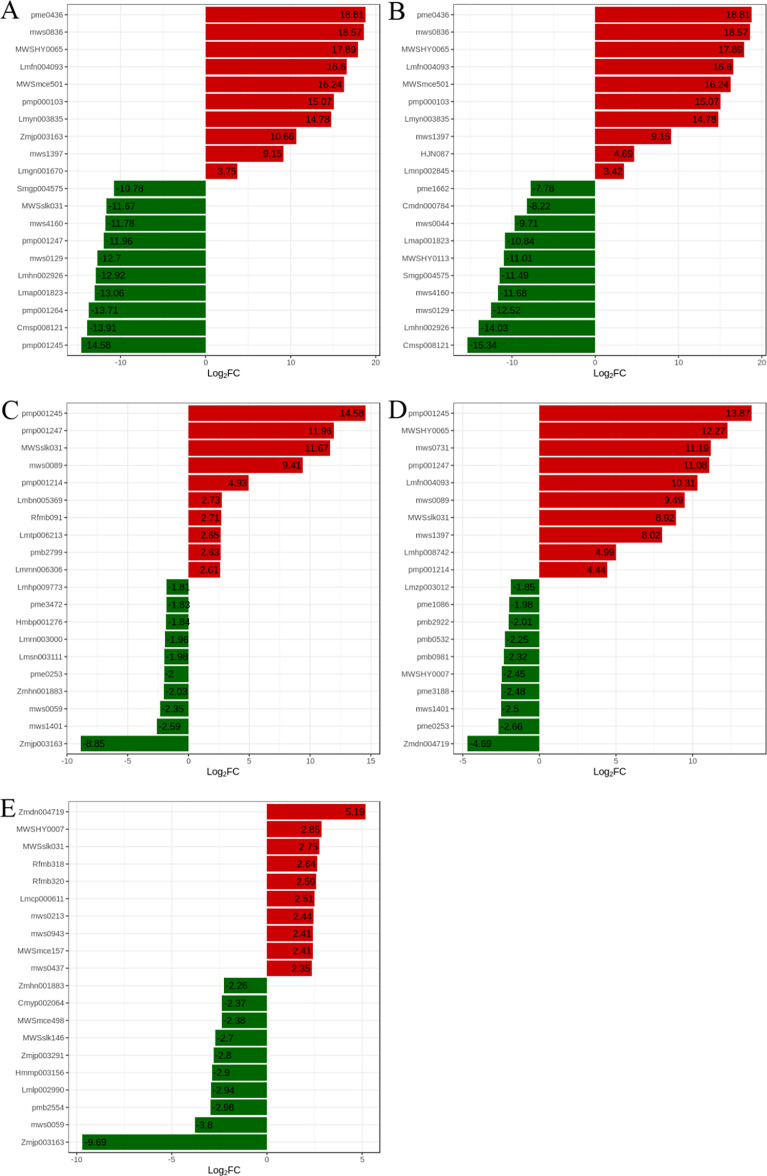
We compared the variation ratios of metabolite quantitative information within each group and subsequently processed these variations (log2FC). The first 20 changes in each group (10 up-regulated and 10 down-regulated) were differentially expressed metabolic components, as shown in Fig. 5. Ten significantly upregulated metabolites in the group of LHR88 vs. LHHG contained three tannins (procyanidin B1, procyanidin B3, arecatannin A2), three flavonoids (catechin, phloretin-4′-O-glucoside (trilobatin), epicatechin gallate), three phenolic acide (protocatechuic acid methyl ester, 4-(3,4,5-trihydroxybenzoxy)benzoic acid, salicylic acid) and one organic acids (jasmonic acid) (Fig. 5A). There are eight metabolites with differences greater than 10 times. The significantly down-regulated metabolites in the group of LHR88 vs. LHHG included four alkaloids (indirubin, feruloylcholine glucoside, hexadecylsphingosine, dhurrin), four flavonoids (quercetin-5-O-β-D-glucoside, wogonin (5,7-Dihydroxy-8-methoxyflavone), feruloylcholine, genkwanin (apigenin 7-methyl ether)), one phenolic acids (p-Coumaroylmalic acid) and one lipids (7-O-methylnaringenin) (Fig. 5A). The differences of these metabolites were more than 10 times.
Figure 5. The highest fold change was observed in the top 10 metabolites that were up-regulated and down-regulated in each comparison group.
Group (A) compared LHR88 to LHHG, Group (B) compared MSD502 to LHHG, Group (C) compared MSD502 to LHR88, Group (D) compared MSD502 to MSR88, and Group (E) compared MSR88 to LHR88. The up-regulated metabolites are represented by red bar charts while the down-regulated metabolites are represented by green bar charts.
Ten significantly upregulated metabolites in the group of MSD502 vs. LHHG contained three tannins (procyanidin B1, procyanidin B3, arecatannin A2), five flavonoids (catechin, phloretin-4′-O-glucoside (trilobatin), epicatechin gallate, naringenin-4′-O-glucoside, Diosmetin-6-C-glucoside), two phenolic acids (protocatechuic Acid Methyl Ester, 4-(3,4,5-Trihydroxybenzoxy)benzoic acid) (Fig. 5B). There are seven metabolites with differences greater than 10 times. The significantly down-regulated metabolites in the group of MSD502 vs. LHHG included seven flavonoids (5,4′-Dihydroxy-7-methoxyflavanone (sakuranetin), dihydroquercetin (Taxifolin), quercetin-3-O-galactoside (Hyperin), quercetin-5-O-β-D-glucoside, wogonin (5,7-Dihydroxy-8-methoxyflavone), genkwanin (Apigenin 7-methyl ether), 7-O-methylnaringenin), one nucleotides and derivatives (uric acid), one alkaloids (dhurrin) and one phenolic acids (p-coumaroylmalic acid) (Fig. 5B). There are seven metabolites with differences greater than 10 times.
Ten significantly upregulated metabolites in the group of MSD502 vs. LHR88 included four alkaloids (feruloylcholine, feruloylcholine glucoside, indirubin, sinapine), three lipids (13(S)-HODE; 13(S)-hydroxyoctadeca-9Z, 11E-dienoic acid, 9S-hydroxy-10E,12Z-octadecadienoic acid, 12,13-Epoxy-9-octadecenoic Acid), one flavonoids (kaempferol-7-O-glucoside), one phenolic acids (3′-Hydroxy-3,4,5-trimethoxybibenzyl) and one others (machilusolide D) (Fig. 5C). There are three metabolites with differences greater than 10 times. The significantly down-regulated metabolites in the group of MSD502 vs. LHR88 included one lipids (1-α-linolenoyl-glycerol-3-O-glucoside), four phenolic acids (arbutin, gallacetophenone, 1-O-p-coumaroyl-β-D-glucose, N-acetyl-L-leucine), one organolic acids (2-hydroxy-3-phenylpropanoic acid), two animo acids and derivatives (vanillic acid-4-O-glucoside, L-theanine), one flavonoids (quercetin-3-O-rutinoside (rutin)) and one organic acids (jasmonic acid) (Fig. 5C).
Ten significantly upregulated metabolites in the group of MSD502 vs. MSR88 comprised four alkaloids (feruloylcholine, feruloylcholine glucoside, indirubin, sinapine), four flavonoids (catechin, phloretin-4′-O-glucoside (trilobatin), kaempferol-7-O-glucoside, epicatechin gallate), one amino acids and derivatives (nicotinuric acid) and one lipids (lysoPC 20:5) (Fig. 5D). There are five metabolites with differences greater than 10 times. The significantly down-regulated metabolites in the group of MSD502 vs. MSR88 included two flavonoids (3-hydroxy-4′,5,7-trimethoxyflavanone, 7-methoxyisoflavone), three amino acids and derivatives (glutathione reduced form, L-theanine, N-Acetyl-L-leucine), four nucleotides and derivatives (uridine 5′-diphospho-D-glucose, inosine 5′-monophosphate, adenosine 5′-monophosphate, uridine 5′-monophosphate) and one others (2-(2′-hydroxypropyl)-5-methyl-7-hydroxychromone) (Fig. 5D).
Ten significantly upregulated metabolites in the group of MSR88 vs. LHR88 contained three others (2-(2′-hydroxypropyl)-5-methyl-7-hydroxychromone, ribitol, D-arabitol), one flavonoids (7-methoxyisoflavone), four alkaloids (indirubin, 2-amino-4,5-dihydro-1H-imidazole-4-acetic acid, 1,4-Dihydro-1-methyl-4-oxo-3-pyridinecarboxamide, stachydrine) and two amino acids and derivatives (D-proline betaine, 1-Methylpiperidine-2-carboxylic acid) (Fig. 5E). The significantly down-regulated metabolites in the group of MSR88 vs. LHRR88 contained two phenolic acids (vanillic acid-4-O-glucoside, 5-O-Feruloyl quinic acid glucoside,), seven flavonoids (yuanhuanin, rhoifolin, 4′-O-Glucosylvitexin, “Vitexin-2”“-O-galactoside”, saponarin (isovitexin-7-O-glucoside), isosaponarin (Isovitexin-4′-O-glucoside), quercetin-3-O-rutinoside (rutin)) and one organic acids (jasmonic acid) (Fig. 5E).
Discussion
In the study, we conducted a widely-targeted metabolomics analysis of 15 rice samples and provided their comprehensive metabolic profile. Previous studies used near infrared spectroscopy and chemometrics to study wheat flour doping and tea origin information, which are relatively easy to achieve, but it is difficult to achieve the qualitative and quantitative components of plant cell metabolism. The metabolome can more accurately reflect the nutrient composition and content of plant cells, so it plays an important role in evaluating food quality (Ana et al., 1998; Xu et al., 2014).
Based on the results of metabolomics, a total of 1,005 metabolites were identified by qualitative and quantitative analysis based on ion pair information of compounds in rice samples. Compared with 732 metabolites identified in colored rice (Zhang et al., 2023) and 672 metabolites identified in millet whole grains (Li et al., 2021), we identified a wider variety of metabolites in rice. The metabolites included 63 nucleotides and their derivatives, 110 amino acids and their derivatives, 87 alkaloids, 80 organic acids, 137 phenolic acids, 21 lignans and coumarins, 172 lipids, 23 terpenoids, five quinones, 184 flavonoids, six tannins, and 117 other metabolites (Table 1). Most of compounds were flavonoids, lipids and phenolic acids. A low percentage of compounds were tannins, quinones. Most of differential metabolites were flavonoids, lipids, and phenolic acids. However, Lignans and coumarins, quinines, and tannins showed a small change not only in different cultivars but also in each treatment (Table 2). Among the different metabolites of the five groups of rice samples, most substances were flavonoids, phenolic acids, lipids, amino acids and their derivatives. LHHG belongs to colored rice, and its phenolic and flavonoid compounds are more highly expressed than white rice. The results show that the metabolites that cause the difference between colored rice and non-colored rice are quite different. Flavonoids exhibit superior antioxidant and anti-inflammatory characteristics (Alves et al., 2016; Min et al., 2012; Min, Mcclung & Chen, 2011) and are important as they contributed to people’s health via gut microbiota regulation (Esfandiar et al., 2023; Li et al., 2023). As an important component of plant phenolic compounds (Ren et al., 2022), they have antiviral, anti-free radical, anti-oxidation, reducing lipid peroxidation, anti-inflammation, anti-aging, anti-cancer and prevention of cardiovascular diseases (Li et al., 2014). The researchers (Kris-Etherton et al., 2002) found that phenolic compounds, including their subflavonoids, are widely found in grains, legumes and nuts. Yang et al. (2016) have demonstrated that flavonoids are mainly present in cotyledon and bran, but not in ground grains. In addition, different metabolites such as lipids, amino acids and their derivatives, organic acids were also more in colored and non-colored rice. In non-colored rice, MSD502 showed that significant differences in different metabolites (such as lipids, phenolic acids, alkaloids and flavonoids) are different from other rice varieties. Different metabolite composition, especially the composition of secondary metabolites, leads to different quality, taste and texture of rice. A previous study showed that the contents of thiamine, oryzanol, total phenols and phyticacid were different, and eating quality, glycemic index, sensory quality were various in three different varieties of raw and cooked rice (Zhao et al., 2024).
In the present study, a total of 406 distinct metabolites were identified in these groups, only 16 unique metabolites (MSD502 vs. MSR88/LHHG/LHR88) were found to be common among them (Fig. 3A). The pathways of different metabolites in different comparison groups showed significant differences. For example, the pathways differential metabolites remarkably enriched in the group of MSD502 vs. MSR88 were “linoleic acid metabolism”, “cutin, suberine and wax biosynthesis”, “citrate cycle (TCA cycle)”, “carbon metabolism”, “arginine biosynthesis” and “alanine, aspartate and glutamate metabolism” (P < 0.05) (Fig. 4B). The pathways differential metabolites remarkably enriched in the group of MSD502 vs. LHHG were “starch and sucrose metabolism”, “propanoate metabolism”, “lysine degradation”, “glutathione metabolism” and “amino sugar and nucleotide sugar metabolism” (P < 0.05) (Fig. 4C). The reasons for these different results may be different ecological environment and climate characteristics. Therefore, it is of great significance to promote the planting of plateau characteristic rice in different places to meet the nutritional and flavor needs of different consumers.
To further analyze the characteristic metabolites between different treatment groups, ten significantly upregulated metabolites and ten significantly downregulated metabolites were chosen. The major upregulated metabolites in the group of LHR88 vs. LHHG were tannins, flavonoids, phenolic acids and organic acids, and the major downregulated metabolites were flavonoids, phenolic acids, alkaloids and lipids. The major upregulated metabolites in the group of MSD502 vs. LHHG were tannins, flavonoids and phenolic acids, and the major downregulated metabolites were nucleotides and derivatives, flavonoids, alkaloids and phenolic acids. The major upregulated metabolites in the group of MSD502 vs. LHR88 were alkaloids, flavonoids, lipids, phenolic acids and others, and the major downregulated metabolites were lipids, phenolic acids, organic acids, amino acids and derivatives, flavonoids. The major upregulated metabolites in the group of MSD502 vs. MSR88 were alkaloids, flavonoids, amino acids and derivatives, flavonoids, lipids, and the major downregulated metabolites were flavonoids, amino acids and derivatives, nucleotides and derivatives, flavonoids, others. The major upregulated metabolites in the group of MSR88 vs. LHR88 were flavonoids, alkaloids, amino acids and derivatives, others, and the major downregulated metabolites were phenolic acids, flavonoids, phenolic acids, organic acids and others. These findings shown that notable variances in the metabolites among distinct rice varieties originating from identical production regions or within the same variety across diverse production areas are common which is consistent with the findings of previous study (Tianxin et al., 2019). It can be seen that compared with non-colored rice, the characteristic flavone in LHHG is proanthocyanidins (Yu et al., 2020) and does not contain anthocyanidins (Finocchiaro, Ferrari & Gianinetti, 2010). Shao & Bao (2015) have shown that proanthocyanidins mainly exist in rice bran. These results are of great significance for preventing the adulteration of the characteristic rice in the plateau and distinguishing the planting areas.
Although this study analysis the metabolomics profiling of different rice in different planting areas, it has not been studied in combination with the environment, soil, flavor and taste of rice in the planting area, and future studies need to further explore their relationship.
Conclusion
In this study, a total of 1,005 metabolites belonging to 12 different classes were detected in the analysis of 15 rice samples. These classes encompassed nucleotides and their derivatives, amino acids and their derivatives, alkaloids, organic acids, phenolic acids, lignans and coumarins, lipids, terpenoids, quinones, flavonoids, tannins as well as other categories. The pathways and enrichment of differential metabolites in the MSD502 vs. MSR88/LHHG/LHR88 comparison groups are significantly different, and only 16 unique metabolites were found to be common among them. The present work is helpful to understand the metabolite composition and flavonoids, phenolic acids and other compounds of different varieties of rice, and provides a basis for the breeders to screen out nutrition-rich and functional quality rice varieties through comparative evaluation. Different varieties of rice planted in different producing area can be clearly separated on PCA plot, which provides a good idea for identifying the adulteration problem of quality rice varieties in Yunnan Province.
Supplemental Information
Funding Statement
This work was funded by the National Natural Science Foundation of China (32060710). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Chao Liu, Email: liuchao_80@163.com.
Fuying Liu, Email: 372243690@qq.com.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Xuheng Nie performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Shuiyan Yang performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Ying Guo analyzed the data, prepared figures and/or tables, and approved the final draft.
Xin Wang analyzed the data, prepared figures and/or tables, and approved the final draft.
Yunman Wen analyzed the data, prepared figures and/or tables, and approved the final draft.
Chao Liu conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Fuying Liu conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files and OMIX: OMIX007768.
References
- Alves et al. (2016).Alves GH, Ferreira CD, Vivian PG, Fernandes Monks JL, Elias MC, Vanier NL, De Oliveira M. The revisited levels of free and bound phenolics in rice: effects of the extraction procedure. Food Chemistry. 2016;208(3):116–123. doi: 10.1016/j.foodchem.2016.03.107. [DOI] [PubMed] [Google Scholar]
- Ana et al. (1998).Ana M, Andrew F, Gerry R, Hill SJ. Preliminary study using trace element concentrations and a chemometrics approach to determine the geographical origin of tea. Journal of Analytical Atomic Spectrometry. 1998;13(6):521–525. doi: 10.1039/a708658j. [DOI] [Google Scholar]
- Catchpole et al. (2005).Catchpole GS, Beckmann M, Enot DP, Mondhe M, Zywicki B, Taylor J, Hardy N, Smith A, King RD, Kell DB. Hierarchical metabolomics demonstrates substantial compositional similarity between genetically modified and conventional potato crops. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(40):14458–14462. doi: 10.1073/pnas.0503955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang et al. (2012).Chang Y, Zhao C, Zhu Z, Wu Z, Zhou J, Zhao Y, Lu X, Xu G. Metabolic profiling based on LC/MS to evaluate unintended effects of transgenic rice with cry1Ac and sck genes. Plant Molecular Biology. 2012;78(4–5):477–487. doi: 10.1007/s11103-012-9876-3. [DOI] [PubMed] [Google Scholar]
- Deng et al. (2015).Deng ML, Xia YP, Zhao B, Tu DW, Yang XS. Application of food omics technology in risk control about food safety. Food and Fermentation Technology. 2015;51:95–99. doi: 10.3969/j.issn.1674-506X.2015.06-021. (in Chinese) [DOI] [Google Scholar]
- Deng et al. (2017).Deng AF, Yang CD, Chen QH, Shi Y, Chen YK, Li CY, Xu SL, Mao GX, Li GY, Xia QM, Zhu HP. Effects of different fertilization methods on yield and photosynthetic material production of direct seeding rice at different densities. China Rice. 2017;23(4):123–129. doi: 10.3969/j.issn.1006-8082.2017.04.025. (in Chinese) [DOI] [Google Scholar]
- Deng et al. (2019).Deng AF, Yang CD, Luo J, Li GY, Zhu HP, Xia QM, Pu YP, Ma SQ. Present situation of rice production in Yunnan province and cuntermeasures for green development. China Rice. 2019;25(3):83–88. doi: 10.3969/j.issn.1006-8082.2019.03.018. (in Chinese) [DOI] [Google Scholar]
- Esfandiar et al. (2023).Esfandiar Z, Hosseini-Esfahani F, Mirmiran P, Azizi F. Higher dietary flavonol and isoflavonoid intakes are associated with lower incidence of type 2 diabetes. International Journal for Vitamin and Nutrition Research. 2023;94(3–4):163–170. doi: 10.1024/0300-9831/a000782. [DOI] [PubMed] [Google Scholar]
- Fiehn (2002).Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Molecular Biology. 2002;48(1/2):155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- Finocchiaro, Ferrari & Gianinetti (2010).Finocchiaro F, Ferrari B, Gianinetti A. A study of biodiversity of flavonoid content in the rice caryopsis evidencing simultaneous accumulation of anthocyanins and proanthocyanidins in a black-grained genotype. Journal of Cereal Science. 2010;51(1):28–34. doi: 10.1016/j.jcs.2009.09.003. [DOI] [Google Scholar]
- Gowda et al. (2008).Gowda GAN, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Review of Molecular Diagnostics. 2008;8(5):617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao et al. (2017).Hao J, Jiang J, Mao T, Sun XD. Research progress of metabonomics in food safety risk monitoring. Journal of Food Safety & Quality. 2017;8(7):2587–2595. (in Chinese) [Google Scholar]
- Kris-Etherton et al. (2002).Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine. 2002;113(9):71–88. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Le Gall et al. (2003).Le Gall G, Dupont MS, Mellon FA, Davis AL, Collins GJ, Verhoeyen ME, Colquhoun IJ. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. Journal of Agricultural & Food Chemistry. 2003;51(9):2438–2446. doi: 10.1021/jf025995e. [DOI] [PubMed] [Google Scholar]
- Li et al. (2023).Li X-Y, Meng L, Shen L, Ji H-F. Regulation of gut microbiota by vitamin C, vitamin E and β-carotene. Food Research International. 2023;169:112749. doi: 10.1016/j.foodres.2023.112749. [DOI] [PubMed] [Google Scholar]
- Li et al. (2014).Li X, Xu YB, Sun J, Qian Y, Shang XJ. Extraction of total flavonoids from brown rice and its scavenging capacity on hydroxyl free radicals. Journal of Jilin Agricultural University. 2014;36(3):347–351. doi: 10.13327/j.jjlau.2014.2141. (in Chinese) [DOI] [Google Scholar]
- Li et al. (2021).Li W, Wen L, Chen Z, Zhang Z, Guo Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chemistry. 2021;357(4):129791. doi: 10.1016/j.foodchem.2021.129791. [DOI] [PubMed] [Google Scholar]
- Min et al. (2012).Min B, Gu L, Mcclung AM, Bergman CJ, Chen MH. Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chemistry. 2012;133(3):715–722. doi: 10.1016/j.foodchem.2012.01.079. [DOI] [Google Scholar]
- Min, Mcclung & Chen (2011).Min B, Mcclung AM, Chen MH. Phytochemicals and antioxidant capacities in rice brans of different color. Journal of Food Science. 2011;76(1):C117–C126. doi: 10.1111/j.1750-3841.2010.01929.x. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development (1993).Organization for Economic Co-operation and Development . Safety evaluation of foods derived through modern biotechnology: concepts and principles. Paris: Organization for Economic Co-operation and Development; 1993. [Google Scholar]
- Ramirez et al. (2013).Ramirez T, Daneshian M, Kamp H, Bois FY, Clench MR, Coen M, Donley B, Fischer SM, Ekman DR, Fabian E. t4 report: metabolomics in toxicology and preclinical research. ALTEX. 2013;30(2):209–225. doi: 10.14573/altex.2013.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren et al. (2022).Ren CY, Lu SW, Hong B, Zhang YL, Guan LJ, Li B, Huang WG, Lu WH. Non-targeted metabolomic analysis of brown rice and white rice. Food Science. 2022;43(20):183–190. doi: 10.7506/spkx1002-6630-20211207-082. (in Chinese) [DOI] [Google Scholar]
- Scalbert et al. (2009).Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, Pujos-Guillot E, Verheij E, Wishart D, Wopereis S. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5(4):435–458. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao & Bao (2015).Shao Y, Bao J. Polyphenols in whole rice grain: genetic diversity and health benefits. Food Chemistry. 2015;180:86–97. doi: 10.1016/j.foodchem.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Tang & Wang (2006).Tang HR, Wang YL. Metabonomics: a revolution in progress. Progress in Biochemistry and Biophysics. 2006;33(12):401–417. [Google Scholar]
- Tebani & Bekri (2019).Tebani A, Bekri S. Paving the way to precision nutrition through metabolomics. Frontiers in Nutrition. 2019;6:373. doi: 10.3389/fnut.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tianxin et al. (2019).Tianxin FU, Yuchao F, Liyuan Z, Xue LI, Changyuan W. Metabonomics study on rice from different geographical areas based on gas chromatography-mass spectrometry. Food Science. 2019;40:176–180. doi: 10.7506/spkx1002-6630-20180621-412. (in Chinese) [DOI] [Google Scholar]
- Xu et al. (2014).Xu YR, Liu CL, Sun XR, Wu SN, Dong XL. Determination of wheat flour adulteration based on Near- and Mid-Infrared spectroscopy. Food Chemistry. 2014;35(12):128–132. doi: 10.7506/spkx1002-6630-201412025. (in Chinese) [DOI] [Google Scholar]
- Yang et al. (2016).Yang Z, Nakabayashi R, Mori T, Takamatsu S, Kitanaka S, Saito K. Metabolome analysis of Oryza sativa (Rice) using liquid chromatography-mass spectrometry for characterizing organ specificity of flavonoids with anti-inflammatory and anti-oxidant activity. Chemical and Pharmaceutical Bulletin. 2016;64(7):952–956. doi: 10.1248/cpb.c16-00180. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2020).Yu XT, Yang T, Qi QQ, Du YM, Shi J, Liu XM, Liu YH, Zhang HB, Zhang ZF, Yan N. Comparison of the contents of phenolic compounds including flavonoids and antioxidant activity of rice (Oryza sativa) and Chinese wild rice (Zizania latifolia) Food Chemistry. 2020;15(344):128600. doi: 10.1016/j.foodchem.2020.128600. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2023).Zhang L, Cui D, Ma X, Han B, Han L. Comparative analysis of rice reveals insights into the mechanism of colored rice via widely targeted metabolomics. Food Chemistry. 2023;399(1):133926. doi: 10.1016/j.foodchem.2022.133926. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2024).Zhao S, Shi J, Cai S, Xiong T, Cai F, Li S, Chen X, Fan C, Mei X, Sui Y. Impact of rice variety, cooking equipment and pretreatment method on the quality of lightly milled rice. Food Chemistry. 2024;451(2):139271. doi: 10.1016/j.foodchem.2024.139271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files and OMIX: OMIX007768.