Abstract
Metronidazole (MTZ) is a broad-spectrum antibiotic with numerous routes of administration, including topical. Topical application of MTZ gel or cream results in very low systemic absorption, resulting in the need for a sensitive extraction method to quantify plasma concentrations. Currently published methods are not suitable for analysis of plasma concentrations after topical application, as undetectable MTZ concentrations commonly occur. We validated a simple extraction method for MTZ recovery from plasma and quantified it using an LC-MS/MS analytical method. Methods: Plasma samples were spiked with MTZ (0.5 – 5 ng/mL) and internal standard (tinidazole, 2 ng/mL). MTZ was extracted by liquid-liquid extraction using ethyl acetate and acetonitrile mixture (4:1) as the extraction solvent. A quadrupole mass spectrometer interfaced with an Acquity H-Class HPLC was used to quantify MTZ concentrations in positive ion mode. A Kinetix C18 analytical column (150 mm × 4.6 mm i. d., 5 μm particle size) was used for separation. The plasma extraction method was validated for various parameters, including % recovery, precision, accuracy, and stability. Results: The extraction method demonstrated high MTZ recovery, ranging from 93.7 – 97.5%. The calibration curve prepared using MTZ samples extracted from plasma (0.5 – 5 ng/mL) had excellent linearity with R2 = 0.999. The extracted samples also showed higher autosampler and freeze-thaw stability over a 72-hr period. The mean intra- and inter-day accuracy and precision of the extraction assay ranged from 97 to 101.6% and 2.7 – 4.8% RSD, respectively. The assay was highly efficient, with a limit of quantification (0.53 ± 0.04 ng/mL) lower than previously published methods (≥5 ng/mL). The extraction method was successfully validated using LC-MS/MS and can be used to extract and detect trace amounts of MTZ in plasma after topical application.
Keywords: Metronidazole, Bioanalytical assay, Human plasma, Extraction, Validation, LC-MS/MS
1. Introduction
Metronidazole (MTZ) is a safe and therapeutically effective antibiotic extensively used to eradicate anaerobic and parasitic infections. As a small molecule (171.15 Da), MTZ can be administered via numerous routes, including intravenous, oral, vaginal, and topical. MTZ doses vary widely depending on route of administration and clinical indication. Consequently a large range of peak serum MTZ concentrations have been reported in human subjects, ranging from 6 μg/mL to 40 μg/mL following single oral doses of various strengths [1]. Bioavailability after oral administration is 100%, followed by extensive hepatic metabolism and renal excretion.
Because of its physicochemical properties (specifically, logP of − 0.15 and 20 mg/mL water solubility), MTZ is very suitable for topical administration. MTZ is commercially available as topical creams, gels, lotions, and jellies, in concentrations ranging from 0.75% to 1.3% [1–3]. As a dermal product, MTZ is clinically indicated for the localized treatment of rosacea, a chronic inflammatory dermatosis. In rosacea treatment, MTZ is applied topically once or twice daily to affected areas and is thought to exert a local anti-inflammatory effect [1,4]. Response to treatment can typically be seen within 3 weeks. Because of the localized nature of topical treatment, serum MTZ concentrations are typically below detectable levels for most patients [4]. This highlights the challenges of analyzing therapeutic compounds in serum or plasma during dermal pharmacokinetic studies of topical products, including MTZ. Drug plasma concentrations are often undetectable, and sometimes in the ng/mL range. However, MTZ has frequently been used as a model drug to develop robust in vitro-in vivo correlation (IVIVC) and bioequivalence studies for comparison of topical products [5]. Thus, detecting low MTZ concentrations in biological samples is of high importance and a simple and highly sensitive plasma extraction process would be valuable.
Many reports have described extraction and quantification of MTZ from human plasma. However, previous extraction methods are complicated and require multiple steps, increasing the risk of losing detectable sample. Additionally, previously published detection methods had high limits of detection or quantification (≥5 ng/mL) that may not be suitable for topical MTZ delivery studies [2,6–8]. The main objective of the present work was to develop a simple and reproducible MTZ plasma extraction method with greater sensitivity than previously reported methods, and validate the method using LC-MS/MS. The long-term goal is to use both methods (extraction and detection) in dermal and transdermal pharmacokinetic studies in healthy human subjects.
2. Materials and methods
2.1. Chemicals and reagents
Analytical standard metronidazole (Cat. M3761) was purchased from Sigma Aldrich (St. Louis, MO, USA). Tinidazole (TND) with more than 98% purity (Cat. T3058) was used as an internal standard and was purchased from Tokyo Chemical Industry (Japan). Pooled human plasma was purchased from Innovative Research (Novi, MI, USA). Ethyl acetate, acetonitrile, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Liquid chromatography tandem mass spectrometry (LC-MS/MS) detection
LC-MS/MS analysis was performed at the University of Iowa High Resolution Mass Spectrometry Core Facility on a Waters Xevo TQ-S Cronos quadrupole mass spectrometer interfaced with an Acquity H-Class HPLC (Waters Corporation, USA). Electrospray ionization (ESI) was operated in positive ion mode. A full scan was conducted to produce the complete mass spectrum and identify the fractioned daughter ions produced during transitions of MTZ and tinidazole (TND). The best m/z transitions for MTZ and TND were 172.03→111 and 248.03→121.01, respectively. The source temperature and desolvation line temperature were maintained at 148 °C and 399 °C, respectively. The analytical column used for separation was a Kinetix C18 column (150 mm × 4.6 mm i.d., 5 μm particle size) with a Phenomenex AJO-4287 C18 guard cartridge (5 mm × 4.6 mm i.d., 5 μm particle size) (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 80:20 ratio of 0.1% v/v formic acid:acetonitrile at a flow rate of 0.5 mL/min. The column temperature was maintained at 30 °C. A sample volume of 20 μL was injected and the total run time was 6.0 min. All settings are shown in Table 1.
Table 1.
Mass spectrometer settings for MTZ and TND analysis.
| Parameter | Values |
|---|---|
|
| |
| Ionization method | Electron spray ionization |
| Ionization mode | Positive ion |
| Capillary voltage | 1.5 KV |
| Cone voltage | 26 Volts |
| Collision energy | 22 Volts |
| Source temperature | 148 °C |
| Desolvation temperature | 399 °C |
| Precursor to product ion transition (MTZ) | 172.03 → 111 |
| Precursor to product ion transition (TND) | →121.01 |
MTZ = metronidazole; TND = tinidazole (internal standard)
2.3. Preparation of calibration standards and quality control samples
A 5000 ng/mL MTZ working solution was prepared in methanol and diluted with HPLC grade water to achieve final MTZ standard spiking solutions at concentrations of 10, 20, 30, 40, 60, 80, and 100 ng/mL. A 1000 μg/mL stock solution of TND in methanol was diluted with HPLC grade water to achieve a final concentration of 2 ng/mL. TND was chosen as an internal standard for these studies because it is a small molecule with a similar structure to MTZ (both are in the nitroimidazole group), has a different daughter ion, and has similar retention to MTZ. The stock solutions of MTZ and TND were stored at 4 °C. Calibration standards for MTZ plasma samples were prepared by spiking blank human plasma with MTZ standard spiking solution to achieve final concentrations of 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 5.0 ng/mL. Quality control samples contained 0.75, 2.5, and 4.5 ng/mL MTZ in plasma or water. All spiked plasma samples were stored at − 80 °C until used for extraction.
2.4. Plasma extraction procedure
Liquid-liquid extraction (protein precipitation) was used to extract MTZ from human plasma. Two-hundred microliters of MTZ-spiked plasma samples and 50 μL of 2 ng/mL TND standard solution (as an internal standard) were mixed with 4:1 ethyl acetate:acetonitrile mixture and vortexed for 60 s. This solvent ratio was determined after several initial trials of single solvent (ethyl acetate vs acetonitrile) vs solvent combinations (data not shown). The internal standard was constant for all the samples and used to improve the accuracy and precision of the method, keeping the MTZ/TND ratio unchanged during extraction. The vortexed mixture was centrifuged at 10,000 rpm for 10 min. After centrifugation, the supernatant organic layer was transferred to 5 mL glass tubes. The extraction step was repeated on the sedimented plasma samples using fresh ethyl acetate:acetonitrile mixture. The collected organic layer was dried under nitrogen gas at 40 °C for 20 min. Finally, the dried residues were reconstituted with 200 μL of 0.1% formic acid and sonicated for 15 min before analysis. The extracted samples were analyzed using the LC-MS/MS method described above. Finally, the ratio of MTZ/TND response was plotted against MTZ concentrations to determine the various bioanalytical method validation parameters.
2.5. Validation of MTZ plasma extraction assay
2.5.1. Stability studies
The freeze-thaw stability and autosampler stability of the method were evaluated to check the effectiveness for eventual pharmacokinetic studies in humans. For freeze-thaw stability analysis, previously frozen MTZ quality control plasma samples were thawed on day one and then immediately frozen again at − 80 °C. The MTZ spiked plasma samples were extracted after three consecutive freeze-thaw cycles, occurring on three sequential days. For autosampler stability testing, the MTZ sample prepared from spiked human plasma samples was stored at 4 °C for 72 hr in the autosampler and then analyzed. These studies were completed because it is important to study stability in the autosampler when the temperature of the autosampler is different than the specified storage condition of the samples (4 °C in the autosampler vs − 80 °C for typical sample storage). Accuracy and precision were calculated for each quality control sample.
2.5.2. Percent recovery and matrix effect
The percent (%) recovery represents the ability of the method to extract trace amounts of a compound from spiked plasma samples. To determine the % recovery, the peak area of MTZ quality control plasma samples spiked before extraction was compared with the peak area of blank plasma samples spiked after extraction. Moreover, peak areas of blank plasma samples spiked after extraction were compared with peak areas of MTZ quality control samples prepared in water to determine a matrix effect and calculate the matrix factor. Both % recovery and matrix factor were calculated using 0.75, 2.5, and 4.5 ng/mL quality control concentration levels. The matrix factor should not be more than ± 15% to meet acceptable criteria [9]. % recovery and matrix factor were calculated according to the following equations:
The matrix factor was calculated based on methods used in previous reports [10].
2.5.3. Specificity
Specificity represents the ability to explicitly detect the analyte (MTZ) in the presence of impurity, matrix, or other components expected to be present in test samples. Samples prepared from the extraction of blank human plasma samples, plasma samples spiked with TND, and plasma samples spiked with MTZ + TND were tested to determine the specificity of the LC-MS/MS method. The results of blank plasma and TND spiked plasma samples were compared with MTZ + TND spiked samples to detect any interference.
2.5.4. Range and linearity
Linearity represents the direct relationship of the response with the concentration of the analyte. The final calibration curves included MTZ concentrations ranging from 0.5 to 5 ng/mL, with 3 replicates per calibration point. Linearity was assessed through linear regression between MTZ concentration, x, and the MTZ to TND peak area ratio, y. The final calibration curve was plotted as MTZ/TND ratio vs MTZ concentration (ng/mL).
2.5.5. Lower limit of detection (LLOD) and quantification (LLOQ)
The LLOD and LLOQ are calculated to validate sensitivity of the method. The LLOD represents the minimum amount of analyte in a test sample that can be detected but cannot be precisely quantified. The standard deviation and slope method was used to calculate the LLOD and LLOQ. In addition, LLOD and LLOQ were also calculated based on the signal to noise ratio to consolidate the data (signal to noise ratio is one of three methods that can be used to calculate LLOD and LLOQ, according to FDA guidelines) [11]. The LLOD was calculated from the calibration curve of MTZ obtained from extracted plasma samples according to the following equation (standard deviation and slope method):
The LLOQ represents the minimum amount of analyte in the test sample that can be accurately and precisely quantified. It was calculated from the calibration curve of MTZ obtained from extracted plasma samples according to the following equation:
2.5.6. Accuracy and precision
Studies of intra- and inter-day accuracy and precision of the plasma extraction method were conducted over a period of three days. Blank plasma, plasma spiked with TND only, plasma spiked with MTZ calibration standards + TND, and plasma spiked with quality control samples + TND were analyzed each day. Five replicates were prepared for each MTZ concentration for all plasma samples. The mean concentration of MTZ in quality control samples was calculated to determine the intra-day and inter-day % accuracy of the plasma extraction method. Finally, the assay accuracy was calculated from the mean accuracy found on each day for each individual quality control sample.
Precision measurements were conducted similarly as above. Percent relative standard deviation (%RSD) was calculated from the mean concentration of quality control samples. Overall precision of the assay was defined by calculating the mean precision achieved on different days for each quality control concentration. Five replicates were prepared for each MTZ concentration for all plasma samples.
3. Results and discussion
3.1. LC-MS/MS method
A Kinetix C18 (150 mm × 4.6 mm i.d., 5 μm particle size) was used for the separation of MTZ and TND during analysis and HPLC parameters were adapted from previous methods, with slight modifications to the composition and flow rate of the mobile phase [12]. The combination of 0.1% formic acid in water and acetonitrile (80:20) at the 0.5 mL/min flow rate comprised the ideal condition for analysis. Under these conditions MTZ and TND had retention times of 3.4 min and 4.5 min, respectively.
3.2. % recovery and matrix effect
The % MTZ recovery from 100 μL of human plasma was evaluated using quality control samples (0.75, 2.5, and 4.5 ng/mL MTZ) with five replicates each. The extraction method exhibited excellent recovery of MTZ from plasma, with mean recovery ranging from 93.7 – 97.5%. The % recovery of TND was similar, with a mean recovery of 96.5 – 98.5%. The biological matrix can either enhance or suppress the response of the analyte. In our study the matrix effect was insignificant: the matrix factor varied from − 4.7 to − 5.3%, indicating that the MTZ ion response was suppressed only slightly in the presence of human plasma matrix. However the suppression was nonsignificant and met acceptance criteria that the matrix factor should not exceed ± 15% [9].
3.3. Stability
The MTZ-spiked plasma samples showed excellent stability at the end of freeze-thaw and autosampler stability studies. The accuracy and precision of the quality control sample autosampler stability study ranged from 98.2 – 104.3% for accuracy and 1.9 – 5.03 %RSD for precision. However, after three freeze-thaw cycles over 72 hrs, both accuracy and precision decreased slightly to 95.8 – 97.1% and 1.3 – 4.1% RSD, respectively. Results from all stability studies are shown in Table 2.
Table 2.
Results from freeze-thaw and autosampler stability studies. Results displayed as mean ± SD, n = 5.
| Validation parameter | Quality control sample concentration in plasma (ng/mL) |
|||
|---|---|---|---|---|
| 0.75 | 2.5 | 4.5 | ||
|
| ||||
| Autosampler stability | MTZ concentration (ng/mL) | 0.78 ± 0.04 | 2.45 ± 0.05 | 4.50 ± 0.09 |
| Accuracy, % | 104.31 ± 5.25 | 98.19 ± 2.11 | 100.10 ± 1.90 | |
| Precision, %RSD | 5.03 | 2.15 | 1.90 | |
| Freeze thaw stability | MTZ concentration (ng/mL) | 0.72 ± 0.03 | 2.40 ± 0.09 | 4.37 ± 0.05 |
| Accuracy, % | 95.76 ± 3.91 | 96.06 ± 3.60 | 97.12 ± 1.21 | |
| Precision, %RSD | 4.09 | 3.74 | 1.25 | |
3.4. Specificity
The validated bioanalytical method was highly selective and the blank plasma samples did not exhibit any matrix or impurity-originated interference. Any peaks created by blank plasma samples at the MTZ retention time (3.4 min) were < 20% of the response observed for the 0.5 ng/mL MTZ samples, which was the lowest MTZ concentration in our studies. Similarly, any peaks observed for blank plasma samples at the TND retention time (4.5 min) were much lower than 5% of the response for the lowest TND concentration tested. The chromatogram of blank human plasma, plasma spiked with TND, and plasma spiked with MTZ and TND are shown in Fig. 1.
Fig. 1.
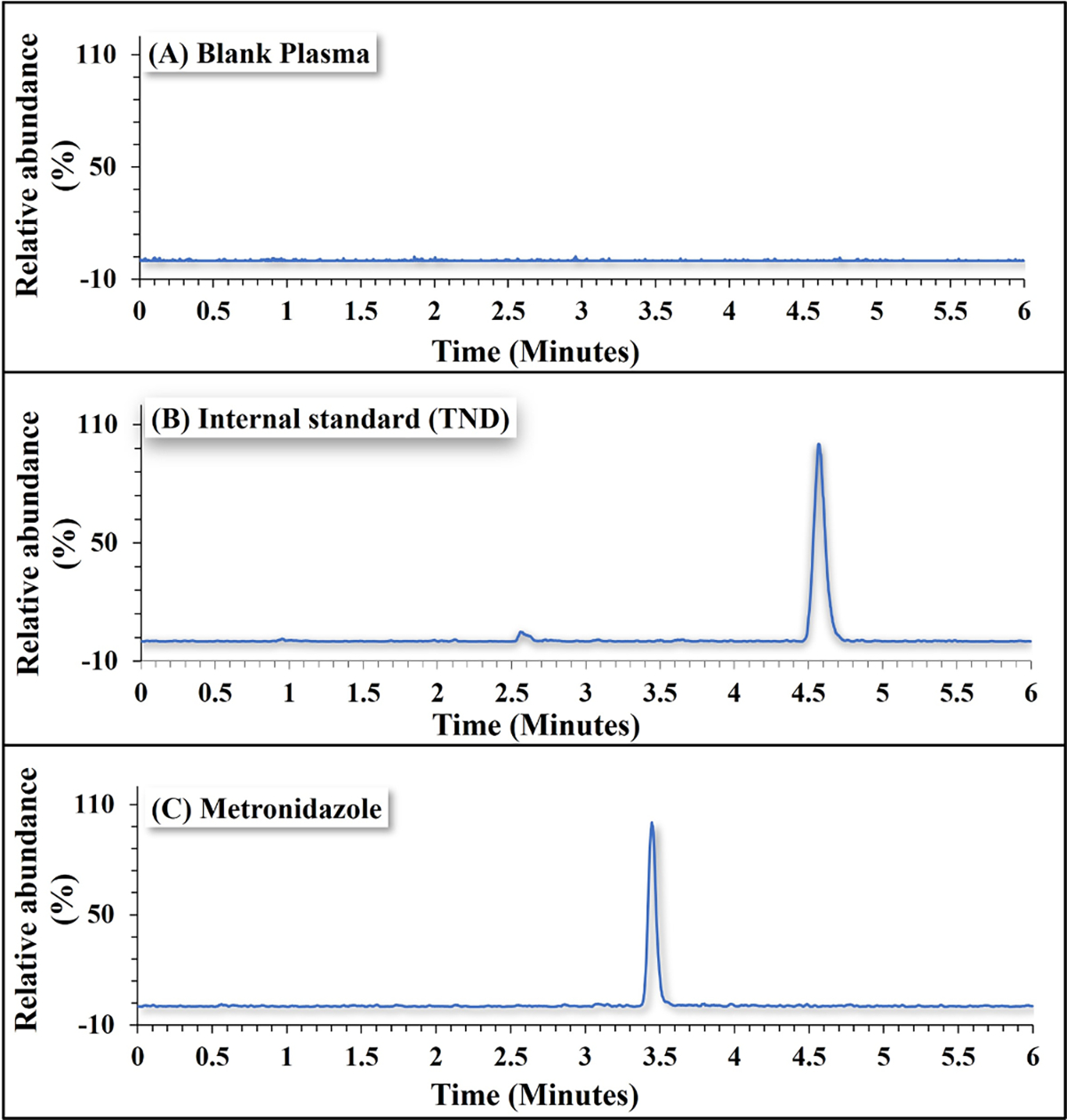
Chromatogram of MTZ (0.5 ng/mL) and internal standard (TND, 2 ng/mL) extracted from spiked human plasma.
3.5. Linearity
The calibration curve prepared from MTZ-spiked plasma samples was linear over the tested concentration range (0.5 – 5 ng/mL). Intra- and inter-day averages (n = 3) were used to plot the calibration graph (MTZ:TND ratio vs time) and calculate the validation parameters. The r2 value of 0.999 confirmed that the plot was linear over the range of calibration standards.
3.6. Lower limit of detection (LLOD) and lower limit of quantification (LLOQ)
The MTZ extraction method was highly efficient and exhibited a LLOD of 0.17 ± 0.01 ng/mL and LLOQ of 0.53 ± 0.04 ng/mL, which is much lower than previously reported bioanalytical methods (Table 3). The signal-to-noise ratio for the 0.5 ng/mL MTZ concentration ranged from 9 – 11, which complies with FDA guidance and supports the above LLOQ result for bioanalytical method validation qualification [13].
Table 3.
Comparison of the current human plasma extraction method with published methods of MTZ quantification.
| Extraction method/Extraction solvent | Detection method | LLOQ | Linearity range | LC flow | LC mobile phase composition | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| LLE / ACN and EA (1:4) | MS/MS | 0.5 ng/ mL | 0.5 – 5 ng/mL | Isocratic | 0.1% FA in water: ACN (80:20) | Current method |
| LLE / ACN | MS | 8 ng/mL | 0.1–6.4 μg/mL | Gradient | 0.1% v/v FA in 1:1 MeOH:water | [6] |
| LLE / 1 M NaOH, MTBE, and DCM | MS/MS | 10 ng/mL | 0.01–10 μg/mL | Isocratic | ACN: 10.0 mm AF in water (80:20) | [15] |
| SPE / Oasis HLB 30 pm, 96-well extraction plates | MS/MS | 15 ng/mL (0.09 μM) | 1 – 3000 μM | Gradient | 0.1% FA in water: 0.1% FA in MeOH (80:20) | [7] |
| LLE / 10 mM NaOH, EA | MS/MS | 50 ng/mL | 50 – 20,000 ng/ mL | Isocratic | 0.1% FA in water:0.1% FA in MeOH (80:20) | [8] |
| LLE / 0.1 N NaOH, 0.1 N pH 9 buffer and EA | MS/MS | 50 ng/mL | 50 – 5000 ng/mL | Gradient | ACN/water/FA (15:85:0.1, v/v/v) | [2] |
| LLE / MTBE | MS/MS | 50 ng/mL | 50 – 500 ng/mL | Gradient | 5 mM AF buffer (pH 3.5) and ACN | [16] |
AA: Ammonium acetate; ACN: Acetonitrile; AF: Ammonium formate; DCM: Dichloromethane; EA: Ethyl acetate; FA: formic acid; LLE: Liquid-liquid extraction; MeOH: Methanol; MTBE: Methyl-tert butyl ether; MS: mass spectrometry; MS/MS: tandem mass spectrometry; SPE: Solid phase extraction
3.7. Accuracy and precision
The % accuracy and precision were calculated from three different runs, completed on three different days, to determine intra- and inter-day accuracy and precision. Table 4 shows a summary of intra-day and inter-day accuracy and precision results calculated from three different quality control samples (0.75, 2.5, and 4.5 ng/mL MTZ). The mean accuracy of the extraction method ranged from 97.0 – 101.6%. The mean precision ranged from 2.7 – 4.8% RSD. These findings of accuracy and precision comply with FDA and ICH guidelines for bioanalytical method development, which suggest that both accuracy and precision should be within the limit of ± 15% [13].
Table 4.
Percent recovery, precision, and accuracy calculated for MTZ quality control samples during validation.
| Validation parameter | MTZ concentration in quality control samples (ng/mL) |
|||
|---|---|---|---|---|
| 0.75 | 2.5 | 4.5 | ||
|
| ||||
| % Recovery (n = 5) | MTZ | 97.53 ± 4.23 | 95.12 ± 1.42 | 93.74 ± 2.78 |
| % Accuracy (n = 5) | Day 1 (Intra-day) | 103.67 ± 2.99 | 96.32 ± 4.32 | 97.48 ± 4.45 |
| Day 2 (Inter-day) | 101.53 ± 4.13 | 95.43 ± 2.58 | 98.65 ± 8.86 | |
| Day 3 (Inter-day) | 99.65 ± 1.25 | 99.24 ± 0.80 | 98.42 ± 0.93 | |
| Mean accuracy (n = 15) | Day 1, 2 and 3 | 101.62 ± 3.45 | 97.00 ± 3.36 | 98.18 ± 5.77 |
| Precision, % RSD (n = 5) | Day 1 (Intra-day) | 2.89 | 4.48 | 4.56 |
| Day 2 (Inter-day) | 4.06 | 2.70 | 8.98 | |
| Day 3 (Inter-day) | 1.26 | 0.80 | 0.94 | |
| Mean precision (n = 15) | Days 1, 2 and 3 | 2.74 | 2.66 | 4.83 |
MTZ = metronidazole
3.8. Comparison with previous methods and future applications
In the current study we aimed to develop a simple extraction method with minimal processing or extraction steps, while also targeting a lower LLOD and LLOQ than what has previously been reported. Our method has a LLOQ of 0.5 ng/mL, which is 16 times lower than the lowest previously reported LLOQ of 8 ng/mL (LLOQ from previous studies ranged from 8 – 50 ng/mL, Table 3). Our detection method uses isocratic LC flow, which is often considered more straightforward than gradient flow. Further, we achieved a highly sensitive extraction (93.7 – 97.5% recovery) with only two short liquid-liquid extraction steps.
Because of its physicochemical properties and availability in multiple topical dosage forms and strengths, MTZ is an ideal compound for dermal pharmacokinetics studies. In particular, its hydrophilic nature makes MTZ especially suitable for studying microneedle-assisted dermal delivery. Microneedles are a physical enhancement technique that allow transdermal delivery of hydrophilic compounds that do not deliver well through the hydrophobic outer skin layers. MTZ has been used as a model compound in prior in vitro studies, in which the formulation type (gel vs cream) significantly impacted the extent of MTZ delivered through excised skin treated with microneedles [14]. The newly developed method we report here will be used in an upcoming clinical trial (ClinicalTrials.gov identifier NCT05929794), in which MTZ will be quantified in plasma after applying MTZ to microneedle-treated skin in healthy human subjects. To maximize the safety profile of the study, very small volumes of 0.75% MTZ cream or gel will be applied to microneedle-treated skin. This further underscores the importance of having a very low LLOD and LLOQ, which we have demonstrated here. Further, our method is very simple, making it well suited for analyzing the high volume of plasma samples that are collected during pharmacokinetic studies. One potential limitation of our method is that we did not specifically determine linearity at concentrations higher than 5 ng/mL. However, we do not expect this to be a significant concern because plasma concentrations in the pharmacokinetic study will likely be low, making our 0.5 – 5 ng/mL range suitable for the study objectives.
4. Conclusions
We developed a precise, reproducible, and sensitive method to extract MTZ from human plasma and used LC-MS/MS to quantify MTZ concentrations. Our simple method has higher recovery, higher sensitivity, and a lower limit of quantification (0.5 ng/mL) than previously published methods. This validated extraction method will be applicable for analyzing MTZ in plasma samples collected during a dermal kinetics study in human subjects, which are likely to contain extremely low MTZ concentrations (below 1 ng/mL).
Acknowledgments
We are grateful to Dr. Lynn Teesch and Dr. Vic Parcell for providing technical assistance during method validation at the University of Iowa Core Research Facility.
This work was funded by the National Institutes of Health grant R35GM124551.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nicole Brogden reports financial support was provided by National Institutes of Health. Nicole Brogden reports a relationship with National Institutes of Health that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Brogden Nicole: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization. Cota Valeria: Writing – review & editing, Methodology, Data curation. Patel Krishna Kumar: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
References
- [1].Metronidazole. In Depth Answers [database on the Internet]. Greenwood Village (CO): IBM Corporation; 2023. [cited 2023 Dec 12]. Available from: 〈www.micromedexsolutions.com〉. Subscription required to view. [Google Scholar]
- [2].Sagan C, Salvador A, Dubreuil D, Poulet PP, Duffaut D, Brumpt I, Simultaneous determination of metronidazole and spiramycin I in human plasma, saliva and gingival crevicular fluid by LC-MS/MS, J. Pharm. Biomed. Anal. (2) (2005) 298–306, 10.1016/j.jpba.2004.12.033. [DOI] [PubMed] [Google Scholar]
- [3].Silva M, Schramm S, Kano E, Koono E, Porta V, Serra C, Development and validation of a HPLC-MS-MS method for quantification of metronidazole in human plasma, J. Chromatogr. Sci. 47 (2009) 781–784, 10.1093/chromsci/47.9.781. [DOI] [PubMed] [Google Scholar]
- [4].Product Information: metronidazole 0.75% topical gel. PruGen Pharmaceuticals (per DailyMed), Scottsdale, AZ, 2015. [Google Scholar]
- [5].Rath S, Kanfer I, A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach, Pharmaceutics 12 (2020) 119, 10.3390/pharmaceutics12020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kathriarachchi UL, Vidhate SS, Al-Tannak N, Thomson AH, da Silva Neto MJJ, Watson DG, Development of a LC-MS method for simultaneous determination of amoxicillin and metronidazole in human serum using hydrophilic interaction chromatography (HILIC), J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1089 (2018) 78–83, 10.1016/j.jchromb.2018.05.012. [DOI] [PubMed] [Google Scholar]
- [7].Stancil SL, van Haandel L, Abdel-Rahman S, Pearce RE, Development of a UPLC-MS/MS method for quantitation of metronidazole and 2-hydroxy metronidazole in human plasma and its application to a pharmacokinetic study, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1092 (2018) 272–278, 10.1016/j.jchromb.2018.06.024. [DOI] [PubMed] [Google Scholar]
- [8].Yoon J, Kang SW, Shim WS, et al. , Quantification of metronidazole in human bile fluid and plasma by liquid chromatography-tandem mass spectrometry, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1138 (2020) 121959, 10.1016/j.jchromb.2019.121959. [DOI] [PubMed] [Google Scholar]
- [9].Bhateria M, Ramakrishna R, Pakala DB, Bhatta RS, Development of an LC-MS/MS method for simultaneous determination of memantine and donepezil in rat plasma and its application to pharmacokinetic study, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1001 (2015) 131–139, 10.1016/j.jchromb.2015.07.042. [DOI] [PubMed] [Google Scholar]
- [10].Koseki N, Kawashita H, Hara H, et al. , Development and validation of a method for quantitative determination of valsartan in human plasma by liquid chromatography-tandem mass spectrometry, J. Pharm. Biomed. Anal. 43 (2007) 1769–1774, 10.1016/j.jpba.2006.12.030. [DOI] [PubMed] [Google Scholar]
- [11].FDA Guidance Document CVM GFI #2088 (VICH GL49). Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies, March 2015: Silver Spring, MD and/or Center for Veterinary Medicine (CVM): Rockville, MD. p. 1–23. [Google Scholar]
- [12].Vanol PG, Sanyal M, Shah PA, Shrivastav PS, Quantification of metronidazole in healthy subjects’ feces using an LC-MS/MS method, Biomed. Chromatogr. 21 (2018) e4265, 10.1002/bmc.4265. [DOI] [PubMed] [Google Scholar]
- [13].FDA Guidance Document. Bioanalytical Method Validation Guidance for Industry. 2018: Silver Spring, MD and/or Center for Veterinary Medicine (CVM): Rockville, MD. p. 1–44. [Google Scholar]
- [14].Patel KK, Brogden NK, Impact of Formulation and Microneedle Length on Transdermal Metronidazole Permeation through Microneedle-Treated Skin, Published online December 22,, Pharm. Res (2023), 10.1007/s11095-023-03640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vanol PG, Sanyal M, Shah PA, Shrivastav PS, Quantification of metronidazole in human plasma using a highly sensitive and rugged LC-MS/MS method for a bioequivalence study, Biomed. Chromatogr. 32 (2018) e4242, 10.1002/bmc.4242. [DOI] [PubMed] [Google Scholar]
- [16].Ilomuanya M, Uboh C, Ciallella J, et al. , Analysis of metronidazole in equine plasma using liquid chromatography/tandem mass spectrometry and high-resolution accurate mass spectrometry, Rapid Commun. Mass Spectrom. 29 (2015) 753–763, 10.1002/rcm.7158. [DOI] [PubMed] [Google Scholar]


