Abstract
We present a versatile nickel-electrocatalytic deaminative cross-coupling platform for the efficient construction of C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds from readily available alkyl bistriflimides. This methodology involves the assembly of two leaving groups on alkyl amines to form alkyl bistriflimides, followed by their effective coupling with a wide range of alkyl halides, alkyl pseudohalides, aryl halides, and alkenyl halides under electrochemical reductive conditions. Moreover, the successful application of electrochemical reductive relay cross-coupling and transition metal–free cross-electrophile coupling further demonstrates the versatility of alkyl bistriflimides as valuable building blocks in organic synthesis. Combined control experiments and density functional theory calculations provide insights into the reaction pathway and the crucial role of iodide in the catalytic process.
Versatile electrocatalytic cross-coupling of alkyl bistriflimides enables efficient C–C bond formation under mild conditions.
INTRODUCTION
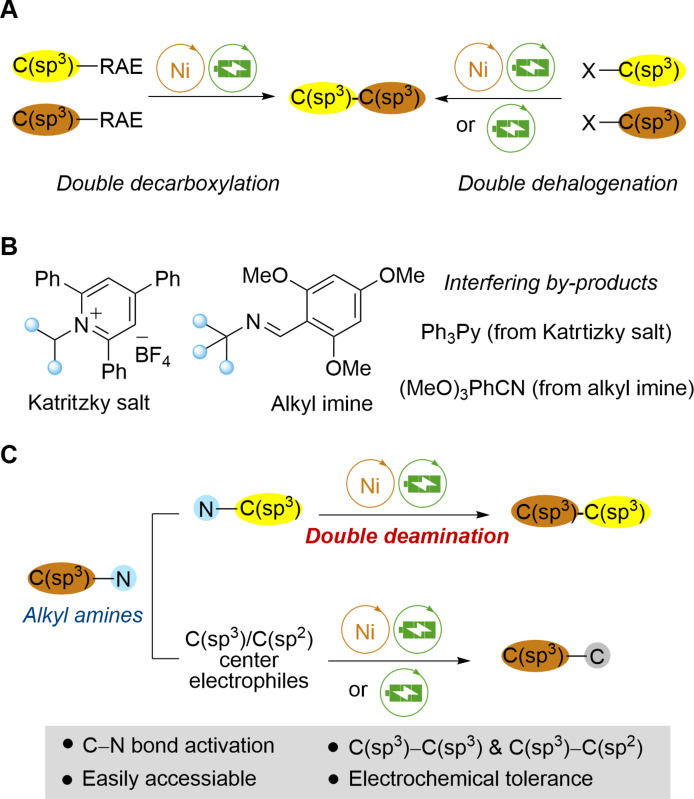
Electrochemically cross-electrophile coupling (e-XEC) protocols have garnered notable attention for their ability to construct complex carbon scaffolds from generally readily available electrophiles (1–5). Compared to the cross-electrophile coupling (XEC) strategies, e-XEC uses renewable and easily accessible electricity to replace traditional redox reagents, promoting more sustainable chemical production (6–14). In the past decade, e-XEC strategies have been used to construct C–C (14) and C–X (15–18) bonds, thereby enriching the compound library. Among these efficient methodologies, the construction of C(sp3)–C(sp3) bonds is in high demand in contemporary organic synthesis (19–25). Recent advances include the Baran group’s nickel-electrocatalytic doubly decarboxylative coupling for C(sp3)–C(sp3) bond formation using redox-active esters derived from aliphatic acids (26, 27), the Qiu group’s C(sp3)–C(sp3) cross-coupling of unactivated alkyl halides under similar catalytic conditions (28), and the Lin group’s e-XEC of different alkyl halides (Fig. 1A) (29). However, there is a notable lack of focus on C(sp3)–C(sp3) e-XEC efforts in deaminative coupling (Fig. 1A) (30, 31).
Fig. 1. Strategies for deaminative C(sp3)–C bond construction.
(A) Electrocatalytic C(sp3)–C(sp3) cross-coupling. (B) Strategies of deaminative cross-coupling. (C) Deaminative cross-coupling of alkyl bistriflimides (this work). RAE, redox active ester.
While approaches to constructing C(sp3)-centered molecules have primarily used aliphatic alcohols (32–35) and carboxylic acids (36–39) as precursors, amines have been less frequently used as cross-coupling partners (40–42). Strategies for deaminative cross-coupling reactions include conversion of amines to pyridinium salts (30, 43–61) and use of redox-active imines (Fig. 1B) (62–65). However, these methods produce neutral molecule by-products that may be captured by radicals, reducing reaction efficiency (66). Inspired by seminal reports from the 1980s concerning bis-sulfonyl amide ion moieties (67, 68), we propose, using trifluoromethanesulfonic anhydride as an activating agent, to form the C–NTf2 structure (69). This approach could facilitate the deamination process of alkyl bistriflimides to form alkyl radicals by single electron reduction, due to the excellent leaving ability of NTf2. To date, the use of alkyl bistriflimides in cross-coupling reactions, particularly those involving double deamination, has not been reported. We present a versatile nickel-electrocatalytic deaminative cross-coupling platform that enables the efficient construction of C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds from readily available alkyl bistriflimides (Fig. 1C).
RESULTS
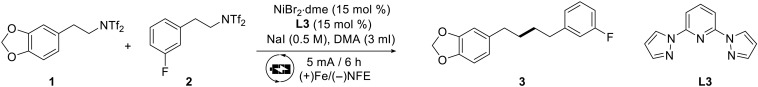
We initiated the assessment of nickel-electrocatalytic double deaminative reaction using alkyl bistriflimides 1 and 2 as model substrates to develop the e-XEC (Table 1). The optimal reaction conditions were identified as follows: Nickel(II) bromide ethylene glycol dimethyl ether complex (NiBr2·dme) as the catalyst, di(1-pyrazolyl)pyridine L3 as the ligand, and NaI as the supporting electrolyte under a constant current (5 mA) in N,N′-dimethylformamide (DMF) with iron plate and Ni foam as electrodes. The reaction was conducted for 6 hours at room temperature, achieving a maximum yield of 70% for the cross-coupling product 3 (entry 1). Other electrode combinations decreased the efficiency or suppressed the transformation (entry 2) (70). The application of alternative electrolytes also diminished the yield (entry 3). Similar outcomes were obtained by changing the electric current to 2.5 or 10 mA (entries 4 and 5). Control experiments revealed that the reaction cannot proceed in the absence of the electricity or catalyst (entries 6 and 7).
Table 1. Optimization of the cross-coupling reaction involving double deamination.
Reaction condition: 1 (0.3 mmol), 2 (0.9 mmol), [Ni] (0.045 mmol), ligand (0.045 mmol), NaI (0.5 M), DMA (3 ml), Ar atmosphere, and rt. Detected by gas chromatography with dodecane as the internal standard. NFE, nickel foam electrode; n.d., not detected.
| Entry | Variation from the standard conditions | Yield [%] |
|---|---|---|
| 1 | None | 74 (70)* |
| 2 | (+)C/(−)NFE, 2.5 equiv of B2pin2 or Et3N | n.d. |
| 3 | TBAB or TBAI or NaBr | 33–62 |
| 4 | 2.5 mA/12 h | 54 |
| 5 | 10 mA/3 h | 65 |
| 6 | No electricity | n.d. |
| 7 | W/o NiBr2 dme | Trace |
*Isolated yield.
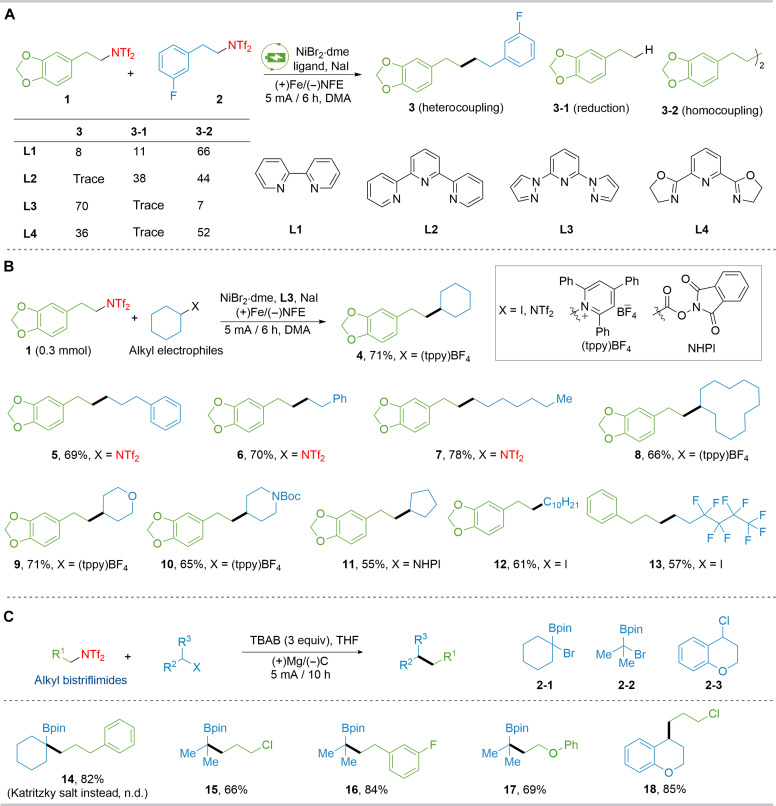
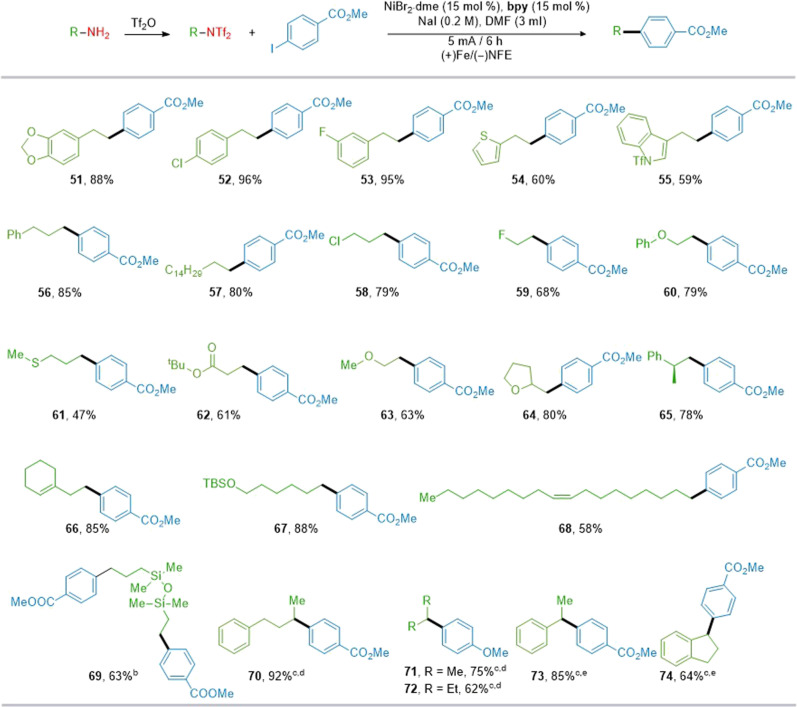
The role of the ligand was further investigated. As shown in Fig. 2A, using bipyridine L1 (bpy) or terpyridine L2 (tpy) as the ligand led to the formation of homocoupling product 3-2 of two alkyl bistriflimides, along with protonation product 3-1 and low efficiency for heterocoupling product 3. L4 gave a lower yield, with only 36% of the heterocoupling product. We then expanded this platform for C(sp3)–C(sp3) bond construction. Cross-coupling products were obtained in 55 to 78% yields, as demonstrated with alkyl bistriflimides, alkyl Katritzky salts, redox-active esters, and alkyl iodides, encompassing both long-chain (5 to 7, 12, and 13) and cyclic structures (8 to 11). These results demonstrate that this electrochemical synthesis platform can use a diverse array of radical precursors from various sources to facilitate C(sp3)–C(sp3) bond formation. Moreover, alkyl bistriflimides in electrochemical reactions not only act as radical precursors but also undergo a pathway involving a sequence of electrochemical-chemical-electrochemical-chemical steps (29, 71). Using tetrabutylammonium bromide (TBAB) as the electrolyte, Mg as the sacrificial anode, carbon as the cathode, non-anhydrous THF as the solvent, and a constant current of 5.0 mA under ambient conditions, we successfully used the e-XEC to synthesize a diverse array of coupling products, demonstrating compatibility with various functional groups, including alkyl chlorides, ethers, and fluorides from alkyl bistriflimides and α-haloboronate esters (14 to 17) as well as benzyl chloride (18). It is noteworthy that the activation method using pyridinium salts is ineffective for obtaining the desired products. These methods highlight the unique advantages of alkyl bistriflimides in the electrochemical construction of C(sp3)–C(sp3) bonds, demonstrating their versatility in being modified to suit different reaction conditions.
Fig. 2. Effect of ligand and scope of various alkyl electrophiles.
Yields of isolated products unless otherwise specified. (A) Ligand effect in C(sp3)-C(sp3) formation. NFE, nickel foam electrode; h, hours. (B) Scope of C(sp3)-C(sp3) formation. (C) Electrochemically driven cross-electrophile coupling of alkyl bistriflimides and alkyl halides. n.d., not detected; tppy, 2,4,6-triphenylpyridine.
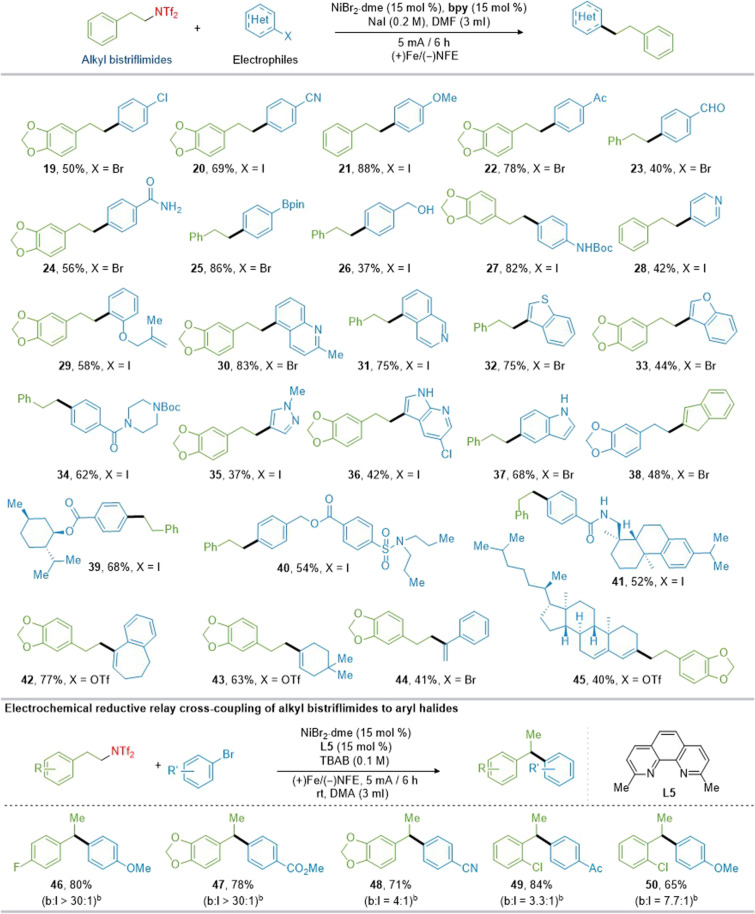
To further prove the applicability of this electrochemical deamination method, we subsequently evaluated the scope of (hetero)aryl halides to construct the C(sp3)–C(sp2) bonds. As shown in Fig. 3, electron-rich and electron-deficient aryl halides could be converted to the corresponding product in high efficiency. Functional groups such as chlorine (19), cyano (20), methoxy (21), and ketone (22) afforded the desired products in 50 to 78% yields. Aldehyde (23), amide (24), boronic acid pinacol ester (Bpin) (25), and Boc-amide (27) on the para position of the benzene ring were tolerated; in particular, the Bpin group provides opportunities for further diversification. The benzyl alcohol group is also compatible but less efficient (26). Allyloxa (29) on the ortho position of the benzene ring could be tolerated here. Heterocycles including pyridine (28), quinoline (30 and 31), benzothiophenyl (32), and benzofuran (33) could be converted to the corresponding products in 42 to 83% yields. This nickel-electrocatalytic deaminative arylation reaction appears to be a general protocol for heterocycles such as N-alkylpyrazoles and azaindoles (35 and 36, 37 and 42% yield, respectively) (72). Unprotected indole motifs exhibited compatibility within this reaction, as evidenced by the efficient coupling of 5-iodoindole, yielding 37 in 68% yield. Probenecid (39), menthol (40), and dehydroabietylamine (41) were successfully alkylated, yielding the desired products in 52 to 83% yields. Notably, the coupling electrophile in this C(sp3)–C(sp2) construction reaction can be extended to alkenyl bromo/triflate coupling partners, yielding the products with moderate efficiency (38 and 42 to 45). To broaden the method’s applicability, we further investigated electrochemical reductive relay cross-coupling of alkyl bistriflimides to aryl halides (73, 74). Using ligand L5, TBAB as the electrolyte, and dimethylamine (DMA) as the solvent, the electrochemical coupling between alkyl bistriflimides and aryl bromides yielded 1,1-diarylalkanes in 65 to 80% yields, with good functional group compatibility and regioselectivity (46 to 50). Next, we examined the scope with respect to the alkyl bistriflimides, as illustrated in Fig. 4. Alkyl bistriflimides bearing a heterocyclic or halogen-containing aromatic ring reacted smoothly to afford the products (51 to 56) in moderate to good yields (49 to 96%). For alkyl amine substrates, long carbon chains (57), and with various functional groups like chloro (58), fluoro (59), ether (60, 63, and 64), thiomethyl (61), and ester (62), were well tolerated under standard reaction conditions, yielding the products in good yields. Other alkyl bistriflimides, such as those derived from cyclic olefin (66), silyl ether (67), and oleylamine (68), were readily applicable in this reaction. The bistriflimide derived from a disiloxane structure also performed well in this catalytic system, producing the desired product with a 63% yield (69). For secondary alkylamines, straightforward modifications to the leaving group enabled successful deaminative arylation under manganese powder reduction conditions. However, under electrochemical conditions, the disubstituted structure exhibited minimal reactivity, resulting in its conversion to a monosubstituted configuration (70 to 74).
Fig. 3. Reaction scope of the electrophiles.
Yields of isolated products unless otherwise specified. bThe ratio of branched-to-linear products (b:l) was determined by 1H NMR. rt, room temperature.
Fig. 4. Reaction scope of alkyl bistriflimides.
Yields of isolated products unless otherwise specified. bMethyl 4-iodobenzoate (2.5 equiv). cNiBr2·dme (0.03 mmol), dtbpy (0.03 mmol), NaI (0.3 mmol), and Mn (0.6 mmol), 60°C, in DMF (1 ml) under Ar atmosphere for 6 hours. d3,5-Difluorobenzenesulfonyl chloride instead of Tf2O. eTsCl instead of Tf2O.
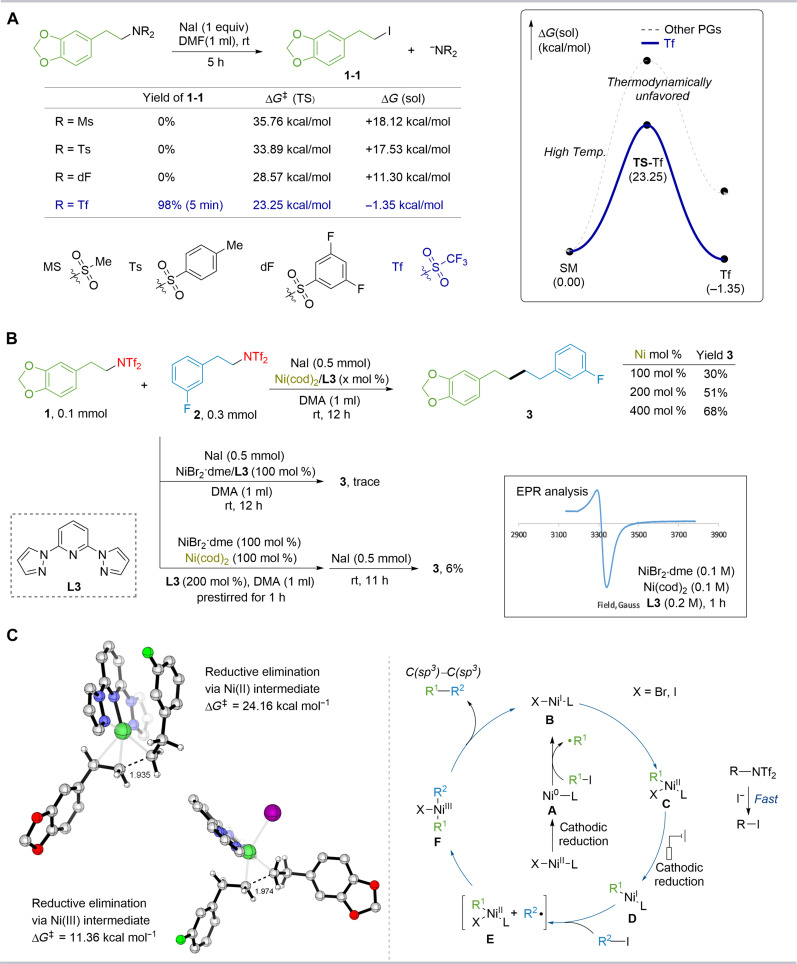
To elucidate the mechanism, a series of controlled experiments was conducted. The intermediate (1-1) rapidly underwent equimolar conversion with alkyl bistriflimide 1 and sodium iodide in DMF within 5 min, showcasing a highly efficient protocol for arylation reactions. The role of NaI was investigated by systematically examining its concentration and corresponding impact on yields (see fig. S2 in the Supplementary Materials for more details). Productivity was found to be linearly related to the content of NaI. Even in the absence of NaI, a substantial product yield was obtained, suggesting that the iodide generated from aryl iodide during the reaction likely plays a central role in enabling the catalytic cycle.
Next, a series of control experiments with the nickel catalyst was performed to elucidate the mechanism in the construction of C(sp3)–C(sp3) bonds. The cross-coupling product was obtained with a 61% yield when Ni(0) was used as a catalyst (see the Supplementary Materials for more details). This result suggests that, under the standard conditions, Ni(0) might be produced from the electroreduction of Ni(II), subsequently enabling the reduction of the substrates. To validate this hypothesis, we conducted control experiments using equimolar amounts of nickel(0) and nickel(II) complexes in the absence of current (Fig. 5B). The efficiency of coupling product formation increased with higher amounts of nickel(0), whereas the use of an equivalent amount of only nickel(II) resulted in no reaction. Premixing nickel(0) and nickel(II) in a 1:1 ratio provided only a 6% product yield. This low yield suggests the formation of a Ni(I)-Br species, which was confirmed by electron paramagnetic resonance (EPR). These findings highlight that the formation of nickel(0) in the initial stage of the reaction mechanism plays a crucial role. In addition, in the cyclic voltammetry (CV) experiments, incorporating NaI and bistriflimide 1 into the current nickel catalytic system revealed a distinct current peak corresponding to Ni(I) (see fig. S1 in the Supplementary Materials), indicating the capability of Ni(0) to reduce iodide 1-1. Radical trapping experiments also suggest that the electrochemical reaction may proceed through a radical pathway (see fig. S3 in the Supplementary Materials for more details). However, we cannot exclude the possibility that Ni(I)-Br species reduces the substrate to generate an alkyl radical (75).
Fig. 5. Effect of leaving groups and DFT calculations.
(A) Effect of leaving groups. (B) Control experiments of [Ni] without using electricity. (C) DFT-calculated free energy barrier of the reductive elimination step and proposed mechanism. Ts, Tosyl; sol, solvent; PGs, protective groups; cod, 1,5-cyclooctadiene.
Further density functional theory (DFT) calculations were performed to elucidate the reaction mechanism. We used quantum chemical methods at the M06-D3/6-311G(d,p)//B3LYP-D3/6-31G** level of theory (Fig. 5). Experimental results revealed that triflate is the only feasible functional group, as indicated by the outcomes of the SN2 reaction (Fig. 5A). For other functional groups, the calculated transition energies were notably high, and these groups were predicted to undergo thermodynamically unfavorable endothermic processes (see fig. S15 in the Supplementary Materials for more details). In contrast, triflate was found to proceed through an exothermic reaction, consistent with our experimental findings. Furthermore, increasing the temperature to 60°C enabled reactions with other leaving groups, enhancing the reliability of our computational results. Next, we calculated the free energy barrier for the reductive elimination step, considering the two possible oxidation states for Ni (see fig. S17 in the Supplementary Materials for more details). The calculations for both Ni(II) and Ni(III) oxidation states indicated that reductive elimination via dialkyl-Ni(III) complexes is preferred by 12.9 kcal mol−1 (Fig. 5C). On the basis of these results and DFT calculations, we proposed a Ni(I–III) catalytic cycle as the plausible mechanism for this nickel-electrocatalyzed cross-coupling reaction. In this mechanism, alkyl bistriflimides react rapidly via an SN2 mechanism to generate alkyl iodide intermediates in the presence of iodide within the system. Concurrently, electroreduction of Ni(II) provides low-valent Ni(0) species A. This Ni(0) species then reduces alkyl halides, generating alkyl radicals and Ni(I) species B. Species B engages in radical addition processes to deliver Ni(II) species C. This Ni(II) species C is electrochemically reduced to Ni(I) species D (76, 77). The alkyl Ni(I) species D then reacts with an additional halide substrate, forming Ni(III) species F. Last, the reaction advances through a reductive elimination step, yielding the desired product. However, based on the literature (28), we cannot exclude the potential involvement of species B in a sequence of consecutive radical addition and reductive elimination processes that ultimately lead to the formation of the cross-coupled products; in addition, the mechanism involving Ni(I) species B in the radical generation process cannot be excluded either (see figs. S10 and S11 in the Supplementary Materials). The mechanisms of other reaction products are discussed in the Supplementary Materials.
DISCUSSION
In conclusion, we have developed a powerful and versatile nickel-electrocatalytic deaminative cross-coupling methodology that enables efficient construction of C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds from readily available alkyl bistriflimides. The key to this approach is the straightforward assembly of leaving groups on alkyl amines to form alkyl bistriflimides, which can then be effectively coupled with a wide range of partners under mild electrochemical reductive conditions. The successful application of electrochemical reductive relay cross-coupling and transition metal–free e-XEC further underscores the versatility of alkyl bistriflimides as valuable building blocks in organic synthesis. The broad substrate scope, functional group tolerance, and mild reaction conditions demonstrate the practicality and utility of this methodology. Mechanistic studies, including control experiments and DFT calculations, have provided insights into the reaction pathway and highlighted the crucial role of the iodine anion in the catalytic process. This work not only expands the toolbox for C–C bond formation but also offers a sustainable approach to using amine-derived precursors in organic synthesis.
MATERIALS AND METHODS
General procedure 1 for the e-XEC reaction of alkyl bistriflimides with C(sp3) electrophiles using electrocatalysis
In a glove box, NaI (90.0 mg, 0.6 mmol), alkyl bistriflimides (0.3 mmol, 1 equiv), alkyl electrophiles (0.9 mmol, 3 equiv), NiBr2·dme (13.8 mg, 0.03 mmol, 15 mol %), L5 (9.4 mg, 0.02 mmol, 15 mol %), and dried DMA (3.0 ml) were added into the flame-dried undivided ElectraSyn vial (5 ml) equipped with a stir bar. The resulting suspension was prestirred for about 1 min to dissolve the electrolyte (if the substrate was solid, it would be added to the vial before adding the solvent; if the substrate was sticky oil, it would be added as a solution in DMA). After the reaction is completed, the mixture was transferred to a separatory funnel. Then, H2O (20 ml) was added and the mixture was extracted three times with ethyl acetate (EtOAc) (20 ml). The combined organic layer was washed with 1.0 M NaOH (20 ml), brine (20 ml), and H2O (20 ml). The organic layer was dried with anhydrous Na2SO4 and then concentrated under vacuum. The product was purified by flash column chromatography on silica gel or Preparative Thin-Layer Chromatography (PTLC) using petroleum ether/EtOAc as the eluent.
General procedure 2 for the e-XEC reaction with alkyl bistriflimides
In a glove box, TBAB (483.0 mg, 1.5 mmol, 3 equiv) and dried THF (2.5 ml) were added into the flame-dried undivided ElectraSyn vial (5 ml) equipped with a stir bar. The resulting suspension was prestirred for about 1 min to dissolve the electrolyte (if the substrate was solid, it would be added to the vial before adding the solvent; if the substrate was sticky oil, it would be added as a solution in THF). Then, the substrate with an anion-stabilizing group (0.5 mmol, 1 equiv) and alkyl bistriflimides (1.5 mmol, 3 equiv) was sequentially added to the mixture. The vial was sealed with the ElectraSyn vial cap equipped with anode (Mg plate) and cathode (graphite plate), and then it was brought out of the glove box. Prestirring the resulting mixture for 2 min, and then the reaction mixture was stirred and electrolyzed at a constant current of 5 mA under room temperature for 10 hours. After the reaction is completed, the mixture was transferred to a separatory funnel, and electrodes were washed with EtOAc. Then, the crude mixture was further diluted with Et2O (if there were too much floc., filter it by Celite). The resulting mixture was washed with brine. The organic layer was dried over with anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel to furnish the desired product.
General procedure 3 for the e-XEC reaction of alkyl bistriflimides with C(sp2) electrophiles using electrocatalysis
In a glove box, NaI (90.0 mg, 0.6 mmol), alkyl bistriflimides (0.3 mmol, 1.5 equiv), electrophiles (0.2 mmol, 1 equiv), NiBr2·dme (9.2 mg, 0.03 mmol, 15 mol %), bpy (4.7 mg, 0.02 mmol, 15 mol %), and dried DMF (3.0 ml) were added into the flame-dried undivided ElectraSyn vial (5 ml) equipped with a stir bar. The resulting suspension was prestirred for about 1 min to dissolve the electrolyte (if the substrate was solid, it would be added to the vial before adding the solvent; if the substrate was sticky oil, it would be added as a solution in DMF). After the reaction is completed, the mixture was transferred to a separatory funnel. Then, H2O (20 ml) was added and the mixture was extracted three times with EtOAc (20 ml). The combined organic layer was washed with 1.0 M NaOH (20 ml), brine (20 ml), and H2O (20 ml). The organic layer was dried with anhydrous Na2SO4 and then concentrated under vacuum. The product was purified by flash column chromatography on silica gel or PTLC using petroleum ether/EtOAc as the eluent.
General procedure 4 for the electrochemical reductive relay cross-coupling of alkyl bistriflimides to aryl halides
In a glove box, TBAB (290.0 mg, 0.6 mmol), alkyl bistriflimides (0.3 mmol, 1.5 equiv), aryl bromide (0.2 mmol, 1 equiv), NiBr2·dme (9.2 mg, 0.03 mmol, 15 mol %), ligand (0.02 mmol, 15 mol %), and dried DMA (3.0 ml) were added into the flame-dried undivided ElectraSyn vial (5 ml) equipped with a stir bar. The resulting suspension was prestirred for about 1 min to dissolve the electrolyte (if the substrate was solid, it would be added to the vial before adding the solvent; if the substrate was sticky oil, it would be added as a solution in DMA). After the reaction is completed, the mixture was transferred to a separatory funnel. Then, H2O (20 ml) was added and the mixture was extracted three times with EtOAc (20 ml). The combined organic layer was washed with 1.0 M NaOH (20 ml), brine (20 ml), and H2O (20 ml). The organic layer was dried with anhydrous Na2SO4 and then concentrated under vacuum. The product was purified by flash column chromatography on silica gel or PTLC using petroleum ether/EtOAc as the eluent.
General procedure 5 for the XEC reaction of alkyl bistriflimides with C(sp2) electrophiles using metal reductant Mn
In a glove box, a dry 8-ml vial equipped with a Teflon-coated magnetic stir bar was charged with electrophiles (0.2 mmol, 1 equiv, if solid), alkyl bistriflimides (0.3 mmol, 1.5 equiv, if solid), NiBr2·dme (9.2 mg, 0.03 mmol, 15 mol %), 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy) (8.0 mg, 0.03 mmol, 15 mol %), NaI (45.0 mg, 0.3 mmol, 1.5 equiv), and Mn (33 mg, 0.6 mmol, 2.5 equiv). Anhydrous and degassed DMF (1.0 ml), electrophiles (0.2 mmol, 1 equiv, if liquid), and alkyl bistriflimides (0.3 mmol, 1.5 equiv, if liquid) were added via a syringe. The vial was capped and sealed with Parafilm. The reaction mixture was stirred for 6 hours at 60°C. After the reaction is completed, the mixture was transferred to a separatory funnel. Then, H2O (20 ml) was added and the mixture was extracted three times with EtOAc (20 ml). The combined organic layer was washed with 1.0 M NaOH (20 ml), brine (20 ml), and H2O (20 ml). The organic layer was dried with anhydrous Na2SO4 and then concentrated under vacuum. The product was purified by flash column chromatography on silica gel or PTLC using petroleum ether/EtOAc as the eluent.
Acknowledgments
We thank the Collaborative Innovation Center of Advanced Microstructures and Jiangsu Provincial Key Laboratory of Photonic and Electronic Materials at Nanjing University and Institute for Basic Science for support.
Funding: This work was supported by the National Natural Science Foundation of China (nos. 21971107, 22071101, and 22271147 to Y.W.), China Postdoctoral Science Foundation (2021T140309 and 2021M691511 to Y.W.), and Institute for Basic Science (IBS-R010-A2 to S.H.).
Author contributions: Methodology: X.T. Investigation: X.T., W.L., Z.X., H.S., and Q.W. Writing—review and editing: X.T., W.L., S.N., Y.P., S.H., and Y.W. Supervision: S.H. and Y.W.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Tables S1 to S7
Figs. S1 to S17
References
REFERENCES AND NOTES
- 1.Corbet J.-P., Mignani G., Selected patented cross-coupling reaction technologies. Chem. Rev. 106, 2651–2710 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Twilton J., Johnson M. R., Sidana V., Franke M. C., Bottecchia C., Lehnherr D., Lévesque F., Knapp S. M. M., Wang L., Gerken J. B., Hong C. M., Vickery T. P., Weisel M. D., Strotman N. A., Weix D. J., Root T. W., Stahl S. S., Quinone-mediated hydrogen anode for non-aqueous reductive electrosynthesis. Nature 623, 71–76 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Li P., Wang Y., Qiu Y., Electroreductive cross-electrophile coupling (eXEC) reactions. Angew. Chem. Int. Ed. Engl. 62, e202306679 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Zubaydi S. A., Waske S., Akyildiz V., Starbuck H. F., Majumder M., Moore C. E., Kalyani D., Sevov C. S., Reductive alkyl-alkyl coupling from isolable nickel-alkyl complexes. Nature 634, 585–591 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinh L. P., Starbuck H. F., Hamby T. B., LaLama M. J., He C. Q., Kalyani D., Sevov C. S., Persistent organonickel complexes as general platforms for Csp2–Csp3 coupling reactions. Nat. Chem. 16, 1515–1522 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen T., Lambert T. H., Electrophotocatalytic diamination of vicinal C–H bonds. Science 371, 620–626 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida J.-i., Kataoka K., Horcajada R., Nagaki A., Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Yuan Y., Lei A., Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 52, 3309–3324 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Francke R., Little R. D., Redox catalysis in organic electrosynthesis: Basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Röckl J. L., Pollok D., Franke R., Waldvogel S. R., A decade of electrochemical dehydrogenative C,C-coupling of aryls. Acc. Chem. Res. 53, 45–61 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Yan M., Kawamata Y., Baran P. S., Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao K.-J., Xing Y.-K., Yang Q.-L., Qiu H., Mei T.-S., Site-selective C–H functionalization via synergistic use of electrochemistry and transition metal catalysis. Acc. Chem. Res. 53, 300–310 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Xiong P., Xu H.-C., Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 52, 3339–3350 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Hamby T. B., LaLama M. J., Sevov C. S., Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 376, 410–416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lou T. S.-B., Kawamata Y., Ewing T., Correa-Otero G. A., Collins M. R., Baran P. S., Scalable, chemoselective nickel electrocatalytic sulfinylation of aryl halides with SO2. Angew. Chem. Int. Ed. Engl. 61, e202208080 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Y.-M., Liu X.-T., Xu L.-L., Shang M., Electrochemical Ni-catalyzed decarboxylative C(sp3)−N cross-electrophile coupling. Angew. Chem. Int. Ed. Engl. 63, e202315222 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Wang Q., Xu J., Xu Z., Wang Z., Tao X., Ni S., Pan Y., Wang Y., Catalyst-free electroreductive carboxylic acid–nitroarene coupling. Green Chem. 25, 7084–7091 (2023). [Google Scholar]
- 18.Li P., Guo C., Wang S., Ma D., Feng T., Wang Y., Qiu Y., Facile and general electrochemical deuteration of unactivated alkyl halides. Nat. Commun. 13, 3774 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X., Yang T., Wang S., Xu H., Gong H., Nickel-catalyzed reductive cross-coupling of unactivated alkyl halides. Org. Lett. 13, 2138–2141 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Ye Y., Sessler J. L., Gong H., Cross-electrophile couplings of activated and sterically hindered halides and alcohol derivatives. Acc. Chem. Res. 53, 1833–1845 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Dai Y., Gong H., Nickel-catalyzed reductive couplings. Top. Curr. Chem. 374, 43 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Weix D. J., Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knappke C. E. I., Grupe S., Gärtner D., Corpet M., Gosmini C., Jacobi von Wangelin A., Reductive cross-coupling reactions between two electrophiles. Chemistry 20, 6828–6842 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Moragas T., Correa A., Martin R., Metal-catalyzed reductive coupling reactions of organic halides with carbonyl-type compounds. Chemistry 20, 8242–8258 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki T., Kambe N., Ni-catalyzed C–C couplings using alkyl electrophiles. Top. Curr. Chem. 374, 66 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Zhang B., Gao Y., Hioki Y., Oderinde M. S., Qiao J. X., Rodriguez K. X., Zhang H.-J., Kawamata Y., Baran P. S., Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling. Nature 606, 313–318 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y., Zhang B., He J., Baran P. S., Ni-electrocatalytic enantioselective doubly decarboxylative C(sp3)–C(sp3) cross coupling. J. Am. Chem. Soc. 145, 11518–11523 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P., Zhu Z., Guo C., Kou G., Wang S., Xie P., Ma D., Feng T., Wang Y., Qiu Y., Nickel-electrocatalysed C(sp3)–C(sp3) cross-coupling of unactivated alkyl halides. Nat. Catal. 7, 412–421 (2024). [Google Scholar]
- 29.Zhang W., Lu L., Zhang W., Wang Y., Ware S. D., Mondragon J., Rein J., Strotman N., Lehnherr D., See K. A., Lin S., Electrochemically driven cross-electrophile coupling of alkyl halides. Nature 604, 292–297 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z., Cernak T., The formal cross-coupling of amines and carboxylic acids to form sp3–sp3 carbon–carbon bonds. Angew. Chem. Int. Ed. Engl. 60, 27293–27298 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Yang T., Wei Y., Koh M. J., Photoinduced nickel-catalyzed deaminative cross-electrophile coupling for C(sp2)–C(sp3) and C(sp3)–C(sp3) bond formation. ACS Catal. 11, 6519–6525 (2021). [Google Scholar]
- 32.Dong Z., MacMillan D. W. C., Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X., MacMillan D. W. C., Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3–sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc. 138, 13862–13865 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y., Ben-zvi B., Diao T., Diastereoselective synthesis of aryl C-glycosides from glycosyl esters via C−O bond homolysis. Angew. Chem. Int. Ed. Engl. 60, 9433–9438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi B. K., Widness J. K., Gilbert M. M., Salgueiro D. C., Garcia K. J., Weix D. J., In-situ bromination enables formal cross-electrophile coupling of alcohols with aryl and alkenyl halides. ACS Catal. 12, 580–586 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez N., Goossen L. J., Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev. 40, 5030–5048 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Zuo Z., Ahneman D. T., Chu L., Terrett J. A., Doyle A. G., MacMillan D. W. C., Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan A. Y., Perry I. B., Bissonnette N. B., Buksh B. F., Edwards G. A., Frye L. I., Garry O. L., Lavagnino M. N., Li B. X., Liang Y., Mao E., Millet A., Oakley J. V., Reed N. L., Sakai H. A., Seath C. P., MacMillan D. W. C., Metallaphotoredox: The merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo Z., Cong H., Li W., Choi J., Fu G. C., MacMillan D. W. C., Enantioselective decarboxylative arylation of α-amino acids via the merger of photoredox and nickel catalysis. J. Am. Chem. Soc. 138, 1832–1835 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath N. A., Brichacek M., Njardarson J. T., A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010). [Google Scholar]
- 41.Sanderson K., Amino acid provides shortcut to drugs. Nature 488, 266–266 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Steiniger K. A., Lamb M. C., Lambert T. H., Cross-coupling of amines via photocatalytic denitrogenation of in situ generated diazenes. J. Am. Chem. Soc. 145, 11524–11529 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker K. M., Lucas Baca D., Plunkett S., Daneker M. E., Watson M. P., Engaging alkenes and alkynes in deaminative alkyl-alkyl and alkyl-vinyl cross-couplings of alkylpyridinium salts. Org. Lett. 21, 9738–9741 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basch C. H., Liao J., Xu J., Piane J. J., Watson M. P., Harnessing alkyl amines as electrophiles for nickel-catalyzed cross couplings via C–N bond activation. J. Am. Chem. Soc. 139, 5313–5316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J., Wang G., Li S., Shi Z., Selective C-N borylation of alkyl amines promoted by Lewis base. Angew. Chem. Int. Ed. Engl. 57, 15227–15231 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Jiang X., Zhang M. M., Xiong W., Lu L. Q., Xiao W. J., Deaminative (carbonylative) alkyl-Heck-type reactions enabled by photocatalytic C-N bond activation. Angew. Chem. Int. Ed. Engl. 58, 2402–2406 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Klauck F. J. R., James M. J., Glorius F., Deaminative strategy for the visible-light-mediated generation of alkyl radicals. Angew. Chem. Int. Ed. Engl. 56, 12336–12339 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Liao J., Basch C. H., Hoerrner M. E., Talley M. R., Boscoe B. P., Tucker J. W., Garnsey M. R., Watson M. P., Deaminative reductive cross-electrophile couplings of alkylpyridinium salts and aryl bromides. Org. Lett. 21, 2941–2946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Montero R., Yatham V. R., Yin H., Davies J., Martin R., Ni-catalyzed reductive deaminative arylation at sp(3) carbon centers. Org. Lett. 21, 2947–2951 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Plunkett S., Basch C. H., Santana S. O., Watson M. P., Harnessing alkylpyridinium salts as electrophiles in deaminative alkyl-alkyl cross-couplings. J. Am. Chem. Soc. 141, 2257–2262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandfort F., Strieth-Kalthoff F., Klauck F. J. R., James M. J., Glorius F., Deaminative borylation of aliphatic amines enabled by visible light excitation of an electron donor-acceptor complex. Chemistry 24, 17210–17214 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Sun S. Z., Cai Y. M., Zhang D. L., Wang J. B., Yao H. Q., Rui X. Y., Martin R., Shang M., Enantioselective deaminative alkylation of amino acid derivatives with unactivated olefins. J. Am. Chem. Soc. 144, 1130–1137 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Sun S. Z., Romano C., Martin R., Site-selective catalytic deaminative alkylation of unactivated olefins. J. Am. Chem. Soc. 141, 16197–16201 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Wang C., Qi R., Xue H., Shen Y., Chang M., Chen Y., Wang R., Xu Z., Visible-light-promoted C(sp3)-H alkylation by intermolecular charge transfer: Preparation of unnatural α-amino acids and late-stage modification of peptides. Angew. Chem. Int. Ed. Engl. 59, 7461–7466 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Hoerrner M. E., Watson M. P., Weix D. J., Nickel-catalyzed synthesis of dialkyl ketones from the coupling of N-alkyl pyridinium salts with activated carboxylic acids. Angew. Chem. Int. Ed. Engl. 59, 13484–13489 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J., Grant P. S., Li X., Noble A., Aggarwal V. K., Catalyst-free deaminative functionalizations of primary amines by photoinduced single-electron transfer. Angew. Chem. Int. Ed. Engl. 58, 5697–5701 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J., He L., Noble A., Aggarwal V. K., Photoinduced deaminative borylation of alkylamines. J. Am. Chem. Soc. 140, 10700–10704 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Yi J., Badir S. O., Kammer L. M., Ribagorda M., Molander G. A., Deaminative reductive arylation enabled by nickel/photoredox dual catalysis. Org. Lett. 21, 3346–3351 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yue H., Zhu C., Shen L., Geng Q., Hock K. J., Yuan T., Cavallo L., Rueping M., Nickel-catalyzed C-N bond activation: Activated primary amines as alkylating reagents in reductive cross-coupling. Chem. Sci. 10, 4430–4435 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng X., Yan W., Zacate S. B., Cai A., Wang Y., Yang D., Yang K., Liu W., Copper-catalyzed deaminative difluoromethylation. Angew. Chem. Int. Ed. Engl. 59, 16398–16403 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Ni S., Li C.-X., Mao Y., Han J., Wang Y., Yan H., Pan Y., Ni-catalyzed deaminative cross-electrophile coupling of Katritzky salts with halides via C─N bond activation. Sci. Adv. 5, eaaw9516 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashley M. A., Rovis T., Photoredox-catalyzed deaminative alkylation via C-N bond activation of primary amines. J. Am. Chem. Soc. 142, 18310–18316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorsheimer J. R., Ashley M. A., Rovis T., Dual nickel/photoredox-catalyzed deaminative cross-coupling of sterically hindered primary amines. J. Am. Chem. Soc. 143, 19294–19299 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorsheimer J. R., Rovis T., Late-stage isotopic exchange of primary amines. J. Am. Chem. Soc. 145, 24367–24374 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchese A. D., Dorsheimer J. R., Rovis T., Photoredox-catalyzed generation of tertiary anions from primary amines via a radical polar crossover. Angew. Chem. Int. Ed. Engl. 63, e202317563 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu C.-H., Zeng L., Hu M., Qin J.-H., Xu X.-H., Li J.-H., Photoreductive alkylative dearomatization of N-alkyl pyridin-1-ium salts: Site selective access to 4-alkyl 1,4-dihydropyridines. European J. Org. Chem. 27, e202301120 (2024). [Google Scholar]
- 67.Scholz R., Hellmann G., Rohs S., Özdemir D., Raabe G., Vermeeren C., Gais H.-J., Enantioselective synthesis, configurational stability, and reactivity of lithium α-tert-butylsulfonyl carbanion salts. European J. Org. Chem. 2010, 4588–4616 (2010). [Google Scholar]
- 68.Müller P., Thi M. P. N., Conversion of amines into phenylsulfides and phenylselenides via ditosylamides. Helv. Chim. Acta 63, 2168–2172 (1980). [Google Scholar]
- 69.Qin Q., Cheng Z., Jiao N., Recent applications of trifluoromethanesulfonic anhydride in organic synthesis. Angew. Chem. Int. Ed. Engl. 62, e202215008 (2023). [DOI] [PubMed] [Google Scholar]
- 70.Sun D., Gong Y., Wu Y., Chen Y., Gong H., Bis(pinacolato)diboron-enabled Ni-catalyzed reductive arylation/vinylation of alkyl electrophiles. Adv. Sci. 11, 2404301 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu L., Wang Y., Zhang W., Zhang W., See K. A., Lin S., Three-component cross-electrophile coupling: Regioselective electrochemical dialkylation of alkenes. J. Am. Chem. Soc. 145, 22298–22304 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palkowitz M. D., Laudadio G., Kolb S., Choi J., Oderinde M. S., Ewing T. E.-H., Bolduc P. N., Chen T., Zhang H., Cheng P. T. W., Zhang B., Mandler M. D., Blasczak V. D., Richter J. M., Collins M. R., Schioldager R. L., Bravo M., Dhar T. G. M., Vokits B., Zhu Y., Echeverria P.-G., Poss M. A., Shaw S. A., Clementson S., Petersen N. N., Mykhailiuk P. K., Baran P. S., Overcoming limitations in decarboxylative arylation via Ag–Ni electrocatalysis. J. Am. Chem. Soc. 144, 17709–17720 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar G. S., Peshkov A., Brzozowska A., Nikolaienko P., Zhu C., Rueping M., Nickel-catalyzed chain-walking cross-electrophile coupling of alkyl and aryl halides and olefin hydroarylation enabled by electrochemical reduction. Angew. Chem. Int. Ed. Engl. 59, 6513–6519 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Jiao K. J., Liu D., Ma H. X., Qiu H., Fang P., Mei T. S., Nickel-catalyzed electrochemical reductive relay cross-coupling of alkyl halides to aryl halides. Angew. Chem. Int. Ed. Engl. 59, 6520–6524 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Wu H., Zhang S.-Q., Hong X., Mechanisms of nickel-catalyzed reductive cross-coupling reactions. Chem. Synth. 3, 39 (2023). [Google Scholar]
- 76.Xu H., Zhao C., Qian Q., Deng W., Gong H., Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant. Chem. Sci. 4, 4022–4029 (2013). [Google Scholar]
- 77.Anderson T. J., Vicic D. A., Direct observation of noninnocent reactivity of ZnBr2 with alkyl halide complexes of nickel. Organometallics 23, 623–625 (2004). [Google Scholar]
- 78.R. G. Parr, Y. Weitao, Density-Functional Theory of Atoms and Molecules (Oxford Univ. Press, 1994). [Google Scholar]
- 79.M. J. Frisch, G. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. Sonnenberg, M. Hada, D. Fox, Gaussian 09 Revision A.1. Gaussian Inc. (2009).
- 80.Lee C., Yang W., Parr R. G., Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37, 785–789 (1988). [DOI] [PubMed] [Google Scholar]
- 81.Grimme S., Antony J., Ehrlich S., Krieg H., A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Marenich A. V., Cramer C. J., Truhlar D. G., Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Moore B. P., Hewlett P. S., Insecticidal synergism with the pyrethrins: Studies on the relationship between chemical structure and synergistic activity in the 3:4-methylenedioxyphenyl compounds. J. Sci. Food Agric. 9, 666–672 (1958). [Google Scholar]
- 84.Komeyama K., Ohata R., Kiguchi S., Osaka I., Highly nucleophilic vitamin B12-assisted nickel-catalysed reductive coupling of aryl halides and non-activated alkyl tosylates. Chem. Commun. 53, 6401–6404 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Su W., Qiao R.-X., Jiang Y.-Y., Zhen X.-L., Tian X., Han J.-R., Fan S.-M., Cheng Q., Liu S., Ligand-Free Iron-Catalyzed Regioselectivity-Controlled Hydroboration of Aliphatic Terminal Alkenes. ACS Catal. 10, 11963–11970 (2020). [Google Scholar]
- 86.Nguyen J., Chong A., Lalic G., Nickel-catalyzed anti-Markovnikov hydroarylation of alkenes. Chem. Sci. 10, 3231–3236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Q.-H., Wei L.-P., Xiao B., Alkyl-GeMe3: Neutral metalloid radical precursors upon visible-light photocatalysis. Angew. Chem. Int. Ed. 61, e202115592 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Koyanagi T., Herath A., Chong A., Ratnikov M., Valiere A., Chang J., Molteni V., Loren J., One-pot electrochemical nickel-catalyzed decarboxylative Sp2–Sp3 cross-coupling. Org. Lett. 21, 816–820 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Fuentes J. A., Smith S. M., Scharbert M. T., Carpenter I., Cordes D. B., Slawin A. M. Z., Clarke M. L., On the functional group tolerance of ester hydrogenation and polyester depolymerisation catalysed by ruthenium complexes of tridentate aminophosphine ligands. Chem. Eur. J. 21, 10851–10860 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Choi J., Laudadio G., Godineau E., Baran P. S., Practical and Regioselective Synthesis of C-4-Alkylated Pyridines. J. Am. Chem. Soc. 143, 11927–11933 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ray Choudhury A., Mukherjee S., Enantioselective dearomatization of isoquinolines by anion-binding catalysis en route to cyclic α-aminophosphonates. Chem. Sci. 7, 6940–6945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Truce W. E., Emrick D. D., Miller R. E., The Internal Condensation of 2,4-Diphenyl-1-butanesulfonylSulfone1. J. Am. Chem. Soc. 75, 3359–3361 (1953). [Google Scholar]
- 93.He Y., Cai Y., Zhu S., Mild and regioselective benzylic C–H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 139, 1061–1064 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Wu X., Gannett C. N., Liu J., Zeng R., Novaes L. F. T., Wang H., Abruña H. D., Lin S., Intercepting hydrogen evolution with hydrogen-atom transfer: Electron-initiated hydrofunctionalization of alkenes. J. Am. Chem. Soc. 144, 17783–17791 (2022). [DOI] [PubMed] [Google Scholar]
- 95.Sun G., Sun H., Wang Z., Zhou M.-M., A novel InCl3/SiO2-catalyzed hydroarylation of arenes with styrenes under solvent-free conditions. Synlett 2008, 1096–1100 (2008). [Google Scholar]
- 96.Basnet P., Thapa S., Dickie D. A., Giri R., The copper-catalysed Suzuki–Miyaura coupling of alkylboron reagents: disproportionation of anionic(alkyl)(alkoxy)borates to anionic dialkylborates prior to transmetalation. Chem. Commun. 52, 11072–11075 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Lin Q., Ma G., Gong H., Ni-catalyzed formal cross-electrophile coupling of alcohols with aryl halides. ACS Catal. 11, 14102–14109 (2021). [Google Scholar]
- 98.Ji C.-L., Han J., Li T., Zhao C.-G., Zhu C., Xie J., Photoinduced gold-catalyzed divergent dechloroalkylation of gem-dichloroalkanes. Nat. Catal. 5, 1098–1109 (2022). [Google Scholar]
- 99.Yang Y., Zhou Q., Cai J., Xue T., Liu Y., Jiang Y., Su Y., Chung L., Vicic D. A., Exploiting the trifluoroethyl group as a precatalyst ligand in nickel-catalyzed Suzuki-type alkylations. Chem. Sci. 10, 5275–5282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hang W., Liang N., Liu Y., Xi C., Cobalt-catalyzed highly regioselective three-component arylcarboxylation of acrylate with aryl bromides and carbon dioxide. ChemSusChem 14, 4941–4946 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Luridiana A., Mazzarella D., Capaldo L., Rincón J. A., García-Losada P., Mateos C., Frederick M. O., Nuño M., Jan Buma W., Noël T., The merger of benzophenone HAT photocatalysis and silyl radical-induced XAT enables both nickel-catalyzed cross-electrophile coupling and 1,2-dicarbofunctionalization of olefins. ACS Catal. 12, 11216–11225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H., Li S., Hu H., Sun R., Liu M., Ding A., Liu X., Luo W., Fu Z., Guo S., Cai H., Visible-light-induced C(sp3)–C(sp3) bond formation via radical/radical cross-coupling. Chem. Commun. 59, 1205–1208 (2023). [DOI] [PubMed] [Google Scholar]
- 103.Yedase G. S., Jha A. K., Yatham V. R., Visible-Light Enabled C(sp3)–C(sp2) Cross-Electrophile Coupling via Synergistic Halogen-Atom Transfer (XAT) and Nickel Catalysis. J. Org. Chem. 87, 5442–5450 (2022). [DOI] [PubMed] [Google Scholar]
- 104.Chowdhury R. R., Crane A. K., Fowler C., Kwong P., Kozak C. M., Iron(iii) amine-bis(phenolate) complexes as catalysts for the coupling of alkyl halides with aryl Grignard reagents. Chem. Commun., 94–96 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Pan Y., Gong Y., Song Y., Tong W., Gong H., Retracted Article: Deoxygenative cross-electrophile coupling of benzyl chloroformates with aryl iodides. Org. Biomol. Chem. 17, 4230–4233 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Tables S1 to S7
Figs. S1 to S17
References