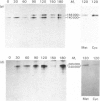
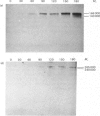
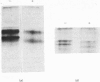
Abstract
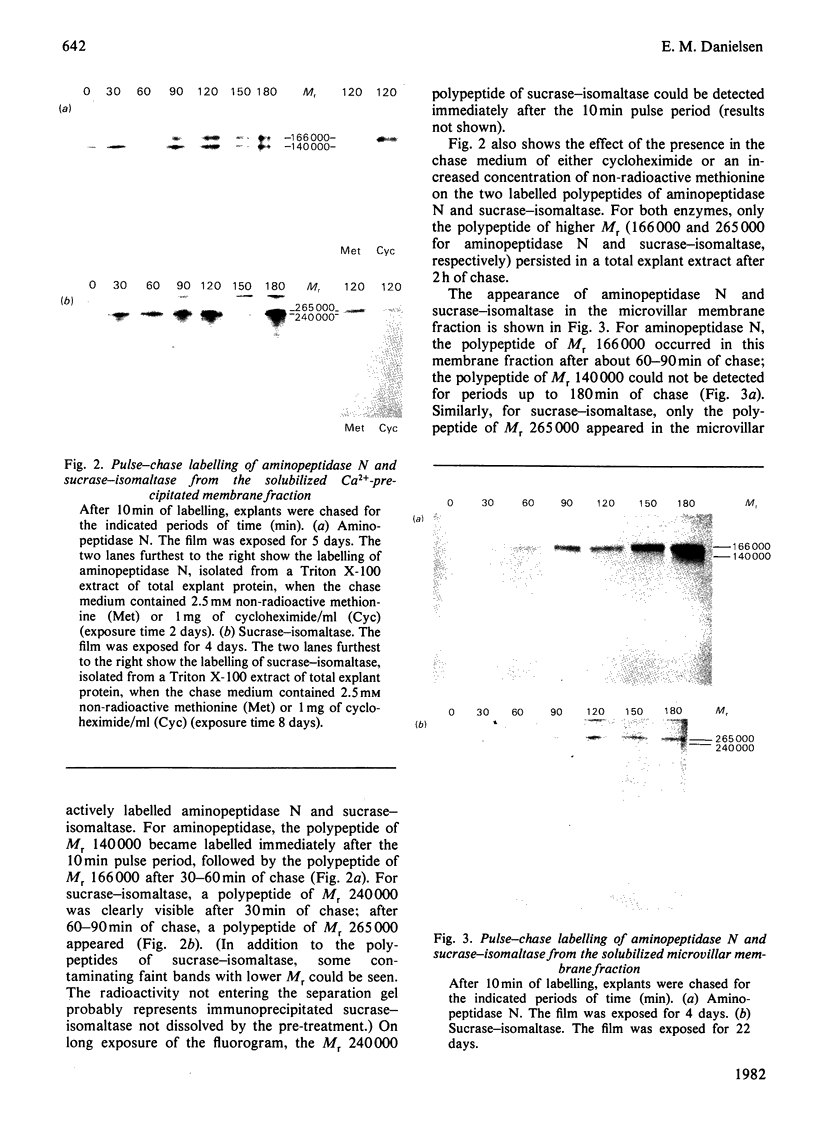
The biogenesis of two microvillar enzymes, aminopeptidase N (EC 3.4.11.2) and sucrase (EC 3.2.1.48)-isomaltase (EC 3.2.1.10), was studied by pulse-chase labelling of pig small-intestinal explants kept in organ culture. Both enzymes became inserted into the membrane during or immediately after polypeptide synthesis, indicating that translation takes place on ribosomes attached to the rough endoplasmic reticulum. The earliest detectable forms of aminopeptidase and sucrase-isomaltase were polypeptides of Mr 140 000 and 240 000 respectively. These polypeptides were susceptible to treatment with endo-beta-N-acetylglucosaminidiase H (EC 3.2.1.96), suggesting that the microvillar enzymes during or immediately after completion of protein synthesis become glycosylated with a 'high-mannose' oligosaccharide structure similarly to other plasma-membrane and secretory proteins. After 20--40 min or 60--90 min of chase, respectively, aminopeptidase N and sucrase-isomaltase were reglycosylated to give the polypeptides of Mr 166 000 (aminopeptidase N) and 265 000 (sucrase-isomaltase). These were expressed at the microvillar membrane after 60--90 min. During the entire process of synthesis and transport to the microvillar membrane the enzymes were bound to membranes, indicating that the biogenesis of aminopeptidase N and sucrase-isomaltase occurs in accordance with the membrane flow hypothesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezard J. P., Conklin K. A., Das B. C., Gray G. M. Incomplete intracellular forms of intestinal surface membrane sucrase-isomaltase. J Biol Chem. 1979 Sep 25;254(18):8969–8975. [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Translational evidence in vitro that aminopeptidase N is synthesized as a Mr-115000 polypeptide. Biochem J. 1982 Apr 15;204(1):323–327. doi: 10.1042/bj2040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Bro B., Dabelsteen E. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J. 1982 Mar 15;202(3):647–654. doi: 10.1042/bj2020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Skovbjerg H., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Nature of precursor forms of microvillar enzymes from Ca2+-precipitated enterocyte membranes. FEBS Lett. 1981 Sep 28;132(2):197–200. doi: 10.1016/0014-5793(81)81159-3. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maze M., Gray G. M. Intestinal brush border aminooligopeptidases: cytosol precursors of the membrane enzyme. Biochemistry. 1980 May 27;19(11):2351–2358. doi: 10.1021/bi00552a011. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Christiansen L., Wacker H., Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem. 1980 Dec 10;255(23):11332–11338. [PubMed] [Google Scholar]
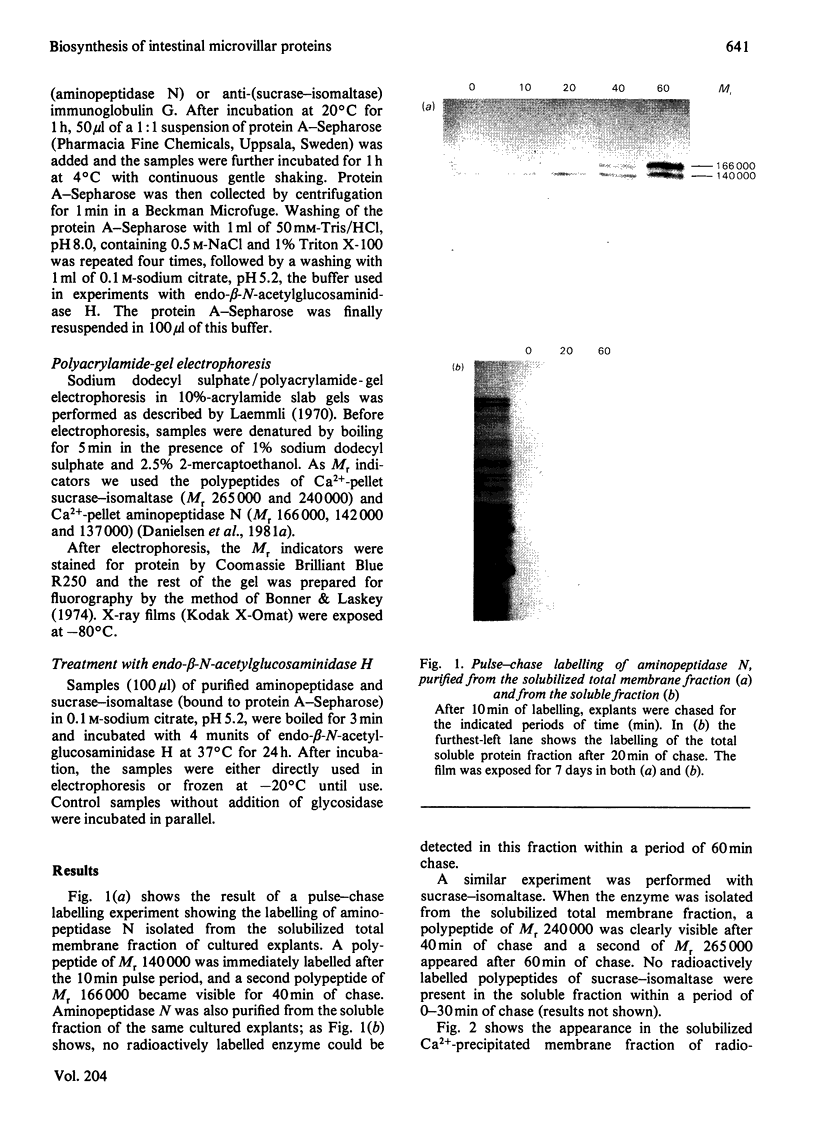
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]