Abstract
Premise
Volatile emissions from flowers and fruits play a key role in signalling to animals responsible for pollination and seed dispersal. Here, we investigated the pollination biology and chemical ecology of reproduction in Apodolirion buchananii, an African amaryllid that flowers in a leafless state soon after grassland vegetation is burnt in the dry late‐winter season.
Methods
Pollinators were identified through field collection and pollen loads were quantified. Floral traits including spectral reflectance and scent chemistry were documented. Bioassays using cup traps were used to test the function of floral volatiles. Fruiting biology was investigated using controlled hand‐pollination experiments and chemical analysis of fruit scent. Seed germination was scored in greenhouse trials. Seed dispersal was monitored using observations and camera trapping.
Results
The sweetly scented white flowers of A. buchananii are pollen‐rewarding and pollinated mainly by a diverse assemblage of bees. Cup‐trap experiments demonstrated that pollinators are attracted to phenylacetaldehyde, the dominant volatile in the floral scent. Plants are shown to be self‐incompatible, and the fleshy fruits were found to emerge from the soil six months after pollination during the peak of the summer rains. Fruits emit a diverse blend of aliphatic and aromatic esters and contain large fleshy recalcitrant seeds which germinate within days of fruits splitting open. Seed dispersal by ants was recorded.
Conclusions
This first account of the reproductive biology of a species in the genus Apodolirion highlights an outcrossing mating system involving bees attracted to color and scent as well as the unusual fruiting biology and ant‐mediated system of seed dispersal.
Keywords: amaryllidaceae, ants, Apodolirion buchananii, fire, functional traits, plant volatiles, phenology, phenylacetaldehyde, seed dispersal, subterranean ovary
In most plants, flowering and seed dispersal are closely coupled phenophases. Interesting exceptions include geophytic species with subterranean ovaries that retain fertilized seeds underground for several months during unfavorable conditions. A subterranean ovary functions as a protective mechanism, particularly in harsh climatic conditions, and is associated with early flowering, the avoidance of pollinator competition, and the emergence of mature fruits in a different season—when conditions are favorable for seed dispersal and seedling establishment (Burtt, 1970; Dafni et al., 1981).
The best‐known example of geophytes with an underground ovary is the genus Crocus L. in the Iridaceae, but examples of this reproductive strategy are also known from the Amaryllidaceae and Colchicaceae. In many cases, flowering occurs very early in spring, even before the development of leaves (Dafni et al., 1981). Geophytes with subterranean ovaries are best known from Mediterranean climates, but some such as Apodolirion Baker (Amaryllidaceae), occur in summer rainfall regions and flower in the dry conditions of spring (Burtt, 1970). In most cases, the dispersal biology of these plants is poorly understood, but seed dispersal by ants (myrmecochory) has been observed in Sternbergia Waldst. & Kit. (Amaryllidaceae) and Colchicum L. (Colchicaceae) (Burtt, 1970; Dafni et al., 1981).
Southern Africa is rich in geophytes and is one of the centres of diversity and endemism for the Amaryllidaceae (Meerow and Snijman, 1998). Many of these species are located in fire‐prone habitats and their flowering phenology is mediated by fire (Keeley, 1993). Apodolirion represents a small group of six bulbous geophytic perennial species in the African tribe Haemantheae (subtribe Gethyllidinae) found in both the winter and summer rainfall regions of southern Africa (Meerow and Snijman, 1998). In the Gethyllideae, Apodolirion is either sister to, or nested in Gethyllis L., which almost exclusively occurs in the winter rainfall region of South Africa (Meerow and Clayton, 2004). Little is known about the pollination biology or seed dispersal system of either Apodolirion or Gethyllis, and this has hindered our understanding of the functional significance and evolution of their distinctive shared traits, such as strongly scented flowers, an underground ovary, and fleshy fruits and seeds. Marloth (1915) asserted that the fragrant white or light‐pink flowers of Gethyllis ciliaris (Thunb.) Thunb. were visited by the hawk‐moth Agrius convolvuli L. [Sphingidae], which probes deeply into the long floral tube. However, this must have been conjecture as Burtt (1970) noted that the narrow diameter of the floral tube is largely dominated by the style, leaving little to no room for nectar probing by a long‐tongued moth. Emerging only after rains when ripe, the fragrant highly sought after fruits of Gethyllis were reported by Marloth (1915) to be dispersed by birds and rodents, but it has been unclear how this could result in effective seed dispersal given that the seeds are soft and ill‐suited to passage through the gut of animals. Kamatou et al. (2008) found that the fruits of G. afra L. and G. ciliaris emit a rich blend of aliphatic and aromatic esters as well as monoterpenes. Flowers and fruits of Apodolirion, by contrast, are not well‐described. The predominantly white flowers of A. amyanum D.Müll.‐Doblies, have been reported as intensely jasmine‐scented, producing an odorless and tasteless reddish club‐shaped berry (Zikishe et al., 2020).
To resolve some of the uncertainties and contradictions that have surrounded the pollination biology and seed dispersal system of the Gethyllidinae for more than a century, we conducted a study of Apodolirion buchananii, a species that occurs in the eastern summer rainfall regions of South African and Eswatini (Pooley, 1998). We sought to establish: (1) the assemblage of pollinators; (2) floral traits, including the chemistry of scent; (3) behavioral responses of pollinators to floral scent compounds; (4) the dependence on pollinators for seed production; (5) the timing of seed germination; (6) fruit traits including the chemistry of scent; and (7) agents of seed dispersal.
MATERIALS AND METHODS
Species
Apodolirion buchananii is known as the “Natal crocus” on account of its underground ovary and flowering in a leafless state shortly after fires in the mid‐ to late‐austral winter and early spring. The actinomorphic flowers (Appendix S1) range from white to light‐pink (Pooley, 1998), with long floral tubes and a rudimentary scape that is shrouded by the bulb neck (Meerow and Snijman, 1998). Floral anthesis occurs in the morning and anthers dehisce between one and two hours after anthesis. Flowers close in the late afternoon and open again during the following morning. Flowers last for 3–5 days. The Gethyllidinae produce a clavate, thin‐walled, indehiscent fruit with soft globose seeds (Meerow and Snijman, 1998), but we found no previous description or herbarium record of the fruit or seeds of A. buchananii or any mention of the time of year when the fruit appears above ground.
Study sites
Studies were conducted in populations at two sites (separated by approximately 31 km) in KwaZulu‐Natal, South Africa. The site at Mount Gilboa Nature Reserve (hereafter MG, 29°17′14″S30°17′33″E, 1760 m a.s.l.), had a population of approximately 200 flowering plants and the site at Fountainhill Nature Reserve (hereafter FH, 29°29′54″S30°30′36″E, 670 m a.s.l.) had approximately 250 plants. A voucher specimen, M. Streicher 20, is lodged in the Bews Herbarium (NU, University of KwaZulu‐Natal, South Africa).
During the mid‐austral winter (June) of 2022, bulbs were collected from the FH site with the permission of the landowner and brought to the greenhouse (University of KwaZulu‐Natal, Pietermaritzburg Campus, Botanical Gardens) for breeding system experiments. Given that the timing of fruit emergence from the soil was unknown, it was deemed essential to conduct these experiments using cultivated plants which could be monitored closely.
Pollinator observation and collection
During the flowering season in July–August of 2022, A. buchananii populations were monitored at MG for seven days (totalling 56 hours), and at FH for 13 days (totalling 104 hours). Observations typically began at 08H00 and ended at 16H00. We recorded the date, time, duration of foraging bouts, behavior (including anther and stigma contact) and identity of each species visiting the flowers. Floral visitors were collected, killed by rapid freezing, and their pollen loads removed by swabbing small blocks of fuchsin gel over the wings and body. Fuchsin gel blocks were melted onto microscope slides and sealed with glass coverslips and pollen grains (including foreign pollen) were counted using a compound microscope and reference slides for comparison.
Floral traits
Floral spectral reflectance (300–700 nm) was measured for 9 flowers, from 9 plants (for each site) using a fibre optic reflection probe (QR‐400‐7‐UV‐VIS; 400 µm) coupled with a S2000 spectrophotometer (Ocean Optics, Dunedin, Florida, USA), as described in detail by Johnson and Anderson (2002). We measured floral dimensions at the FH site (10 flowers, from 10 plants), recording: (1) Total flower length (above ground); (2) floral tube length (ground to corolla base); (3) floral tube width (at ground level); (4) floral width (broadest part of the flower); (5) tepal width and length (inner and outer tepals); (6) floral entrance width; (7) anther and stigma protrusion (taken as the distance from the tip of the flower); (8) herkogamy (stigma to nearest anther); (9) stigma width; (10) anther length and width (upper and lower anthers); (11) stamen length (upper and lower stamens); (12) and corolla depth. We dissected and examined flowers under a compound microscope (Zeiss Axio Imager.A2; Carl Zeiss, Jena, Germany) for nectar and similarly conducted a Sudan IV test for floral oils. Floral traits were measured shortly after flowers had fully opened and prior to anther dehiscence.
To determine the composition of floral scent we collected dynamic headspace samples from three flowers from the MG site and from 11 flowers from the FH site. Flowers were sampled singly in acetate bags (Kalle, Wiesbaden, Germany) for 45 minutes using a Spectrex PAS‐500 personal air sampler (Spectrex Corp. Redwood City, California, USA) at an airflow rate of 200 ml/min. Scent compounds were collected in glass cartridges filled with 1.5 mg each of Carbotrap® B (20–40 mesh; Merck KGaA, Darmstadt, Germany) activated charcoal and Tenax® TA (60/80; Supelco, Bellefonte, Pennsylvania, USA). Control samples from empty bags were run concurrently to identify and exclude compounds that did not originate from flowers. Gas chromatography‐mass spectrometry (GC‐MS) analysis of volatiles was conducted on a Varian CP‐3800 GC (Varian Corp., Palo Alto, California, USA) coupled to a Bruker 300 single quadrupole MS. Volatiles were thermally desorbed from cartridges using a Varian 1079 PTV injector port modified with a ChromatoProbe device. Volatiles were separated on an SGE SolGel Wax polar capillary column (30 m × 0.25 mm ID, 0.25 µm film thickness; SGE Analytical Science, Milton Keynes, England). The GC temperature programme started at 40°C with a 3 min hold, increased to 240°C at 10°C min−1 and was then held at 240°C for 12 min. The MS was operated in electron‐impact ionization mode at 70 eV. Compounds were identified using the NIST 2020 mass spectral library (website: http://www.nist.gov) through a combination of mass spectral matching and comparisons of calculated linear (non‐isothermal) n‐alkane retention times relative to average values reported in NIST 2020 for the same type of column.
Floral scent bioassays
To test the role of scent in the pollination system, cup traps were deployed at the FH site from July to September and from August to September at the MG site. We used commercially available plastic Redtop® Fly Catchers (website: www.redtop-flytraps.com) Cup Traps (19 × 9 × 7 cm) sunk into the ground such that the lids (sans the square cover) were presented at the height of natural flowers (see Appendices S2, S3). We painted the funnel‐shaped 10 cm diameter lids (1 cm entrance diameter) with white acrylic paint (white 01; Genuine Heritage Craft Products, Johannesburg, South Africa) that matched the spectral reflectance of the flowers (Appendix S4). Small scent vials containing either pure unscented liquid paraffin (control) or an artificial scent consisting of a phenylacetaldehyde (Sigma‐Aldrich, St. Louis, Missouri, USA) and paraffin mixture were strapped to the inside of the cup traps (below the lid) using a black Duct tape (3 M® 389 Duct Tape; website: www.3m.co.uk). We used phenylacetaldehyde in these experiments because it is the dominant compound in the floral scent (see Results). Artificial scent was made by adding 0.12 g of phenylacetaldehyde to 10 ml of pure paraffin oil. The mixture was vortexed on high for 15 minutes to ensure thorough homogenisation. 1 ml of artificial scent or pure paraffin were pipetted into each vial and kept in the refrigerator until used. Cup traps were deployed in pairs at two meters apart, one with a paraffin control and one with artificial scent. Each pair was set out at least 10 m from the next. Traps were left in the field for a maximum of five days to ensure that the odor did not deteriorate significantly. Scented traps were still strongly perfumed to the human nose at time of collection. To test whether emission rate of scent from the cup traps was similar to that of actual flowers, we took headspace samples from cup traps (using the same method as described previously for flowers) on the first, third and fifth day of exposure to field conditions. Nine trapping bouts were deployed at FH (totalling 40 days) site and six trapping bouts (totalling 27 days) were conducted at MG. After the trapping period, insects were collected and swabbed for pollen in the same way as described for field‐collected specimens.
Controlled hand‐pollination experiments
To conduct controlled hand‐pollination experiments and to study the fruiting biology of A. buchananii, plants were located in dry grassland in the winter by means of their withered leaves. With the permission of the landowner, 43 bulbs were removed from the FH site along with soil from the surrounding area which consisted mainly of fine sand and little organic matter. Bulbs had a mean width of 21.78 ± 2.00 mm and a mean length of 21.82 ± 0.70 mm (n = 43). Bulbs were placed into cool, dark, well‐ventilated plastic storage boxes lined with screened substrate. Bulbs were allowed to sit at ambient temperature (10–25°C) for 24 hours before being cleaned, weighed (after removal of any dead leaves) and measured. Circular brown 25 cm plastic pots were lined with course nylon mesh (to prevent substrate loss and allow adequate drainage) followed by a 4 cm thick layer of vermiculite. Pots were then filled with substrate and plants covered such that a 5 cm thick layer of substrate covered each bulb (one bulb per pot). Pots were housed in the greenhouse under 40% shade, receiving rainfall and additional water as needed. Plants were not fertilized. Flowers took an average of 19 ± 0.3 days to emerge from the soil from the time of planting.
To test for self‐incompatibility and the dependence of A. buchananii on pollinators for seed set, we performed controlled hand‐pollination using plants that flowered in cultivation. Of the 43 collected bulbs, 34 flowered, producing 36 flowers (2 bulbs each produced 2 flowers). Flowers were randomly allocated to one of three treatments: (1) Unmanipulated control for autogamy (8 flowers, 8 plants); (2) self‐pollination (9 flowers, 9 plants); (3) cross‐pollination (19 flowers, 17 plants). Plants producing two flowers were assigned to a single treatment. All hand‐pollination treatments were carried out twice: Once shortly after anther dehiscence (approximately one hour after flowers fully opened; usually at 11H00) on the first day, and at the same time on the second day. Anthers were collected, marked, and kept in the fridge (6°C) until use (discarded after two days). Pots were kept isolated in small fine mesh nylon pollination exclosures during the duration of flowering and fruits were monitored for emergence throughout their development. Fruits were collected for seed scoring once they had fully emerged from the soil when ripe and splitting (see Figure 1H,I).
Figure 1.

Reproductive biology of Apodolirion buchananii. (A) Immature buds emerging from the soil surrounded by freshly opening flowers – FH site. (B) Apis mellifera scutellata attempting unsuccessfully to move deeper into the corolla during foraging bout – MG site. (C) Stomorhina sp. (Rhiniidae) feeding on pollen from an anther – FH site. (D) Two Pseudapis sp. (Halictidae) at the FH site after collecting pollen (insects not collected), together with an unidentified Coleoptera species. (E) Seladonia sp. (Halictidae) clutching an anther following a lengthy pollen collection bout deep within the corolla – FH site. (F) Allograpta sp. (Syrphidae) feeding on pollen – FH site. (G) Ceratina sp. (Apidae) leaving a flower with a large pollen load on its ventral surface – MG site. (H) A fully emerged, ripe fruit, the day before dehiscence (greenhouse potted plant). (I) Two fruits (from a single bulb), each bearing many seeds, illustrating the typical splitting and progression of fruit senescence (greenhouse potted plant – atypical production of two fruits). (J) A ripe fruit following dehiscence and seed removal by ants. A single seed remains wedged between grass culms – FH site. (K) Myrmicaria sp. (likely natalensis) attempting to extract a seed from an old fruit (four days after splitting) – FH site. (L) Apodolirion buchananii seedlings, 10 days after seeds were sown. (M) Myrmicaria natalensis carrying A. buchananii seeds to their nest. Scale bars = 10 mm (Photographs: I. Kiepiel).
Fruit traits and seed germination
We recorded the time taken for fruit development and emergence from the ground. The width and length of the emergent parts of 11 intact fruits were measured the day before fruit dehiscence (i.e., splitting). Scent emissions from fruits were sampled and analysed as described above for flowers. We planted 20 seeds each from six fruits (six plants) in an 80% shaded greenhouse using the method described above. Seeds were removed from the fruit and gently wiped clean with tissue paper and placed on the surface of the substrate. An additional 10 seeds from five fruits were planted at a depth of 1 cm below the soil surface. Pots were watered as needed with a fine water spray and germination was scored daily. The dimensions of 98 seeds were also measured (20 seeds each from four fruits, and 18 from one fruit). We measured the height of 10 randomly selected seedlings from each pot 10 days after planting and again 24 days after planting.
Seed dispersal
We used camera traps (Bushnell® 14 mp NatureView Cam HD; website: www.bushnell.com) to detect animals that may be responsible for seed dispersal at the FH site. A two‐meter diameter circular exclusion area was set up using 80% nylon shade cloth wired to steel poles. The shade cloth was positioned at a height of 30 cm above the ground and measured 1.2 m in height. This allowed for smaller animals such as rodents to enter whilst excluding antelope and larger animals. We reasoned that rodents may disperse seeds while dragging and manipulating fruits, while larger animals would simply destroy seeds by ingesting them along with whole fruits. We began by sinking a single potted plant bearing a single unsplit ripe fruit (i.e., Figure 1H) into the ground such that the soil in the pot was at ground level (the rim of the pot was cut away). Three cameras, each at different heights and visual angles (to maximize the probability of recording interactions) were set on a single fruit at 09H00 in the morning. Cameras were checked at the same time in subsequent mornings. This was carried out twice for 72‐hour periods each. Given that few fruits could be located in the natural population, we then opted to take whole ripe unsplit fruits from the greenhouse into the field. Whole fruits were gently removed from potted plants and placed into the ground so that they protruded to the same height as when they were harvested. Cameras were set (as above) and the experiment carried out using six fruits for a period of 48 hours per fruit.
After observing seed removal from fruits by ants, we removed the seeds from each fruit and placed them in the centre of the circular exclusion area. Seeds were placed equidistant from one another in a 10‐cm diameter circular pattern with the empty fruit in the centre. Seed movement was monitored in the exclusion area for a period of two hours, after which cameras (n = 3) were programmed to time lapse (set to record for one minute at 10‐minute intervals) and the surrounding area and ant trail networks were searched for dropped seeds. Untaken and dropped seeds quickly germinated such that that any residual seeds from a previous trial were easily distinguishable from a subsequent one. The experiment was carried out with the seeds from seven fruits. Cameras were left running for 48 hours after which the number of seeds remaining were recorded.
We used a bioassay to test the behavioral responses of ants to volatile compounds on the seeds using three different solvent extractions of whole seeds that were freshly removed from fruits. In a small glass vial, we submerged 12 seeds (four seeds each from three separate fruits) in gas chromatography‐grade solvents either hexane, dichloromethane, or a mixture of 70% ethanol and 30% distilled water for one hour, after which the seeds were removed. Each bioassay used a single solvent. Using a paper punch, we punched out small disks of uniformly sized filter paper. A pair of disks, one a control and one an extract, were placed 10 cm apart after being immersed in their respective solutions. Each bioassay was allowed to run for 20 minutes and was repeated with new disks a total of 10 times for each of the three solvents (Merck KGaA, Darmstadt, Germany).
Statistical analysis
Data were analysed using generalized linear models in SPSS 28 (IBM Corp., Armonk, New York, USA). Mean fruit set was compared among pollination treatments using Generalized Linear Models (GLMs), with a binomial model distribution and a logit link function. Data for the number of seeds per flower (count measure) were analysed using negative binomial models with a log link function. Significance was assessed using likelihood ratios. Cup‐trap data were analysed using Generalized Linear Mixed Models (GLMM) using a negative binomial model and log link function. Cup‐trap pairs (control and phenylacetaldehyde) were treated as the subject, with the response variable designated as the number of insects collected per trap for the duration of a trapping bout. We included an offset consisting of the natural log of days of exposure to obtain a rate of insects trapped per day. Each trapping bout was considered as an additional random effect, with site, treatment and the interaction of site and treatment assigned as fixed effects. Marginal means and asymmetrical standard errors from GLM and GLMM analysis were obtained through back‐transformation of values from the linear scale. If GLMs failed to run owing to a lack of variance (e.g., zero fruit set for unmanipulated treatment) we substituted a single value (e.g., 0 replaced by 1) to allow model convergence. This results in a slightly more conservative test since the differences among means is reduced.
RESULTS
Pollinator observation and collection
We observed the behavior of 107 insects on A. buchananii flowers and collected 71 of these insects at the FH site (Appendix S5). Flowers open in the mid‐morning and most insects were observed and collected between 11h00 and 14h00. Visiting insect numbers tapered off sharply towards the mid‐afternoon and flowers closed overnight, thus precluding nocturnal visits. Insects alighted on the tepals and collected pollen from the anthers or pollen scattered on the tepals. Bees were the most commonly observed floral visitors and accounted for approximately 71% of all visits, followed by flies (ca. 22%, Table 1; Appendix S5). Apidae comprised ca. 82% of the bees visiting the flowers, with Halictidae making up the remaining 18% (Appendix S5). Apis mellifera scutellata Lepeletier was the most common floral visitor, followed by Ceratina nyassensis Strand, then Braunsapis otavica Cockerel (Table 1). Apis mellifera had the highest A. buchananii pollen loads and carried the least amount of foreign pollen of any bee species (Table 1). Bees were actively engaged in pollen collection and moved considerably within the flowers, making stigma contact far more frequently than other insects (Appendix S5). Flies typically fed on scattered pollen on the tepals, with the consequence that they did not usually venture far into the corolla to feed (Figure 1C, F). This led to poor stigma contact (mean proportion: 0.33 ± 0.1 in comparison with bees (mean proportion: 0.92 ± 0.1; Appendix S5). Pollen accumulated mainly on the head and proboscis of flies, whilst on bees it accumulated over their entire body and large pollen loads were also observed in their corbiculae or scopae, indicating active collection. Bees carried the highest A. buchananii pollen loads of all studied insects (mean 817 ± 394.1, excluding scopal loads), followed by flies (mean 53 ± 17.5), then lepidopterans (11 ± 5.8; Appendix S5).
Table 1.
Ten most abundant insect species visiting Apodolirion buchananii flowers and the number of each species recorded at the FH and MG sites during the 2022 flowering season (n = 99, see Appendix S5 for full list). Insects and pollen loads were collected only at the FH site. Values represent means ± SE, followed by the sample size in parenthesis (number captured). Pollen purity calculated as the mean percentage of A. buchananii pollen loads found on each insect species relative to foreign pollen.
| Number recorded | |||||
|---|---|---|---|---|---|
| Order, Family | Species | FH | MG | A. buchananii pollen load | A. buchananii pollen purity (%) |
| Hymenoptera | |||||
| Apidae | Apis mellifera | 15 | 14 | 3737 ± 2395.1 (7) | 100 |
| Braunsapis otavica | 16 | 68 ± 33.7 (14) | 92 | ||
| Ceratina nyassensis | 17 | 198 ± 76.0 (12) | 95 | ||
| Halictidae | Lasioglossum sp. 1 | 5 | 971 ± 813.1 (5) | 55 | |
| sp. 3 | 3 | 25 ± 16.0 (3) | 77 | ||
| Seladonia sp. 2 | 4 | 259 ± 104.4 (4) | 98 | ||
| Diptera | |||||
| Rhiniidae | sp. 1 | 10 | 85 ± 47.2 (6) | 97 | |
| Syrphidae | Eupeodes corollae | 3 | 36 ± 19.5 (3) | 40 | |
| Ischiodon aegyptius | 7 | 38 ± 14.4 (5) | 34 | ||
| Lepidoptera | |||||
| unknown | sp. 1 | 5 | 18 ± 7.2 (3) | 100 | |
Floral traits
Flowers are white with strong UV absorption and the spectra of cup traps closely matched those of the flowers (Appendix S4). Some flowers were tinged pink (Appendix S4). Apodolirion buchananii flowers did not emerge more than several centimetres from the soil and bloomed close to the ground (Appendix S2), with tepals orientated horizontally (Figure 1D) and flowers showing slight heliotropism. Flowers exhibit herkogamy, with the anthers encircling the style on two levels just beneath the stigma (Appendix S2). No nectar or floral oils were detected in the flowers. The floral scent was dominated by aromatic compounds and phenylacetaldehyde made up more than 50% of total floral scent emissions at both sites (Table 2).
Table 2.
Chemical composition of the scent of Apodolirion buchananii flowers. Proportion values are means ± SE with the number of samples in which a compound was recorded indicated in parentheses.
| Compound | Linear retention index | MG (n = 3) | FH (n = 11) |
|---|---|---|---|
| Aromatics | |||
| Benzaldehyde | 1545 | 45.96 ± 1.22 (3) | 20 ± 7.49 (11) |
| Phenylacetaldehyde | 1667 | 50.54 ± 0.92 (3) | 62.21 ± 10.25 (11) |
| Benzyl formate | 1714 | 0.06 ± 0.03 (2) | 0.06 ± 0.07 (3) |
| Phenylethyl acetate | 1839 | 0.04 ± 0.06 (1) | 2.89 ± 1.41 (11) |
| Benzyl alcohol | 1899 | — | 2.94 ± 1.35 (11) |
| Phenylethyl alcohol | 1938 | 2.9 ± 1.88 (3) | 8.53 ± 3.33 (11) |
| Methyleugenol | 2031 | 0.14 ± 0.24 (1) | — |
| Eugenol | 2187 | 0.03 ± 0.05 (1) | 0.09 ± 0.14 (2) |
| Elemicin | 2240 | 0.02 ± 0.03 (1) | — |
| 4‐Methoxy‐2‐phenylethanol | 2347 | — | 0.11 ± 0.13 (4) |
| Nitrogen compounds | |||
| 2‐Phenylacetonitrile | 1954 | — | 2.84 ± 1.33 (7) |
| Irregular terpenes | |||
| 4‐Oxoisophorone | 1720 | 0.33 ± 0.25 (2) | 0.08 ± 0.08 (3) |
| Sesquiterpenes | |||
| (E,E) α‐farnesene | 1771 | — | 0.15 ± 0.24 (2) |
| (E)‐Nerolidol | 2054 | — | 0.09 ± 0.15 (2) |
Floral scent bioassays
We captured a total of 156 insects in cup traps at the FH site and 114 insects in cup traps at the MG site. At both sites, the mean number of insects captured in cup traps with phenylacetaldehyde was approximately three‐fold greater than those lacking phenylacetaldehyde (F1,268 = 55.99, P < 0.01, Figure 2). There was a slightly higher overall rate of capture at the MG site (F1,268 = 6.09, P = 0.014, Table 3, Figure 2) and no significant interaction between treatment and site (F1,268 = 0.26, P = 0.61).
Figure 2.
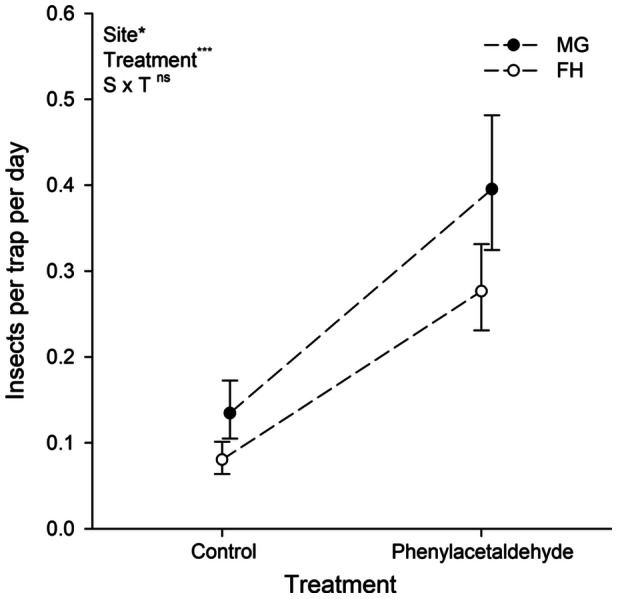
Mean number of insects trapped per day at the MG (solid symbol) and FH (open symbol) site during the 2022 flowering season (see text for sample size and trapping duration).
Table 3.
Cup trap collected insects from the FH (n = 156) and MG site (n = 114) during the 2022 flowering season. Values represent the number of individuals of a species collected in control (unscented) or scented cup traps, followed by the percentage of individuals found carrying A. buchananii pollen. Formicidae not shown (FH, n = 1; MG, n = 4). Species represented by no more than two individuals from both sites are omitted from the table. Species marked with * were also collected on A. buchananii flowers (see Appendix S5).
| Order, Family | Species | FH | MG | ||||
|---|---|---|---|---|---|---|---|
| Control | Scented | A. buchananii pollen (%) | Control | Scented | A. buchananii pollen (%) | ||
| Hymenoptera | |||||||
| Apidae | Allodape rufogastra | 0 | 2 | 100 | 1 | 6 | 0 |
| Apis mellifera* | 2 | 4 | 83 | 3 | 4 | 71 | |
| Braunsapis otavica* | 4 | 9 | 31 | ||||
| B. nyassensis* | 16 | 27 | 53 | ||||
| Halictidae | Lasioglossum sp. 1* | 0 | 1 | 0 | 5 | 9 | 21 |
| sp. 2 | 3 | 9 | 50 | 11 | 37 | 52 | |
| sp. 3* | 1 | 21 | 41 | ||||
| sp. 4* | 4 | 8 | 42 | 5 | 11 | 31 | |
| Seladonia sp. 1 | 1 | 8 | 78 | ||||
| sp. 2* | 1 | 20 | 43 | 0 | 1 | 100 | |
| sp. 3 | 1 | 9 | 30 | 0 | 2 | 100 | |
The assemblage of Hymenoptera captured in the cup traps was similar to that observed on A. buchananii and insects captured in the cup traps were found to have adherent pollen of A. buchananii, but in smaller amounts compared to field caught insects (Appendix S5).
The rates of scent emission from the cup traps were very close to flowers of A. buchananii. We recorded mean (±SE) emission rates (ng/hr) from traps of 3192.5 ± 1014.8 on day one, 679.9 ± 265.5 on day two and 546.3 ± 188.2 on day three. Emission rates recorded from flowers were 2747.2 ± 962.3 at FH and 660.1 ± 41.7 at MG.
Controlled hand‐pollination experiments
Unmanipulated bagged flowering plants failed to set fruit indicating that plants are incapable of autonomous selfing (Figure 3). Approximately 84% of cross‐pollinated flowers set fruit (Figure 3). A single self‐pollinated flower set a fruit containing just two seeds, while flowers in the cross‐pollination treatment produced a mean of 25 seeds per fruit (Figure 3). The time taken from pollination to the emergence of the fruit (see Figure 1I) was approximately 30 weeks. Fruits all ripened within three weeks of one another. The 30‐week period from flowering to fruiting was not an artifact of cultivation as we found that fruit emergences in natural populations coincided with those of cultivated plants. There was a positive correlation between the number of seeds produced per fruit and bulb mass in cross pollination treatments, with bigger bulbs generally producing a greater number of seeds per fruit than smaller bulbs (Appendix S6).
Figure 3.

Fruit set (A) and seeds per flower (B) of Apodolirion buchananii following control (unmanipulated), self‐ and cross‐pollination treatments. Values represent means (±SE), followed by sample size (number of flowers and number of plants in parentheses). Dissimilar lettering indicates significant differences between treatments.
Fruit traits and seed germination
Fruits took more than six months to develop and began to emerge during the peak of the summer rainy season, either with or without leaves. Fruits had a mean ± (SE) width of 12.2 ± 0.80 mm and stood 34.0 ± 2.87 mm above the soil. The scent of fruits was strongly dominated by a very diverse range of aliphatic and aromatic esters (Table 4). Remarkably, there was almost no overlap between the compounds emitted by the flowers and fruits (Tables 2, 4).
Table 4.
Chemical composition of the scent of Apodolirion buchananii fruits. Proportion values are means ± SE with the number of samples in which a compound was recorded indicated in parentheses.
| Compound | Linear retention index | FH (n = 6 samples) |
|---|---|---|
| Aliphatic acid | ||
| Acetic acid | 1397 | 5.6 ± 6.96 (3) |
| Aliphatic alcohols | ||
| Heptan‐2‐ol | 1286 | 14.39 ± 7.33 (4) |
| Nonan‐2‐ol | 1471 | 0.19 ± 0.46 (1) |
| Octan‐1‐ol | 1535 | 0.36 ± 0.4 (2) |
| Aliphatic esters | ||
| Ethyl hexanoate | 1223 | 0.18 ± 0.45 (1) |
| 2‐Heptyl acetate | 1256 | 11.05 ± 6.65 (4) |
| 2‐Heptyl butyrate | 1321 | 1.65 ± 1.88 (2) |
| 2‐Heptyl hexanoate | 1527 | 2.38 ± 2.18 (3) |
| Decyl acetate | 1628 | 0.13 ± 0.32 (1) |
| Aliphatic ketone | ||
| Heptan‐2‐one | 1158 | 32.75 ± 5.97 (6) |
| Aromatic esters | ||
| Isopropyl benzoate | 1636 | 1.94 ± 1.16 (4) |
| Benzyl acetate | 1702 | 1.4 ± 1.55 (2) |
| Benzyl isobutyrate | 1757 | 0.74 ± 1.02 (2) |
| Benzyl propanoate | 1766 | 0.33 ± 0.8 (1) |
| Benzyl butyrate | 1842 | 2.86 ± 1.76 (4) |
| 2‐Heptanol, benzoate | 1996 | 0.22 ± 0.26 (2) |
| Benzyl n‐hexanoate | 2043 | 1.35 ± 1.05 (3) |
| Benzyl tiglate | 2080 | 0.03 ± 0.07 (1) |
| (E)Cinnamyl acetate | 2115 | 0.05 ± 0.06 (2) |
| Benzyl octanoate | 2251 | 0.3 ± 0.23 (3) |
| Benzyl benzoate | 2596 | 2.56 ± 1.11 (6) |
| Aromatic alcohols | ||
| 1‐phenylethanol | 1778 | 0.31 ± 0.29 (3) |
| Benzyl alcohol | 1826 | 2.25 ± 1.49 (4) |
| 3‐Phenylpropanol | 2000 | 0.23 ± 0.57 (1) |
| m‐cresol | 2015 | 0.14 ± 0.16 (2) |
| Cinnamyl alcohol | 2235 | 0.04 ± 0.1 (1) |
| Aromatic acid | ||
| Benzoic acid | 2390 | 0.24 ± 0.6 (1) |
| Monoterpenes | ||
| β‐myrcene | 1146 | 0.95 ± 0.62 (4) |
| Limonene | 1173 | 1.96 ± 2.95 (2) |
| (‐)‐‐α‐Terpineol | 1669 | 0.08 ± 0.09 (2) |
| Nitrogen‐containing compounds | ||
| Oxime‐, methoxy‐phenyl‐ | 1720 | 6.1 ± 3.87 (4) |
| 2‐Phenylacetonitrile | 1885 | 1.84 ± 1.33 (3) |
| Sesquiterpenes | ||
| (E)‐Farnesyl acetate | 2229 | 3.97 ± 2.32 (5) |
| Farnesol | 2307 | 0.48 ± 0.19 (5) |
| Unknown | ||
|
69, 55, 70, 56, 83, 41, 97, 57, 43, 84 |
1240 | 0.94 ± 1.03 (2) |
Seeds were 4.7 ± 0.05 mm in length and 3.2 ± 0.04 mm in width. These began germinating two days after planting and most of seeds (ca. 85%) had germinated within 12 days (Appendix S7). Seeds planted below the surface of the soil failed to emerge from the ground. Seedling growth was rapid. After 10 days seedlings had grown an average of 25.1 ± 1.36 mm in height and were 121.6 ± 2.01 mm tall after 24 days.
Seed dispersal
In the second period of monitoring potted bulbs in fruit, the plant was dug up by a Cape porcupine (Hystrix africaeaustralis Peters, Hystricidae) which appeared disinterested in the fruit or seeds (these were later recovered intact). A small piece of the bulb was bitten off and presumably eaten as this could not be located. Harvested fruits were subsequently untouched by porcupines despite camera footage revealing regular visitation to the enclosure area. Camera traps captured videos of several Natal multimammate mice (Mastomys natalensis Smith, Muridae) passing the fruits (potted and harvested) and seeds at various times during the night without showing interest.
Observations and camera footage revealed consistent seed dispersal by ants. We observed both Camponotus niveosetosus Mayr and Myrmicaria natalensis F. Smith (Formicidae) carrying small pieces of fruit and seeds, but the majority of seed‐carrying behavior involved M. natalensis (Figure 1M). A third smaller unidentified ant species was also observed on the fruits, carrying off minute pieces of the fruit wall. Ants ignored fruits until the fruit wall began to split open and seeds were revealed (Figure 1I), at which point they showed noticeable excitement and a marked increase in activity. Ants found it difficult to penetrate the fruit and relied on the natural dehiscence of the fruit to access the seeds. The time taken from the initial fruit splitting to opening of the fruit was between two and three days, depending on temperature (i.e., more rapid during hot, sunny days). Once fully open, fruits were generally completely stripped of their seeds by ants over a period of 24 hours (i.e., Figure 1J). Seed movement by M. natalensis was observed only during daytime hours and ants were not active during periods of cold or rain. Though ants did remove parts of the fruit and often the entire fruit was gradually taken, seed removal was prioritised, and fruit collection occurred mainly after seed removal (i.e., Figure 1J). When an individual seed became dislodged from a fruit and fell to the ground or when placed on the ground, in the case of our trials, the ants were observed spending a few minutes examining the seed with their antennae and forelegs before picking it up. This behavior was also observed in small groups of ants which surrounded a seed in a similar manner. Once the decision was made to pick up a seed, it usually took frequent attempts for an individual ant to grasp the smooth unwieldy seed with its mandibles. Once clasped, seeds were swiftly carried along predefined ant trail networks with one seed carried by each ant. Ants were recorded carrying many seeds toward their nest and several seeds were seen being carried into the nest entrance (Figure 1M). Worker ants would often come to meet seed‐bearing individuals just before the nest entrance (Figure 1M).
Seeds that were not gathered in the first few hours after placement were subsequently usually ignored by the ants and seen germinating within a few days (see Appendix S7). Seeds that were removed from fruit and placed into paper envelopes the day before a trial were also ignored by ants, which evidently strongly favored seeds freshly removed from a fruit. During seed placement trials, the fate of 54 seeds from three fruits were closely monitored. Almost 41% of these seeds were taken out of the exclosure by M. natalensis individuals (i.e., for a distance of at least one meter, and likely further as we thoroughly searched the surrounding area) whilst approximately 32% of the seeds were ignored by the ants. Just over 20% of the seeds were dropped within the exclosure, at a mean distance of 100.4 mm. During these trials we were able to find only one dropped seed outside the exclosure at 3.1 m from the placement site owing to the dense grassland vegetation. On two separate occasions we tracked the fate of a seed collected from the placement site and taken into the nest, a distance of 8.6 m which took a mean time of 23 minutes and 44 seconds.
On several occasions, when a M. natalensis colony was disturbed, ants were observed emerging from the nest carrying both larvae and seeds at the same time (see Appendix S8, Supplementary Video). Myrmicaria natalensis ant larvae were found to be a mean of 4.71 mm in length with a width of 2.91 mm (n = 3). These are remarkably similar in size, shape and coloration to A. buchananii seeds which, on average, were only 0.05 mm smaller in length and 0.31 mm larger in width.
In the bioassays using solvent extracts from seeds, C. niveosetosus and M. natalensis did not show any interest in either the control or the seed extractions, despite passing in close proximity.
DISCUSSION
This study sheds light on the reproductive biology of the Gethyllidinae, a clade of Amaryllidaceae characterized by a crocus‐like reproductive strategy involving a subterranean ovary that allows for uncoupling of flowering and fruiting phenophases. The flowers of A. buchananii are nectarless, strongly scented (Table 2), open only during the day, and are pollinated mainly by pollen‐collecting bees (Table 1). Phenylacetaldehyde, an aromatic compound dominating the scent was found to play a key functional role in attraction of a broad assemblage of bee pollinators (Table 3, Figure 2). Flower color also plays a role as some insects carrying pollen of A. buchananii were captured in the unscented control traps that matched the spectral reflectance of the flowers. By cultivating plants of A. buchananii and conducting controlled hand‐pollination experiments (Figure 3), we were able to establish that the species is self‐incompatible and thus fully pollinator dependent. Furthermore, the first detailed information about the fruiting biology and the mode of seed dispersal was obtained.
Flowering of A. buchananii in its natural habitat, occurs only after fire. We were able to induce flowering through bulb shock during translocation, as has been previously documented in other plants (Norden and Kirkman, 2004; Brewer et al., 2009). The exact physiology behind fire‐stimulated flowering remains uncertain (Hulbert, 1988; Lamont and Downes, 2011; Pyke, 2017). In another southern African amaryllid, a Cape fire‐lily Cyrtanthus ventricosus Willd., smoke was found to initiate flower production after just three days and flowers opened ten days following exposure (Keeley, 1993).
Flowering in a recently burnt landscape increases the visibility of flowers, particularly those that are geoflorous (i.e., presented at ground level) and may reduce pollinator competition. Insect assemblages present in a landscape that has been burnt just one or two weeks previously could be depauperate and this may favor the evolution of relatively generalized pollination systems, particularly in plants such as A. buchananii that are fully dependent on pollinators for seed production (Figure 3). Fire can also increase pollinator abundance and diversity (Ponisio et al., 2016; Burkle et al., 2019). Accordingly, Hymenoptera have been shown to benefit from fire particularly shortly after a burn (Carbone et al., 2019), as do other generalist feeders that are able to return to burned areas faster than specialists (Geerts et al., 2012; García et al., 2018). Wildfires, even high intensity ones, are known to create conditions favorable to sustain bee populations (Potts et al., 2003; Lazarina et al., 2016; Burkle et al., 2019). Much of the research focusing on the effects of fire on pollination is focused on bees (Ne'eman et al., 2000; Potts et al., 2001; Lazarina et al., 2016), and this is likely to reflect the high incidence of bee pollination in pyrogenic habitats.
The nectarless flowers of A. buchananii have a relatively generalized pollination system exploiting solitary bees, honeybees and to a lesser extent, flies, foraging on pollen. Observations of various Gethyllis species in the Cape region have also identified pollen‐collecting bees as the most likely pollinators (S. D. Johnson, unpublished data, W. R. Liltved (South African Museum, Cape Town, South Africa), personal communication), but formal studies of these species have not yet been conducted. Similar floral architecture and pollinator behavior occurs in Sternbergia clusiana Ker Gawl. ex Schult.(Amaryllidaceae), a nectar‐producing eastern Mediterranean amaryllid pollinated by syrphids, honeybees (Apis mellifera) and solitary bees (Anthophora sp. [Apidae]) and Halictus sp. [Halictidae]) (Dafni and Ella, 1982). Apodolirion buchananii pollen which falls from the anthers and collects at bottom of floral tube is collected only by small bee species as honeybees are too large to enter the corolla. Smaller bee species readily visit older pollen‐depleted flowers in search of this pollen and may as a result be more effective pollinators than honeybees. In a community‐ level study conducted at the MG site, Stanley et al. (2020) found that honeybees were among the most common floral visitors in the community but were important pollinators of only a small fraction of the plant species studied (Stanley et al., 2020).
Floral scent functions as an important advertising signal mediating pollinator interactions in many pollination systems (see Raguso, 2008). Scent emission in flowers of A. buchananii may elicit innate attraction responses in bees (Dobson, 1987), but its primary role is probably to reinforce learning (Marden, 1984; Muth et al., 2016). The attraction of bees to phenylacetaldehyde recorded in this study (Table 3, Figure 2) may reflect either or both processes. Knauer and Schiestl (2015) found that naïve Bombus terrestris L. (Apidae) were not initially attracted to phenylacetaldehyde, but after a period of learning through visits to flowers of Brassica rapa L. [Brassicaceae] they quickly learned to associate the compound with the presence of rewards and thereafter developed a preference for the compound. This highlights the importance of honest signalling by plants, but also the potential for learning of associations between volatiles and rewards at the whole community level where bees sample numerous food plants. Phenylacetaldehyde has been recorded in the floral scent of many plant species (Knudsen 1994), including bee‐pollinated taxa (Theis and Raguso, 2005, Mas et al., 2020). In a recent study, Dunlap et al. (2023) found that addition of phenylacetaldehyde to pan traps led to a significant increase in capture of Hymenoptera, which is consistent with earlier studies by Meagher (2002) and El‐Sayed et al. (2008), while other volatiles such as benzaldehyde, geraniol, limonene, and linalool did not have this effect. This suggests that the emission of phenylacetaldehyde is an effective strategy for generalized bee pollination in A. buchananii.
Seed dispersal mechanisms in the amaryllid tribe Haemantheae are particularly interesting because the seeds are baccate (fleshy and berry‐like) and recalcitrant (lacking dormancy) and thus almost certainly unable to withstand gut passage. These physiological traits impose significant constraints on dispersal and seedling establishment. Studies in the subtribes Haemanthinae and Cliviinae have shown that seeds are dispersed by primates, rodents, and birds that consume the edible outer layer of the fruit and then either spit or drop the seeds (Kiepiel and Johnson, 2019; Butler and Johnson, 2022, 2023).
Our chemical analysis of the scent of A. buchananii fruits (Table 4) revealed a complex blend of aliphatic and aromatic esters that is very different from the flower scent (Table 2) and remarkably similar to that described previously from analysis of the scent of fruits of two other Gethyllis species (Kamatou et al., 2008). It is therefore likely that Gethyllis and Apodolirion share the same seed dispersal mechanism. The chemical profile of the scent of fruit of A. buchananii and related Gethyllis species is similar to that of sugar‐rich scented fruits such as those dispersed by bats and other frugivorous mammals (Nevo and Ayasse 2020; Nevo et al., 2019, 2020). We initially expected to find a role for small mammals in the dispersal of A. buchananii fruits, given their scent and position close to the ground. Given that the fruit tissue is palatable (I. Kiepiel, personal observation), we speculated that small rodents may eat the fruits and, in the process, scatter the seeds without ingesting them. However, camera trapping provided no evidence of a role for vertebrates in the dispersal of A. buchananii seeds.
We regularly observed dispersal of the seeds by ants. Myrmicaria natalensis and to a lesser extent C. niveosetosus were observed carrying seeds to their nest (Figure 1M). A wide range of ant species are known to disperse seeds in South Africa and C. niveosetosus is a particularly important disperser of seeds in the Cape region (Slingsby, 2017), whilst other Myrmicaria species have been reported to carry seeds to their nests where they are buried (Bond and Breytenbach, 1985). We observed that both ant species are enthusiastic scavengers but will also hunt live prey and are additionally attracted to floral nectar and sugars such as those found in fruit (I. Kiepiel, personal observation).
Myrmecochory is found in ca. 4.5% of plants globally and is concentrated in Australia, South Africa and the northern temperate regions (Lengyel et al., 2010). The Amaryllidaceae are reported to contain eight myrmecochorous genera, totalling 289 species (Lengyel et al., 2010). Fruits (and seeds) of myrmecochorous plant species are not usually scented to the human nose and are considered to deploy fatty food rewards (eliasomes) and non‐volatile hydrocarbons that elicit innate behavior by ants (Brew et al., 1989; Davidson et al., 1990), possibly through mimicry of the hydrocarbon profiles of ant larvae. Several myrmecophiles including butterflies, beetles, and spiders are able to manipulate the recognition cues of ants and either produce similar hydrocarbons themselves or obtain these through their interactions with their ant hosts (see Hölldobler and Kwapich, 2022). Seeds of A. buchananii were attractive to ants for just a few hours after being released from the fruit and while we cannot rule out a role for non‐volatile hydrocarbons in eliciting ant seed‐carrying behavior we found that solvent extracts of seeds placed on filter paper did not result in behavioral responses by ants. Another possibility is that the ants were attracted to the ester‐rich scent of the fruits (see Yamada et al., 2021) and hence simply perceive the seeds as a food source. However, observations of disturbed ant colonies, with ants emerging from nests carrying both larvae and seeds coupled with the remarkably similar appearance of ant larvae to seeds, suggest that seeds may be treated as young rather than food.
Perhaps the most puzzling aspect of the seed dispersal system in A. buchananii is that the recalcitrant seeds must be left above ground for successful establishment. Germination occurs within days of the release of seeds by the fruit (Figure 1L). The belowground storage and subsequent release of seeds at the peak of the rainy season is clearly related to their sensitivity to desiccation, a trait common to recalcitrant seeds (Berjak and Pammenter, 2008). We did observe that ants often discard the seeds en route to their nest. One possibility is that the profile of volatile compounds on the seed surface changes rapidly, such that once the seed is removed from the fruit or starts germinating, the odor becomes increasingly less comparable to that of ant larvae. It is even possible that seeds are removed from the nest by ants once the scent changes in this manner. However, we did not record or observe ants removing seeds from the nest other than during a disturbance event and we found no traces of seeds on nest waste dumps, nor could we find any seedlings or mature A. buchananii plants in close proximity to existing ant nests. We found that seeds that were freshly removed from their fruit were highly attractive to ants, whereas seeds that had been artificially cleaned (by removing the mesocarp and endocarp) on the previous day were completely ignored by ants. The seeds of desiccated fruit remained attractive to ants provided that a layer of endocarp still covered the seeds (Figure 1K).
CONCLUSIONS
Decoupling of flowering and seed dispersal allows A. buchananii to exploit the most favorable conditions for these phenophases. Bulbs protect plants from fire and allow for rapid pyrogenic flowering in late winter and early spring to exploit post‐burn periods where flowers at ground level are not obscured by vegetation and competition for pollinators is low. However, the dry conditions in late winter and early spring are not suitable for establishment of seedlings and the retention of seeds in an underground ovary for six months allows for the recalcitrant seeds to be released at the peak of the rainy season in mid‐summer. The arrangement of the above‐ground flowers and underground ovary of A. buchananii has been termed geophytic geocarpy (Barker, 2005). However, given that the fruits do not remain in the soil and are pushed out of the ground (see Burtt, 1970), “emergent geocarpy” may be a more accurate description of the fruiting biology of A. buchananii.
Both flowers and fruits of A. buchananii are scented. Phenylacetaldehyde, the main floral volatile is attractive to a diverse suite of bees (and other insect groups) and likely enables rapid associative learning in combination with the ample pollen rewards. Our observations of ant‐dispersal of seeds were unexpected and raise a number of new questions. Are the ants attracted by the fruit odors? Once the ants locate the fruits, are they deceived into carrying the seeds to their nest because the seeds chemically mimic ant larvae or is the interest of ants in the seeds purely food‐related? Given that deep burial of seeds is not conducive to seedling establishment, does A. buchananii rely on ants discarding seeds above‐ground when they no longer smell like larvae or food? It will require more chemical analyses and experimentation to answer these questions.
AUTHOR CONTRIBUTIONS
I.K.: Conceptualization, data acquisition, analysis, visualization, photographic and video documentation, writing—original draft, review and editing; S.D.J.: Conceptualization, funding, data acquisition, analysis, visualization, chemical analyses, writing—review and editing.
Supporting information
Appendix S1. Apodolirion buchananii floral shape variation ‐ FH site. Photographs taken during the 2022 flowering season.
Appendix S2. Apodolirion buchananii floral traits at the FH site (n = 10 flowers, from 10 plants). Values are grand means (mm ± SE).
Appendix S3. Example of cup trap deployment (one of a pair) in the field ‐ FH site.
Appendix S4. Spectral reflectance of Apodolirion buchananii flowers at the (A) FH and (B) MG site along with cup traps (see Appendix S3). Spectra represent mean reflectance: n = 9 flowers, from 9 plants (for each site) and n = 5 for cup traps.
Appendix S5. Foraging behavior of insects visiting Apodolirion buchananii flowers at the FH (n = 93 observations) and MG site (n = 14 observations) during the 2022 flowering season (July to September). Insects and pollen loads collected only at the FH site (n = 71). Values represent means ± SE, followed by the sample size in parenthesis. NR = time not recorded.
Appendix S6. Relationship between the number of seeds per fruit (cross‐pollination treatment) and bulb mass in Apodolirion buchananii (n = 19 fruit from 19 bulbs).
Appendix S7. Cumulative proportion of germinated Apodolirion buchananii seeds (n = 20 seeds each from 6 fruits) over a 12‐day period (greenhouse trial). Sigmoidal curve derived using nonlinear regression.
Appendix S8. Supplementary video.
ACKNOWLEDGMENTS
Thanks to Mondi Shanduka's Mount Gilboa forestry estate for permission to work in the Mount Gilboa Nature Reserve. Thanks to Ed Gevers and Fountain Hill Estate for assistance and permission to work in the Fountain Hill Nature Reserve. Thanks to Daniella Egli, Julian Z. Kiepiel, and Hannah Butler for assistance with field work. Thanks to Connal Eardley, Daniella Egli, Terry Olckers, John Midgley, and Adam Shuttleworth for assistance with insect identification. Thanks to Caswell Munyai for assistance with ant identification and discussion on the ecology of Myrmicaria natalensis and Camponotus niveosetosus. Thanks to Alison Young for discussion and horticultural support. Thanks to William R. Liltved for comments on the manuscript. Thanks to Kathleen Kay and two anonymous reviewers for their comments on the manuscript. This study was funded by the National Research Foundation (grant 46372 to S.D.J.).
Kiepiel, I. , and Johnson S. D.. 2024. Scent‐mediated bee pollination and myrmecochory in an enigmatic geophyte with pyrogenic flowering and subterranean development of fleshy fruits. American Journal of Botany 111(11): e16421. 10.1002/ajb2.16421
DATA AVAILABILITY STATEMENT
Data available at Zenodo: https://doi.org/10.5281/zenodo.12724835.
REFERENCES
- Barker, N. P. 2005. A review and survey of basicarpy, geocarpy, and amphicarpy in the African and Madagascan flora. Annals of the Missouri Botanical Garden 92: 445–462. [Google Scholar]
- Berjak, P. , and Pammenter N.. 2008. From Avicennia to Zizania: Seed recalcitrance in perspective. Annals of Botany 101: 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, W. , and Breytenbach G. J.. 1985. Ants, rodents and seed predation in Proteaceae. African Zoology 20: 150–154. [Google Scholar]
- Brew, C. R , O'Dowd D. J., and Rae I.. 1989. Seed dispersal by ants: Behaviour‐releasing compounds in elaiosomes. Oecologia 80: 490–497. [DOI] [PubMed] [Google Scholar]
- Brewer, J. S. , Cunningham A. L., Moore T. P., Brooks R. M., and Waldrup J. L.. 2009. A six‐year study of fire‐related flowering cues and coexistence of two perennial grasses in a wet longleaf pine (Pinus palustris) savanna. Plant Ecology 200: 141–154. [Google Scholar]
- Burkle, L. A. , Simanonok M. P., Durney J. S., Myers J. A., and Belote R. T.. 2019. Wildfires influence abundance, diversity, and intraspecific and interspecific trait variation of native bees and flowering plants across burned and unburned landscapes. Frontiers in Ecology and Evolution 7: 1–14. [Google Scholar]
- Burtt, B. L. 1970. The evolution and taxonomic significance of a subterranean ovary in certain monocotyledons. Israel Journal of Botany 19: 77–90. [Google Scholar]
- Butler, H. C. , and Johnson S. D.. 2022. Seed dispersal by monkey spitting in Scadoxus (Amaryllidaceae): Fruit selection, dispersal distances and effects on seed germination. Austral Ecology 47: 1029–1036. [Google Scholar]
- Butler, H. C. , and Johnson S. D.. 2023. Directed vertebrate‐mediated seed dispersal of a fleshy‐fruited amaryllid in a heterogenous habitat. Biotropica 55: 888–896. [Google Scholar]
- Carbone, L. M. , Tavella J., Pausas J. G., and Aguilar R.. 2019. A global synthesis of fire effects on pollinators. Global Ecology and Biogeography 28: 1487–1498. [Google Scholar]
- Dafni, A. , and Ella W.. 1982. Pollination ecology of Sternbergia clusiana (Ker‐Gawler) Spreng. (Amaryllidaceae). New Phytologist 91: 571–577. [Google Scholar]
- Dafni, A. , Shmida A., and Avishai M.. 1981. Leafless autumnal‐flowering geophytes in the Mediterannean region ‐ phytological, ecological and evolutionary aspects. Plant Systematics and Evolution 137: 181–193. [Google Scholar]
- Davidson, D. , Seidel J., and Epstein W.. 1990. Neotropical ant gardens II. Bioassays of seed compounds. Journal of Chemical Ecology 16: 2993–3013. [DOI] [PubMed] [Google Scholar]
- Dobson, H. E. M. 1987. Role of flower and pollen aromas in host‐plant recognition in solitary bees. Oecologia 72: 618–623. [DOI] [PubMed] [Google Scholar]
- Dunlap, M. M. , Casey C. R., and VanOverbeke D. R.. 2023. Adding Scent: Exploring improvements in pan trapping to monitor pollinators. Frontiers in Bee Science. 1: 1274989. [Google Scholar]
- El‐Sayed, A. , Byers J., Manning L., Jürgens A., Mitchell V., and Suckling D.. 2008. Floral scent of Canada thistle and its potential as a generic insect attractant. Journal of Economic Entomology 101: 720–727. [DOI] [PubMed] [Google Scholar]
- García, Y. , Clara Castellanos M., and Pausas J. G.. 2018. Differential pollinator response underlies plant reproductive resilience after fires. Annals of Botany 122: 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts, S. , Malherbe S. D., and Pauw A.. 2012. Reduced flower visitation by nectar‐feeding birds in response to fire in Cape fynbos vegetation, South Africa. Journal of Ornithology 153: 297–301. [Google Scholar]
- Hölldobler, B. , and Kwapich C. L.. 2022. The guests of ants: How myrmecophiles interact with their hosts, Harvard University Press, Cambridge, Massachusetts. [Google Scholar]
- Hulbert, L. C. J. E. 1988. Causes of fire effects in tallgrass prairie. Ecology 69: 46–58. [Google Scholar]
- Johnson, S. D. , and Andersson S.. 2002. A simple field method for manipulating ultraviolet reflectance of flowers. Canadian Journal of Botany‐Revue canadienne de botanique 80: 1325–1328. [Google Scholar]
- Kamatou, G. , Viljoen A. M., Őzek T., and Başer K.. 2008. Head‐space volatiles of Gethyllis afra and G. ciliaris fruits (“kukumakranka”). South African Journal of Botany 74: 768–770. [Google Scholar]
- Keeley, J. E. 1993. Smoke‐induced flowering in the fire‐lily Cyrtanthus ventricosus . South African Journal of Botany 59: 638. [Google Scholar]
- Kiepiel, I. , and Johnson S. D.. 2019. Spit it out: Monkeys disperse the unorthodox and toxic seeds of Clivia miniata (Amaryllidaceae). Biotropica 51: 619–625. [Google Scholar]
- Knauer, A. C. , and Schiestl F. P.. 2015. Bees use honest floral signals as indicators of reward when visiting flowers. Ecology Letters 18: 135–143. [DOI] [PubMed] [Google Scholar]
- Knudsen, J. T. 1994. Floral scent variation in the Pyrola rotundifolia complex in Scandinavia and Western Greenland. Nordic Journal of Botany 14: 277–282. [Google Scholar]
- Lamont, B. B. , and Downes K. S. J. P. E.. 2011. Fire‐stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecology 212: 2111–2125. [Google Scholar]
- Lazarina, M. , Sgardelis S., Tscheulin T., Kallimanis A., Devalez J., and Petanidou T.. 2016. Bee response to fire regimes in Mediterranean pine forests: The role of nesting preference, trophic specialization, and body size. Basic and Applied Ecology 17: 308–320. [Google Scholar]
- Lengyel, S. , Gove A. D., Latimer A. M., Majer J. D., and Dunn R. R.. 2010. Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: A global survey. Perspectives in Plant Ecology, Evolution and Systematics 12: 43–55. [Google Scholar]
- Marden, J. H. 1984. Remote perception of floral nectar by bumblebees. Oecologia 64: 232–240. [DOI] [PubMed] [Google Scholar]
- Marloth, R. 1915. Flora of South Africa: IV. Monocotyledones. Darter, Cape Town, South Africa. [Google Scholar]
- Mas, F. , Horner R. M., Brierley S., Butler R. C., and Suckling D. M.. 2020. Selection of key floral scent compounds from fruit and vegetable crops by honey bees depends on sensory capacity and experience. Journal of Insect Physiology 121: 104002. [DOI] [PubMed] [Google Scholar]
- Meagher, R. L. 2002. Trapping noctuid moths with synthetic floral volatile lures. Entomologia Experimentalis et Applicata 103: 219–226. [Google Scholar]
- Meerow, A. W. , and Clayton J. R.. 2004. Generic relationships among the baccate‐fruited Amaryllidaceae (tribe Haemantheae) inferred from plastid and nuclear non‐coding DNA sequences. Plant Systematics and Evolution 244: 141–155. [Google Scholar]
- Meerow, A. W. , and Snijman D. A.. 1998. Amaryllidaceae. In Kubitzki K. [ed.], Families and genera of vascular plants,3, 83–110. Springer Verlag, Berlin, Germany. [Google Scholar]
- Muth, F. , Papaj D. R., and Leonard A. S.. 2016. Bees remember flowers for more than one reason: Pollen mediates associative learning. Animal Behaviour 111: 93–100. [Google Scholar]
- Ne'eman, G. , Dafni A., and Potss S. G.. 2000. The effect of fire on flower visitation rate and fruit set in four core‐species in east Mediterranean scrubland. Plant Ecology 146: 97–104. [Google Scholar]
- Nevo, O. , and Ayasse M.. 2020. Fruit scent: Biochemistry, ecological function, and evolution. In Mérillon J.‐M., and Ramawat K. G. [eds], Co‐evolution of secondary metabolites, 403–425. Springer Nature, Cham, Switzerland. [Google Scholar]
- Nevo, O. , Razafimandimby D., Valenta K., Jeffrey J. A. J., Reisdorff C., Chapman C. A., J. U.Ganzhorn , and Ayasse M.. 2019. Signal and reward in wild fleshy fruits: Does fruit scent predict nutrient content? Ecology and Evolution 9: 10534–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo, O. , Valenta K., Kleiner A., Razafimandimby D., Jeffrey J. A. J., Chapman C. A., and Ayasse M.. 2020. The evolution of fruit scent: Phylogenetic and developmental constraints. BMC Evolutionary Biology 20: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden, A. H. , and Kirkman L. K.. 2004. Factors controlling the fire‐induced flowering response of the federally endangered Schwalbea americana L. (Scrophulariaceae). Journal of the Torrey Botanical Society 131: 16–22. [Google Scholar]
- Ponisio L.C., Wilkin K., M'Gonigle L. K., Kulhanek K., Cook L., Thorp R., Griswold T., and C. Kremen. 2016. Pyrodiversity begets plant–pollinator community diversity. Global Change Biology 22: 1794–1808. [DOI] [PubMed] [Google Scholar]
- Pooley, E. 1998. A Field guide to wild flowers of KZN and the eastern region. Natal Flora Publications, Durban, South Africa. [Google Scholar]
- Potts, S. G. , Dafni A., and Ne'eman G.. 2001. Pollination of a core flowering shrub species in Mediterranean phrygana: Variation in pollinator diversity, abundance and effectiveness in response to fire. Oikos 92: 71–80. [Google Scholar]
- Potts, S. G. , Vulliamy B., Dafni A., Ne'eman G., O'Toole C., Roberts S., and Willmer P.. 2003. Response of plant‐pollinator communities to fire: Changes in diversity, abundance and floral reward structure. Oikos 101: 103–112. [Google Scholar]
- Pyke, G. H. 2017. Fire‐stimulated flowering: A review and look to the future. Critical Reviews in Plant Sciences 36: 179–189. [Google Scholar]
- Raguso, R. A. 2008. Wake up and smell the roses: The ecology and evolution of floral scent. Annual Review of Ecology, Evolution, and Systematics 39: 549–569. [Google Scholar]
- Slingsby, P. 2017. Ants of southern Africa: The ant book for all. Slingsby Maps. [Google Scholar]
- Stanley, D. A. , Msweli S. M., and Johnson S. D.. 2020. Native honeybees as flower visitors and pollinators in wild plant communities in a biodiversity hotspot. Ecosphere 11: 1–12. [Google Scholar]
- Theis, N. , and Raguso R. A.. 2005. The effect of pollination on floral fragrance in thistles. Journal of Chemical Ecology 31: 2581–2600. [DOI] [PubMed] [Google Scholar]
- Yamada, M. , Hojo M. K., and Imamura A.. 2021. Odor of achlorophyllous plants’ seeds drives seed‐dispersing ants. Ecology and Evolution 11: 9308–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikishe, V. , Sgatya P., and Dold T.. 2020. Rescue mission for a rare amaryllis. Veld & Flora 106: 38–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Apodolirion buchananii floral shape variation ‐ FH site. Photographs taken during the 2022 flowering season.
Appendix S2. Apodolirion buchananii floral traits at the FH site (n = 10 flowers, from 10 plants). Values are grand means (mm ± SE).
Appendix S3. Example of cup trap deployment (one of a pair) in the field ‐ FH site.
Appendix S4. Spectral reflectance of Apodolirion buchananii flowers at the (A) FH and (B) MG site along with cup traps (see Appendix S3). Spectra represent mean reflectance: n = 9 flowers, from 9 plants (for each site) and n = 5 for cup traps.
Appendix S5. Foraging behavior of insects visiting Apodolirion buchananii flowers at the FH (n = 93 observations) and MG site (n = 14 observations) during the 2022 flowering season (July to September). Insects and pollen loads collected only at the FH site (n = 71). Values represent means ± SE, followed by the sample size in parenthesis. NR = time not recorded.
Appendix S6. Relationship between the number of seeds per fruit (cross‐pollination treatment) and bulb mass in Apodolirion buchananii (n = 19 fruit from 19 bulbs).
Appendix S7. Cumulative proportion of germinated Apodolirion buchananii seeds (n = 20 seeds each from 6 fruits) over a 12‐day period (greenhouse trial). Sigmoidal curve derived using nonlinear regression.
Appendix S8. Supplementary video.
Data Availability Statement
Data available at Zenodo: https://doi.org/10.5281/zenodo.12724835.


