Abstract
Premise
To survive climate change and habitat loss, plants must rely on phenotypic changes in response to the environment, local adaptation, or migration. Understanding the drivers of intraspecific variation is critical to anticipate how plant species will respond to climate change and to inform conservation decisions. Here we explored the extent of local adaptation and phenotypic plasticity in Heteromeles arbutifolia, toyon, a species endemic to the California Floristic Province.
Methods
We collected leaves from 286 individuals across toyon's range and used seeds from 37 individuals to establish experimental gardens in the northern and southern parts of toyon's range. We measured leaf functional traits of the wild‐collected leaves and functional and fitness traits of the offspring grown in the experimental gardens. We then investigated the relationships between traits and source environment.
Results
Most traits we investigated responded plastically to the environment, and some traits in young seedlings were influenced by maternal effects. We found strong evidence that variation in leaf margins is a result of local adaptation to variation in temperature and temperature range. However, the source environment was not related to fitness traits or survival in the experimental gardens.
Conclusions
Our findings reiterate the adaptive role of toothed leaf margins in colder and more seasonally variable environments. Additionally, we provide evidence that fitness of toyon is not dependent on where they are sourced, and thus toyon can be sourced across its range for restoration purposes.
Keywords: chaparral, conservation management, functional traits, intraspecific variation, maternal effects, phenotypic plasticity, restoration, Rosaceae, seed sourcing
Plant species are increasingly at risk of local or global extinction due to the rapidly changing climate (Ceballos et al., 2015). Climate change has shifted the biotic and abiotic factors with which plant populations interact (Parry et al., 2007; McNichol and Russo, 2023), and these changes are expected to accelerate in the future (Gardiner, 2008; Callaghan et al., 2010). Habitat destruction and fragmentation, invasive species, and pollution further threaten plant populations, and their detrimental effects are exacerbated by the changing climate (Oliver and Morecroft, 2014). To persist in the face of these threats, plant populations must rely on phenotypic plasticity, local adaptation, or movement to a more favorable climate (Aitken et al., 2008; de Lafontaine et al., 2018). While the potential for plants to survive predicted climate change has been studied in some species (e.g., Chamaecrista fasciculata, Etterson, 2004a, b; Arachis hypogaea, Kumar et al., 2012; Quercus lobata, Sork et al., 2016; Populus fremontii, Hultine et al., 2020), the potential of survival under changing climatic conditions is unknown for most plant species (Münzbergová et al., 2017); extinction research has been strongly biased toward animals (Nic Lughadha et al., 2020).
The source and structure of intraspecific trait variation influences the fate of species confronting anthropogenic climate change. Intraspecific trait variation can arise from genetic differences due to local adaptation or genetic drift, phenotypic plasticity, and/or maternal effects (Palacio‐López et al., 2015; Auge et al., 2017). Trait variation caused by genetic differences can result in individuals that are better adapted to the changed climate. If there is sufficient gene flow across populations, these adaptive alleles can make their way to individuals living in locations where the new climate matches the climate conditions to which they are adapted (Bolnick et al., 2011; Ceballos et al., 2015; Skelton et al., 2019). Further, if these well‐adapted individuals are identified, they can be used for restoration, conservation, and assisted migration efforts (Hufford and Mazer, 2003). However, if trait differences are due to local adaptation, only some individuals will be able to thrive under the altered climate regime, limiting the seed sources for restoration or assisted migration efforts (Van Andel, 1998; Broadhurst et al., 2008).
Trait variation due to phenotypic plasticity and maternal effects have different consequences for the ability of a species to survive in a changed climate. If trait variation is driven by plastic responses to the environment, any individual might be equally likely to survive in a new climate regime, assuming the new climate regime is within the range of current conditions (Van Andel, 1998). Variation due to environmental maternal effects (i.e., the environment of an organism's mother affects that organism's phenotype; Roach and Wulff, 1987) can likewise increase the environmental range over which an individual is able to survive. Environmental maternal effects are well documented in plants (reviewed by Roach and Wulff, 1987), and in many cases are driven by changes to seed size (maternal provisioning, Roach and Wulff, 1987). While maternal effects mediated through seed size are often limited to early life stages, the direct effects of maternal environment on offspring phenotype can be longer lasting. In fact, maternal effects can have an impact across multiple generations (Suter and Widmer, 2013; Akkerman et al., 2016; Meier et al., 2022). Environmental maternal effects have been shown to affect many aspects of offspring phenotype (e.g. maternal herbivory inhibits leaf, stolon, and root growth in Alternanthera philoxeroides; Dong et al., 2017) and life history traits (annual vs. biennial habit in Campanula americana; Galloway, 2005).
Despite these varied sources of trait variation, conservation managers often assume that trait variation is due to local adaptation and thus that locally sourced seeds are the most appropriate for habitat restoration to maximize local adaptation and reduce outbreeding depression (Linhart and Grant, 1996; Broadhurst et al., 2008, Havens et al., 2015). Further, there is concern that introducing variation from seeds sourced elsewhere can risk introducing maladapted genes to an already well‐adapted population (Otto and Lenormand, 2002; Agrawal, 2006; Otto, 2009). However, this assumption can be problematic because relying only on locally sourced seeds can reduce the quantity and quality of seeds (Broadhurst et al., 2008). Limiting seed sources to only those populations adapted to environments matching the current or future climates may result in a limited gene pool, which could lead to demographic challenges such as genetic bottlenecks or inbreeding depression (Schemske and Lande, 1985; Fenster and Galloway, 2000). If trait variation is due to plasticity or environmental maternal effects, conservation managers could introduce seeds from across a species' range and increase the potential for more adapted genotypes to spread and prevent population declines due to climate change (Bolnick et al., 2011; Ceballos et al., 2015; Skelton et al., 2019). Broadhurst et al. (2008) advocates ending the assumption “local is best” to facilitate easier restoration efforts and increase access to genetically diverse and high‐quality seeds. If trait variation is due to phenotypic plasticity or environmental maternal effects, not local adaptation, there is less concern about introducing maladapted genes and maximizing local adaption, so seeds intended for restorations could be sourced from across a species’ range with equal if not greater success. Thus, conservation managers need to understand the underlying cause of the trait variation in their species of interest to make informed conservation decisions.
Here we focused on trait variation of leaf functional traits because these traits mediate interactions between a plant and its abiotic and biotic environment and are relatively easy to measure in the field and in herbarium specimens. Leaves are responsible for carbon fixation, transpiration, and herbivory prevention (Wright et al., 2004; Liu et al., 2017; Shi et al., 2019). Traits such as leaf shape (e.g., length to width ratio), leaf thickness (e.g., SLA), and leaf area are well known to vary along environmental gradients, both between (Reich et al., 1999; Wright et al., 2004; Liu et al., 2017) and within species (e.g., Carlson et al., 2011; Prunier et al., 2012). Leaf margin shape (toothed vs. entire) also varies with environment; plant species with more toothed leaves are more likely to be found in places with cooler climates than in warmer climates (Bailey and Sinnott, 1916). The biological explanation for the global relationship between leaf margin shape and temperature is debated (Baker‐Brosh and Peet, 1997; Royer and Wilf, 2006; Adams et al., 2008; Zohner et al., 2019). However, toothed leaf margins do reduce herbivory (Brown et al., 1991), increase positive root pressure reducing flooding of the roots and freeze–thaw embolisms (Feild et al., 2005), and increase rates of transpiration and photosynthesis, particularly at the beginning of the growing season (Baker‐Brosh and Peet, 1997). Analyses of leaf functional traits are generally based on species‐wide trait averages, which obscure any intraspecific variation (Wright et al., 2005; Bolnick et al., 2011). Thus, in addition to informing conservation policy, studies of intraspecific variation including the understudied relationship between intraspecific leaf margin variation and environmental variation add to our understanding of the relationships between plant functional traits and the environment.
In this study, we used common gardens to explore the extent to which trait variation in an iconic California species, toyon [Heteromeles arbutifolia (Lindl.) M. Roem. (Rosaceae)] is due to phenotypic plasticity, local adaptation, and/or maternal effects. Toyon inhabits a highly heterogenous environment ranging from coastal chaparral to oak woodlands and displays substantial phenotypic variation, particularly in its leaf morphology (Figure 1). While toyon is not itself endangered, it is a key part of chaparral restorations (Roy, 2009; Riordan et al., 2018), so understanding the contributions of these three sources of trait variation can be immediately applied to restoration efforts. In particular, we addressed the following questions: (1) How do toyon traits vary along environmental gradients? (2) How do genetic differences and environmental maternal effects contribute to trait variation between individuals grown in a common environment, and how does this trait variation relate to the source environment? (3) Are toyon individuals adapted to their local environment? (4) Are some traits more plastic than others, and do maternal lines vary in plasticity?
Figure 1.

Leaf margin variation for wild and offspring toyon (Heteromeles arbutifolia) individuals. From left to right: Sample 267 from Lower Lake, northern California; Sample 199 from central coastal California; and Sample 278 from Rubio Canyon, Altadena, southern California. From top to bottom: toyon individual in the wild, leaf from wild‐collected individual scanned at a standardized distance, and leaf from offspring of the wild‐collected individual collected on 31 August 2022 from the Stunt Ranch common garden. Ruler units are centimeters.
MATERIALS AND METHODS
Study species
Toyon is an evergreen shrub endemic to the California Floristic Province (CFP, Montalvo et al., 2018), a biodiversity hotspot (Cunningham and Beazley, 2018) with a mediterranean climate having cool, wet winters and hot, dry summers (Baldwin, 2014). Toyon is widespread and common in the CFP, found in lower mountain slopes in the coastal and Sierra Nevada Mountain ranges and in coastal regions where it is an often‐dominant component of both chaparral and oak woodland ecosystems (Montalvo et al., 2018). In the late fall and winter, toyon's clusters of pomes (the fruits of plants in the subfamily Maloideae with a central core surrounded by a layer of flesh, Rohrer et al., 1991) mature to a dramatic red and are an important winter food source for birds and small mammals (Montalvo et al., 2018). Toyon has highly variable leaf margins (Figure 1), can grow in shade and full sun, and can have a shrub or tree‐like growth habit.
Material collection
To investigate leaf trait variation across toyon's range, we collected leaves from 286 toyon individuals from October 2020 to January 2022 across the range of the species in California (Figure 2) and used observations from the iNaturalist database accessed through the Global Biodiversity Information Facility (GBIF.org, 2020) to identify individuals to sample. While toyon's range extends into Baja California in Mexico, we were unable to sample south of the United States–Mexico border. When possible, we collected the second fully expanded leaf from a branch in the sun two thirds of the way up the plant. However, some plants were growing in the understory, and only shaded leaves were available to sample. We immediately pressed collected leaves in an herbarium press for later analysis and deposit in the University of California, Los Angeles (UCLA) herbarium (see Appendix S1 for accessions). The source environment of our collections spans a mean annual temperature (MAT) from approximately 10°C to 18°C, mean annual precipitation (MAP) from 228 mm to 1748 mm, and elevation from 11 m to 1430 m a.s.l.
Figure 2.
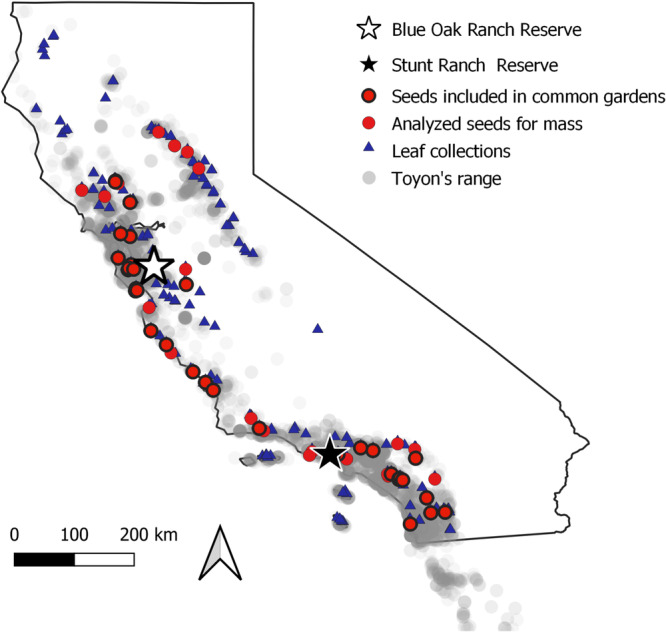
Geographic range of Heteromeles arbutifolia overlain by the locations where leaf samples (N = 286) and pomes were collected. Pomes collected only for seed mass are indicated with a red circle (N = 62), and those included in the common garden are indicated with red circles with a black outline (N = 37).
Of the 286 individuals from which we collected leaves, we also collected fresh pomes from 51 maternal lines from November 2021 to late January 2022, as well as dried pomes from 21 of the herbarium samples that had been collected in the winter of 2020–2021 (Figure 2). For the fresh pomes, we collected two umbels of fully ripened toyon pomes from one maternal plant and stored them in a brown paper bag at 4°C until we processed them for germination and planting.
Wild‐leaf characterization
To measure the functional traits of the wild‐collected leaves, we removed one leaf from the herbarium collection for each of the 286 collected toyon individuals. We weighed each herbarium leaf and then scanned it on a flatbed scanner to measure leaf area and leaf length to width ratio using ImageJ (Version 1.53t; Schneider et al., 2012). We calculated specific leaf area (SLA) by dividing the measured leaf area in ImageJ by the mass of the herbarium leaf. We quantified the tooth patterns on the leaf margins in three ways: by counting the total number of teeth (including major and minor teeth), by measuring the total tooth area (by tracing the inside of the leaf along the teeth and subtracting the area excluding teeth from the total leaf area), and by measuring the average length of three major teeth (not minor teeth, see Appendix S2). We measured stomatal density on the dried leaves by painting a thin layer of clear nail polish on the left center portion of the abaxial leaf surface (Zhu et al., 2018) and removing it with a piece of clear tape. To obtain stomatal density, we counted the number of stomata visible at 400× magnification in three different locations on the stomatal peel for an average number of stomata per frame and divided that number by the area of the field of view (1.76475 mm2, Zhu et al., 2018).
Seed processing
We manually extracted seeds from the pomes to avoid damaging the seed coats. For the fresh pomes, we refrigerated the pomes from the time of collection until we extracted the seeds. To make the dried pomes from herbarium collections more pliable, we soaked the pomes for 2–3 days in deionized water before extracting the seeds. Once we extracted and cleaned the seeds of any remaining fruit material, we stored them at 4°C in a sealed plastic container. We then weighed all the seeds collected for each maternal line together and divided the mass by the total number of seeds to obtain the average seed mass for each maternal line.
Germination and propagation
We began germinating processed seeds in March 2022 at UCLA. We rinsed 50 seeds per maternal line in a 10% bleach solution to discourage mold growth and then distributed the seeds between two sterile petri dishes lined with sterilized filter paper wetted with deionized water. Because optimal germination conditions for toyon are unknown, we placed one petri dish from each maternal line in a closed box in the refrigerator at 4°C, and the other petri dish in a closed box and kept it in a cool dark corner in the lab. We moved the seeds from the lab into the refrigerator between 5 and 20 days after propagations to slow the mold that had begun growing on the seeds. We checked the petri dishes daily until mid‐May 2022 to identify germinated seeds ready for planting, to determine average germination time per maternal line, and to monitor moisture levels. After 1 month, we added gibberellic acid to the water to assist germination, but it did not appear to have an effect.
After each seed germinated, we planted it using sterilized tweezers either immediately or the following day into a DeepPot (Ray Leach Containers, Corvallis, OR, USA) 6.35 cm in diameter and 25.4 cm deep in the UCLA Plant Growth Center (Los Angeles, CA, USA). We used a soil mixture consisting of washed plaster sand (18.75%), loam (18.75%), peat moss (37.5%), perlite (12.5%), and vermiculite (12.5%). We planted one replicate seed from each maternal line into each of 10 blocks. We also planted extra seedlings from the maternal lines with the highest germination rates to ensure we had enough seedlings in the common garden experiments, and we used two extras to bring our total to 12 individuals per maternal line to transplant.
We watered the seedlings every day with tap water on a mist setting for 1 month, then with fertilizer water (200 ppm of Grow More [20‐20‐20] water soluble fertilizer; Gardena, CA, USA) three times a week on the mist setting. Approximately 60 of our 540 total seedlings either died or did not grow after being planted, so a new germinated seed was planted in its place.
Greenhouse data collection
After seedlings had grown a maximum of 3 months in the DeepPots and before transplanting into the common gardens, we collected trait data on all living seedlings in the greenhouse in June 2022. We measured the height of the seedlings from the soil line to the apical meristem and counted the number of fully expanded leaves and the number of branches. We defined branches as the parts of the plant (other than the apical meristem) where new leaves were growing. We calculated the relative growth rate by dividing the height by the number of days between the planting date and data collection. The second fully expanded leaf of each seedling was also gently pressed between two pieces of plexiglass with a ruler for reference, then photographed (Appendix S3). We processed leaf images as described for maternal leaves.
Planting the common gardens, maintenance, and data collection
To quantify trait plasticity, we established two common gardens beginning in May 2022, one at Stunt Ranch Reserve (Los Angeles County, CA, USA 34.0908, 118.6575, elevation 390.74 m a.s.l.) and one at Blue Oak Ranch Reserve (Santa Clara County, CA, USA 37.3815, –121.7367, 554.15 m a.s.l.). Stunt Ranch, in the southern portion of toyon's range, has less precipitation than Blue Oak Ranch in the northern half of toyon's range (Appendix S4). At Stunt Ranch, we cleared plant material, rototilled, and dug trenches in preparation of planting. At Blue Oak Ranch, the soil was much softer, so we were able to dig trenches without the use of a rototiller. We then covered the trenches with cardboard to suppress weeds and retain moisture.
We planted seedlings on 23 June 2022 at Stunt Ranch and on 28 June 2022 at Blue Oak Ranch. At each garden, we planted one seedling from each maternal line into each of six completely randomized blocks. After the germination and propagation process, we had enough seedlings from 37 maternal lines for a total of 444 individuals. We planted the seedlings 20 cm apart into 16 rows, each 3 m long. Immediately after planting, we measured the heights of all the plants to account for changes in height due to variable planting depths. We protected the gardens from herbivores with a 5‐foot‐tall fence of 0.5‐inch (12.7 mm) hardware cloth and T‐posts. To further prevent herbivory and to shade the seedlings, we secured unused, open cardboard milk cartons over each plant. We watered the transplanted seedlings three times a week for the first month (July), twice a week for the second month (August), and once a week from September to mid‐December 2022, then stopped watering. We assessed survival every week and measured height, leaf number, and number of branches on the living plants every other week, from 13 July 2022, until 14 September 2022. We collected the second fully expanded new leaf from each surviving plant at both gardens on 31 August 2022 to analyze leaf functional traits. We used the data collected on 14 September for our analysis of height, leaf number, branching, and relative growth rate to maximize the number of maternal lines included in the analysis; a large heat wave in mid‐September 2022 caused widespread mortality at both gardens (6% of the surviving plants). We collected data once more at Stunt Ranch on 5 November 2022 and at Blue Oak Ranch on 23 November 2022, which we used in our survival analysis.
Data analyses
All analyses were performed in R version 4.2.2 (R Core Team, 2022) unless otherwise stated.
How do toyon traits vary along environmental gradients?
To investigate the relationships between traits measured on the 286 wild collected leaves and climatic variables we extracted 14 of 19 Bioclim variables (Fick and Hijmans, 2017) by intersecting the 30‐s resolution Bioclim raster layers with the coordinates of the 286 collected toyon individuals in QGIS version 3.28.3 (QGIS.org, 2022). We excluded the 5 Bioclim variables related to seasonality. We also extracted the elevations from the Elevation Point Query Service Digital Elevation Model (DEM) using the elevator package (version 0.4.2) in R (Hollister et al., 2021). To generate environmental variables to use in our linear models, we compressed the number of predictor variables using a PCA. We created two sets of PC axes, one summarizing variation between collection site in the seven temperature variables (mean annual temperature, maximum temperature of the warmest month, minimum temperature of the coldest month, mean temperature of the wettest quarter, mean temperature of the driest quarter, mean temperature of the warmest quarter, mean temperature of the coldest quarter) and the other summarizing variation between collection sites in the seven precipitation variables (annual precipitation, precipitation of the wettest month, precipitation of the driest month, precipitation of the wettest quarter, precipitation of the driest quarter, precipitation of the warmest quarter, precipitation of the coldest quarter). Together, the first and second PCs for each PCA set captured more than 90% of the environmental variation between source locations. We interpreted temperature PC axis 1 (TPC‐1, 52.1% of variance) as temperature range because variables related to max temperatures loaded positively and variables related to min temperatures loaded negatively onto this axis (Appendix S5). We interpreted temperature PC axis 2 (TPC‐2, 43.8% of variance) as temperature because all of the temperature variables loaded positively on the temperature PC axis 2. For precipitation PC axis 1 (PPC‐1, 78.7% of variance), all variables loaded positively, so we interpreted PPC‐1 as total rainfall. We interpreted precipitation PC axis 2 (PPC‐2, 17.1% of variance) as precipitation range because the variables measuring precipitation of the driest times of year loaded negatively and variables measuring precipitation of the wetter times of the year loaded positively. We then used TPC‐1, TPC‐2, PPC‐1, PPC‐2, and elevation as predictor variables in our linear models estimating trait–environment relationships; these variables were not strongly correlated with each other. The highest correlation between environmental variables was 0.61 (Appendix S6).
We investigated the relationships between the environmental variables and the following traits measured on wild‐collected seeds and leaves: average seed mass (mg), germination time (days), leaf mass (mg), leaf area (cm2), specific leaf area (mg/cm2), leaf length to width ratio, number of teeth, teeth area (cm2), average length of major teeth (cm), and stomatal density (stomata/mm2). We visually inspected the distribution of each trait to ensure that the data were normally distributed and log‐transformed those variables for which the transformation made the distribution more normal: leaf mass, leaf area, specific leaf area, leaf length to width ratio, and average length of major teeth. We fit linear models of the form: trait ~ TPC‐1 + TPC‐2 + PPC‐1 + PPC‐2 + elevation for each trait; for average seed mass, we also included seed source (herbarium vs fresh) as a covariate. To model germination time, we used a linear mixed model with the same predictor variables, including maternal line as a random effect to account for the lack of independence between seeds from the same maternal line. The mixed model was fitted using the lme function in the R package nlme version 3.1‐162 (Pinheiro et al., 2023).
How do genetic differences and environmental maternal effects contribute to trait variation between individuals grown in a common environment, and how does this trait variation relate to the source environment?
We used seedlings from 37 maternal lines represented in the greenhouse and in both common gardens to understand whether any observed trait variation in offspring was a result of genetic differentiation (and thus likely adaptation) or maternal effects. For the analysis of traits measured in the greenhouse, our response variables were leaf area (cm2), leaf length to width ratio, number of teeth, teeth area (cm2), and average length of major teeth (cm). In the common gardens, we also measured stomatal density (stomata/mm2), leaf mass (mg), and specific leaf area (mg/cm2). To better understand patterns in each location, we used separate linear models to estimate trait–environment relationships for traits measured in the greenhouse and the two common gardens.
We first determined whether offspring traits varied between maternal lines by running a linear mixed model with an intercept and a random effect for maternal line. We then determined whether the trait variance attributed to maternal line was significantly different from zero by estimating 95% confidence intervals (intervals.lme function in the nlme package; Pinheiro et al., 2023). If the confidence interval did not overlap zero, we concluded that maternal line contributed to variation in that offspring trait. For traits which had a confidence interval that did overlap zero, we concluded that maternal line was not a significant predictor and did not investigate whether maternal environment explained offspring variation. Maternal line was NOT a significant predictor of offspring trait variation in four traits at the Stunt Ranch garden (leaf area, leaf mass, specific leaf area, teeth area; Appendix S7) and two traits at the Blue Oak Ranch garden (height, number of leaves), so we did not further investigate these traits at those locations.
With only those trait–garden combinations for which we found a significant effect of maternal line, we then investigated whether the maternal plant's environment explained offspring trait variation. We used mixed linear regressions including the maternal line as a random effect to account for the lack of independence between the individuals from the same maternal line. In addition to the environmental predictors, we included three key covariates: (1) planting date of the germinated seed to account for differences in the trait due to length of growing time, (2) the corresponding maternal trait value to estimate heritability, and (3) seed size to account for differences in maternal provisioning (maternal effects; as done by Singh et al., 2017). This analysis was implemented in the nlme package (Pinheiro et al., 2023). Because we conducted these analyses on traits in both 1‐ to 2‐month‐old seedlings in the greenhouse and 4‐ to 5‐month‐old seedlings in the two common gardens, we were able to compare the effect size of maternal effects (i.e., seed mass) between younger and older seedlings and test the expectation that maternal effects would be stronger at an earlier developmental stage. We estimated broad sense heritability to be 2× the estimated slope between the maternal trait values and offspring trait values (expected genetic relationship between maternal and offspring plants is 0.5). While traits measured in 1–6‐month‐old seedlings are unlikely to have the same values as the traits measured on adult leaves due to developmental differences, any similarities we detect are thus more remarkable.
Are toyon individuals adapted to their local environment?
We explored local adaptation in toyon using two approaches. First, to detect selection, we investigated the relationships between each fitness trait (height [cm], relative growth rate [cm/day], leaf number, and branch number) and the traits for which we found significant trait–environment relationships. We included planting date (to account for any developmental differences between individuals) and maternal line (as a random effect to account for a lack of independence between half‐sibs) as covariates (Lande and Arnold, 1983). This analysis was conducted using the lme function in the nlme package (Pinheiro et al., 2023).
We then determined whether toyon individuals sourced from environments similar to the common garden environments had higher fitness there. If toyon populations are locally adapted to their environment, we predicted that plants with the highest fitness in a common garden are likely from mothers that grew in similar environments. We used two tests: (1) For each common garden, we investigated the relationship between fitness traits and environmental dissimilarity between the garden and the source site, and (2) we conducted a survival analysis. Our fitness traits were height (cm), relative growth rate (cm/day), number of new leaves, and number of new branches. For each fitness trait separately, we used a linear mixed model with the environmental differences (along the four environmental PC axes and elevation) between the seed source location and the common garden as predictors. We again included planting date into the garden and average seed mass as covariates and maternal line as a random effect.
We conducted the survival analysis for plants in the two common gardens using the weekly survival data. We quantified the length of time each plant survived as its death date minus its planting date into the garden. If a plant did not die, its survival time was 140 days. We also created an event variable that assigned a 1 to all individuals that died, and a 0 to individuals that did not die. We used the survival and coxme packages in R (version 3.5‐7, Therneau, 2023; version 2.2‐18.1, Therneau, 2022) to perform a survival analysis for each common garden with the differences in climate variables between the source and garden as fixed effects, the maternal line as a random effect, and the survival object including the event and the time as the response variable.
Are some traits more plastic than others, and do maternal lines vary in plasticity?
In the context of this experiment, trait plasticity is the difference in mean trait value between the half‐sibling groups grown in the two common gardens. For the plasticity analyses, we included only those maternal lines for which we had data from at least three offspring in each garden. Because traits differed in the number of individuals for which we had data, we included 29–35 maternal lines per trait in the plasticity analysis. Before analysis, we normalized each trait to a mean of zero and a standard deviation of one which allows us to compare the magnitude of variation of traits measured on different scales. To determine whether the traits differed in plasticity, we used a Bayesian mixed model approach implemented in the R package RstanArm version 2.32.1 (Goodrich, 2023) to run two models, one which allowed the effect of garden on the standardized trait values to vary with trait (garden × trait interaction) and one which included garden and trait as fixed effects with no interaction. In both models, we also estimated a random intercept for maternal line and a garden × maternal line interaction. We then used leave‐one‐out (LOO) cross validation to determine whether the model that included a garden × trait interaction had better predictive value (Vehtari et al., 2016). If the difference in the generic (expected) log‐predictive density (ELPD) is more than 4 and the difference is much larger than the standard error of the difference, we can conclude that the model with the higher ELPD is preferred (Vehtari et al., 2016).
To determine whether plasticity varied between maternal lines (G × E interaction), we ran two models for each trait and compared them with LOO cross validation as above. The first model included a fixed effect of garden (E) and a random slope (G × E interaction) and intercept (G) for maternal lines, while the second included only a fixed garden effect (E) and a random intercept for maternal line (G). We again used LOO cross validation to compare the models.
RESULTS
How do toyon traits vary along environmental gradients?
From the wild‐collected leaves and seeds, we determined that plants from different source environments varied in leaf margin traits, SLA, and seed mass. Of the traits we measured, leaf margin traits were most likely to be related to the temperature variables (Table 1, Figure 3). We found that the number of teeth and the length of the major teeth increased as temperature range (TPC‐1) increased, that the number of teeth decreased as overall temperature (TPC‐2) increased, and that leaves had fewer and shorter teeth as the elevation increased (Figure 3). As total rainfall (PPC‐1) increased, SLA increased, and seed mass decreased (Table 1).
Table 1.
Significant relationships between traits measured on wild‐collected Heteromeles arbutifolia individuals and environmental variables where the individuals were collected. Specific leaf area (SLA) and mean length of major teeth were log‐transformed for the analyses.
| Fixed effects | Mean seed mass (mg) N = 62 | SLA (cm2/g) N = 286 | No. teeth N = 279 | Mean length of major teeth (cm) N = 278 |
|---|---|---|---|---|
| Trait range | 0.894–13.688 | 22.626–79.571 | 7–79 | 0.0203–0.2153 |
| Temperature range | — | — | 2.071*** | 0.112*** |
| Temperature | — | — | –1.783** | — |
| Precipitation | –0.781** | 0.0279** | — | — |
| Elevation (m a.s.l.) | — | — | –0.0145*** | –0.000318** |
| Herbarium collection | –1.332* | NA | NA | NA |
Note: Trait‐environmental variable combinations without a significant relationship are indicated with a dash (—); traits and environmental variables that did not have any significant relationships are not included in the table. Effect sizes are reported for significant relationships.
Abbreviation: N, number of individuals.
Signifiance levels: ***P < 0.001, **P < 0.01, *P < 0.05.
Figure 3.
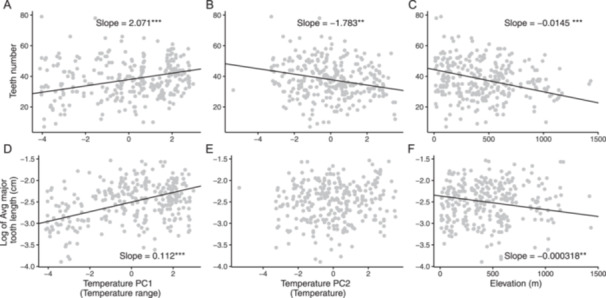
Relationships between leaf margin traits and the environment for 286 wild‐collected Heteromeles arbutifolia individuals. Slopes are reported for significant relationships. (A–C) Relationships between number of teeth and the climatic variables. (D–F) Relationships between the log of average length of major teeth and the climatic variables. Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05.
How do genetic differences and environmental maternal effects contribute to trait variation between individuals grown in a common environment, and how does this trait variation relate to the source environment?
Two of the traits were heritable, but only in some of the locations. Average major tooth length of offspring increased with the length of the maternal major teeth when measured in the greenhouse (slope = 0.323, f = 3.162, P = 0.0037) and in individuals grown at Stunt Ranch (slope = 0.393, f = 3.058, P = 0.0049). Leaf length to width ratio increased with maternal leaf length to width ratio in individuals grown at Stunt Ranch (slope = 0.123, f = 2.240, P = 0.0329) (Appendix S8: Tables 2 and 3). Because we expect the genetic similarity between a parent and offspring to be 0.5, heritability is the slope/0.5; the heritability estimate for average major teeth length was 0.646 in the greenhouse and 0.786 in Stunt Ranch, and 0.246 for leaf length width ratio in Stunt Ranch.
Table 2.
Relationships between environmental variables at the seed source and leaf traits of 12 individuals of 37 maternal lines of Heteromeles arbutifolia after 3 months of growth in a greenhouse.
| Fixed effects | Leaf area (cm2) N = 37 | L/W N = 37 | No. teeth N = 37 | Teeth area (cm2) N = 37 | Mean length of major teeth (cm) N = 37 |
|---|---|---|---|---|---|
| Trait range | 0.977–28.756 | 0.724–2.240 | 9–74 | 0.096–4.075 | 0.058–0.392 |
| Maternal trait | — | — | — | — | 0.323** |
| Germination day | –0.202*** | –0.00516*** | –0.419*** | –0.0194*** | –0.000865*** |
| Temperature | — | — | 1.527* | — | — |
| Precipitation | — | — | 1.769* | — | — |
| Precipitation range | 1.202* | — | — | 0.152* | — |
| Elevation (m a.s.l.) | ‐ | — | — | — | 0.0000422* |
| Mean seed mass | 0.564** | — | 0.792* | 0.0551* | — |
Note: Trait‐environmental variable combinations without a significant relationship are indicated with a dash (—); traits and environmental variables that did not have any significant relationships are not included in the table. Effect sizes are reported for significant relationships.
Abbreviations: L/W, length to width ratio; N, number of maternal lines.
Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05.
Table 3.
Relationships between environmental variables at the seed source and traits of Heteromeles arbutifolia individuals after 3 months of growth in Stunt Ranch and Blue Oak Ranch common gardens. Stunt Ranch values are listed before the slash and Blue Oak Ranch values are listed after.
| Fixed effects | Leaf mass (g) N = 37 | L/W N = 37 | SLA (cm2/g) N = 37 | No. teeth N = 37 | Mean length of major teeth (cm) N = 36 |
|---|---|---|---|---|---|
| Trait range | 0.0089–0.261/0.0049–0.220 | 1.260‐2.999/1.279–3.091 | 47.426–186.233/40.989–201.771 | 16–64/9–74 | 0.0697–0.324/0.0407–0.397 |
| Maternal trait | NA/– | 0.123*/– | NA/– | –/– | 0.393**/– |
| Temperature range | NA/– | –/– | NA/6.285* | –/– | –/– |
| Precipitation range | NA/– | –/– | NA/– | 3.0924**/2.987* | –/– |
| Elevation (m a.s.l.) | NA/0.0000438** | –/– | NA/– | –/0.00849* | 0.0000760**/0.0000543* |
Note: Trait–environmental variable combinations without a significant relationship are indicated with a dash (—); traits and environmental variables that did not have any significant relationships are not included in the table. Effect sizes are reported for significant relationships.
Abbreviations: L/W, length to width ratio; N, number of maternal lines; NA, maternal line was not a significant random effect for the trait (Supplemental Table 3).
Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05.
Variation in leaf margin traits we measured on offspring grown in common environments was predicted by variation of the source environment (Figure 4). At Blue Oak Ranch and Stunt Ranch, individuals sourced from regions with more precipitation range (PPC‐2, Figure 4A) tended to have more teeth. At Blue Oak Ranch, we also observed more teeth on the leaves of individuals sourced from regions that have higher temperature (TPC‐2, Figure 4B). Confusingly, in the three common environments, the mean length of major teeth was higher in individuals sourced from higher elevations (Figure 4C, Tables 2, 3), which was the opposite of what we observed in the wild‐collected leaves (Figure 3, Table 1).
Figure 4.
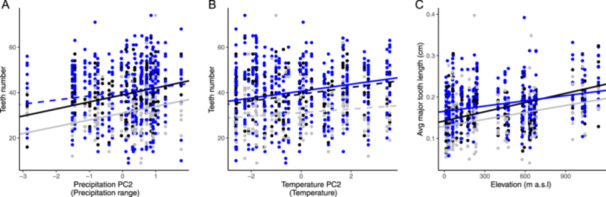
Relationships between leaf margin traits and the environment at the seed source for individuals from 37 Heteromeles arbutifolia maternal lines. There are 12 individuals per maternal line in the greenhouse (blue), six of which were then randomly assigned to Blue Oak Ranch (gray) and six to Stunt Ranch (black). Significant relationships are indicated by solid lines and nonsignificant relationships by dashed lines. Slopes for significant lines: (A) Blue Oak Ranch slope = 2.989*; Stunt Ranch slope = 3.092*; (B) greenhouse slope = 1.527*; (C) greenhouse slope = 0.0000422*; Blue Oak Ranch slope = 0.0000543*; Stunt Ranch slope = 0.0000760**. Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05.
We only detected environmental maternal effects (via differences in seed mass) on traits measured in the greenhouse. In the greenhouse, average seed mass was a significant predictor of functional traits (leaf area, tooth number, and tooth area) (Table 2, 4).
Table 4.
Relationship between number of teeth number and mean length of major teeth on fitness traits of Heteromeles arbutifolia measured in the greenhouse and two common gardens. The models also included germination date and leaf area as fixed effects.
| Trait | Location | RGR (cm/day) | Height (cm) | No. branches | No. leaves |
|---|---|---|---|---|---|
| Mean length major teeth (cm) | Greenhouse | — | — | — | — |
| Blue Oak Ranch | 0.0176** | — | — | — | |
| Stunt Ranch | — | –29.527** | –13.602* | –107.326** | |
| No. teeth | Greenhouse | 0.000124** | 0.0270*** | — | 0.0845* |
| Blue Oak Ranch | — | — | — | — | |
| Stunt Ranch | 0.00154** | — | — | — |
Note: Leaf trait‐fitness trait combinations without a significant relationship are indicated with a dash (—); effect sizes are reported for significant relationships.
Abbreviation: RGR, relative growth rate.
Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05.
Are toyon individuals adapted to their local environment?
Tooth number and average major tooth length were related to fitness traits. However, the trends were not all in the same direction, nor did we detect them at all locations. Tooth number was positively associated with faster growth, height, and leaf number in the greenhouse, faster growth at Stunt Ranch, and we did not detect any trends at BOR (Table 4). Average major tooth length was associated with a higher growth rate at BOR, but a decrease in height, branches, and number of leaves in Stunt Ranch, and we did not detect any trends in the greenhouse (Table 4).
We found no significant effects of environmental similarity on the fitness components height, relative growth rate, leaf number, and branch number in the two common gardens. Neither maternal line nor differences in the climatic variables between the source environments and the common gardens affected whether individuals survived in the common gardens. More plants died in the southern garden location Stunt Ranch Reserve (29.4% mortality), which is in the southern part of the range and has less annual precipitation and higher annual mean temperature than Blue Oak Ranch in the northern part of toyons range (13.6% mortality at Blue Oak Ranch) (though the plants that did survive at Stunt grew more, Appendix S9). The survival analysis combined with our other results suggests that there were not significant fitness differences between individuals sourced from different localities, but that across all maternal lines, the fitness of toyon transplanted in the summer declined in the warmer and drier southern common garden site.
Are some traits more plastic than others, and do maternal lines vary in plasticity?
We found that traits did vary in plasticity. The model that included a trait × garden interaction was strongly preferred by the LOO cross validation (ELPD difference 47.9, SE of difference 10.7). Most traits varied between gardens (i.e., they had plastic responses to the environment); however, leaf length/width ratio, tooth area, and number of new branches on 14 September 2022, did not vary between gardens (Figure 5). The remaining traits we measured were all greater at Stunt Ranch, except for stomatal density, which was lower at Stunt Ranch (Appendix S9). We found no evidence of G × E interactions: We were unable to detect differences between maternal lines in plasticity.
Figure 5.
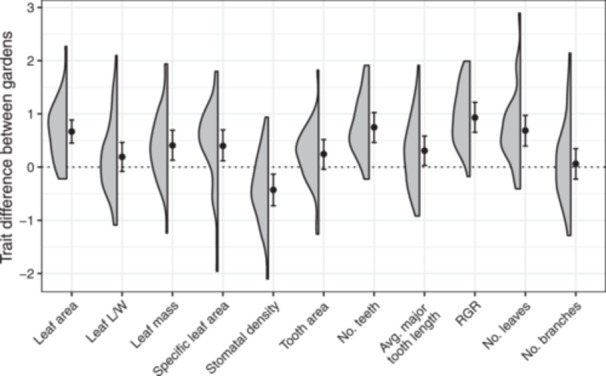
Heteromeles arbutifolia functional and fitness traits measured in two common gardens differ in plasticity. Points represent the mean of the posterior distribution of the effect of being in the Stunt Ranch garden (environmental effect) on each trait; error bars are 95% credible intervals. Bars that overlap zero indicate traits for which we did not detect plasticity. Half violin plots show the observed distribution of the mean trait differences between maternal half‐sib families grown in the two common gardens.
The table in Appendix S10 summarizes the results of all of our analyses.
DISCUSSION
In this study, we aimed to determine the extent to which toyon's trait variability across the California Floristic Province is due to phenotypic plasticity, maternal effects, local adaptation, or a combination thereof to best inform conservation and restoration efforts. We found that for most of the functional traits we measured, the variation we observed had a large plastic component, that maternal effects were stronger in younger plants, and that they mediated some, but not all, trait–environment relationships. We did find evidence of local adaptation in the wild‐collected leaves and in traits from plants grown in the greenhouse and common gardens. However, we did not find any relationships between source environment and fitness in the common gardens, suggesting that local sourcing of toyon is not critical for conservation efforts.
For most of the traits we measured, we found a strong plastic response to the environmental differences between our gardens. In particular, we found that leaf area and SLA were very plastic. Plasticity in these traits is documented in many species (e.g., Reich et al., 1999; Rosbakh et al., 2015; Liu et al., 2017) likely because leaf shape and thickness can respond quickly to changing environmental conditions such as light (Marenco and Vieira, 2005), climate (Rosbakh et al., 2015; Liu et al., 2017), and nutrient availability (Ordoñez et al., 2009). Our gardens varied in temperature, soil moisture, and likely soil nutrients (R. Prunier, personal observations), which might explain the plasticity we observed in these leaf traits. We also found that the number of teeth was more plastic than other leaf margin traits, which might help to explain why we observed conflicting relationships between teeth number and elevation in our analysis of traits measured on wild‐collected leaves and those of plants grown in the common garden (Another potential factor is that the wild‐collected leaves spanned a larger elevation gradient than offspring leaves in the gardens) (Figures 3, 4). Fitness traits (except for branch number) were also very plastic in our study. Theoretical work predicts (Kingsolver et al., 2012) and empirical work has shown (Palacio‐López et al., 2015) that fitness traits should be less plastic than functional traits because they are under stronger selection than other traits. Our results are not consistent with this expectation, perhaps because the plastic responses we see in functional traits are not sufficient to maintain similarly high levels of fitness in the two common gardens. Overall, the strong plastic responses we found in toyon suggest that toyon genotypes are likely able to survive in a variety of environmental conditions. Plasticity is essential for surviving changes in the environment for long‐lived perennial plants (Auge et al., 2017; Donelson et al., 2018; Yin et al., 2019), and high levels of plasticity have been found in toyon in prior studies (Valladares and Pearcy, 1997, 1998). Further, plasticity is often selected for because it can help facilitate the process of plants adapting to new environments (Ghalambor et al., 2007; De Kort et al., 2020), making plasticity critical for plant populations to survive and adapt to the changing climate.
Branching patterns and most leaf margin traits did not vary between our two experimental gardens, which raises the question of why some traits responded plastically to the environmental differences between our experimental gardens while these did not. Traits may vary in plasticity due to differential selection pressures of the landscape such as the scale or predictability of variation (Balaguer et al., 2001; Donohue, 2003), differences in limitations on plasticity (e.g., genetic constraints cost of plasticity; Givnish, 2002) for each trait, or a combination of the two (Valladares et al., 2007). Plasticity has been demonstrated both theoretically (Lande, 2009, 2015) and empirically (Leung et al., 2020) to decrease as the environment becomes less predictable and more heterogenous. It is beyond the scope of this study to fully parse the potential causes of variation in plasticity between traits, but it seems probable that the lack of plasticity in these traits might be due to genetic constraints. For example, while branching patterns in toyon have been shown to respond plastically to light differences (Valladares and Pearcy, 1998), both of our gardens were in the full sun. It might be the case that plasticity in branching pattern is constrained in our study because it only responds to variation in light level. The lack of plasticity in most of the leaf margin traits might be due to their high heritability. Theory suggests that traits that have heritability higher than the degree of plasticity are more likely to adapt to environmental differences (Scheiner and Lyman, 1989), potentially explaining why we observed less plasticity in tooth area and average major tooth length than in other traits. As the climate becomes more variable and extreme in California, it is possible that the plasticity in toyon will be selected against because of the increasingly heterogeneous environment and the greater costs of plasticity associated with more stressful environments. Limitation on plasticity would allow for greater genetic divergence and the potential for toyon to experience more adaptive divergence as the California Floristic Province becomes more heterogenous. Alternatively, if plasticity is adaptive for toyon individuals, plants able to respond to the environment plastically might be favored by selection, allowing adaptive plasticity to facilitate the assimilation of new trait values necessary in the changed climate.
In toyon, maternal effects are an important contributor to trait variation, especially early in development, but not in the expected traits. We found that maternal effects (using seed mass as a proxy as done by Singh et al., 2017) were stronger and more prevalent in younger seedlings. This finding is consistent with theoretical expectations (Roach and Wulff, 1987; Donohue, 2009; Auge et al., 2017) and empirical observations (Singh et al., 2017) that maternal effects should become weaker as offspring develop because the predictive accuracy of maternal cues declines over time. Interestingly, we did not observe any maternal effects on the less‐plastic tooth traits, and we observed more maternal effects in the more‐plastic traits (e.g., leaf area and tooth number; Figure 5, Table 2), in contrast to what is expected from theory. In general, maternal effects should be stronger when traits are less plastic because within‐generation plasticity can mask maternal effects (Kuijper and Hoyle, 2015; Auge et al., 2017). A further factor affecting maternal effects is the degree of selection pressure on the progeny. When individuals experience strong selection and higher levels of competition, maternal effects are more likely to be observed and be maintained into adulthood than when there is weaker selection (Galloway, 1995; Uller and Pen, 2011; Kuijper and Hoyle, 2015). Again, our results are in contrast to this expectation: For the trait that we found the strongest evidence of differential selection (average length of major teeth), we found no evidence of maternal effects. Our observed patterns might be due to the role of leaf shape traits in responding rapidly to the changing environment. Some traits might be more tightly tied to fitness (e.g., SLA and fitness components), and thus their responses to environmental cues in the maternal and progeny environment allow the plants to respond rapidly to the changing climate; rapid changes in leaf margin traits might be less important to survival. If so, that might be why we did not detect maternal effects on tooth length. Because we observed both maternal effects and plasticity in the same traits, there is the opportunity for within and across generational plasticity to operate in the same direction and bring the phenotype of toyon closer to a new optimum with a unidirectional changing environment from climate (Hoyle and Ezard, 2012; Kuijper and Hoyle, 2015; Auge et al., 2017).
We found evidence for local adaptation in toyon, though it did not cause survival differences in the common garden. The trait for which we have the strongest evidence of local adaptation is the average length of the major teeth. Variation in tooth length is likely due to genetic differences; we did not detect phenotypic plasticity in this trait, and we also found evidence of heritable genetic variation in tooth length by regressing the offspring trait on the maternal trait (Wright, 1920). However, because our estimate of heritability is confounded by maternal effects (Lande and Price, 1989), our estimate is likely an overestimate and should be interpreted with caution. That said, these heritable trait differences are related to the maternal environment, both when measured in wild‐collected plants and those grown in the experimental gardens. The pattern we observed (longer and more teeth in plants sourced from regions that were cooler and had larger temperature ranges) mirrors the global pattern (Bailey and Sinnott, 1916; Baker‐Brosh and Peet, 1997; Royer and Wilf, 2006; Adams et al., 2008). Our findings are also consistent with intraspecific patterns detected in other woody species (Royer et al., 2005).
This tooth–temperature pattern within and between species suggests that toothiness is an important adaptation to temperature. While the biological explanation for the global relationship between leaf margin shape and temperature is debated (Baker‐Brosh and Peet, 1997; Royer and Wilf, 2006; Adams et al., 2008; Zohner et al., 2019), some biological explanations for why leaf margins are beneficial in colder more temperate climates include teeth increase positive root pressure reducing flooding of the roots and freeze–thaw embolisms (Feild et al., 2005), and teeth increase rates of transpiration and photosynthesis, particularly at the beginning of the growth season (Baker‐Brosh and Peet, 1997). With climate change, rain in California is predicted to become less plentiful overall but more concentrated in the winter (Cayan et al., 2008); thus, leaf margins in toyon and other toothed species might play an important role in mitigating the effects of a changing climate. Toyon individuals with more teeth might have an adaptive advantage as precipitation range increases with the more irregular and extreme rainfall events.
Tooth length also appears to be under differential natural selection in our two gardens. Plants with shorter teeth had higher fitness (taller, more branches, and more leaves) at Stunt Ranch, but those with longer teeth had higher fitness (higher RGR) at Blue Oak Ranch. This pattern is consistent with the trend of selection favoring shorter teeth in warmer climates and longer teeth in cooler climates because Stunt Ranch was considerably warmer than BOR in the summer of 2022 (Appendix S4). Further, if the relationships between our fitness traits (growth, leaf number) and leaf margin traits were the result of allometric patterns or developmental changes, we would expect the relationships to be in the same direction in the three locations (Baumgartner et al., 2020; Chitwood et al., 2021). Because the direction of the relationship varies with garden location, they likely reflect different selection pressures in the two gardens, as expected given the environmental differences between our two common gardens. Similar patterns have been found in other widely distributed plant species (e.g., Carlson et al., 2011, 2016).
Despite the strong evidence of local adaptation in leaf margin traits, we found no evidence that any of these trait differences resulted in survival differences in the experimental gardens. Because we found that traits differed between individuals sourced from different environments and evidence for divergent selection between the two common gardens, we expected that toyon individuals sourced from environments similar to each of the two common gardens would have higher survival there. However, we found no significant relationships between environmental similarity (between source environment and common garden locations) and fitness traits measured there, nor did source environmental similarity to each garden increase survival there. The lack of relationship between source environment similarity and fitness suggests that toyon does not need to be sourced locally for restoration. While sourcing widely could theoretically lead to the introduction of maladapted genotypes (Otto and Lenormand, 2002; Agrawal, 2006; Otto, 2009), our study shows limited local adaptation. Widely sourcing seeds increases the quantity and quality of seeds (Broadhurst et al., 2008) and reduces genetic demographic problems caused by a lack of genetic diversity (Schemske and Lande, 1985; Fenster and Galloway, 2000).
CONCLUSIONS
Our findings are relevant to the conservation community and more broadly to those interested in the role of leaf margin traits in local adaptation. Toyon is a commonly used plant in restoration throughout California (Roy, 2009; Riordan et al., 2018) and plays a large ecosystem role as a winter food source for birds throughout many different California ecosystems, so our findings that toyon leaf margins are shaped by differential selection, but that fitness is not significantly impacted by source environment will inform the restoration and conservation community in California. Small‐scale restoration projects without the capacity to collect and propagate local seeds are encouraged to source plants from the most convenient plant nursery. Further, our methods for investigating the contributions of genetic differentiation, plasticity, and maternal effects can be used by those interested in conserving plant species that cover large, heterogenous ranges, which will help to fill a gap in our understanding of plant responses to climate change.
AUTHOR CONTRIBUTIONS
L.T. and R.P. developed and implemented the project and collected, processed, and analyzed the data. L.T. wrote the manuscript with guidance from R.P.
Supporting information
Appendix S1. Accessions for maternal Heteromeles arbutifolia individuals collected across California (Figure 2).
Appendix S2. Tracing leaf perimeter at “base” of teeth to calculate total teeth area. A is a major tooth and B is a minor tooth.
Appendix S3. Plexiglass configuration to nondestructively capture leaf data. A is a major tooth, and B is a minor tooth.
Appendix S4. Weather data at Stunt Ranch and Blue Oak Ranch weather stations from June to September 2022 when the common gardens were established.
Appendix S5. Loadings of the Bioclim environmental variables onto the first two PC axes for the PC analyses performed on the 286 locations where leaves were collected. PC1 and PC2 summarize over 95% of the total variance for both PC analyses. Cumulative variance explained is in parentheses.
Appendix S6. Correlation matrix between the five climate variables used throughout data analysis: bioclimatic water variables PC1 and PC2, bioclimatic temperature variables PC1 and PC2, and elevation. Strength of correlation is indicated by the size of the circle and the intensity of the color; red is for negative relationships, blue for positive.
Appendix S7. Standard deviation with 95% confidence intervals in parentheses for the effect of maternal line on traits in the greenhouse and two common gardens.
Appendix S8. Relationships between maternal and offspring for (A) average major tooth length and (B) length to width ratio in the greenhouse (blue), Blue Oak Ranch (gray), and Stunt Ranch (black). Average major tooth length heritability is 0.786 at Stunt Ranch and 0.646 when estimated in the greenhouse. Length to width ratio heritability at Stunt Ranch is 0.246. Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05; NS, P < 1.
Appendix S9. Slope variation between maternal lines for different traits in the common gardens. Reaction norms are drawn for the average trait value for each maternal line.
Appendix S10. Summarized results for the sources of variation. The table contains all traits measured and collected data with each finding categorized as maternal effects, genetic differentiation, plasticity, or a combination. Asterisks indicate significant results. NA, analysis was not conducted for that trait.
ACKNOWLEDGMENTS
The authors thank numerous sources for funding, mentorship, and research assistance. This project was funded by multiple UCLA awards to L.T., multiple California Native Plant Society awards to L.T., the La Kretz Center and Stunt Ranch Reserve Fellowship to L.T., and CCGP award 86 to R.P. The authors thank the U.S. Forest Service, Bureau of Land Management, California State Parks, and the UC Reserve System for granting permission to collect toyon. The authors thank the UC Reserve System for their generous support with special thanks to Z. Harlow and Z. Tuthill at Blue Oak Ranch and G. Bucciarelli, B. Shaffer, and D. Blake at Stunt Ranch for supporting the logistics of planting and maintaining two common gardens. The authors thank N. Nartz, C. Fuson, K. Heine, R. Cuellar, A. King, N. Moss, and especially N. Liu for lab, greenhouse, and field help. The authors thank C. Kremer for statistical consulting and V. Sork and the Sork lab at UCLA for providing research space, logistical support, help with experimental design, and help with manuscript preparation. Lastly, the authors thank the reviewers and Associate Editor for thoughtful comments that allowed us to significantly improve this manuscript.
Thomas, L. G. , and Prunier R.. 2024. Local adaptation and phenotypic plasticity drive leaf trait variation in the California endemic toyon (Heteromeles arbutifolia). American Journal of Botany 111(11): e16430. 10.1002/ajb2.16430
DATA AVAILABILITY STATEMENT
The data sets and code are available at Dryad: DOI:10.5061/dryad.gqnk98sx2 (Thomas, 2024).
REFERENCES
- Adams, J. M. , Green W. A., and Zhang Y.. 2008. Leaf margins and temperature in the North American flora: Recalibrating the paleoclimatic thermometer. Global and Planetary Change 60: 523–534. [Google Scholar]
- Agrawal, A. F. 2006. Evolution of sex: Why do organisms shuffle their genotypes? Current Biology 16: R696–R704. [DOI] [PubMed] [Google Scholar]
- Aitken, S. N. , Yeaman S., Holliday J. A., Wang T., and Curtis‐McLane S.. 2008. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications 1: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman, K. C. , Sattarin A., Kelly J. K., and Scoville A. G.. 2016. Transgenerational plasticity is sex‐dependent and persistent in yellow monkeyflower (Mimulus guttatus). Environmental Epigenetics 2: dvw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge, G. A. , Leverett L. D., Edwards B. R., and Donohue K.. 2017. Adjusting phenotypes via within‐ and across‐generational plasticity. New Phytologist 216: 343–349. [DOI] [PubMed] [Google Scholar]
- Bailey, I. W. , and Sinnott E. W.. 1916. The climatic distribution of certain types of angiosperm leaves. American Journal of Botany 3: 24–39. [Google Scholar]
- Baker‐Brosh, K. F. , and Peet R. K.. 1997. The ecological significance of lobed and toothed leaves in temperature forest trees. Ecology 78: 1250–1255. [Google Scholar]
- Balaguer, L. , Martínez‐Ferri E., Valladares F., Pérez‐Corona M. E., Baquedano F. J., Castillo F. J., and Manrique E.. 2001. Population divergence in the plasticity of the response of Quercus coccifera to the light environment. Ecology 15: 124–135. [Google Scholar]
- Baldwin, B. G. 2014. Origins of plant diversity in the California Floristic Province. Annual Review of Ecology, Evolution, and Systematics 45: 347–369. [Google Scholar]
- Baumgartner, A. , Donahoo M., Chitwood D. H., and Peppe D. J.. 2020. The influences of environmental change and development on leaf shape in Vitis . American Journal of Botany 107: 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick, D. I. , Amarasekare P., Araújo M. S., Bürger R., Levine J. M., Novak M., Rudolf V. H. W., et al. 2011. Why intraspecific trait variation matters in community ecology. Trends in Ecology and Evolution 26: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst, L. M. , Lowe A., Coates D. J., Cunningham S. A., McDonald M., Vesk P. A., and Yates C.. 2008. Seed supply for broadscale restoration: maximizing evolutionary potential. Evolutionary Applications 1: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, V. K. , Lawton J. H., and Grubb P. J.. 1991. Herbivory and the evolution of leaf size and shape [and Discussion]. Philosophical Transactions of the Royal Society, B, Biological Sciences 333: 265–272. [Google Scholar]
- Callaghan, T. V. , Bergholm F., Christensen T. R., Jonasson C., Kokfelt U., and Johansson M.. 2010. A new climate era in the sub‐Arctic: Accelerating climate changes and multiple impacts. Geophysical Research Letters 37: L14705. [Google Scholar]
- Carlson, J. E. , Adams C. A., and Holsinger K. E.. 2016. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Annals of Botany 117: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, J. E. , Holsinger K. E., and Prunier R.. 2011. Plant responses to climate in the Cape Floristic Region of South Africa: Evidence for adaptive differentiation in the Proteaceae. Evolution 65: 108–124. [DOI] [PubMed] [Google Scholar]
- Cayan, D. R. , Maurer E. P., Dettinger M. D., Tyree M., and Hayhoe K.. 2008. Climate change scenarios for the California region. Climatic Change 87: 21–42. [Google Scholar]
- Ceballos, G. , Ehrlich P. R., Barnosky A. D., García A., Pringle R. M., and Palmer T. M.. 2015. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances 1: e1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , Mullins J., Migicovsky Z., Frank M., VanBuren R., and Londo J. P.. 2021. Vein‐to‐blade ratio is an allometric indicator of leaf size and plasticity. American Journal of Botany 108: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C. , and Beazley K.. 2018. Changes in human population density and protected areas in terrestrial global biodiversity hotspots, 1995–2015. Land 7: 136. [Google Scholar]
- De Kort, H. , Panis B., Helsen K., Douzet R., Janssens S. B., and Honnay O.. 2020. Pre‐adaptation to climate change through topography‐driven phenotypic plasticity. Journal of Ecology 108: 1465–1474. [Google Scholar]
- de Lafontaine, G. , Napier J. D., Petit R. J., and Hu F. S.. 2018. Invoking adaptation to decipher the genetic legacy of past climate change. Ecology 99: 1530–1546. [DOI] [PubMed] [Google Scholar]
- Donelson, J. M. , Salinas S., Munday P. L., and Shama L. N. S.. 2018. Transgenerational plasticity and climate change experiments: Where do we go from here? Global Change Biology 24: 13–34. [DOI] [PubMed] [Google Scholar]
- Dong, B. C. , Fu T., Luo F. L., and Yu F. H.. 2017. Herbivory‐induced maternal effects on growth and defense traits in the clonal species Alternanthera philoxeroides . Science of the Total Environment 605–606: 114–123. [DOI] [PubMed] [Google Scholar]
- Donohue, K. 2009. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society, B, Biological Sciences 364: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue, K. 2003. Setting the stage: Phenotypic plasticity as habitat selection. International Journal of Plant Sciences 164: S79–S92. [Google Scholar]
- Etterson, J. R. 2004a. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. I. Clinal patterns of selection along an environmental gradient in the Great Plains. Evolution 58: 1446–1456. [DOI] [PubMed] [Google Scholar]
- Etterson, J. R. 2004b. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. II. Genetic architecture of three populations reciprocally planted along an environmental gradient in the Great Plains. Evolution 58: 1459–1471. [DOI] [PubMed] [Google Scholar]
- Feild, T. S. , Sage T. L., Czerniak C., and Iles W. J. D.. 2005. Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation‐induced flooding of the mesophyll. Plant, Cell and Environment 28: 1179–1190. [Google Scholar]
- Fenster, C. B. , and Galloway L. F.. 2000. Inbreeding and outbreeding depression in natural populations of Chamaecrista fasciculata (Fabaceae). Conservation Biology 14: 1406–1412. [Google Scholar]
- Fick, S. E. , and Hijmans R. J.. 2017. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Galloway, L. F. 1995. Response to natural environmental heterogeneity: maternal effects and selection on life‐history characters and plasticities in Mimulus guttatus . Evolution 49: 1095–1107. [DOI] [PubMed] [Google Scholar]
- Galloway, L. F. 2005. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytologist 166: 93–100. [DOI] [PubMed] [Google Scholar]
- Gardiner, G. 2008. Accelerating climate change. Research Paper No. 2, 2008, Parliament Library Research Service, Melbourne, Australia. [Google Scholar]
- GBIF.org . 2020. GBIF occurrence download. Website: 10.15468/dl.j4s7ny [accessed 13 August 2020]. Global Biodiversity Information Facility, Copenhagen, Denmark. [DOI]
- Ghalambor, C. K. , McKay J. K., Carroll S. P., and Reznick D. N.. 2007. Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21: 394–407. [Google Scholar]
- Givnish, T. J. 2002. Ecological constraints on the evolution of plasticity in plants. Evolutionary Ecology 16: 213–242. [Google Scholar]
- Goodrich, B. 2023. rstanarm: Bayesian applied regression modeling via Stan. R package version 2.32.1. Website: https://cran.r-project.org/web/packages/rstanarm/index.html
- Havens, K. , Vitt P., Still S., Kramer A. T., Fant J. B., and Schatz K.. 2015. Seed sourcing for restoration in an era of climate change. Natural Areas Journal 35: 122–133. [Google Scholar]
- Hollister, J. W. , Robitaille A. L., Beck M. W., Johnson‐NOAA M., and Shah T.. 2021. elevatr: Access elevation data from various APIs. R package version 0.99.0, Website: https://github.com/jhollist/elevatr/
- Hoyle, R. B. , and Ezard T. H. G.. 2012. The benefits of maternal effects in novel and in stable environments. Journal of the Royal Society Interface 9: 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford, K. M. , and Mazer S. J.. 2003. Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends in Ecology & Evolution 18: 147–155. [Google Scholar]
- Hultine, K. R. , Allan G. J., Blasini D., Bothwell H. M., Cadmus A., Cooper H. F., Doughty C. E., et al. 2020. Adaptive capacity in the foundation tree species Populus fremontii: implications for resilience to climate change and non‐native species invasion in the American Southwest. Conservation Physiology 8: coaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver, J. G. , Diamond S. E., Siepielski A. M., and Carlson S. M.. 2012. Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evolutionary Ecology 26: 1101–1118. [Google Scholar]
- Kuijper, B. , and Hoyle R. B.. 2015. When to rely on maternal effects and when on phenotypic plasticity? Evolution 69: 950–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, U. , Singh P., and Boote K. J.. 2012. Effect of climate change factors on processes of crop growth and development and yield of groundnut (Arachis hypogaea L.). Advances in Agronomy 116: 41–69. [Google Scholar]
- Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. Journal of Evolutionary Biology 22: 1435–1446. [DOI] [PubMed] [Google Scholar]
- Lande, R. 2015. Evolution of phenotypic plasticity in colonizing species. Molecular Ecology 24: 2038–2045. [DOI] [PubMed] [Google Scholar]
- Lande, R. , and Arnold S. J.. 1983. The measurement of selection on correlated characters. Evolution 37: 1210–1226. [DOI] [PubMed] [Google Scholar]
- Lande, R. , and Price T.. 1989. Genetic correlations and maternal effect coefficients obtained from offspring‐parent regression. Genetics 122: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, C. , Rescan M., Grulois D., and Chevin L.. 2020. Reduced phenotypic plasticity evolves in less predictable environments. Ecology Letters 23: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart, Y. B. , and Grant M. C.. 1996. Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics 27: 237–277. [Google Scholar]
- Liu, M. , Wang Z., Li S., Lü X., Wang X., and Han X.. 2017. Changes in specific leaf area of dominant plants in temperate grasslands along a 2500‐km transect in northern China. Scientific Reports 7: 10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco, R. A. , and Vieira G.. 2005. Specific leaf area and photosynthetic parameters of tree species in the forest understorey as a function of the microsite light environment in central Amazonia. Journal of Tropical Forest Science 17: 265–278. [Google Scholar]
- McNichol, B. H. , and Russo S. E.. 2023. Plant species’ capacity for range shifts at the habitat and geographic scales: A trade‐off‐based framework. Plants 12: 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, H. S. , Schuman I. J., Layden T. J., Ritz A., Kremer C. T., and Fey S. B.. 2022. Temperature‐mediated transgenerational plasticity influences movement behaviour in the green algae Chlamydomonas reinhardtii . Functional Ecology 36: 2969–2982. [Google Scholar]
- Montalvo, A. M. , Riordan E. C., and Beyers J. L.. 2018. Plant profile for Heteromeles arbutifolia. Native plant recommendations for Southern California ecoregions. Website: https://www.fs.usda.gov/research/treesearch/57250
- Münzbergová, Z. , Hadincová V., Skálová H., and Vandvik V.. 2017. Genetic differentiation and plasticity interact along temperature and precipitation gradients to determine plant performance under climate change. Journal of Ecology 105: 1358–1373. [Google Scholar]
- Nic Lughadha, E. , Bachman S. P., Leão T. C. C., Forest F., Halley J. M., Moat J., Acedo C., et al. 2020. Extinction risk and threats to plants and fungi. Plants, People, Planet 2: 389–408. [Google Scholar]
- Oliver, T. H. , and Morecroft M. D.. 2014. Interactions between climate change and land use change on biodiversity: attribution problems, risks, and opportunities. WIREs Climate Change 5: 317–335. [Google Scholar]
- Ordoñez, J. C. , Van Bodegom P. M., Witte J. M., Wright I. J., Reich P. B., and Aerts R.. 2009. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography 18: 137–149. [Google Scholar]
- Otto, S. P. 2009. The evolutionary enigma of sex. American Naturalist 174: S1–S14. [DOI] [PubMed] [Google Scholar]
- Otto, S. P. , and Lenormand T.. 2002. Resolving the paradox of sex and recombination. Nature Reviews Genetics 3: 252–261. [DOI] [PubMed] [Google Scholar]
- Palacio‐López, K. , Beckage B., Scheiner S., and Molofsky J.. 2015. The ubiquity of phenotypic plasticity in plants: A synthesis. Ecology and Evolution 5: 3389–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry, M. L. , Canziani O., Palutikof J. P., van der Linden P., and Hanson C. E. [eds.]. 2007. Climate change 2007: Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland. [Google Scholar]
- Pinheiro, J. , Bates D., and Team R Core. 2023. nlme: Linear and nonlinear mixed effects models. R package version 3.1–164. Website: https://CRAN.R-project.org/package=nlme [Google Scholar]
- Prunier, R. , Holsinger K. E., and Carlson J. E.. 2012. The effect of historical legacy on adaptation: do closely related species respond to the environment in the same way? Journal of Evolutionary Biology 25: 1636–1649. [DOI] [PubMed] [Google Scholar]
- QGIS.org . 2022. QGIS Geographic Information System. QGIS Association. Website: http://www.qgis.org
- R Core Team . 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Website: https://www.r-project.org
- Reich, P. B. , Ellsworth D. S., Walters M. B., Vose J. M., Gresham C., Volin J. C., and Bowman W. D.. 1999. Generality of leaf trait relationships: a test across six biomes. Ecology 80: 1955–1969. [Google Scholar]
- Riordan, E. C. , Montalvo A. M., and Beyers J. L.. 2018. Using species distribution models with climate change scenarios to aid ecological restoration decision making for southern California shrublands. U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, Placerville, CA, USA. [Google Scholar]
- Roach, D. A. , and Wulff R. D.. 1987. Maternal effects in plants. Annual Review of Ecology and Systematics 18: 209–235. [Google Scholar]
- Rohrer, J. R. , Robertson K. R., and Phipps J. B.. 1991. Variation in structure among fruits of Maloideae (Rosaceae). American Journal of Botany 78: 1617–1635. [Google Scholar]
- Rosbakh, S. , Römermann C., and Poschlod P.. 2015. Specific leaf area correlates with temperature: new evidence of trait variation at the population, species and community levels. Alpine Botany 125: 79–86. [Google Scholar]
- Roy, C. L. 2009. Restoration of A. fasciculatum at Rocky Canyon Granite Quarry, San Luis Obispo, CA. Master's thesis, California Polytechnic University, San Luis Obispo, CA, USA. [Google Scholar]
- Royer, D. L. , and Wilf P.. 2006. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. International Journal of Plant Sciences 167: 11–18. [Google Scholar]
- Royer, D. L. , Wilf P., Janesko D. A., Kowalski E. A., and Dilcher D. L.. 2005. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany 92: 1141–1151. [DOI] [PubMed] [Google Scholar]
- Scheiner, S. M. , and Lyman R. F.. 1989. The genetics of phenotypic plasticity I. Heritability. Journal of Evolutionary Biology 2: 95–107. [Google Scholar]
- Schemske, D. W. , and Lande R.. 1985. The evolution of self‐fertilization and inbreeding depression in plants. II. Empirical observations. Evolution 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Schneider, C. A. , Rasband W. S., and Eliceiri K. W.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, P. , Liu M., Yu X., Gielis J., and Ratkowsky D.. 2019. Proportional relationship between leaf area and the product of leaf length and width of four types of special leaf shapes. Forests 10: 178. [Google Scholar]
- Singh, J. , Clavijo Michelangeli J. A., Gezan S. A., Lee H., and Vallejos C. E.. 2017. Maternal effects on seed and seedling phenotypes in reciprocal F1 hybrids of the common bean (Phaseolus vulgaris L.). Frontiers in Plant Science 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton, R. P. , Anderegg L. D. L., Papper P., Reich E., Dawson T. E., Kling M., Thompson S. E., et al. 2019. No local adaptation in leaf or stem xylem vulnerability to embolism, but consistent vulnerability segmentation in a North American oak. New Phytologist 223: 1296–1306. [DOI] [PubMed] [Google Scholar]
- Sork, V. L. , Squire K., Gugger P. F., Steele S. E., Levy E. D., and Eckert A. J.. 2016. Landscape genomic analysis of candidate genes for climate adaptation in a California endemic oak, Quercus lobata . American Journal of Botany 103: 33–46. [DOI] [PubMed] [Google Scholar]
- Suter, L. , and Widmer A.. 2013. Environmental heat and salt stress induce transgenerational phenotypic changes in Arabidopsis thaliana . PLoS One 8: e60364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T. M. 2022. coxme: Mixed effects cox models. R package version 2.2‐18.1. Website: https://cran.r-project.org/web/packages/coxme/index.html
- Therneau, T. M. 2023. survival: Survival analysis. R package version 3.5‐7. Website: https://CRAN.R-project.org/package=survival
- Thomas, L. 2024. Local adaptation and phenotypic plasticity drive leaf trait variation in the California endemic toyon dataset (Version 4) [Data set]. Dryad. 10.5061/DRYAD.GQNK98SX2 [DOI] [PMC free article] [PubMed]
- Uller, T. , and Pen I.. 2011. A theoretical model of the evolution of maternal effects under parent‐offspring conflict. Evolution 65: 2075–2084. [DOI] [PubMed] [Google Scholar]
- Valladares, F. , Gianoli E., and Gómez J. M.. 2007. Ecological limits to plant phenotypic plasticity. New Phytologist 176: 749–763. [DOI] [PubMed] [Google Scholar]
- Valladares, F. , and Pearcy R. W.. 1997. Interactions between water stress, sun–shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia . Plant, Cell & Environment 20: 25–36. [Google Scholar]
- Valladares, F. , and Pearcy R. W.. 1998. The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia 114: 1–10. [DOI] [PubMed] [Google Scholar]
- Van Andel, J. 1998. Intraspecific variability in the context of ecological restoration projects. Perspectives in Plant Ecology, Evolution and Systematics 1: 221–237. [Google Scholar]
- Vehtari, A. , Mononen T., Tolvanen V., Sivula T., and Winther O.. 2016. Bayesian leave‐one‐out cross‐validation approximations for Gaussian latent variable models. Journal of Machine Learning Research 17: 1–38. [Google Scholar]
- Wright, I. J. , Reich P. B., Cornelissen J. H. C., Falster D. S., Garnier E., Hikosaka K., Lamont B. B., et al. 2005. Assessing the generality of global leaf trait relationships. New Phytologist 166: 485–496. [DOI] [PubMed] [Google Scholar]
- Wright, I. J. , Reich P. B., Westoby M., Ackerly D. D., Baruch Z., Bongers F., Cavender‐Bares J., et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright, S. 1920. The relative importance of heredity and environment in determining the piebald pattern of guinea‐pigs. Proceedings of the National Academy of Sciences, USA 6: 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, J. , Zhou M., Lin Z., Li Q. Q., and Zhang Y.. 2019. Transgenerational effects benefit offspring across diverse environments: a meta‐analysis in plants and animals. Ecology Letters 22: 1976–1986. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Yu Q., Xu C., Li J., and Qin G.. 2018. Rapid estimation of stomatal density and stomatal area of plant leaves based on object‐oriented classification and its ecological trade‐off strategy analysis. Forests 9: 616. [Google Scholar]
- Zohner, C. M. , Ramm E., and Renner S. S.. 2019. Examining the support–supply and bud‐packing hypotheses for the increase in toothed leaf margins in northern deciduous floras. American Journal of Botany 106: 1404–1411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Accessions for maternal Heteromeles arbutifolia individuals collected across California (Figure 2).
Appendix S2. Tracing leaf perimeter at “base” of teeth to calculate total teeth area. A is a major tooth and B is a minor tooth.
Appendix S3. Plexiglass configuration to nondestructively capture leaf data. A is a major tooth, and B is a minor tooth.
Appendix S4. Weather data at Stunt Ranch and Blue Oak Ranch weather stations from June to September 2022 when the common gardens were established.
Appendix S5. Loadings of the Bioclim environmental variables onto the first two PC axes for the PC analyses performed on the 286 locations where leaves were collected. PC1 and PC2 summarize over 95% of the total variance for both PC analyses. Cumulative variance explained is in parentheses.
Appendix S6. Correlation matrix between the five climate variables used throughout data analysis: bioclimatic water variables PC1 and PC2, bioclimatic temperature variables PC1 and PC2, and elevation. Strength of correlation is indicated by the size of the circle and the intensity of the color; red is for negative relationships, blue for positive.
Appendix S7. Standard deviation with 95% confidence intervals in parentheses for the effect of maternal line on traits in the greenhouse and two common gardens.
Appendix S8. Relationships between maternal and offspring for (A) average major tooth length and (B) length to width ratio in the greenhouse (blue), Blue Oak Ranch (gray), and Stunt Ranch (black). Average major tooth length heritability is 0.786 at Stunt Ranch and 0.646 when estimated in the greenhouse. Length to width ratio heritability at Stunt Ranch is 0.246. Significance levels: ***P < 0.001, **P < 0.01, *P < 0.05; NS, P < 1.
Appendix S9. Slope variation between maternal lines for different traits in the common gardens. Reaction norms are drawn for the average trait value for each maternal line.
Appendix S10. Summarized results for the sources of variation. The table contains all traits measured and collected data with each finding categorized as maternal effects, genetic differentiation, plasticity, or a combination. Asterisks indicate significant results. NA, analysis was not conducted for that trait.
Data Availability Statement
The data sets and code are available at Dryad: DOI:10.5061/dryad.gqnk98sx2 (Thomas, 2024).


