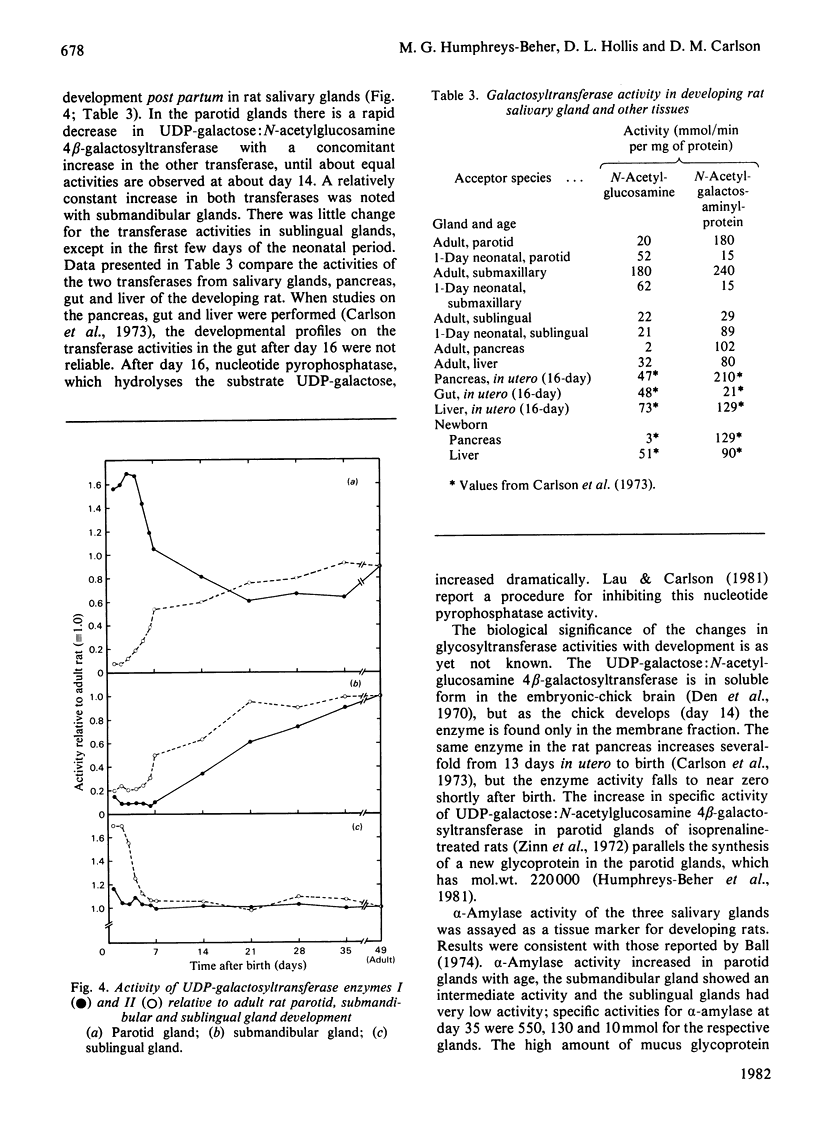
Abstract
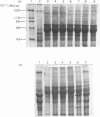
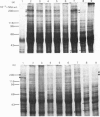
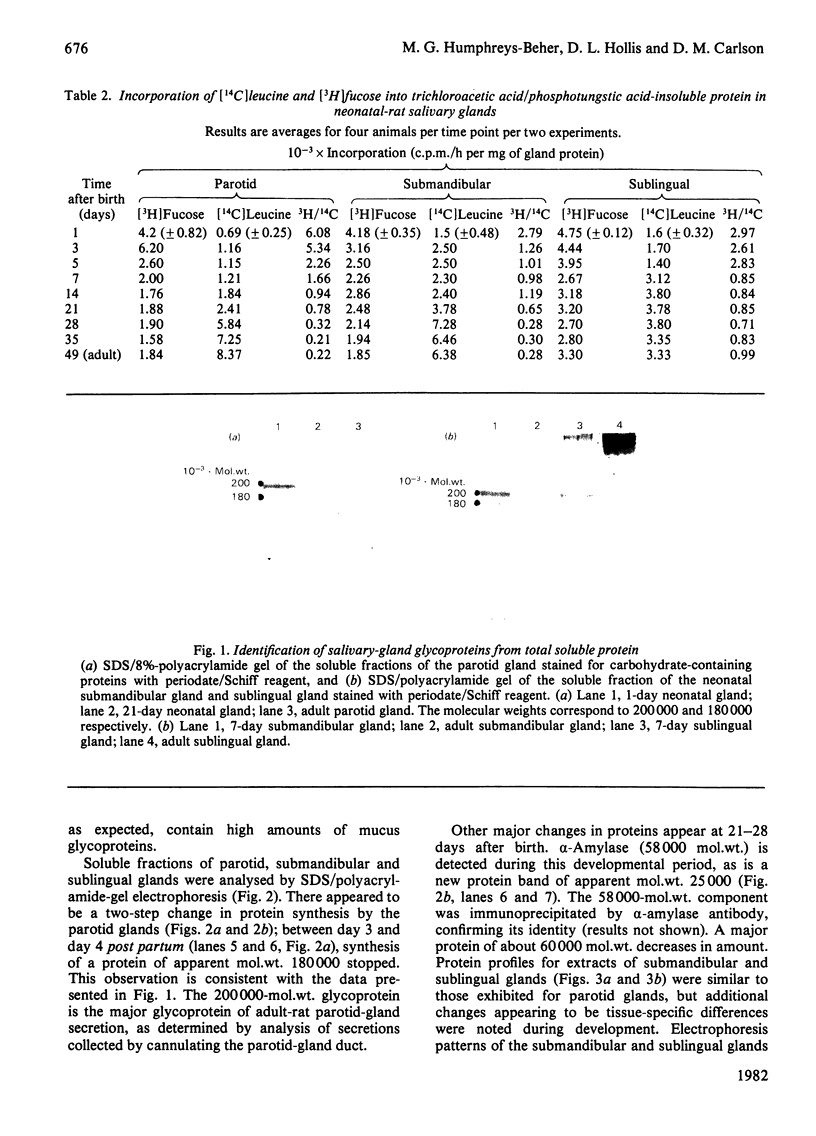
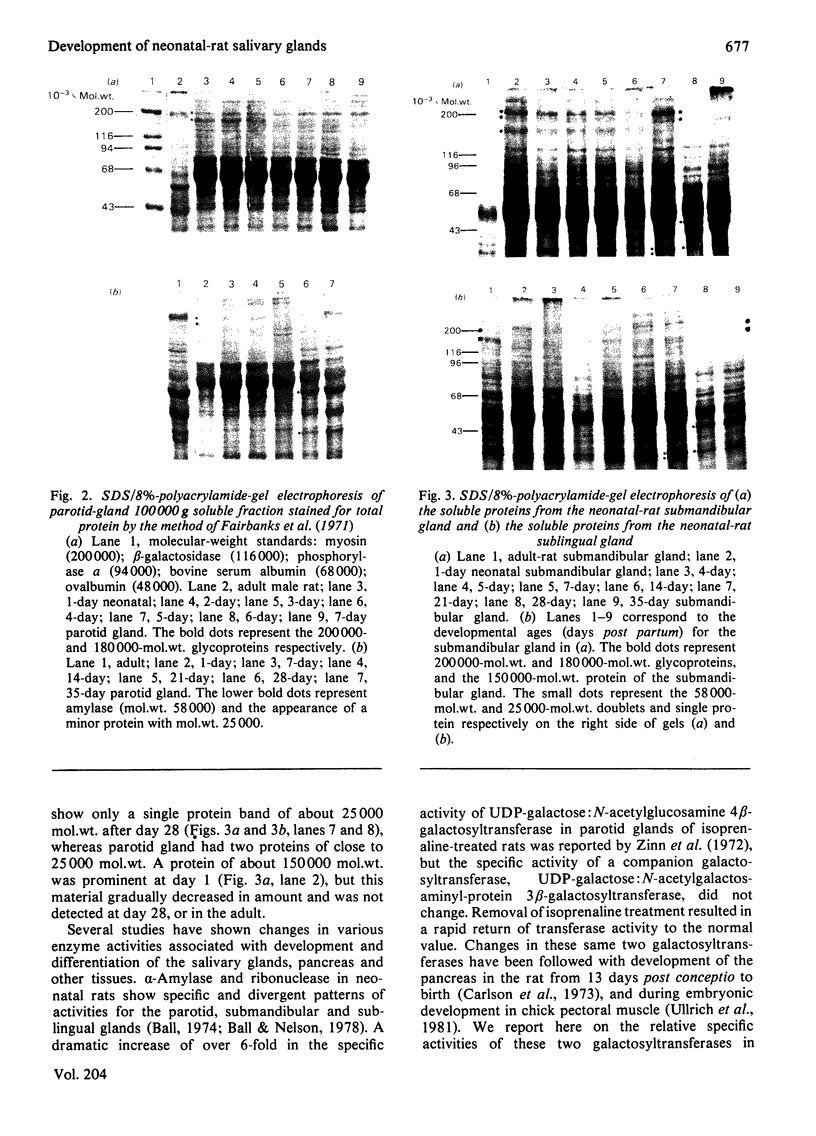
Analysis of the soluble protein fractions from the rat parotid, submandibular and sublingual glands by polyacrylamide-gel electrophoresis reveals similarities in overall patterns of protein synthesis at birth. Tissue-specific changes in protein and glycoprotein synthesis occur shortly after birth and again at the time of weaning, 21--28 days later. Incorporation of [3H]thymidine into DNA was at its highest after birth and gradually decreased in both the parotid and submandibular gland, whereas [3H]thymidine incorporation in the sublingual gland was low throughout the time of neonatal development. [14C]Leucine incorporation into total protein increased in all glands with age after birth, showing an accelerated rate 21--28 days later. Trichloroacetic acid/phosphotungstic acid-precipitable [3H]fucose in glycoproteins declined over the time of neonatal development in the parotid and submandibular gland, but its incorporation remained higher in the sublingual gland. alpha-Amylase (EC 3.2.1.1) in the salivary glands increased at the time of weaning, as judged by detectability in sodium dodecyl sulphate/polyacrylamide gels and by immune precipitation. Two membrane-bound enzymes, UDP-galactose:2-acetamido-2-deoxy-D-glucosamine 4 beta-galactosyltransferase (EC 2.4.1.22) and UDP-galactose:2-acetamido-2-deoxy-D-galactosaminyl-protein 3 beta-galactosyltransferase (no EC number), undergo tissue-specific change rather than changes induced by physiological stimulation of the salivary glands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball W. D. Development of the rat salivary glands. IV. Amylase and ribonuclease activity during embryonic and neonatal development of the parotid and submaxillary glands. Dev Biol. 1974 Dec;41(2):267–277. doi: 10.1016/0012-1606(74)90305-4. [DOI] [PubMed] [Google Scholar]
- Ball W. D., Nelson N. J. A stage-restricted secretory system in the submandibular gland of the neonatal rat. Differentiation. 1978 May 26;10(3):147–158. doi: 10.1111/j.1432-0436.1978.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., David J., Rutter W. J. Galactosyltransferase activities in pancreas, liver and gut of the developing rat. Arch Biochem Biophys. 1973 Aug;157(2):605–612. doi: 10.1016/0003-9861(73)90680-2. [DOI] [PubMed] [Google Scholar]
- Den H., Kaufman B., Roseman S. Properties of some glycosyltransferases in embryonic chicken brain. J Biol Chem. 1970 Dec 25;245(24):6607–6615. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sorensen A., Carlson D. M. Isolation of a "proline-rich" protein from rat parotid glands following isoproterenol treatment. Biochem Biophys Res Commun. 1974 Sep 9;60(1):249–256. doi: 10.1016/0006-291x(74)90198-3. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Holden K. G., Yim N. C., Griggs L. J., Weisbach J. A. Gel electrophoresis of mucous glycoproteins. II. Effect of physical deaggregation and disulfide-bond cleavage. Biochemistry. 1971 Aug 3;10(16):3110–3113. doi: 10.1021/bi00792a020. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau J. T., Carlson D. M. Galactosyltransferase activities in rat intestinal mucosa. Inhibition of nucleotide pyrophosphatase. J Biol Chem. 1981 Jul 25;256(14):7142–7145. [PubMed] [Google Scholar]
- Redman R. S., Ball W. D. Cytodifferentiation of secretory cells in the sublingual gland of the prenatal rat: a histological, histochemical and ultrastructural study. Am J Anat. 1978 Nov;153(3):367–389. doi: 10.1002/aja.1001530304. [DOI] [PubMed] [Google Scholar]
- SCHNEYER C. A., SCHNEYER L. H. Secretion by salivary glands deficient in acini. Am J Physiol. 1961 Nov;201:939–942. doi: 10.1152/ajplegacy.1961.201.5.939. [DOI] [PubMed] [Google Scholar]
- SHACKELFORD J. M. Histochemical comparison of mucous secretions in rodent, carnivore, ungulate, and primate major salivary glands. Ann N Y Acad Sci. 1963 Mar 30;106:572–582. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Growth pattern of postnatally developing rat parotid gland. Proc Soc Exp Biol Med. 1969 Feb;130(2):603–607. doi: 10.3181/00379727-130-33617. [DOI] [PubMed] [Google Scholar]
- Ullrich S. J., Kent C., Carlson D. M. Changes in galactosyltransferase activity in chick pectoral muscle during embryonic development. Biochem J. 1981 Apr 15;196(1):17–23. doi: 10.1042/bj1960017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S. Metabolism and isoenzyme alterations in experimental hepatomas. Fed Proc. 1973 Dec;32(12):2162–2167. [PubMed] [Google Scholar]
- Yamashina S., Mizuhira V. Postnatal development of acinar cells in rat submandibular gland as revealed by electron microscopic staining for carbohydrates. Am J Anat. 1976 Jul;146(3):211–235. doi: 10.1002/aja.1001460302. [DOI] [PubMed] [Google Scholar]