Abstract
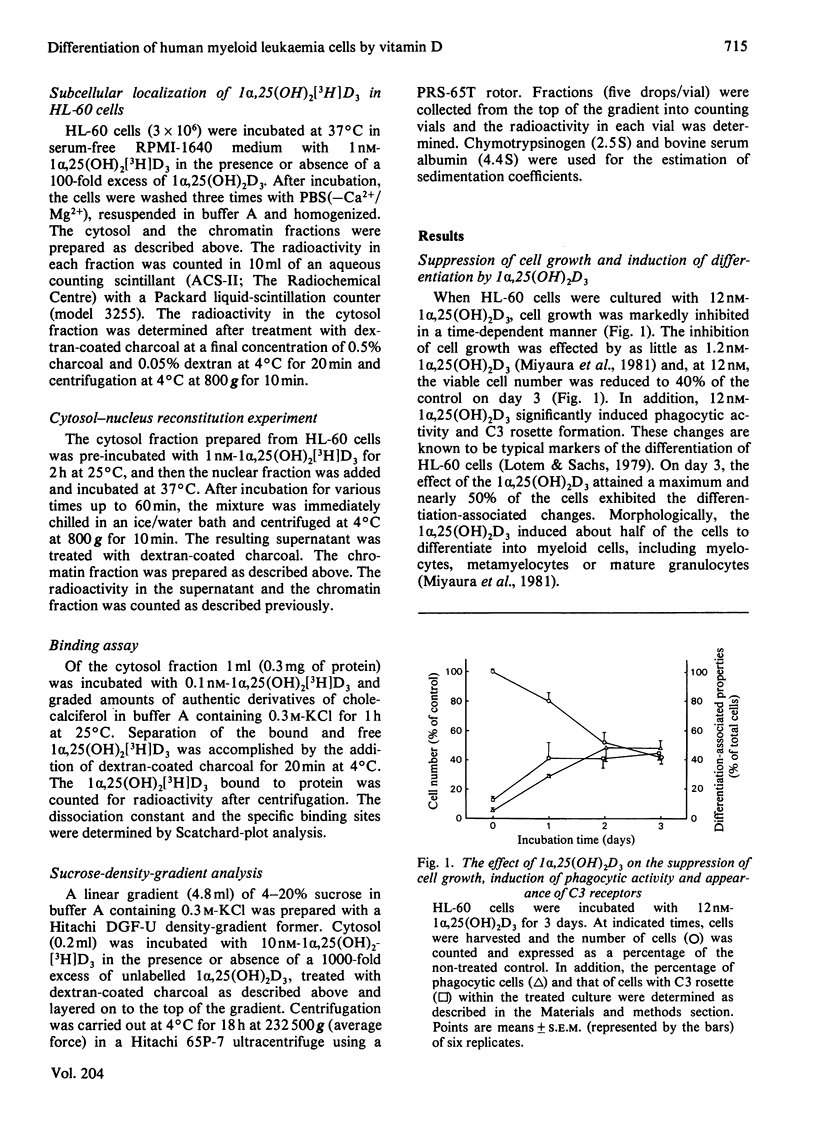
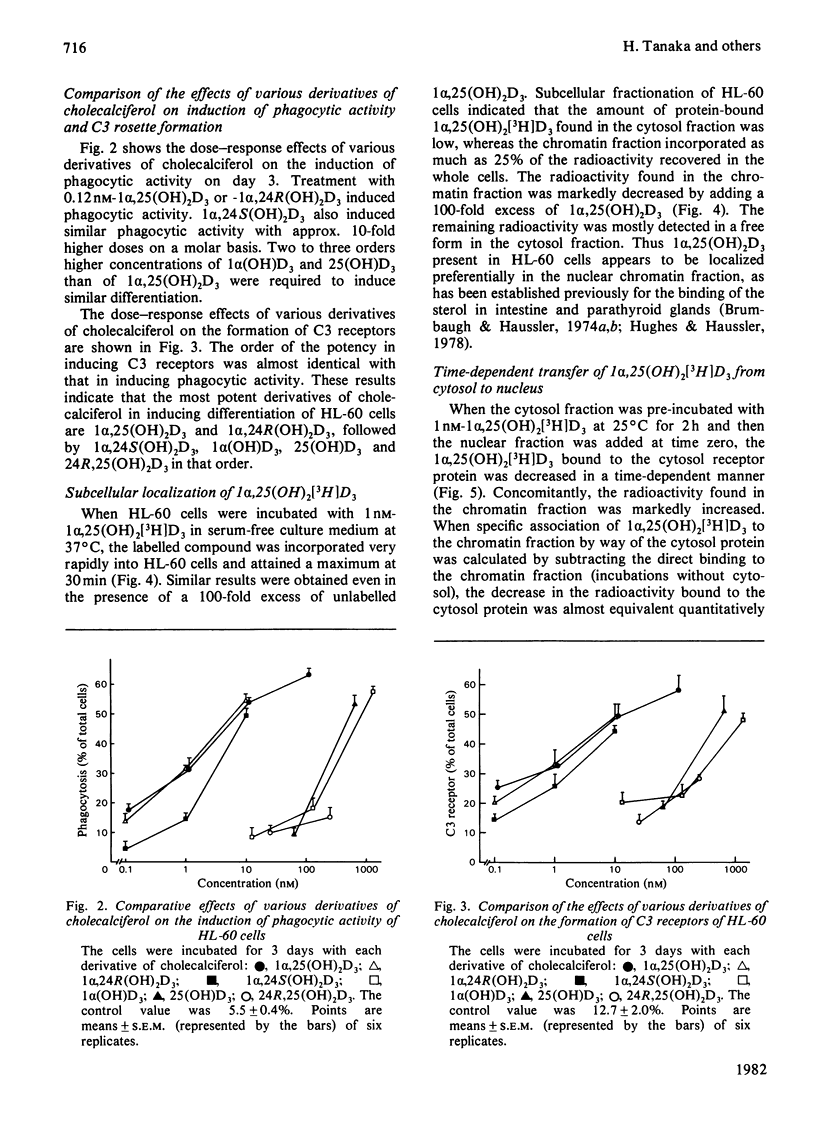
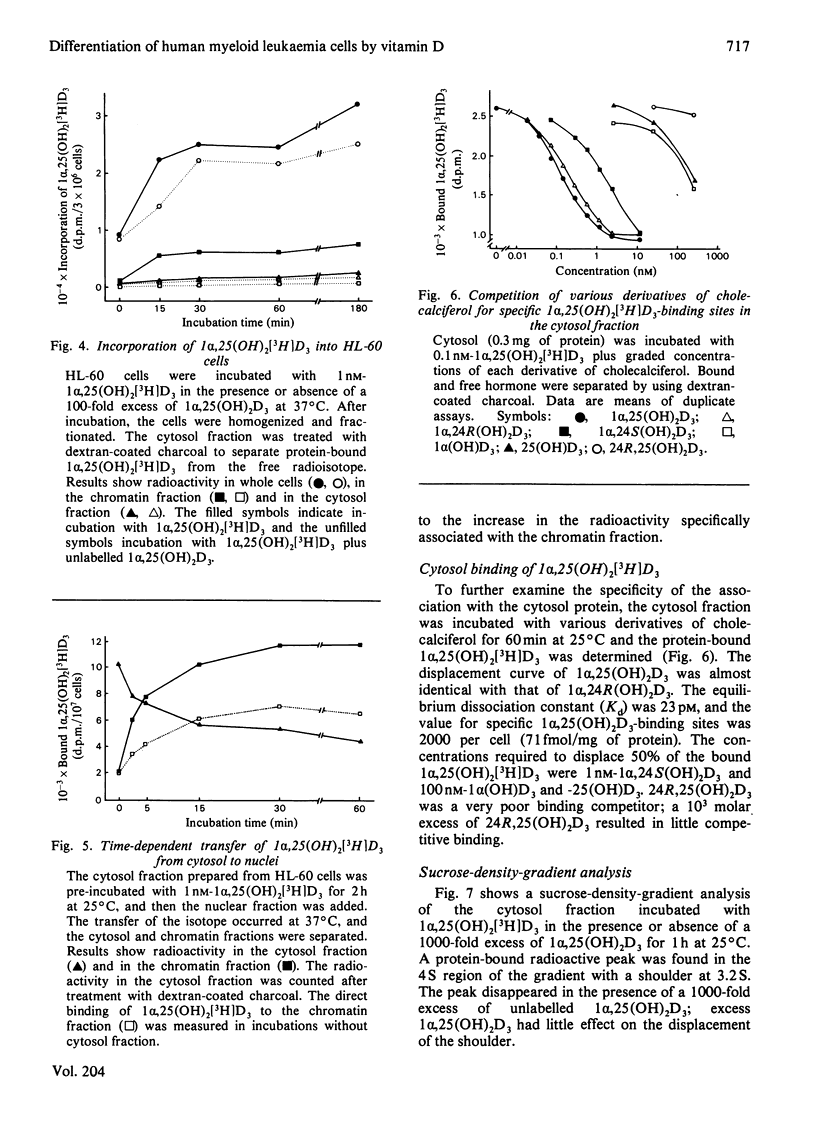
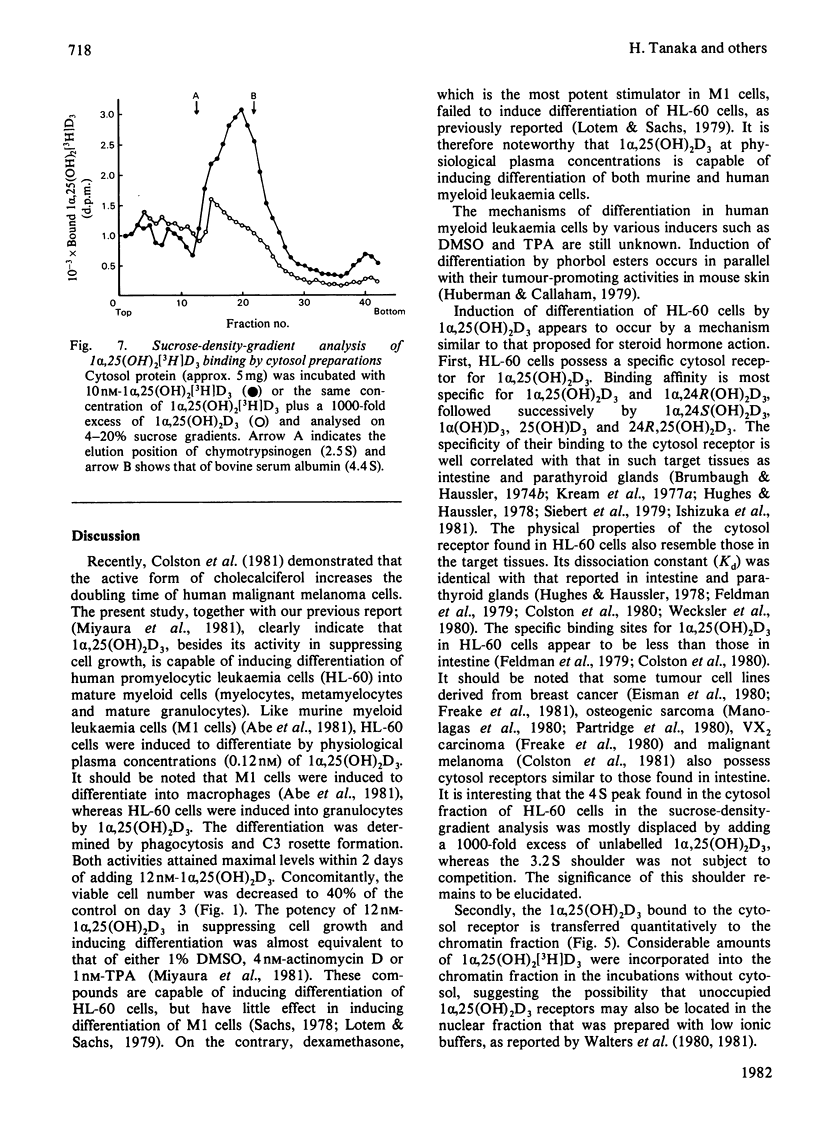
Human promyelocytic leukaemia cells (HL-60) can be induced to differentiate into mature granulocytes in vitro by 1 alpha,25-dihydroxycholecalciferol [1 alpha,25(OH)2D3], the active form of cholecalciferol. The differentiation-associated properties, such as phagocytosis and C3 rosette formation, were induced by as little as 0.12 nM-1 alpha,25(OH)2D3, and, at 12 nM, about half of the cells exhibited differentiation on day 3 of incubation. Concomitantly the viable cell number was decreased to less than half of the control. Among various derivatives of cholecalciferol examined, 1 alpha,25(OH)2D3 and 1 alpha,24R-dihydroxycholecalciferol were the most potent in inducing differentiation, followed successively by 1 alpha,24S-dihydroxycholecalciferol, 1 alpha-hydroxycholecalciferol, 25-hydroxycholecalciferol and 24R,25-dihydroxycholecalciferol. A cytosol protein specifically bound to 1 alpha,25 (OH)2D3 was found in HL-60 cells. Its physical properties closely resembled those found in such target tissues as intestine and parathyroid glands. 1 alpha,25(OH)2D3 bound to the cytosol receptor was transferred quantitatively to the chromatin fraction. The specificity of various derivatives of cholecalciferol in inducing differentiation was well correlated with that of their association with the cytosol receptor. These results are compatible with the hypothesis that the active form of cholecalciferol induces differentiation of human myeloid leukaemia cells by a mechanism similar to that proposed for the classical concept of steroid hormone action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974 Feb 25;249(4):1251–1257. [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. II. Temperature-dependent transfer of the hormone to chromatin via a specific cytosol receptor. J Biol Chem. 1974 Feb 25;249(4):1258–1262. [PubMed] [Google Scholar]
- Chandler J. S., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3 receptors in rat kidney cytosol. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1057–1063. doi: 10.1016/0006-291x(79)91933-8. [DOI] [PubMed] [Google Scholar]
- Christakos S., Norman A. W. Studies on the mode of action of calciferol. XVIII. Evidence for a specific high affinity binding protein for 1,25 dihydroxyvitamin D3 in chick kidney and pancreas. Biochem Biophys Res Commun. 1979 Jul 12;89(1):56–63. doi: 10.1016/0006-291x(79)90942-2. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston K., Colston M. J., Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981 Mar;108(3):1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- Colston K., Hirt M., Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980 Dec;107(6):1916–1922. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- Eil C., Marx S. J. Nuclear uptake of 1,25-dihydroxy[3H]cholecalciferol in dispersed fibroblasts cultured from normal human skin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2562–2566. doi: 10.1073/pnas.78.4.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman J. A., Martin T. J., MacIntyre I., Frampton R. J., Moseley J. M., Whitehead R. 1,25-Dihydroxyvitamin D3 receptor in a cultured human breast cancer cell line (MCF 7 cells). Biochem Biophys Res Commun. 1980 Mar 13;93(1):9–15. doi: 10.1016/s0006-291x(80)80238-5. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Hirst M., Colston K., Karasek M., Cone C. Demonstration of 1,25-dihydroxyvitamin D3 receptors in human skin biopsies. J Clin Endocrinol Metab. 1980 Dec;51(6):1463–1465. doi: 10.1210/jcem-51-6-1463. [DOI] [PubMed] [Google Scholar]
- Feldman D., McCain T. A., Hirst M. A., Chen T. L., Colston K. W. Characterization of a cytoplasmic receptor-like binder for 1 alpha, 25-dihydroxycholecalciferol in rat intestinal mucosa. J Biol Chem. 1979 Oct 25;254(20):10378–10384. [PubMed] [Google Scholar]
- Freake H. C., Marcocci C., Iwasaki J., MacIntyre I. 1,25-dihydroxyvitamin D3 specifically binds to a human breast cancer cell line (T47D) and stimulates growth. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1131–1138. doi: 10.1016/0006-291x(81)91565-5. [DOI] [PubMed] [Google Scholar]
- Freake H. C., Spanos E., Eisman J. A., Galasko C. S., Martin T. J., MacIntyre I. Specific binding of 1,25 dihydroxyvitamin D3 in the VX2 carcinoma. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1505–1511. doi: 10.1016/s0006-291x(80)80036-2. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Manolagas S. C., Deftos L. J. Evidence for a 1,25-dihydroxyvitamin D3 receptor-like macromolecule in rat pituitary. J Biol Chem. 1980 Jun 10;255(11):5007–5010. [PubMed] [Google Scholar]
- Honma Y., Takenaga K., Kasukabe T., Hozumi M. Induction of differentiation of cultured human promyelocytic leukemia cells by retinoids. Biochem Biophys Res Commun. 1980 Jul 31;95(2):507–512. doi: 10.1016/0006-291x(80)90813-x. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. R., Haussler M. R. 1,25-Dihydroxyvitamin D3 receptors in parathyroid glands. Preliminary characterization of cytoplasmic and nuclear binding components. J Biol Chem. 1978 Feb 25;253(4):1065–1073. [PubMed] [Google Scholar]
- Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1969 Dec;74(3):223–234. doi: 10.1002/jcp.1040740303. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Bannai K., Naruchi T., Hashimoto Y. Studies on the mechanism of action of 1 alpha, 24-dihydroxyvitamin D3. II. Specific binding of alpha, 24-dihydroxyvitamin D3 to chick intestinal receptor. Steroids. 1981 Jan;37(1):33–43. doi: 10.1016/0039-128x(81)90005-2. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Jose M. J., DeLuca H. F. The chick intestinal cytosol binding protein for 1,25-dihydroxyvitamin D3: a study of analog binding. Arch Biochem Biophys. 1977 Mar;179(2):462–468. doi: 10.1016/0003-9861(77)90134-5. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Jose M., Yamada S., DeLuca H. F. A specific high-affinity binding macromolecule for 1,25-dihydroxyvitamin D3 in fetal bone. Science. 1977 Sep 9;197(4308):1086–1088. doi: 10.1126/science.887939. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Induction of specific changes in the surface membrane of myeloid leukemic cells by steroid hormones. Int J Cancer. 1975 May 15;15(5):731–740. doi: 10.1002/ijc.2910150504. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas S. C., Haussler M. R., Deftos L. J. 1,25-Dihydroxyvitamin D3 receptor-like macromolecule in rat osteogenic sarcoma cell lines. J Biol Chem. 1980 May 25;255(10):4414–4417. [PubMed] [Google Scholar]
- Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981 Oct 15;102(3):937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- Moore G. E., Gerner R. E., Franklin H. A. Culture of normal human leukocytes. JAMA. 1967 Feb 20;199(8):519–524. [PubMed] [Google Scholar]
- Partridge N. C., Frampton R. J., Eisman J. A., Michelangeli V. P., Elms E., Bradley T. R., Martin T. J. Receptors for 1,25(OH)2-vitamin D3 enriched in cloned osteoblast-like rat osteogenic sarcoma cells. FEBS Lett. 1980 Jun 16;115(1):139–142. doi: 10.1016/0014-5793(80)80744-7. [DOI] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Ohnuma N., Norman A. W. Studies on the mode of action of calciferol. XIX. A 24R-hydroxyl-group can replace the 25-hydroxyl-group of 1 alpha, 25-dihydroxyvitamin D3 for optimal binding to the chick intestinal receptor. Biochem Biophys Res Commun. 1979 Dec 14;91(3):827–834. doi: 10.1016/0006-291x(79)91954-5. [DOI] [PubMed] [Google Scholar]
- Simpson R. U., DeLuca H. F. Characterization of a receptor-like protein for 1,25-dihydroxyvitamin D3 in rat skin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5822–5826. doi: 10.1073/pnas.77.10.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggles A. W., Spelsberg T. C., Glasser S. R., O'Malley B. W. Soluble complexes between steroid hormones and target-tissue receptors bind specifically to target-tissue chromatin. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1479–1482. doi: 10.1073/pnas.68.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Abe E., Tanabe R., Suda T. A high-affinity cytosol binding protein for 1 alpha,25-dihydroxycholecalciferol in the uterus of Japanese quail. Biochem J. 1980 Sep 15;190(3):513–518. doi: 10.1042/bj1900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Norman A. W. Apparent nuclear localization of unoccupied receptors for 1,25-dihydroxyvitamin D3. Biochem Biophys Res Commun. 1981 Feb 27;98(4):990–996. doi: 10.1016/0006-291x(81)91208-0. [DOI] [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Norman A. W. Unoccupied 1,25-dihydroxyvitamin D3 receptors. Nuclear/cytosol ratio depends on ionic strength. J Biol Chem. 1980 Jul 25;255(14):6799–6805. [PubMed] [Google Scholar]
- Wecksler W. R., Ross F. P., Mason R. S., Posen S., Norman A. W. Biochemical properties of the 1 alpha, 25-dihydroxyvitamin D3 cytoplasmic receptors from human and chick parathyroid glands. Arch Biochem Biophys. 1980 Apr 15;201(1):95–103. doi: 10.1016/0003-9861(80)90491-9. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Ross F. P., Norman A. W. Characterization of the 1 alpha,25-dihydroxyvitamin D3 receptor from rat intestinal cytosol. J Biol Chem. 1979 Oct 10;254(19):9488–9491. [PubMed] [Google Scholar]
- Zerwekh J. E., Lindell T. J., Haussler M. R. Increased intestinal chromatin template activity. Influence of 1alpha,25-dihydroxyvitamin D3 and hormone-receptor complexes. J Biol Chem. 1976 Apr 25;251(8):2388–2394. [PubMed] [Google Scholar]


