Abstract
Background:
The present study aimed to compare the efficacy, safety, and cost-effectiveness of quick penetrating solution (QPS) heparin, QPS diclofenac, and heparin gel in the prevention of superficial thrombophlebitis (ST).
Materials and Methods:
This randomized controlled trial was conducted after approval from the Institutional Ethics Committee and registration to Clinical Trial Registry of India. Patients of 18–60 years age, American Society of Anesthesiologists I/II, and who needed venous cannulation for at least 72 h were included in the study. Patients were randomly divided into three groups receiving study drugs (heparin gel, QPS heparin, and QPS diclofenac) every 8 hourly for a period of 72 h. Venous cannulation site was graded using the Visual Infusion Phlebitis Scale. Patients developing no ST, mean time to reach ST Grade 1 and 2, prevention of ST probability, and cost-effectiveness of interventions during the study period were assessed.
Results:
Out of 219 included patients, development of no ST in the study groups at 72 h of treatment were heparin gel (11%), QPS heparin (9.6%), and QPS diclofenac (2.7%). The mean time (hours) to develop any grade ST in the study arms was heparin gel (36.2 [11.9]), QPS heparin (40.0 [13.4]), and QPS diclofenac (37.0 [13.2]). The Kaplan–Meier analysis did not reveal significant differences for the prevention of any grade ST or severe ST in three treatment arms. The average cost-effectiveness ratio for preventing thrombophlebitis was 14.2 in heparin gel-, 13.2 in QPS heparin-, and 95.6 in QPS diclofenac-treated patients.
Conclusion:
Based on efficacy, safety, and cost-effectiveness, heparin gel or QPS heparin can be used to prevent ST due to intravenous cannulation in surgical patients. QPS diclofenac is not a cost-effective option to prevent ST.
Keywords: Diclofenac, heparin, quick penetrating solution diclofenac, quick penetrating solution heparin, quick penetrating solution, superficial thrombophlebitis, topical heparin
INTRODUCTION
Peripheral intravenous (IV) line cannulation is the most frequently performed procedure on all hospitalized patients.[1] An IV line may be put in place for many indications including fluid and antibiotic administration, blood transfusion, or drug administration. Infusion-associated phlebitis or superficial thrombophlebitis (ST) is the most frequent complication linked to IV cannulation.[2,3] In earlier studies, the incidence of ST varied from 5% to 70%.[4,5] Phlebitis can cause deep vein thrombosis if it persists. The association between ST and deep vein thrombosis has been found to be 6%–44% in previous studies.[4] Therefore, it is crucial to prevent ST to reduce patient discomfort and its complications.[6]
ST is characterized by the combination of thrombosis and inflammation in a superficial vein.[7] Drugs having anticoagulant and anti-inflammatory property could be effective in preventing and treating cannulation-associated ST. There have been many studies done for the effective treatment of ST using low-dose unfractionated heparin and low molecular weight heparin either topically or intravenously.[8,9,10] Studies have also been conducted to investigate the role of topical diclofenac in the treatment of ST.[11] It is suggested that if topical anticoagulants or anti-inflammatory agent is started prophylactically with cannula insertion, it can prevent or postpone ST more effectively.[12] Moreover, quick penetrating solution (QPS) of heparin and diclofenac are available. They assert efficient medication transport through the stratum corneum and thus suggested to be more effective.[13] Recently, few studies have been conducted for the prevention of ST and investigated the role of different preparation of topical heparin including QPS heparin, heparin gel/cream, and QPS diclofenac in its prevention.[13,14,15] These studies suggest trends of promising outcomes with QPS preparations in terms of prevention of ST and delaying its onset. Earlier studies conducted by Bansal et al.[14] compared placebo with QPS heparin in emergency surgical patients belonging to the American Society of Anesthesiologists (ASA) class I/II, while Akhileshwar and Singh[13] compared QPS heparin with QPS diclofenac among patients admitted to intensive care unit or surgery belonging to ASA class I/II/III. Both studies used different study populations. These studies had a high risk of bias in terms of lack of information about allocation concealment,[13,14] blinded assessment,[13,14] and prior trial registration (possibility of selective outcome reporting).[14] None of earlier studies had explored pharmacoeconomic aspect.[13,14] There is no randomized controlled trial conducted comparing QPS heparin and QPS diclofenac against common control heparin gel in a similar study population for the prevention of ST. The aim of the present study was to compare the efficacy, safety, and cost-effectiveness of QPS heparin, QPS diclofenac, and heparin gel in the prevention of ST.
MATERIALS AND METHODS
Study design
This single-site, parallel, 3-arm, 1:1:1 allocation ration, randomized, open-labeled controlled trial was conducted at All India Institute of Medical Sciences, Gorakhpur. The study protocol was approved by the Institutional Human Ethics Committee. Informed consent was obtained from all enrolled patients or legally authorized representatives. The clinical trial was registered with Clinical Trial Registry of India (CTRI/2022/04/041915).
Study participants
The patients who fulfilled the selection criteria and admitted to surgical wards between May 2022 and May 2023 were assessed for eligibility. Patients were included if they were newly admitted for any surgical intervention, between the ages of 18 and 60, in ASA classes I or II, and needed venous cannulation for at least 72 h. Patients were excluded if they were unwilling to participate, had preexisting phlebitis at any other cannulation site, or had a history of hypersensitivity to heparin or coagulation disorders. Patients who were unconscious or comatose, had signs of systemic infection and bacteremia, pregnant and lactating females, and receiving irritant IV drugs or anticoagulants were also excluded from the study.
Randomization and blinding
All included patients were randomized to treatment arms (heparin gel, QPS heparin, and QPS diclofenac) through computer-generated simple randomization (1:1:1 ratio). Using sealed, opaque envelopes, the allocation was concealed. The three study medications were available in different formulations, making blinding challenging for the study team’s nursing staff involved in medication administration and patients who were aware of the treatment allocation after the informed consent. Trained nursing staff who were not part of the investigator team assessed the patients for the development of thrombophlebitis. Treatment codes were not revealed to the outcome assessors and data entry team, who were blinded to the intervention.
Study procedures
After cleaning the site of cannulation (dorsum of either hand) with a surgical spirit swab, an 18-G cannula (B Braun Vasofix) was inserted under aseptic precaution. The nursing staff applied drug as per randomization code along the length of the cannula before securing the cannula with waterproof dressing (Tegaderm IV dressing) and that time was marked as “0” h. The nursing staff kept constant for the study purpose.
Patients in group TH received heparin gel 1 g (200 IU/g - Thrombophob gel, Zydus Lifesciences Ltd., India), group QH patients received QPS heparin 6–8 drops (1000 IU/Ml - Phlebotroy QPS, Troikaa Pharmaceuticals Ltd., India), and group QD patients received QPS diclofenac 10 sprays (4 mg/spray-Dynapar QPS, Troikaa Pharmaceuticals Ltd., India) every 8 hourly for a period of 72 h. A trained nurse assessor who was not aware of the treatment group evaluated the same location for phlebitis at 8, 16, 24, 32, 40, 48, 56, 64, and 72 h using the Visual Infusion Phlebitis Scale. The following data were recorded: demography, medications (past and current), local symptoms of ST (pain, tenderness, redness, local temperature, and venous induration), grades of phlebitis, its onset time, and adverse drug events. The ST was graded using the Visual Infusion Phlebitis Scale [Table 1].[16,17]
Table 1.
Visual infusion phlebitis scale
| Grade | Appearance of cannulation site | Stage and action required |
|---|---|---|
| 0 | Appears healthy | No signs of phlebitis |
| I | One of the following is evident | Possibly first signs of phlebitis |
| Slight pain near IV site or | ||
| Slight redness near IV site | ||
| II | Two of the following are evident | Discontinue the patient and recannulate at other site |
| Pain at IV site | ||
| Erythema around site | ||
| Swelling | ||
| III | All of the following signs are evident | Medium stage of phlebitis |
| Pain along path of cannula | Discontinue the patient and recannulate at other site as well as consider treatment of phlebitis | |
| Erythma around site | ||
| Induration | ||
| IV | All of the following signs are evident | Advanced stage of phlebitis or start of thrombophlebitis |
| Pain along path of cannula | Discontinue the patient and recannulate at other site as well as treat the thrombophlebitis | |
| Erythma around site | ||
| Induration | ||
| Palpable venous cord | ||
| V | All of the following signs are evident | Advanced stage thrombophlebitis. Discontinue the patient and recannulate at other site as well as treat the thrombophlebitis |
| Pain along path of cannula | ||
| Erythma around site | ||
| Induration | ||
| Palpable venous cord | ||
| Pyrexia |
Outcomes
The primary outcome of this study was to compare proportion of patients developing no ST (Grade 0) and first signs of infusion thrombophlebitis (Grade 1 and 2) at the end of 72 h of treatment period. The other outcomes were to compare mean time to reach infusion thrombophlebitis Grade 1 and 2 and prevention of thrombophlebitis probability during the 72 h of the treatment period. Number needed to treat (NNT) for the prevention of ST (Grade 0) and first signs of infusion thrombophlebitis (Grade 1 and 2) at the end of 72 h were calculated in three treatment arms. The pharmacoeconomic aspect was also explored in terms of comparing the cost-effectiveness of three treatment arms for the prevention of thrombophlebitis (any grade and severe) and the mean time to develop thrombophlebitis (any grade and severe). The cost-effectiveness grid was also illustrated to plot comparative cost (lower, same, and high) and effectiveness (lower, same, and high) outcomes. The cost (₹) of medications in December 2023 (https://www.mims.com/India) was used for the cost outcomes.
Statistical analysis
According to the previous studies, the prevalence of ST ranged from 5% to 70%.[4,5] We anticipated 50% of incidence of ST in the absence of any interventions. Null hypothesis (p[H0]) of clinically significant reduction of 15% was expected in each group. The alternative hypothesis (p[H1]) of 15% reduction in heparin gel, 20% in QPS diclofenac, and 30% reduction in QPS heparin was considered to calculate the sample size.[13,15] The other parameters considered were α error – 5% and power – 80%. The estimated sample size with G*power software (3.1.9.7) was 199. With the addition of 10% dropout rate, the estimated total sample size was 219.
The data were analyzed using Statistical Package for MedCalc® Statistical Software version 22.007 (MedCalc Software Ltd, Ostend, Belgium). The normality of continuous data was assessed by the Kolmogorov–Smirnov test. They were compared using unpaired t-test. The categorical data were compared using the Chi-square test. A Kaplan–Meier analysis was conducted to assess the prevention of any grade thrombophlebitis or severe thrombophlebitis (Grade 2 or above) in each group. P < 0.05 was considered statistically significant.
RESULTS
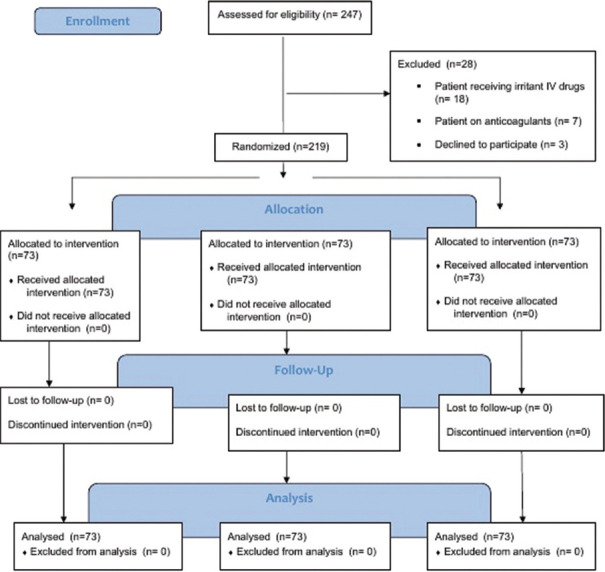
Out of 247 patients screened, 219 eligible patients were recruited between May 2022 and May 2023. The reasons for ineligibility were patients receiving irritant IV drugs (n = 18), anticoagulants (n = 7), and declined to participate (n = 3) [Figure 1].
Figure 1.
Consort flow diagram of patient enrolment
Patients’ baseline characteristics are given in Table 2. There was no difference in mean age of years in patients receiving heparin gel (37.6 ± 11.2), QPS heparin (34.2 ± 10.0), and QPS diclofenac (36.5 ± 10.6). All three treatment groups had comparable gender, body mass index, and ASA scores [Table 2].
Table 2.
Baseline characteristics of study participants
| Variables | Heparin gel (n=73) | QPS heparin (n=73) | QPS diclofenac (n=73) | P |
|---|---|---|---|---|
| Age (years) | 37.6 (11.2) | 34.2 (10.0) | 36.5 (10.6) | 0.14 |
| Female (%) | 31 (42.5) | 39 (53.4) | 39 (53.4) | 0.31 |
| BMI (kg/m2) | 23.4 (3.5) | 23.2 (2.9) | 24.2 (3.6) | 0.18 |
| ASA grade Grade 1 (%) | 73 (100) | 73 (100) | 72 (98.6) | 0.36 |
Values are expressed as mean±SD or n (%). QPS: Quick penetrating solution, ASA: American Society of Anesthesiologists, BMI: Body mass index
Proportion of patients developing no ST (Grade 0) within 72 h of IV treatment was 11% in heparin gel, 9.6% in QPS heparin, and 2.7% in QPS diclofenac group [Table 3]. Development of any type of thrombophlebitis (Grade 1/2 or above) was 89% in heparin gel, 90.4% in QPS heparin group, and 97.2% in QPS diclofenac. The incidence of Grade 0 and any type of thrombophlebitis (Grade 1/2 or above) was higher in QPS diclofenac-treated patients, but the difference was not significant [Table 3].
Table 3.
Comparison of development of thrombophlebitis at the end of 72 h in three treatment groups
| Grade of thrombophlebitis | Heparin gel (n=73) | QPS heparin (n=73) | QPS Diclofenac (n=73) | |||||
|---|---|---|---|---|---|---|---|---|
| Grade 0 | 8 (11.0) | 7 (9.6) | 2 (2.7) | |||||
| Grade 1 | 1 (1.4) | 0 | 2 (2.7) | |||||
| Grade 2 and above | 64 (87.7) | 66 (90.4) | 69 (94.5) | |||||
Values are expressed as n (%). QPS: Quick penetrating solution
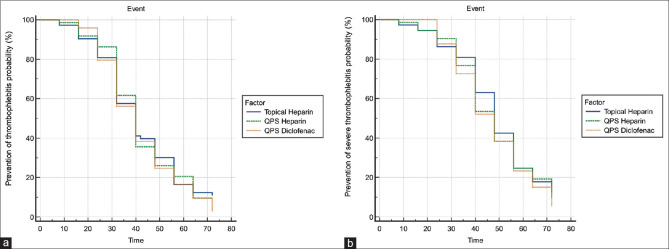
As shown in Figure 2, the Kaplan–Meier analysis did not reveal statistically significant differences for the prevention of any grade thrombophlebitis (long-rank test P = 0.62) or severe thrombophlebitis (long-rank test P = 0.55) at the end of 72 h in three treatment groups [Figure 2]. The mean time (hours) to develop ST of Grade 1 in heparin gel group was 36.2 (11.9) h, 40.0 (13.4) h in QPS heparin group, and 37.0 (13.2) h in QPS diclofenac group. Difference among all groups was statistically insignificant. The mean time (h) to develop ST of Grade 2 was 45.2 (15.2) h, 45.1 (15.3) h, and 45.7 (14.8) h in heparin gel, QPS heparin, and QPS diclofenac group, respectively [Table 4].
Figure 2.
(a) Prevention of thrombophlebitis probability using Kaplan–Meier survival analysis at the end of 72 h in three treatment groups. (b) Prevention of severe thrombophlebitis (Grade 2 and above) probability using Kaplan–Meier survival analysis at the end of 72 h in three treatment groups. QPS: Quick penetrating solution
Table 4.
Mean time (h) to develop superficial thrombophlebitis in three groups
| Grade of thrombophlebitis | Heparin gel | QPS heparin | QPS diclofenac | P | |||
|---|---|---|---|---|---|---|---|
| Grade 0 | 36.1±18.3 | 35.7±17.5 | 33.5±15.8 | 0.63 | |||
| Grade 1 | 36.2±11.9 | 40.0±13.4 | 37.0±13.2 | 0.49 | |||
| Grade 2 and above | 45.2±15.2 | 45.1±15.3 | 45.7±14.8 | 0.97 | |||
Values are expressed as mean±SD. QPS: Quick penetrating solution, SD: Standard deviation
NNT for the prevention of ST (Grade 0) at the end of 72 h was 10.0 in heparin gel-, 11.0 in QPS heparin-, and 37.0 in QPS diclofenac-treated patients [Table 5].
Table 5.
Number needed to treat for the prevention of thrombophlebitis at the end of 72 h in three treatment groups
| Variables | Heparin gel (95% CI) | QPS heparin (95% CI) | QPS diclofenac (95% CI) |
|---|---|---|---|
| NNT for any grade thrombophlebitis | 10.0 (5.5–26.4) | 11.0 (6.1–35.3) | 37.0 (−15.4–99.5) |
| NNT for Grade 2 and above thrombophlebitis | 9.0 (5.0–20.9) | 11.00 (6.1–35.3) | 19.0 (9.3–386.3) |
NNT: Number needed to treat, QPS: Quick penetrating solution, CI: Confidence interval
As shown in Table 6, the average cost-effectiveness ratio for the prevention of thrombophlebitis was 14.2 in heparin gel-, 13.2 in QPS heparin-, and 95.6 in QPS diclofenac-treated patients. The average cost-effectiveness ratio for mean time to develop thrombophlebitis was 4.33 in heparin gel-, 3.17 in QPS heparin-, and 6.97 in QPS diclofenac-treated patients. The cost-effectiveness grid was illustrated to demonstrate the cost-effectiveness of heparin gel and QPS heparin over QPS diclofenac [Figure 3].
Table 6.
Cost-effectiveness analysis of three treatment groups
| Outcome | Heparin gel | QPS heparin | QPS diclofenac |
|---|---|---|---|
| Cost-consequence analysis | |||
| Cost outcome per patient (₹) | 156.0 | 126.8 | 258.0 |
| Prevention of thrombophlebitis (%) | 11.0 | 9.6 | 2.7 |
| Prevention of severe thrombophlebitis (%) | 13.3 | 9.6 | 5.5 |
| Mean time to develop thrombophlebitis (h) | 36.0 | 40.0 | 37.0 |
| Mean time to develop severe thrombophlebitis (h) | 45.2 | 45.1 | 45.7 |
| Average cost-effectiveness ratio | |||
| Prevention of thrombophlebitis (₹/%) | 14.2 | 13.2 | 95.6 |
| Prevention of severe thrombophlebitis (₹/%) | 11.73 | 13.21 | 46.91 |
| Mean time to develop thrombophlebitis (₹/h) | 4.33 | 3.17 | 6.97 |
| Mean time to develop severe thrombophlebitis (₹/h) | 3.45 | 2.81 | 5.65 |
QPS: Quick penetrating solution
Figure 3.
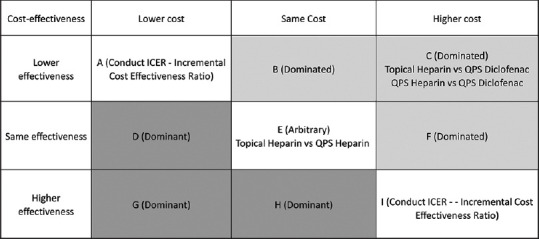
Cost-effectiveness grid for all three treatment pair comparisons. QPS: Quick penetrating solution
DISCUSSION
We found no significant differences regarding prevention of thrombophlebitis (any grade) or severe thrombophlebitis with heparin gel, QPS heparin, or QPS diclofenac. Prophylactic treatment with heparin gel, QPS heparin, or QPS diclofenac can delay the development of thrombophlebitis. Heparin gel and QPS heparin seem cost-effective over QPS diclofenac for the prevention of thrombophlebitis.
Heparin-based topical formulations showed a trend of better outcomes at a lesser cost as compared to diclofenac topical preparation in preventing the incidence of thrombophlebitis in surgical patients. Similarly, NNT patients for the prevention of ST was maximum in QPS diclofenac (37.0) as compared to heparin gel (10.0) and QPS heparin (11.0) [Table 5]. This is in line with a previous study. Akhileshwar and Singh in a randomized controlled trial compared QPS formulation of heparin and diclofenac in preventing thrombophlebitis and found QPS heparin is more effective than QPS diclofenac for the same.[13] In this study, 23% of patients developed Grade 1 thrombophlebitis in diclofenac QPS, but no patient had developed Grade 1 thrombophlebitis in QPS heparin (P = 0.034)-treated patients.[13]
Our findings of high incidence of thrombophlebitis in heparin gel, QPS heparin, and QPS diclofenac suggest that prophylactic use could mainly delay the development of thrombophlebitis and prophylactic agent could not completely prevent it. This is in line with the earlier study.[15]
Our findings suggest no advantage of quick penetrating technology in topical treatment with heparin or diclofenac to delay the onset or decrease the incidence of ST after cannulation. Average time to appear Grade 1 ST after cannulation was around 36–40 h in heparin gel-, QPS heparin-, and QPS diclofenac-treated patients. Average time to develop Grade 2 ST after cannulation was around 45 h in all three intervention groups in our study. The literature suggests that ST usually appears 12–36 h after cannulation.[18,19,20] Our findings of no difference in delay of onset of thrombophlebitis with heparin gel and QPS heparin are in line with a previous study done by Saini et al. In a study done by Saini et al., the mean time to develop Grade 1 thrombophlebitis (QPS heparin - 59.7 h vs. heparin gel - 58.46 h; P = 0.949) and Grade 2 (QPS heparin - 62.4 h vs. heparin gel - 61.17 h; P = 0.732) was comparable and nonsignificant in QPS heparin- and heparin gel-treated patients. However, our findings regarding no difference in the incidence of thrombophlebitis with heparin gel and QPS heparin are contradictory to this study.[15] Saini et al. compared these two preparations of heparin in 84 patients. They observed less incidence of ST at the end of the study period in QPS heparin- than heparin gel-treated patients (32.4% vs. 9.4%; P = 0.00019). Furthermore, the proportion of patients who developed and progressed to Grade 2 infusion-related phlebitis was significantly lesser in QPS heparin as compared to heparin gel (13.5% vs. 22.9%; P = 0.0279) treated patients.[15] An earlier study has demonstrated effectiveness of both preparation of heparin either gel or QPS as compared to no intervention in the prevention of ST.[14,21,22]
The present study has compared effectiveness and cost implications of gel and QPS-based medications in 219 patients. These newer preparations such as QPS heparin and QPS diclofenac developed by adding nonaqueous and nonvolatile solvents along with added permeability enhancers increase the penetration of the drug either heparin or diclofenac across the skin resulting in to more delivery of drug at the site of action. These drugs seem to be promising in better prevention of ST due to novel technology and few earlier studies have also found the same results. Use of QPS heparin helps heparin to penetrate the skin effectively without any systemic absorption or risk of bleeding.[23] Contradictory to previous studies, the present study does not report any significant benefit of either of the QPS preparations over heparin gel.
This study has highlighted the fact that either of the heparin preparation can be used for prevention of ST with the same efficacy safety and cost-effectiveness. Preference for selection of either of heparin ointment or QPS heparin can be decided depending upon other factors. QPS solutions allow hands-free application and thus improve compliance of patients as well as nursing staff.
Use of topical heparin is a standard practice for the treatment of ST and can be considered for prevention also. Use of QPS diclofenac for the prevention of ST can be considered in patients having contraindications of heparin use and are at high risk of developing thrombophlebitis like use of irritant drugs or chemotherapy as compared to no intervention.
This study has several limitations. This study included surgical patient cohort, so result of this study cannot be generalized for all group of patients. Confounding factors like individual body response to IV medications, frequency of stat drugs and IV infusions, and movements of hand due to dexterity of the patient could potentially affect the development of ST and thereby overall result of the study.
CONCLUSION
Based on efficacy, safety, and cost-effectiveness, either heparin gel or QPS heparin can be used to prevent ST due to IV cannulation in surgical patients. QPS diclofenac is not a cost-effective option to prevent ST.
Data availability
Full-trial protocol and dataset are available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank all the patients for their participation and the study team members including nursing officers (Ms. Vineeta and Ms. Nandini), Mr. Nitesh Sinha, and junior resident (Dr. Astha) who worked in collaboration with the authors to make this clinical trial possible. The authors would also like to thank Troikaa Pharmaceuticals Ltd., India, for making study drugs available for the study.
REFERENCES
- 1.Oliveria AS, Parreira PM. Nursing interventions and peripheral venous catheter related phlebitis- systemic literature review. Nursing. 2010;3:137–47. [Google Scholar]
- 2.Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114:845–54. doi: 10.7326/0003-4819-114-10-845. [DOI] [PubMed] [Google Scholar]
- 3.Uslusoy E, Mete S. Predisposing factors to phlebitis in patients with peripheral intravenous catheters: A descriptive study. J Am Acad Nurse Pract. 2008;20:172–80. doi: 10.1111/j.1745-7599.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 4.Macrone LS, Winston BY, Sidnei L. Superficial thrombophlebitis: Epidemiology, physiopathology, diagnosis and treatment. J Vasc Bras. 2008;7:131–43. [Google Scholar]
- 5.Jackson A. Infection control –A battle in vein: Infusion phlebitis. Nurs Times. 1998;94:68–71. [PubMed] [Google Scholar]
- 6.Singh N, Kalyan G, Kaur S, Jayashree M, Ghai S. Quality improvement initiative to reduce intravenous line-related infiltration and phlebitis incidence in pediatric emergency room. Indian J Crit Care Med. 2021;25:557–65. doi: 10.5005/jp-journals-10071-23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascella M, Viscardi D, Bifulco F, Cuomo A. Postoperative massive pulmonary embolism due to superficial vein thrombosis of the upper limb. J Clin Med Res. 2016;8:338–41. doi: 10.14740/jocmr2362w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcaro G, Cesarone MR, Dugall M, Feragalli B, Ippolito E, Corsi M, et al. Topical formulation of heparin is effective in reducing the symptoms of superficial venous thrombosis: A monocenter, observer-blind, placebo-controlled randomized study. Panminerva Med. 2011;53:3–11. [PubMed] [Google Scholar]
- 9.Subnis BM, Rao MR, Panchal VH, Lakhani RJ, Mehtalia B, Maroo SK, et al. Novel topical quick penetrating solution of Heparin in management of superficial thrombophlebitis: Results of randomized active controlled trial. Int J Pharm Sci Res. 2013;4:4442–7. [Google Scholar]
- 10.Vilardell M, Sabat D, Arnaiz JA, Bleda MJ, Castel JM, Laporte JR, et al. Topical heparin for the treatment of acute superficial phlebitis secondary to indwelling intravenous catheter. A double-blind, randomized, placebo-controlled trial. Eur J Clin Pharmacol. 1999;54:917–21. doi: 10.1007/s002280050575. [DOI] [PubMed] [Google Scholar]
- 11.Becherucci A, Bagilet D, Marenghini J, Diab M, Biancardi H. Effect of topical and oral diclofenac on superficial thrombophlebitis caused by intravenous infusion. Med Clin (Barc) 2000;114:371–3. doi: 10.1016/s0025-7753(00)71300-5. [DOI] [PubMed] [Google Scholar]
- 12.Arun Babu T, Sharmila V. Prophylactic topical heparin can prevent or postpone intravenous cannula induced superficial thrombophlebitis. Med Hypotheses. 2010;74:857–8. doi: 10.1016/j.mehy.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Akhilesh, Singh S. Comparison of heparin quick penetrating solution and diclofenac quick penetrating solution for the prevention of superficial thrombophlebitis caused by peripheral venous cannulation: A randomized double-blind study. Anesth Essays Res. 2019;13:155–7. doi: 10.4103/aer.AER_189_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal T, Kumar P, Vashisht G. A study to evaluate the efficacy of topical quick penetrating solution of heparin in preventing thrombophlebitis. Indian Anaesth Forum. 2019;20:95–8. [Google Scholar]
- 15.Saini V, Samra T, Ahuja N, Sethi S. A prospective randomized study to evaluate safety and efficacy of heparin topical solution (1000 IU/ml) compared to heparin topical gel (200 IU/g) in prevention of infusion-associated phlebitis. Indian J Pharmacol. 2018;50:344–9. doi: 10.4103/ijp.IJP_201_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallant P, Schultz AA. Evaluation of a visual infusion phlebitis scale for determining appropriate discontinuation of peripheral intravenous catheters. J Infus Nurs. 2006;29:338–45. doi: 10.1097/00129804-200611000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson J. Royal College of nursing's standards for infusion therapy: An overview. Br J Nurs. 2018;27:S12–4. doi: 10.12968/bjon.2018.27.2.S12. [DOI] [PubMed] [Google Scholar]
- 18.Di Nisio M, Peinemann F, Porreca E, Rutjes AW. Treatment for superficial infusion thrombophlebitis of the upper extremity. Cochrane Database Syst Rev. 2015;2015:CD011015. doi: 10.1002/14651858.CD011015.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaukroger PB, Roberts JG, Manners TA. Infusion thrombophlebitis: A prospective comparison of 645 Vialon and Teflon cannulae in anaesthetic and postoperative use. Anaesth Intensive Care. 1988;16:265–71. doi: 10.1177/0310057X8801600305. [DOI] [PubMed] [Google Scholar]
- 20.Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144:1373–5. [PubMed] [Google Scholar]
- 21.Agarwal S, Verma NC, Singam A. Efficacy of topical heparin in prevention of superficial thrombophlebitis before peripheral venous cannulation. J Evol Med Dent Sci. 2020;9:1734–8. [Google Scholar]
- 22.Pandya JM, Gupta S, Chouhan A, Shah H, Shah S, Jain A. Evaluation of safety and efficacy of quick penetrating heparin solution (1000 IU/ml) in prevention of intravenous cannula related thrombophlebitis: A prospective, randomized, comparative, parallel group clinical study. Indian J Anesth Analg. 2019;6:2129–32. [Google Scholar]
- 23.Vecchio C, Frisinghelli A. Topically applied heparins for the treatment of vascular disorders: A comprehensive review. Clin Drug Investig. 2008;28:603–14. doi: 10.2165/00044011-200828100-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full-trial protocol and dataset are available from the corresponding author on reasonable request.