Abstract
Current models of language processing do not address mechanisms at the neurotransmitter level, nor how pharmacologic agents may improve language function(s) in seemingly disparate disorders. L-Glutamate, the primary excitatory neurotransmitter in the human brain, is extensively involved in various higher cortical functions. We postulate that the physiologic role of L-Glutamate neurotransmission extends to the regulation of language access, comprehension, and production, and that disorders in glutamatergic transmission and circuitry contribute to the pathogenesis of neurodegenerative diseases and sporadic-onset language disorders such as the aphasic stroke syndromes. We start with a review of basic science data pertaining to various glutamate receptors in the CNS and ways that they may influence the physiological processes of language access and comprehension. We then focus on the dysregulation of glutamate neurotransmission in three conditions in which language dysfunction is prominent: Alzheimer’s Disease, Fragile X-associated Tremor/Ataxia Syndrome, and Aphasic Stroke Syndromes. Finally, we review the pharmacologic and electrophysiologic (event related brain potential or ERP) data pertaining to the role glutamate neurotransmission plays in language processing and disorders.
Keywords: Language, Glutamate Neurotransmission, Event-Related Potentials, Alzheimer’s Disease, Fragile X-associated Tremor/Ataxia Syndrome, Stroke Aphasia, Pharmacology
Our understanding of human language and language disorders has steadily increased in the past several decades. The advent of functional neuroimaging has significantly expanded upon the classical lesion-based neuroanatomical models of language processing to include widely-distributed functional language networks. However, these network models do not address language regulation at a neurotransmitter level, or how pharmacologic agents such as the NMDA antagonist memantine may improve language function(s) in disorders as seemingly disparate as Alzheimer’s disease and aphasic stroke syndromes.
In this article, intended for clinicians and scientists, we offer a complementary perspective to current network models by reviewing language processing and its regulation at the neurotransmitter level. We focus on the primary excitatory neurotransmitter in the human brain, L-Glutamate, and pharmacologic and electrophysiologic data pertaining to its role in language disorders. Glutamate neurotransmission has long been implicated in synaptic plasticity and memory formation1 (Traynelis 2010) and is extensively involved in various higher cortical functions. We postulate that the physiologic role of glutamate neurotransmission extends to the regulation of language access, comprehension, and production*, and that disorders in glutamatergic transmission and circuitry contribute to the pathogenesis of neurodegenerative and sporadic-onset language disorders such as the aphasic stroke syndromes.
In this review, we use ‘language processing’ to denote the general access, comprehension, and production of language. We define language ‘access’ as the retrieval of stored semantic and lexical information, and ‘comprehension’ as the ability to manipulate lexical and semantic linguistic components (e.g., syntax, context, and word meaning) to decode and interpret incoming information. Language ‘production’ is defined as the ability to generate novel phrases or sentences de novo or by manipulating existing clauses (motor aspects of speaking and writing are outside the scope of this discussion).
As there are no comparable animal or basic neuroscience models to establish a direct link between glutamatergic neurotransmission and language, we support their hypothesized association by combining clinical data with relevant basic science data (Section I) and data from preclinical investigations (Section II). We focus on three disorders in which language dysfunction is prominent: Alzheimer’s Disease (AD), Aphasic Stroke Syndromes, and Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) to make a case that dysregulation in glutamate neurotransmission, which seems to play a role in the pathogenesis of these diseases, also may be responsible for their disorders of language processing. We also evaluate clinical data from N-Methyl-D-Aspartate (NMDA) receptor antagonists memantine and ketamine in event-related brain potential (ERP) studies (Section III) showing that glutamate-modulating drugs may improve outcome in language disorders. We conclude with a brief discussion of future directions in evaluating the role of glutamate neurotransmission in language disorders and introduce other diseases and pharmacologic agents which also may prove useful in assessing this hypothesized association (Section IV).
To our knowledge, this is the first article to combine basic science, pre-clinical, and clinical data from ERP and pharmacologic studies linking glutamate neurotransmission to language processing and disorders. Our aim is to spur more research interest in the proposed association between glutamate and language, so as to provide new insights and more effective therapies for the language disorders.
*Glutamate’s involvement is particularly likely in distributed language network models. One early example is Quillian’s 1967 spreading activation model, in which semantic memory and search are conceptualized as a cortically spreading wave of excitation from two or more concept nodes in a semantic network; activation propagates until an intersection representing the concept/word of interest is found.2,3 (Quillian 1967, Collins & Loftus 1975). A similar model of “automatic spreading activation” suggests that a word or concept may prime a distributed network of semantically related concepts by spreading outward from a prime concept to the rest of the semantic network in a hub and spoke fashion. Kiefer gives the example of the word “sour” priming the word “lemon” and supports it with data from an N400 congruity paradigm.4 (Kiefer 2002) Although neither Quillian nor Kiefer mention neurotransmitters, as the most prominent excitatory neurotransmitter in the human cortex, glutamate is the ideal candidate for propagating such a wave of spreading activation.
1. Overview of Glutamate Receptors
Glutamate is the principal excitatory neurotransmitter in the central nervous system (CNS); it is involved in a variety of higher functions including memory, learning, and attention.1 (Traynelis 2010) The precise role of glutamatergic neurotransmission in language, however, has not been well established. This is in part because language is almost exclusively a human form of communication and research has been limited by the lack of an animal model. Despite these challenges, studies have begun to shed light on the role of glutamatergic neurotransmission in language. Here, we review the literature and posit that the physiologic regulation of glutamate neurotransmission is essential to the proper access, comprehension, and production of linguistic information.
We begin with a review of the four classes of glutamate receptors. We describe the putative mechanisms by which these receptors are involved in language processing using predominantly data from basic research. We will elaborate on these using specific disease models in section two.
1.1. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) Receptor
Endogenous glutamate receptors include three major families of glutamate-gated ion channels and a family of G protein-coupled metabotropic receptors that act through cytoplasmic secondary messengers or by direct influence of membrane-bound ion channels.5 (Meldrum 2000)
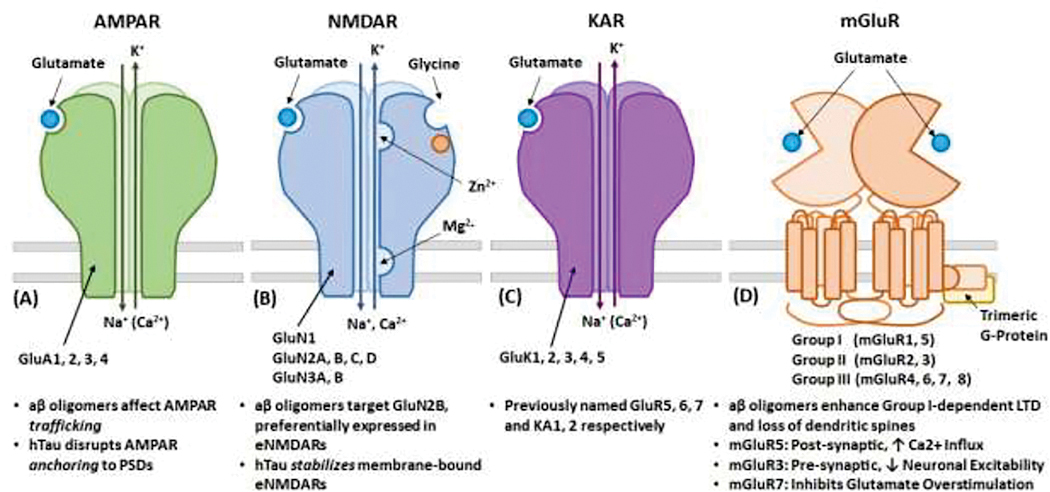
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) are ubiquitous in the adult human brain. They are the primary mediators of fast excitatory synaptic transmission in the CNS. AMPARs are predominantly located postsynaptically on pyramidal neurons and inhibitory interneurons, but may also be expressed on astrocytes.5 (Meldrum 2000) Structurally, AMPARs are tetrameric ligand-gated sodium (Na+) and potassium (K+) channels assembled from subunits GluA1, 2, 3, and 4. (Figure 1a) AMPAR subunit compositions (and therefore roles) vary based on anatomic location as well as stage of cortical development.
Figure 1. Glutamate Receptor Compositions.
(A) α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) Receptor (B) N-Methyl-D-Aspartate (NMDA) Receptor, (C) Kainate Receptor, and (D) Metabotropic Glutamate (mGlu) Receptor
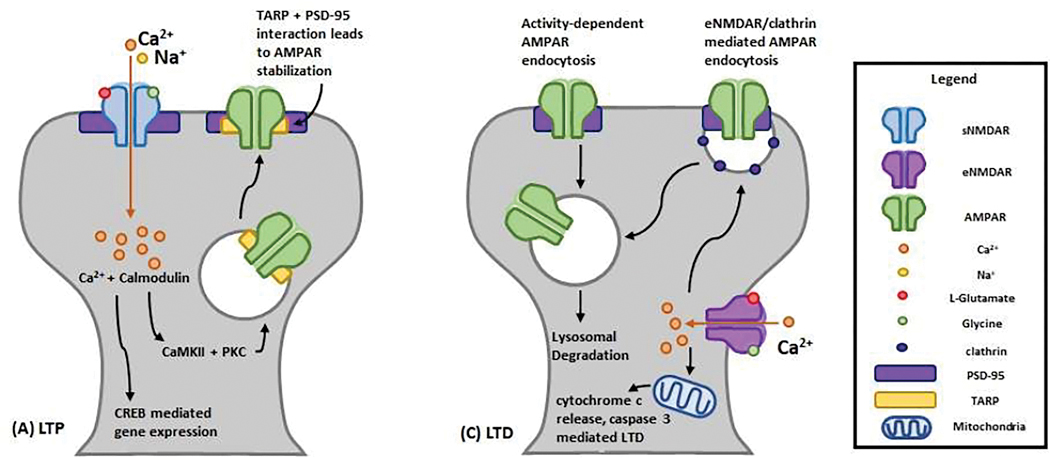
Learning induces changes in the number of AMPARs at synapses via synaptic plasticity. Activity-induced trafficking of AMPARs in and out of synapses form the basis of long-term potentiation (LTP), a persistent increase in synaptic strength, and long-term depression (LTD), a persistent decrease in synaptic strength. During the induction of LTP, AMPARs are inserted into the post-synaptic membrane from a combination of lateral diffusion from extrasynaptic sites and the exocytosis of AMPARs from intracellular membrane compartments.6 (Makino 2009 Secondary stabilization of newly inserted AMPARs on the postsynaptic membrane (and hence LTP stabilization) is achieved through interactions between activated transmembrane AMPAR regulatory proteins (TARPs) and scaffolding proteins in the postsynaptic density.7 (Chen 2000) AMPARs also undergo post-translational modifications at synapses; CaMKII and protein kinase A (PKA) can phosphorylate GluA1 subunits of synaptic AMPARs, increasing channel conductance and opening probabilities.8 (Banke 2000) Dephosphorylation of AMPARs leads to receptor endocytosis and lysosomal degradation in an activity-dependent manner, which is thought to play a role in LTD.9 (Fortin 2010) Together, these post-translational modifications and receptor trafficking mechanisms are crucial to the LTP and LTD processes.
Diseases that interrupt AMPAR trafficking or modification naturally disrupt homeostasis between LTP and LTD. For example, in Alzheimer’s disease synaptic AMPAR density is reduced; Aβ oligomers bind to AMPAR subunits disrupting receptor trafficking and hyperphosphorylated tau (hTau) disrupts receptor anchoring to post synaptic density proteins (PSD)10. (D’Amelio 2011)
1.1.1. Calcium-Permeable AMPA Receptors
Most AMPARs are impermeable to Ca2+ ions due to the presence of the GluA2 subunit. This is because the mRNA for the GluA2 subunit becomes edited post-transcriptionally resulting in a positively-charged arginine (R) in the ion channel that blocks Ca2+.11,12 (Huettner 2015, Wollmuth 2018) Thus, GluA2-lacking AMPARs are permeable to Ca2+ and have been identified in various brain regions.13 (Henley 2016) Although the precise function of these calcium-permeable AMPARs (CP-AMPARs) remains poorly understood, there is accumulating evidence for their roles in synaptic plasticity and pathologic disease states such as AD and ischemic stroke.
CP-AMPARs may be involved in the early phases of synaptic plasticity.14 (Shi 2001) Phosphorylation of membrane-bound, peri-synaptic CP-AMPAR leads to preferential integration at PSD sites and enhanced synaptic calcium entry, contributing to the induction of LTP.9,15 (Yang 2010, Fortin 2010) CP-AMPARs may also be involved in the induction of late phase-LTP, as Ca2+ entry via CP-AMPARs has been shown to trigger de novo protein synthesis of new AMPARs via a PI3K (phosphoinositide 3-kinases) and MAPK (mitogen-activated protein kinase) mediated pathway.16 (Park 2018) In the lateral amygdala, physiologic increases in CP-AMPAR expression may play a role in the consolidation of emotional (but not neutral) memories and memory retrieval and updating.17 (Torquatto 2019)
On the other hand, excessive increases in CP-AMPAR activity contribute to the pathogenesis of multiple diseases such as AD, ischemic/hypoxic brain injury, fragile X syndrome, and epilepsy.18 (Twomey 2018) In early Alzheimer’s disease, intracellular oligomeric Aβ may induce overexpression of synaptic CP-AMPARs, which may lead to excitotoxicity. 19 (Whitcomb 2015) This may be further amplified by disruption of Ca2+ homeostasis, resulting in accelerated disease progression. Epidemiologic research has demonstrated a correlation between low serum vitamin D (specifically 25-Hydroxyvitamin D) levels and increased risk of Alzheimer’s Disease and cognitive decline with aging.20 (Miller 2015) Although the pathogenic link between vitamin D and AD is not fully understood, disruption of Ca2+ homoeostasis may be one among a host of potential mechanisms. Vitamin D is integral to maintaining Ca2+ homeostasis in addition to regulating certain epigenetic processes21 (Berridge 2016) and low serum vitamin D levels can lead to dysregulation of intracellular Ca2+ in neurons as well as accompanying glial cells.22 (Brawek 2014)
In ischemic stroke, increased CP-AMPAR expression increases neuronal oxidative stress contributing to post-stroke excitotoxicity, cell damage, and degeneration.18,23 (Noh 2005, Twomey 2018) These mechanisms likely work in concert with NMDAR dysregulation and contribute to the pathogenesis of language dysfunction in these and other diseases. More on this in the upcoming Alzheimer’s Disease and Ischemic Stroke Syndromes sections.
1.2. N-Methyl-D-Aspartate (NMDA) Receptors
N-Methyl-D-Aspartate receptors (NMDAR) are ligand-gated calcium (Ca2+) channels that are crucially involved in the regulation of synaptic strength. NMDARs are heterotetramers of two obligatory GluN1 subunits and two additional regulatory subunits. These regulatory subunits are primarily GluN2 subunits, of which there are four genes GluN2 (A, B, C, or D), or GluN3 (A or B) 24 (Sanz-Clemente 2012) (Figure 1b) Like AMPARs, NMDAR function is dictated by its subunit composition, and expression of specific subunits is based on CNS location and changes throughout stages of brain development from embryo to adulthood, as well as synaptic versus extrasynaptic location.25 (Henson 2008)
In addition to ligand binding, NMDAR function is regulated by voltage as the channel pore is blocked at rest by a Mg2+ ion removed only upon postsynaptic depolarization24 (Sanz-Clemente 2012), allowing NMDARs to act as a coincidence detector for both presynaptic and postsynaptic activity. NMDARs are also unique in being the only receptor to require a co-ligand, which can be either glycine or D-serine, though the mechanisms regulating co-ligand availability are not well understood.24 (Sanz-Clemente 2012) Due to these additional gating mechanisms, NMDARs possess a higher activation threshold than other glutamate receptors, but once activated they trigger a cascade of downstream effects that mediate synaptic plasticity and help maintain neuronal health, though overactivation and influx of Ca2+ can lead to excitotoxicity.
1.2.1. Synaptic NMDARs
At most excitatory synapses in the brain, NMDAR activation is important for both LTP and LTD. This bidirectional regulation of synaptic strength is thought to be the neurochemical basis of learning and memory.26 (Broutman 2001) (Figure 2a) Synaptic NMDARs (sNMDARs) are clustered in large macromolecular signaling complexes, held together by scaffolding proteins such as postsynaptic density-95 (PSD-95). Activation of sNMDARs results in an influx of Ca2+ into the dendritic spine, triggering various intracellular signaling cascades, notably, Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC)-mediated pathways, as well as changes in the number of postsynaptic AMPARs.27 (Lau 2009)
Figure 2. LTP and LTD.
N-Methyl-D-Aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) Receptor- mediated (A) Long-Term Potentiation (LTP) and (B) Long-Term Depression (LTD).
A separate but related role for sNMDARs is to promote activity-dependent neuronal survival and protection against cell death. Synaptic NMDAR-dependent Ca2+ influx promotes gene transcription via activation of the transcription factor cyclic-AMP responsive element binding (CREB) protein which in turn upregulates pro-cell survival genes such as brain-derived neurotrophic factor (BDNF).28 (Hardingham 2010) BDNF has been implicated in rescuing neurons from NMDAR blockade-induced neuronal death and in regulating all phases (induction, maintenance, and synaptic consolidation) of LTP.29 (Panja 2014) The sNMDAR-CREB pathway also upregulates the expression of activity-regulated inhibitors of death (AID) genes, which may render mitochondria more resistant to cellular stress and toxic insults.30 (Zhang 2009)
Synaptic NMDARs also suppress expression of pro-apoptotic mechanisms in a CREB-independent manner. One such mechanism is via suppression of the pro-apoptotic Bcl2 homology domain 3 (BH3)-only member gene (Puma), which is upstream of a cytochrome C release-mediated, p53-independent, apoptotic cascade.31 (Leveille 2010) Another sNMDAR pathway leads to suppression of the transcription factor forkhead box protein O (FOXO), which promotes neuronal death following excitotoxic injury and oxidative stress.32 (Dick 2010).
These transcription-dependent changes work in concert with the receptor level-changes to maintain activity-dependent synapses and in turn, promotes consolidation of LTP and memory. Failure to maintain synaptic connections may lead to a breakdown of globally-distributed cortical networks, including those responsible for language storage and access; such disruptions may present clinically as a failure to retrieve relevant semantic information.
1.2.2. Extrasynaptic NMDARs
In contrast to synaptic NMDARs, extrasynaptic NMDARs (eNMDAR) are typically located in clusters at the peri-synaptic zone or the dendritic shaft, usually in a contact area between the dendrite and adjacent astrocytes.33 (Petralia 2010) eNMDARs preferentially express the GluN2B subunit, which as we discuss later, may be a target for beta-amyloid (Aβ) oligomers in Alzheimer’s Disease.34,35 (Papouin 2014, Ferreira 2012) From an electrophysiological perspective, eNMDARs were initially defined as NMDARs not activated by low frequency afferent stimulation, or lacking in response to spontaneous glutamate release producing miniature EPSPs.34 (Papouin 2014) This distinction is not entirely accurate* but is useful in thinking of eNMDAR activation as the product of excessive synaptic stimulation and the resultant glutamate spillover at synaptic junctions.
Selective activation of eNMDARs has been associated with inducing LTD via signaling cascades that lead to destabilization of AMPAR-binding membrane anchoring proteins and clathrin-mediated AMPAR endocytosis.36,37 (Liu 2013, Collingridge 2010) (Figure 2b) eNMDAR activation also seems to favor neuronal death by suppressing pro-survival gene expression - inactivating CREB and extracellular signal-regulated kinases Erk1/234 (Papouin 2014) and promoting apoptotic cell-death signaling through upregulation of caspase-3.28 (Hardingham 2010)
That there is a receptor dedicated to promoting neuronal death and attenuating synaptic transmission seems counterintuitive, but this pathway may serve as a last-ditch effort to prevent runaway propagation of excitotoxic impulses throughout the CNS by curtailing aberrantly firing synapses at its source. Indeed, data suggest that extrasynaptic NMDARs trigger glutamate-induced cell death only when co-activated with pathologically activated synaptic NMDARs (e.g. by excessive synaptic stimulation), but that isolated eNMDAR activation does not result in excitotoxicity.38 (Zhou 2015) This seems to support the idea of eNMDAR-associated neuronal death as a fail-safe mechanism that specifically affects neurons at risk for excessive activation.
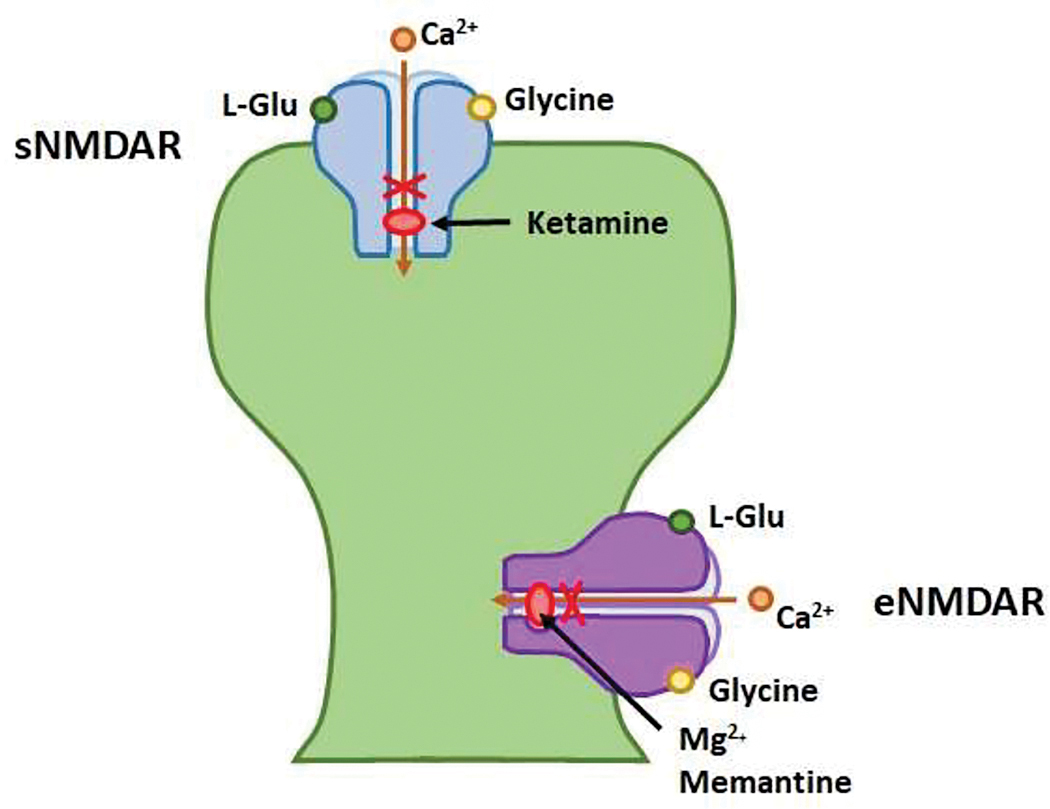
Taken together, NMDARs may contribute to global language processing and the integrity of semantic information storage and retrieval: activation of synaptic NMDARs is largely beneficial to neuronal survival and maintenance of LTP, whereas extrasynaptic NMDAR activation plays an antithetical role, promoting LTD and neuronal death. It is reasonable to assume that in certain disease processes, selective suppression of eNMDARs is neuroprotective and ameliorates or prevents symptom progression, e.g. language deficits. This hypothesis provides a basis for clinical studies using the selective NMDAR antagonists, ketamine and memantine. Both agents are noncompetitive open-channel blockers of NMDARs; memantine possesses a low affinity for the Mg2+ binding pocket and is purportedly selective for extrasynaptic receptors.39 (Lipton 2004) (Figure 3) These studies are reviewed in Part III – Pharmacologic and ERP Evidence from Clinical Research.
Figure 3. Binding site of NMDAR antagonists Memantine and Ketamine.
Memantine has a low affinity for the Mg2+ binding site and is thought to be relatively selective for extrasynaptic NMDAR receptors.
*In practice, eNMDARs possess a tonic and a phasic calcium current. The tonic low-amplitude Ca2+ influx current is regulated by glial-dependent modulation and reuptake of ambient extrasynaptic glutamate.40 (Fleming 2011) The phasic slow inward large-amplitude Ca2+ current is thought to be induced by vesicular release of glutamate from astrocytes into the extrasynaptic space, possibly mediated by astrocytic metabotropic glutamate receptors.41 (Fellin 2004) This implies a substantially more complicated role for physiologic firing and regulation of eNMDAR than as a excitotoxic failsafe, and as such is an area of active on-going research, some of which we will touch on in subsequent sections.
1.3. Kainate Receptors
Kainate, or Kainic Acid Receptors (KAR) are the third family of ionotropic glutamate receptors, named after the receptor agonist Kainate, a neurotoxin isolated from the red algae Diagenea simplex used in epilepsy research.42 (Laycock 1989) Compared to fast excitatory signaling by AMPARs, synaptic KARs’ excitatory post-synaptic currents (EPSC) possess slower decay kinetics, which make them especially well-suited for regulation of metaplasticity (via temporal and amplitude modulation of pre- to post-synaptic firing patterns).43–45 (Castillo 1997, Yang 2007, Sachidhanandam 2009)
Structurally, KARs are tetramers composed of the subunits GluK1, GluK2, GluK3, GluK4, and GluK5 (previously named GluR5-GluR7 and KA1 and KA2, respectively). (Figure 1c) Of these subunits, GluK1, GluK2, and GluK3 can form functional homomeric and heteromeric receptors, but GluK4 and GluK5 require one of the other subunits to form functional receptors. Subunit composition and function of receptors are highly location specific and compartmentalized; CA3 and CA1 KARs produce vastly different effects.46 (Carta 2014)
Synaptic KARs at mossy fiber to pyramidal cell synapses in layer CA3 of the hippocampus act as excitatory ionotropic receptors at physiologic levels of glutamate release. However, with higher frequency stimulation, synaptic KARs act metabotropically, potentiating EPSPs by suppressing the inhibitory K+ afterhyperpolarization current via a protein kinase C (PKC)-mediated signaling pathway.47 (Ruiz 2005) In this synaptic coupling, KAR’s slow decay kinetics allows it to act as a conditional amplifier for inputs to CA3 pyramidal cells, leading to modulation of short-term plasticity in the setting of repetitive stimulation by mossy fibers.44 (Sachidhanandam 2009)
Presynaptic KARs on the other hand, induce γ-aminobutyric acid (GABA) release from CA1 interneurons under physiologic conditions (e.g. ambient glutamate spillover), likely via a metabotropic signaling pathway, producing small inhibitory postsynaptic currents in postsynaptic pyramidal cells.48 (Jiang 2001) Under higher intensity stimulation, KAR activation results in short term facilitation of IPSPs.49 (Lourenco 2010) In this GABAergic synaptic coupling, KAR’s slow decay kinetics allows it to reset the phase of spontaneously firing CA1 interneurons43 (Yang 2007), which may play a critical role in modulating neuronal network oscillations and information transfer.46,50 (Goldin 2007, Carta 2014)
How KAR regulation at the synaptic level affects the hippocampus, both in physiologic and disease states is an area of active research. Consistent with KAR’s role in metaplasticity and fine tuning of synaptic firing patterns, GluK2 knockout mice, with affected CA3 KARs and therefore dentate gyrus to CA3 synapses, have demonstrated moderate memory deficits in pattern completion and spatial tuning of CA1 cells.51 (Nakashiba 2008) CA3 KARs have been implicated in the modulation of cortical gamma oscillations in laboratory conditions and therefore may play a role in modulating and/or propagating thalamic oscillatory activity.52 (Fisahn 2004) The relationship between KARs and language has not been established. However, gamma-range oscillatory activity has been implicated in binding different sensory representations into a cohesive semantic memory, including object-word associations.53 (Hart 2007)
1.4. Metabotropic Glutamate (mGlu) Receptors
Metabotropic glutamate receptors (mGluRs) are dimeric class-C G protein-coupled receptors (GPCR) found throughout the central and peripheral nervous systems and peripheral organs. There are eight receptor subtypes (mGluR1–8) each with its own splice variants, broken into 3 groups based on sequence homology, G-protein coupling, and ligand selectivity.54 (Niswender 2010) Here we briefly discuss characteristics of each group and focus on mGluR3, 5, and 7 given their role in synaptic plasticity and neurodegenerative diseases. (Figure 1d)
Group I receptors include mGluR1 and 5, are generally located post-synaptically, and are linked to the regulation of synaptic plasticity via activation of mammalian target of rapamycin (mTOR) and ERK pathways.55 (Page 2006) mGluR5 is expressed preferentially in the hippocampus, cortex, and striatum56 (Romano 1995) and has been shown to be functionally linked to the GluN2 subunit of NMDARs via Homer and PSD proteins.57 (Attucci 2001) Activation of mGluR5 leads to phosphorylation of linked NMDARs and potentiation of Ca2+ influx.57 (Attucci 2001) mGluR5 is also involved in striatal synaptic plasticity underlying habit memory and motor learning58 (Gubellini 2004) and is linked to increasing NMDAR excitability and conductance in the indirect basal ganglia loop/pathway.59 (Awad 2000) Conversely, Group I mGluR-dependent LTD can be induced by low frequency pair-pulse stimulation, resulting in postsynaptic AMPAR endocytosis via a PKC mediated pathway.60 (Luscher 2011) mGluR-dependent LTD is thought to play a physiologic role in encoding novelty and hippocampal learning60 (Luscher 2011), but may be pathologically enhanced in Alzheimer’s Disease by soluble Aβ oligomers, leading to shrinkage and loss of dendritic spines.61 (Li 2009)
mGluR5s on astrocytes have been associated with vesicular release of glutamate and regulation of phasic slow inward calcium currents in extrasynaptic NMDARs. There is evidence that this type of channel activation can occur synchronously across multiple neurons in the hippocampus and does so in an oscillatory fashion based on astrocytic intracellular Ca2+ concentrations.62 (Fellin 2004) mGluR5s may therefore contribute to the propagation of thalamic gamma oscillations in a GABA receptor-independent manner63 (Whittington 1995), which (as mentioned in the KAR section) may play a role in maintaining the binding of different aspects of semantic memory, including object-word associations.53 (Hart 2007)
Group II receptors, which include mGluR2 and 3, are known to modulate synaptic plasticity by reducing neuronal excitability.64,65 (Hermes 2011, Davidson 2016) Presynaptic mGluR2/3 generally act as autoreceptors that decrease the probability of glutamate release by inhibiting voltage-gated calcium channels.66 (Nicoletti 2011) Postsynaptic group II mGluRs can inhibit depolarization by activating G protein‐coupled inwardly rectifying K+ channels (GIRKs).67 (Knoflach 1998) Glial mGluR3 in particular has been associated with neuroprotection against NMDAR-associated excitotoxicity, possibly through production of pro-survival factors in astrocytes including transforming growth factor-β (TGF-β).68 (Corti 2007)
Group III receptors include mGluR4, 6, 7, and 8, are localized presynaptically at the active zone of neurotransmitter release, and are involved in autoregulation of synaptic glutamate concentration and release.66 (Nicoletti 2011) mGluR7 is unique due to its comparatively low affinity for glutamate compared to the rest of Group III receptors and has been proposed to serve as a feedforward inhibitor of glutamate overstimulation.54 (Niswender 2010) Under high frequency stimulation, mGluR7 interacts with presynaptic GABA and adenosine receptors, suppressing presynaptic Ca2+ influx and glutamate-vesicle release, subsequently facilitating LTD in the hippocampus.69 (Pelkey 2005) More recent studies have suggested a positive regulatory role for mGluR7 as well. Under targeted activation, mGluR7 was shown to facilitate presynaptic glutamate release through a separate G-protein coupled mechanism involving phospholipase C70 (Martin 2010) and may be essential to the induction of LTP at Schaffer collateral-CA1 synapses.71 (Gogliotti 2017) In addition to AD, mGluRs as a class are implicated in various neurologic and psychiatric diseases, including genetic epilepsies, Fragile X Syndrome, Parkinson’s Disease, and schizophrenia.
In the broadest terms, AMPARs are essential for fast excitatory transmission and expression of LTP and LTD. NMDARs are responsible for the induction of LTP and LTD, the regulation of neuronal health and apoptosis, and the propagation of excitotoxic damage. KAR and mGluR are associated with the maintenance of synchronous neuronal oscillations and metaplasticity (i.e. the fine tuning of plasticity processes via timing and amplitude of receptor activation).72 (Abraham 2008) In the following section, we build on this foundation to demonstrate the role glutamate receptors may play in the pathogenesis of AD, FXTAS, and ischemic strokes. We posit that receptor dysfunction is associated with the development of language disorders, and that physiologic glutamate transmission through these receptors plays a key role in the regulation and maintenance of language processing.
2. Disorders of Language and Glutamate in Three Conditions (AD, Ischemic Stroke, FXTAS)
In this section, we review the preclinical data for language dysfunction and disorders of glutamate neurotransmission in Alzheimer’s disease, Aphasic Stroke Syndromes, and Fragile X-associated tremor/ataxia syndrome (FXTAS). Alzheimer’s disease is perhaps the most thoroughly studied of these three and provides a neurodegenerative model of an insidious language decline. Aphasic stroke syndromes have been categorized extensively by their various forms of language dysfunction, but the role of glutamate neurotransmission therein is less well understood. Finally, FXTAS is a relatively newly described disorder. While FXTAS is thought to be a neurodegenerative disorder, synaptic level changes may start early in neurodevelopment. Most studies referenced in this section provide pre-clinical data from in-vitro and animal models; for clinical pharmacologic and ERP studies involving these diseases, please refer to the section titled Clinical Evidence in Pharmacologic and ERP Studies.
2.1. Alzheimer’s Disease
Alzheimer’s Disease (AD) is a progressive disease characterized by prominent memory and learning impairments, generally followed by language, visuospatial, and executive dysfunction.73 (McKhann 2011) Language dysfunction can occur in all stages of AD, although a breakdown in verbal fluency and naming together with normal articulation and syntactic abilities can precede diagnosis by years, manifesting during prodromal AD and perhaps even the preclinical stages.74,75 (Auriacombe 2006, Taler and Phillips 2008)
It is postulated that early language dysfunction in AD is due to semantic memory breakdown, rather than a nonspecific consequence of global cognitive decline76 (Verma 2012), and can occur independently of changes in mini-mental state examination (MMSE) scores.77 (Chan 2001) Naming and comprehension, as demonstrated by decreased performance in object naming (e.g. on Boston Naming Tests (BNT)) and semantic fluency (category fluency)78 (Henry 2004) decline at a faster rate than phonemic fluency79 (Clark 2009).
Language degradation in AD occurs in a hierarchical manner; early errors tend to be superordinate replacements (e.g. using the generic “bird” instead of a more specific “dodo”) rather than intrusions (e.g. listing “carpenter” in an animal naming task) or repetitions (e.g. using the word “oyster” twice in the same list). There is a gradual erosion of peripheral followed by core concepts: language forms learned last deteriorate first (e.g. an AD patient may have a harder time articulating or processing the concept of “death and taxes” compared to a more central concept such as “hunger”).80 (Auriacombe 2006) Some early AD patients also demonstrate a breakdown of conceptual boundaries leading to a loosening of category associations (e.g. “cafeteria” becomes associated with “noon” because of the association with eating and having lunch.).81 (Laisney 2011)
The semantic errors in AD likely represent a breakdown in semantic stores and impaired access and search function.76 (Verma 2012). The neuroanatomic distribution for the semantic language network is an area of active research: there is evidence for a left-hemisphere predominant, widely distributed cortical semantic network82 (Joubert 2010) (more on the distributed model in the thalamic aphasia section), modality specific regions acting in parallel83 (Binder 2009), and amodal representations localized within bilateral temporal lobes84 (Lambon Ralph 2013). These models are in part supported by functional MRI (fMRI) studies in early AD patients. Olichney et al. reported decreased activation of the left medial (and lateral) temporal lobe in fMRI word repetition/semantic category decision tasks85 (Olichney 2010) and Peelle et al. demonstrated reduced activation of bilateral ventral temporal cortices and the left dorsolateral prefrontal cortex in mild AD patients during a fMRI semantic judgment task86. (Peelle 2014)
2.1.1. NMDAR in Alzheimer’s Disease
AD is a disease characterized by the accumulation of extracellular beta-amyloid (Aβ) plaques and hyperphosphorylated Tau-proteins within the central nervous system. Pathologic changes can occur years to decades before the onset of clinical symptoms.87 (Danysz 2012)
Oligomeric Aβ has been shown to interact with GluN2B subunits which are preferentially expressed on extrasynaptic NMDARs (eNMDAR). Activation of extrasynaptic NMDARs inhibits CREB-mediated pro-survival pathways and blocks BDNF gene expression, decreasing cellular resiliency against neurotoxicity.88,89 (Hardingham 2002, Li 2011) eNMDAR activation also causes sustained rather than transient mitochondrial calcium (Ca2+) elevations, which leads to mitochondrial dysfunction, cytochrome c release, ATP depletion and failure of energy production, and oxidative stress leading to cell death.90,91 (Bading 2017, Zhang 2016) Increased intracellular Ca2+ also directly promotes genomic death responses through cytosol to nucleus translocation of Forkhead transcription factor FoxO3a32 (Dick 2010) and promotes beta-secretase activity and cleavage of amyloid precursor protein (APP) into Aβ, creating a positive feedback loop of runaway glutamate associated excitotoxicity.35,92 (Ferreira 2012, Rush 2014)
Aβ oligomers promote LTD via endocytosis and decreased expression of synaptic NMDA receptors through striatal-enriched phosphatase 61 (STEP)93 (Snyder 2005) and proteasome mediated EphB2 degradation94 (Hu 2017). This may lead to reduced sNMDAR-associated BDNF activation and downstream delivery of GluA4 subunit containing AMPARs to synapses, and acquisition of classic conditioned responses95 (Li 2009)
Although the total number of synaptic NMDARs are reduced, Aβ fibrils may still facilitate excitotoxicity by enhancing the activity and sensitivity of the remaining sNMDARs through β-integrin/Src Kinase activation.96 (Uhasz 2010) Intrasynaptic Aβ fibrils further cause mild membrane depolarization in a receptor independent fashion, which may partially relieve NMDAR from their Mg2+ block, resulting in receptor hyperexcitability (and may contribute to the increased incidence of late life-onset seizures in AD patients).97 (Minkeviciene 2009) In light of this, endocytosis of sNMDAR and associated LTD may serve a physiological rather than pathological role as a limited, last-ditch neuroprotective attempt against Aβ-mediated excitotoxicity.
Hyperphosphorylated Tau-proteins also may contribute to Aβ and glutamate-mediated excitotoxicity by stabilizing membrane bound extrasynaptic NMDA receptors. hTau is mistargeted and accumulates in the somatodendritic compartment of neurons and is critical for the localization of tyrosine-kinase Fyn to dendritic spines. Fyn phosphorylates and stabilizes the GluN2B subunit with PSD-95, and further facilitates interaction between Aβ and GluN2B-containing NMDARs, promoting downstream excitotoxicity (that is rescued by knockout Tau), but without directly affecting synaptic NMDAR-mediated EPSCs.*98 (Ittner 2010)
*A recent phase 2a clinical trial investigated the effects of the Fyn kinase inhibitor saracatanib (AZD0530) in AD patients with daily treatments for 52 weeks versus a placebo. It failed to detect significant effects on the rate of cerebral glucose metabolism decline in AD-related regions of interest or cognitive decline (as scored by ADAS-Cog, MMSE, etc). However, secondary volumetric MRI analysis did detect a trend toward slowing of hippocampal volume decline in the study group compared to placebo.99 (van Dyck 2018)
2.1.2. AMPAR in Alzheimer’s Disease
Synaptic AMPAR density and hence AMPA mediated LTP is attenuated by Aβ. Aβ protein accumulation has been shown to reduce AMPAR-mediated EPSC and hippocampal dendritic spine density, leading to memory impairments in mice models through direct interaction with GluA1/2 subunit containing AMPAR.10 (D’Amelio 2011). Some proposed mechanisms underlying these changes include loss of synaptic AMPAR via interference of non-canonical wnt signaling pathways100 (Cerpa 2010) and clathrin-dependent endocytosis of AMPAR by preferential binding of Aβ-oligomeric assemblies to the GluA2 (calcium-impermeable) subunit101 (Zhao 2010).
In contrast, rapid synaptic insertion of CP-AMPARs has been found to be induced by intracellular Aβ oligomers through selective protein kinase A (PKA) phosphorylation of the GluA1 subunit. Increased CP-AMPAR density led to increased AMPAR mediated EPSCs, leading to the hypothesis that intracellular Aβ oligomer neurotoxicity in early stages of AD is in part mediated by AMPAR associated excitotoxicity.19 (Whitcomb 2015)
Aβ oligomers may contribute to network-wide hyperexcitability and decreased seizure threshold through a presynaptic mechanism. This is thought to be due to Aβ-induced GABAergic disinhibition at the level of interneurons, leading to increased synchronous firing at excitatory synapses, rather than a direct effect on AMPA or NMDA receptors.102 (Palop and Mucke 2010)
AMPAR trafficking and anchoring (and synaptic function) may be disrupted by hyperphosphorylated tau (hTau) in dendritic spines, potentially in a PSD-95 associated fashion similar to its effect on NMDARs.103 (Hoover 2010) Aβ activation of Caspase-3, and downstream phosphorylation of tau proteins by glycogen synthase kinase-3β (GSK) have been demonstrated to attenuate AMPA mediated LTP, positing a three-way interaction between Aβ, hTau, and glutamate receptors.10,104 (D’Amelio 2011, Jo 2011).
2.1.3. KAR in Alzheimer’s Disease
No clear association has been made between KARs and Aβ or hTau. However, KARs have been shown to modulate EPSPs at CA3 mossy-fiber pyramidal cell synapses, which demonstrate a unique NMDAR-independent form of presynaptic short and long-term potentiation.24 (Nicoll 2005) Further understanding of this process may open venues for compensatory therapies to offset the loss of NMDAR-mediated synaptic plasticity.
Alternatively, KAR-mediated LTD via membrane receptor endocytosis105 (Chamberlain 2013) and transcription level alterations106 (Evans 2017) have been demonstrated, but how this effect regulates synaptic circuits in the mature hippocampus is not fully understood. If KAR endocytosis can reduce post-synaptic cellular excitability from repeated presynaptic stimulation105 (Chamberlain 2013), then antagonism at KAR receptors may improve neuronal resiliency against excitotoxicity.
2.1.4. mGluR in Alzheimer’s Disease
Migration of postsynaptic mGluR between synaptic and extrasynaptic locations is impaired by Aβ binding to neuronal membranes, resulting in receptor clustering at excitatory neuronal synapses. Activation of pathologically clustered mGluR5 results in sharp rises of intracellular Ca2+, ultimately contributing to synaptic dysfunction and NMDAR dysregulation.107 (Renner 2010) Activation of presynaptic mGluR2 triggers selective release of highly aggregating Aβ42 (but not Aβ40) peptides from nerve terminals; and chronic mGluR2 suppression is associated with a reduction in amnestic behavior and decreased levels of brain Aβ peptides in transgenic mice models.108 (Kim 2014) In contrast, glial mGluR3 activation appears to be neuroprotective by increasing secretion of TGF-β and paracrine-mediated induction of cyclin-dependent kinase inhibitors and anti-apoptotic cascades.68,109 (Caraci 2011, Corti 2007)
Collectively, interactions between Aβ and hTau and glutamate receptors can attenuate LTP, increase LTD, destabilize synaptic integrity, and induce neurotoxicity and cell death.110 (Hu 2012) (Table 1) We postulate that this dysregulation in glutamate receptor homeostasis plays a key role in early semantic dysfunction in AD. Indeed, genome-wide association studies have revealed a link between worsening language performance and GLI3 allelic variants in patients at risk for and with AD (GLI family zinc finger 3, a developmental transcription factor in patterning brain structures), language performance in this population was also found to be associated with in pathways involving glutamate receptor trafficking and function.111 (Deters 2017)
Table 1.
Glutamate Neurotransmission in Alzheimer’s Disease
| NMDAR | AMPAR | KAR | mGluR | |
|---|---|---|---|---|
|
|
||||
| Beta Amyloid (Aβ) | ||||
| Selective eNMDAR activation through GluN2b interaction. • Decreased Resilience Against Neurotoxicity by inhibiting CREB and BDNF expression. • Enhances NMDAR activity and sensitivity via β-integrin/Src Kinase, promoting excitotoxicity. |
Rapid membrane insertion of CP-AMPAR via PKA phosphorylation of GluA1. • Excitotoxicity in early stages of AD. Loss of synaptic AMPAR via interference of non-canonical wnt signaling pathways and clathrin-dependent endocytosis of receptors. |
No clear association demonstrated but may provide compensatory therapies in the future.
NMDAR-independent presynaptic STP and LTP. KAR endocytosis may reduce post-synaptic cellular excitability from repeated presynaptic stimulation. |
mGluR5 clustering at excitatory neuronal synapses. • Rise in intracellular Ca2+ and synaptic dysfunction. • NMDAR dysregulation. Presynaptic mGluR2 activation Selective release of highly aggregating Aβ42 |
|
| • Promotes Mitochondrial Dysfunction via sustained Mitochondrial Ca2+ current. • Promotes Genomic Death Responses via FoxO3a. • Promotes Cleavage of APP into Aβ. Endocytosis and decreased expression of sNMDAR via STEP and EphB2 degradation. • Promotes LTD |
• Reduction of AMPA-mediated EPSC, attenuating LTP. • Decrease Dendritic Spine Density • Memory Impairments Aβ induced Caspase-3 activation leads to downstream phosphorylation of Tau. |
|||
| Hyperphosphorylated Tau (hTau) | ||||
| Stabilizes membrane bound eNMDAR via Fyn pathway. Facilitates Aβ and NMDAR interactions leading to excitotoxicity. |
Disrupts AMPAR trafficking and membrane anchoring. • Attenuates LTP |
Selective activation of mGluR3 may be neuroprotective against Aβ-driven apoptotic cascade by inducing TGF-β secretion and cyclin-dependent kinase inhibitors. | ||
NMDAR = N-Methyl-D-Aspartate receptors, AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, KAR = Kainate receptors, mGluR = metabotropic glutamate receptors, eNMDAR = extrasynaptic NMDAR, sNMDAR = synaptic NMDAR, CP-AMPAR = Calcium Permeable AMPAR, EPSC = Excitatory Post-Synaptic Currents, STP = Short Term Potentiation, LTP = Long Term Potentiation
Some potential mechanisms underlying the semantic dysfunction include disruption of the distributed cortical language network through excitotoxicity and cell death, leading to disrupted synaptic integrity and a failure to access relevant semantic associations — particularly at semantic integration centers with high concentrations of NMDAR and AMPAR (e.g., the hippocampus112 (Piai 2016)) In the upcoming sections, we examine the clinical evidence for this glutamate hypothesis in ERP and pharmacologic studies.
Imbalance between glutamate and the inhibitory neurotransmitter GABA also may contribute to semantic dysfunction. Indeed, higher concentrations of GABA in the left anterior temporal lobe (ATL) of healthy subjects have been associated with improved semantic performance (increased accuracy) and efficiency (lower task-induced ATL activity) in a semantic association task.113 (Jung 2017). Similarly, higher GABA to glutamate concentration ratio in the left lateral prefrontal cortex was associated with reduced reaction time in a word selection task.114 (Vega 2014) Glutamate-GABA (excitatory/inhibitory) imbalance may therefore lead to hindered word selection and semantic processing; it is also possible that impaired GABAergic inhibitory frameworks may lead to the loosening of semantic associations seen in AD.115 (Amieva 2004)
2.2. Aphasic Stroke Syndromes
The classic neuroanatomical models of language are founded on lesion studies of aphasic stroke patients. Compared to neurodegenerative diseases such as AD, acquired language disorders due to ischemic strokes serve as relatively discrete events in time and space, which offer important insights into the effects of glutamatergic neurotransmission on language. Here we focus on Broca’s and Wernicke’s aphasia as classic examples of expressive and receptive language deficits, respectively, and subcortical aphasia to highlight some aspects of anomic and mixed-transcortical aphasias. The role of glutamate in stroke aphasia has not been studied based on specific aphasia subtypes, therefore, additional aphasia syndromes including global, conduction, and transcortical expressive and receptive aphasias will not be individually discussed.
2.2.1. Broca’s Aphasia
In 1861, French surgeon Pierre Paul Broca first noted, “in all probability, both hemispheres collaborated in language and could more or less substitute for each other according to the conditions, although the third frontal convolution of the left hemisphere was the principal site of the function.”116 (Berker 1986) Classic Broca’s Aphasia (BA), due to a lesion in Brodmann’s Areas 44 and 45, presents as dense expressive aphasia with varying degrees of (less appreciated) language comprehension deficits, such as the processing of syntactic ambiguity.117 (Kirshner 2016) “The old man the boat” is an example of a syntactically ambiguous phrase, the word “old” can serve as either an adjective or a noun, and the word “man’ can be either a noun or a verb. The ‘best’ interpretation would read similarly to “the old (people) man the boat; however, patients with BA would have difficulty arriving at such an interpretation, if they succeeded at all.
Wassenaar and Hagoort suggest that these comprehension deficits may arise from an impairment in real time sentence and syntax construction rather than loss of linguistic knowledge.118 (Wassenaar 2005) Deriving from a computational lexical-parsing model, syntactic processing can be thought of as the building up of binding links of sufficient strength between competing word forms, until one set of interpretations prevails (“winning binding frame”).119 (Hagoort 2003) BA patients are capable of initiating this so-called syntactic unification process. However, when confronted with a syntactically complex or ambiguous sentence, they are unable to create the winning binding link of sufficient strength in an adequate timeframe, because the lexical store, although intact, is not readily available. This can lead to parsing errors and syntactic violations or a complete failure to bind with a lexical frame despite initiation attempts, presenting clinically as delayed and/or erroneous language comprehension of syntactic information.118 (Wassenaar and Hagoort 2005)
2.2.2. Wernicke’s Aphasia
Wernicke’s Aphasia (WA) is a predominantly sensory/receptive aphasia characterized by severe deficits in auditory language comprehension and repetition with relatively fluent, albeit largely meaningless, language production.120 (Hillis 2007) Comprehension deficits in Wernicke’s Aphasia are primarily a combination of acoustic-phonologic and lexical-semantic errors, and can include nonverbal processes such as color-to-picture matching, picture-to-picture matching, and drawing from memory.121 (Robson 2012)
Lesions in the pSTG and surrounding areas, particularly the superior temporal sulcus, are thought to be responsible for acoustic-phonologic errors (e.g. impaired repetition and auditory discrimination).121 (Robson 2012) The level of impaired auditory discrimination in WA patients has been shown to be sensitive to the degree of verbal comprehension impairments, whereas no correlations have been found between nonverbal semantic impairments and auditory comprehension. This suggests that impaired phonologic analysis of speech is likely the primary cause of comprehension deficits and that auditory and non-auditory language comprehension, while often found in conjunction, are to some extent distinct processes in WA.122 (Robson 2012)
Based on recent studies of chronic WA patients123 (Ogar 2011), functional neuroimaging83 (Binder 2009), and diffusion tensor imaging (DTI)124 (Saur 2010), lesions in the pMTG are now thought to be the primary source of semantic comprehension impairment in WA. Damage to pMTG can lead to a dysregulation of modal percepts (e.g. visual features, olfactory, somatosensory, etc) associated with a concept, resulting in semantically inappropriate responses. For example, a WA patient may say “I forked my hair” instead of “I combed my hair”, because of an erroneous association between the visual percept of a fork’s tines and the teeth of a comb.
In addition, studies have demonstrated that language performance in WA, which was otherwise insensitive to changes in word familiarity/frequency and naming performance on the Boston Naming Test (BNT), improved considerably with phonemic cues.125 (Jefferies and Ralph 2006) This supports the hypothesis that the core semantic representations in the anterior temporal gyrus, which acts an amodal hub among various cortical association areas, are spared in WA (lesions in the anterior temporal gyrus are primarily associated with semantic dementia).123,126 (Robson 2014, Ogar 2011)
Taken together, these findings suggest that language comprehension deficits in WA reflect issues with access at the level of auditory discrimination and to intact modal precepts, and not with semantic knowledge stores, which are preserved.127 (Thompson 2015)
2.2.3. Subcortical Aphasia
Since the days of Broca and Wernicke, our understanding of language processes has expanded to include a network of cortical and subcortical structures hypothesized to encompass different components of language comprehension and production. In the topographically distributed model of semantic language formation, the thalamus acts as a putative binder of different aspects of semantic information.128 (Kraut 2003) Bilateral thalamic depth electrode and scalp recordings during a semantic memory recall task demonstrate a decrease in theta range power but an increase in the power of spatially specific, phase-locked gamma oscillations in the thalamus and occipital leads, suggesting that thalamus may drive the formation of semantic object associations and semantic memory recall.129 (Slotnick 2002) This finding is consistent with the idea that thalamic relay neurons modulate selective engagement by switching between gamma rhythm-associated active engagement during language processing and a relatively disengaged state that minimizes attention to irrelevant stimuli.130 (Nadeau 1997)
In thalamic aphasia, semantic paraphasias (e.g. substituting the word “cat” with “dog”) and anomia predominate; semantic word-finding can be severely affected such that verbal output deteriorates to jargon similar to the presentation of acute WA, while auditory-verbal comprehension and repetition remain relatively intact.131 (Crosson 2013) These thalamic lesions can result in decreased neuronal metabolism and cerebral blood flow in anatomically connected language cortices, a la von Monakow’s connectional diaschisis theory, further supporting the thalamus as a hub for cortically-distributed language processes.130,132 (Nadeau 1997, Carrera 2014) Furthermore, cortical infarcts due to middle cerebral artery occlusions can result in a reduction of neurons and severe gliosis in ipsilateral, but not contralateral, thalamic nuclei133 (Qü 1998), suggesting that thalamic disruption or disconnection may play a role in cortical aphasic syndromes as well.
The basal ganglia (BG) is another subcortical structure thought to play a role in language functions, specifically, in fine tuning lexical search and selection from existing lexical stores by modulating a cortico-striatal-pallido-thalamic (CSPT) circuit.131 (Crosson 2013) Patients with chronic BG infarcts are not typically impaired on most classical tests of aphasia (e.g. the Western Aphasia Battery (WAB)) but do demonstrate difficulties with more complex language functions such as resolving meanings of semantically ambiguous passages and producing word lists, definitions, synonyms, and antonyms.134 (Copland 2000)
Crosson hypothesizes that the BG modulates signal-to-noise ratios during frontal lobe-mediated word selection tasks, allowing words to be produced more quickly and with fewer errors.131 (Crosson 2013) Adapting Nambu’s model of a cortical-BG loop, Crosson describes the frontal lobe-BG interaction as follows: Upon initiation of a new word selection task, the hyperdirect BG loop first suppresses any active thalamic language connections, turning off any primed lexical-semantic networks from prior tasks. The direct BG loop then assists in the selection of a new semantic concept by enhancing the signal-to-noise ratio of the most contextually appropriate lexical item. Finally, the indirect BG loop reduces the activity level of competing semantic alternatives so that only the relevant semantic concept is selected.135,136 (Nambu 2004, Bohsali & Crosson 2016) Thus, damage to the BG may lead to difficulty with complex language tasks that demand high efficiency in lexical selection whereas simple language processes such as those tested by the WAB may be spared.
2.2.4. Receptor Fingerprints, Language Networks, and Stroke
Despite the differences in topography and symptoms of the aphasic stroke syndromes, there may be a common link in the form of glutamate receptor expression and density. Balance between the densities of different neurotransmitter receptors, including the glutamate receptor subtypes and glutamate to GABA receptor ratios, form patterns unique to specific cortical functions which can span discontinuous regions, which Zilles et al. termed “receptor fingerprint”.137 (Zilles 2002).
Select left hemispheric language regions (including the inferior frontal gyrus and the pars opercularis in Broca’s region and the pSTG in Wernicke’s region) have been shown to have similar receptor fingerprints despite cytoarchitectural differences and topographical distributions. These receptor fingerprints were different from the three primary sensory regions, including at the level of kainite receptor density. In the right hemisphere, structures within Broca’s region demonstrated a cluster of similar receptor densities, including NMDARs, forming a receptor fingerprint that differed from ipsilateral (right hemispheric) motor mouth, prefrontal, and temporal regions. Additional structures involved in language processing, including Brodmann’s areas 7, 9, 46, and 32 and regions of the fusiform gyrus, also shared receptor fingerprint similarities.138 (Zilles 2015)
It is conceivable that alterations in a region’s receptor density/fingerprint, also may affect its function. For example, changes in glutamate receptor expression in a language-related structure post stroke, even without gross structural injury, may still affect language processing. In the following sections, we review how glutamate receptor expression is altered in the lesion and perilesional regions during the acute133,139,140 (Qü a 1998, Pellegrini 1992, Liu 2007) and chronic phases141 (López-Valdés 2014) following a stroke. Strokes in or near language processing areas may alter the region’s receptor fingerprint, even in perilesional regions with no neuronal cell death (i.e. the ischemic penumbra). It is also possible that changes in glutamate receptor density in structures involved in distributed language networks such as the temporal-frontal network, may contribute to language processing deficits on a more global scale.
2.2.5. NMDAR in Aphasic Stroke Syndromes
Following an ischemic stroke, NMDARs are upregulated in the cortical rim surrounding the ischemic core (i.e. the penumbra) (along with a down-regulation of GABA receptors). This upregulation of excitatory receptors localizes to the superficial cortical layers II-IV, which include the supragranular layers where NMDAR-associated neurons are preferentially situated.133 (Qü a 1998) Receptor upregulation may acutely contribute to NMDAR-associated excitotoxicity, and administration of NMDAR antagonists (MK-801 and memantine) within 30–45 minutes after a penetrating vessel occlusion significantly reduces microinfarct volumes in acute stroke. (this is also a likely mechanism to explain some therapeutic consequences of NMDAR modulation in vascular dementia142 (Barbancho 2015)).143 (Shih 2013)
Abnormal glutamate activity extends beyond the acute phase of ischemic strokes; NMDAR antagonism by memantine for 28 days following cortical middle cerebral artery occlusion has been shown to decrease reactive astrogliosis and increase vascular density around the infarcted cortex.141 (López-Valdés 2014) In contrast, there is also evidence of increased BDNF pathway signaling in the peri-infarct cortex (likely associated with NMDAR activation). The BDNF pathway has been associated with promoting activity-dependent neuron survival and synaptogenesis and protects against excitotoxicity through downstream activation of antioxidant enzymes such as Mn-SOD and anti-apoptoic proteins such as Bcl-2.144 (Mattson 2008)
NMDARs are also implicated in cortico-subthalamic nucleus (STN) transmission, which is likely closely related to a CSPT circuit via a STN to globus pallidus glutamatergic synapse.131 (Crosson 2013) Blockade of NMDAR in the STN reduces early excitation of the globus pallidus interna (GPi) and prevents downstream suppression of thalamocortical activity.145 (Nambu 2000) NMDAR dysregulation in this setting may lead to language dysfunction due to impaired cortico-basal ganglia loops causing increased noise-to-signal during word selection tasks, inadequate suppression of competing semantic alternatives, and/or deficits in set-switching between new words or concepts.131 (Crosson 2013)
2.2.6. AMPAR in Aphasic Stroke Syndromes
Ischemic injury to neurons induces a change in AMPAR expression at synaptic sites within 24 hours of injury. In the hippocampus, GluA2-containing calcium impermeable AMPARs are dissociated from PSD sites and endocytosed through the physiologic clathrin-mediated pathway. At the same time, CP-AMPARs become preferentially expressed at the receptor surface, leading to increased amplitudes of miniature EPSCs.140 (Liu 2007) Moreover, GluA2 mRNA expression is also preferentially reduced compared to GluA1 and GluA3 within 24 hours of ischemic insult, indicating transcriptional level changes in addition to alterations in receptor trafficking.139 (Pellegrini 1992)
How much of these changes in AMPAR expression is independent of NMDAR dysregulation is unclear. However, studies have demonstrated that selective blockade of CP-AMPAR is neuroprotective in ischemic stroke models23 (Noh 2005), indicating that direct rescue of AMPAR homeostasis may be beneficial to ameliorating post-stroke excitotoxic injury.
2.2.7. in Aphasic Stroke Syndromes
Kainate receptors have so far not been identified in structures implicated in subcortical aphasic strokes (e.g., the thalamus). However, hippocampal layer-CA3 KARs have been implicated in the modulation of cortical gamma oscillations in laboratory conditions52 (Fisahn 2004), oscillations which may be associated with subcortical language functions.
As noted in the subcortical aphasia section, spatially specific, phase-locked gamma-range oscillations recorded in the thalamus and occipital leads increase in power during semantic memory tasks; this has led to the hypothesis that the thalamus may bind different aspects of semantic information.53,146 (Hart 2007, Slotnick 2002) Moreover, Nadeau hypothesized that thalamic relay neurons may alternate between selective engagement and a relatively disengaged state by switching between theta and gamma frequency oscillations.130 (Nadeau 1997)
Taken together these findings raise the possibility that hippocampal KARs may act in tandem with thalamus-driven gamma range oscillations, to recruit the hippocampus as a part of a globally distributed language network, and/or to maintain selective engagement of hippocampus during semantic recall/language tasks. KAR dysfunction in the hippocampus also may be associated with language dysfunction in hippocampal sclerosis and medial temporal lobe epilepsy147 (Hamberger 2007).
2.2.8. mGluR in Aphasic Stroke Syndromes
mGluR1 mediates post-synaptic activation in thalamic relay neurons and demonstrates high levels of receptor expression in this cell population. Furthermore, cortico-thalamic input may be enhanced by modulating mGluR1 activation; and tonic activation of mGluR1 may potentiate synaptic NMDAR response.148 (Salt 2012) Although these data are derived from a purely somatosensory model, it is plausible to infer that mGluR may play a similar role in the topographically distributed, cortico-thalamo-cortical model of language processing, and that modulation of mGluR may have an effect in thalamic aphasia syndromes.
Taken together, cortical and subcortical aphasic syndromes are likely linked by a distributed network of semantic and lexical representations. Failure to activate and bind the appropriate lexical-semantic constructs leads to various presentations of aphasia in Broca’s, Wernicke’s, and Subcortical Aphasic syndromes. NMDAR, CP-AMPAR, and mGluR may play a role in modulating language deficits by promoting synaptic signaling and protecting against ischemic excitotoxicity. (Table 2) Seemingly disparate structures involved in language processing also may share a similar receptor fingerprint, including the density of glutamate receptors.138 (Zilles 2015) Alterations in glutamate receptor expression post-stroke also may change the receptor fingerprint unique to these language processing regions, thereby contributing to both local and global language dysfunction.
Table 2.
Glutamate Neurotransmission in Post-Stroke Aphasia
| NMDAR | AMPAR | KAR | mGluR | |
|---|---|---|---|---|
|
|
||||
| Post-Stroke Aphasia | ||||
|
Upregulation in the stroke penumbra following acute ischemic stroke (particularly in cortical layers II-IV) may contribute to post-stroke excitotoxicity.
NMDAR antagonists administered within 30–45 minutes of a penetrating vessel occlusion can significantly reduce microinfarct volumes. |
AMPAR expression at synaptic sites are altered within 24 hours of ischemic injury. • CI-AMPARs are preferentially expressed, leading to increased amplitudes of EPSCs. • CP-AMPARs are endocytosed via a clarithin-mediated pathway. • GluA2 mRNA expression is also reduced within 24 hours of insult. |
Hippocampal KARs modulate cortical gamma oscillations and may act in tandem with the thalamus in modulating language functions. • Thalamus-driven gamma oscillations may be essential for binding different aspects of semantic information and the selective engagement of cortically distributed language components. |
mGluR1 mediates postsynaptic activation in thalamic relay neurons. Tonic activation of mGluR1 may potentiate sNMDAR responses in cortico-thalamo-cortical models of language formation. • Modulation of mGluR may have an effect in thalamic aphasia syndromes. |
|
|
NMDAR are involved in cortico-STN transmission in CSPT circuits. • NMDAR blockade in the STN prevents downstream suppression of thalamocortical activity • Receptor dysregulation may affect subcortical language processing. |
Selective blockade of CP-AMPAR is neuroprotective in ischemic stroke models. | |||
NMDAR = N-Methyl-D-Aspartate receptors, AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, KAR = Kainate receptors, mGluR = metabotropic glutamate receptors, CP-AMPAR = Calcium Permeable AMPAR, CI-AMPAR = Calcium Impermeable AMPAR, STN = Subthalamic Nucleus, CPST = Cortico-Striatal-Pallido-Thalamic
2.3. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS)
FXTAS is a neurodegenerative disorder that may affect premutation carriers of the Fragile X Mental Retardation 1 (FMR1) gene. FMR1 premutation, or carrier status, is usually defined by 55–200 CGG repeat nucleotide expansions at the 5’ untranslated region of position q27.3 in the X chromosome.149 (Brouwer 2009) The syndrome affects approximately 40150(1)-80151(2)% of older male and 8150(1)-20151(2)% of female FMR1 premutation carriers, with the females displaying more variable and milder disease phenotypes. (Yang 2014 (1), Hagerman 2016 (2))
Symptoms of FXTAS may include both Alzheimer’s and Parkinson’s disease-like phenotypes, with executive impairments usually being most prominent. Attention, memory, and visuospatial deficits are also typically present, in addition to psychiatric symptoms and progressively debilitating intention tremor and ataxia.152 (Liu 2012) Premutation carriers also may exhibit language153 disorders such as decreased phonemic fluency on the Controlled Oral Word Association Test154 (Seritan 2014) and increased pragmatic language violations on the Pragmatic Rating Scale155 (Losh 2012).
At the molecular level, FXTAS is characterized by an overexpression of the FMR1 mRNA156 (Tassone 2007) and a normal to moderately decreased (36–74%) concentration of the FMR1 protein (FMRP) product157,158, indicating deficient translation of mRNA into protein (Tassone 2000, Kenneson 2001). Overexpression of aberrant FMR1 mRNA leads to a gain of function toxicity, involving sequestration of RNA binding proteins by expanded CGG repeats, production of cytotoxic FMRpolyG peptide products, and direct DNA damage due to formation of co-transcriptional R loops in the non-template DNA strand.151 (Hagerman 2016)
How these molecular changes lead to the FXTAS phenotype is still an area of active research; FMRP levels at or below 70% of normal controls (1 standard deviation) demonstrate a linear correlation with reduced global IQ (R2 = 0.481, p < 0.0001) for both genders.159 (Kim 2019) Selective deficits in inhibition (Hayling Sentence Completion Test category B errors) may be seen in male premutation carriers who do not meet the diagnostic criteria for FXTAS (i.e. asymptomatic carriers) and has an dose dependent association with mutation burden (CGG repeat length) and increasing age.160,161 (Cornish 2011, Hunter 2012) Even non-FXTAS (asymptomatic) adult female carriers of the Fragile X premutation show subtle but significant cognitive impairments in relation to their age and number of CGG repeats.162 (Goodrich-Hunsaker 2011)
Although symptoms typically present late in life (in men older than 50 years of age), young premutation carriers (age 5–23 years) have demonstrated increased prevalence of anxiety disorders (76.9% in males and 50% in females) compared to the general population (9.8%), with higher rates (81.8%) in those with concomitant intellectual disability.163 (Cordeiro 2015) Boys with the premutation also have a higher incidence of attention deficit-hyperactivity disorder and/or autism spectrum disorder (ASD) (93% and 79%, respectively, in one proband group, compared to 13% and 0%, respectively, in sibling controls)164, and ASD in boy premutation carriers is associated with increased rates of seizures (28% in probands)165. (Farzin 2006, Chonchaiya 2011) All this suggests that molecular changes due to increased CGG repeats in FXTAS may begin during early neuronal development.164 (Farzin 2006)
2.3.1. Glutamate in FXTAS
Dysfunction of glutamate neurotransmission is thought to play a central role in both FXTAS and Fragile X Syndrome (FXS) despite differences in underlying molecular pathology.166 (Berman 2014) Glutamate application to FXTAS neurons produce sustained Ca2+ currents of higher amplitude compared to wild-type (WT) neurons which suggests altered glutamate signaling in the affected FXTAS population.152 (Liu 2012) Synaptic glutamate clearance is also affected, as reduced expression of glutamate transporters GLAST and GLT-1 in FXTAS hippocampal astrocytes results in more persistent synaptic glutamate concentrations.167 (Cao 2013)
FXTAS neurons demonstrate decreased dendritic complexity and synaptic architecture, and increased expression of stress proteins alpha B-crystallin, Hsp27 and Hsp70 during early neuronal maturation, thought to be a result of aberrant glutamate neurotransmission.168 (Chen 2010) In a separate study, hippocampal neurons in FXTAS patients also demonstrated gain-of-function mutations in Group-I-mGluRs and deficiencies in vesicular GABAA transporters. The imbalance between glutamatergic and GABAnergic neurotransmission is thought to lead to excessive clustered burst firing and glutamate activation.169 (Cao 2012) As discussed for Alzheimer’s disease, persistent glutamate activation and increased Ca2+ currents have been associated with involution of AMPAR and NMDAR, and may lead to reduced LTP and increased LTD, which likely plays a role in the reported learning and memory deficits.170 (Hunsaker 2012) (Table 3)
Table 3.
Glutamate Neurotransmission in Fragile X-Tremor/Ataxia Syndrome
| NMDAR | AMPAR | KAR | mGluR | |
|---|---|---|---|---|
|
|
||||
| FXTAS | ||||
|
Involution of synaptic NMDAR and AMPAR due to persistent glutamate activation and increased Ca2+ currents may lead to reduced LTP and increased LTD. • Glutamate application to FXTAS-phenotype neurons produce sustained Ca2+ currents of higher amplitude compared WT. |
Aberrant glutamate neurotransmission may be the cause of decreased dendritic complexity and synaptic architecture, and increased expression of stress proteins alpha B-crystallin and Hsp27 and Hsp70 during early neuronal maturation process. |
Hippocampal neurons demonstrate gain-of-function mutations in Group-I-mGluRs and deficiencies in vesicular GABAA transporters. • Imbalanced GABA/Glutamate neurotransmission leads to excessive clustered burst firing and glutamate activation. |
||
| • Reduced expression of glutamate transporters GLAST and GLT-1 in hippocampal astrocytes results in morepersistent synaptic glutamate concentrations. | • Reduced FMRP may lead to increased mGluR5 synthesis, internalization of synaptic AMPAR and increased LTD. | |||
NMDAR = N-Methyl-D-Aspartate receptors, AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, KAR = Kainate receptors, mGluR = metabotropic glutamate receptors, LTP = Long Term Potentiation, LTD = Long Term Depression, GABA = γ-aminobutyric acid, FMRP = Fragile X Mental Retardation Protein
In the cerebellum, postmortem studies of human male FXTAS patients demonstrate a reduction of both GLAST1 and Group-I-mGluR5. mGluR5 downregulation in this case is thought to be downstream of GLAST1 dysfunction and the resultant pathologic accumulation of glutamate in the synaptic cleft.171 (Pretto 2014) Given the cerebellum’s role in speech production and comprehension, such as semantic processing and verbal working memory, these cerebellar-glutamatergic pathologies could potentially contribute to language and dysexecutive deficits seen in FXTAS.172 (Moberget 2016)
Administration of mGluR1 and mGluR5 antagonists (CPCOOEt and MPEP) or GABAA receptor modulator (allopregnanlone) have been shown to ameliorate clustered burst firing in FXTAS neurons. 169 (Cao 2012) Similarly, mGluR antagonists in FMR1 knockout (FXS, rather than FXTAS, phenotype) mice have been shown to reduce socially aberrant and repetitive behavior173 (Burket 2011), and rescue abnormal dendritic spine density and immature/underdeveloped pyramidal neurons174,175 (Su 2011, Michalon 2012). Due to this association between glutamate dysregulation and FXTAS pathogenesis, the NMDAR antagonist memantine has been trialed to decrease or modulate the symptoms of FXTAS.
3. Clinical Evidence from ERP and Pharmacologic Studies
In this section, we review the clinical data on glutamate receptor-modulating drugs on language processing. Specifically, we will discuss these effects in AD, Aphasic Stroke Syndromes, FXTAS – diseases in which we have established a preclinical link between glutamate dysregulation and language dysfunction. We will also briefly evaluate the effect of glutamate modulation in healthy individuals.
To better characterize the (potentially transient) electrophysiologic changes in receptor function, and to capture any subclinical alterations in language processing that may not be reflected in behavioral or language batteries, we focus our review on studies utilizing ERPs to evaluate language-associated changes. As ERP studies are less commonly encountered compared to structural and functional imaging and language batteries, we begin with a brief overview of language-associated ERP components.
3.1. Overview of Selected ERP Components
Event-related brain potentials or ERPs are transient time-locked changes in electrical brain activity reflecting post-synaptic activity during the performance of various mental tasks. ERPs are composed of summed EPSP/IPSPs, instantaneously conducted to the scalp primarily from neocortical and/or deep CNS sources, manifest as a series of voltage deflections in bipolar EEG recordings.176 (Luck 2014) Early deflections (~50–200 ms post-stimulus onset) are associated with sensory processing, selective attention, and gating of information177 (Naatanen 1988) and later deflections (> 250 ms post-stimulus onset) reflect controlled, integrative “cognitive” processes, such as those involved in language processing178 (Kutas 2011) and memory179 (Friedman 2000). Of particular relevance are the ‘N400’, ‘Anterior Negativity (AN)’, and ‘P600’ components which have been linked to semantic and syntactic aspects of the language processing system (see Kappenman and Luck’s ERP Handbook for reviews of other language components180 (Kappenman & Luck 2012)).
As ERPs can reflect synaptic or neuronal circuit changes before anatomic, metabolic, or behavioral changes are evident. They can be particularly useful in studying early disease states where clinical changes are subtle, such as mild cognitive impairment (MCI). ERPs have an unsurpassed temporal resolution, superior to functional imaging modalities that rely on changes in blood flow or oxygenation181 (Duncan 2009) and are not influenced heavily by cultural background or educational level compared to neuropsychological tests182 (Polich 2005). Certain ERP effects may have an increased sensitivity to changes in glutamate transmission (and other pharmacologic manipulations), as demonstrated by the significant positive correlation between frontal lobe P3-auditory amplitude and glutamate/glutamine ratios in the anterior cingulate gyrus.183 (Hall 2015) We posit that pharmacologic modulation of glutamate receptors should result in both electrophysiological (e.g. ERP) and behavioral changes.
3.1.1. N400
The N400 is a scalp negativity (relative to a mastoid reference) peaking at approximately 400 ms after stimulus-onset with a posterior (centro-parietal) maximum; alterations in N400 topography have been suggested to be a potential biomarker for detection of early dementia184,185 (Olichney 2008, Horvath 2018) N400 amplitude is a sensitive marker of semantic language processing load, which is dependent on both the complexity of the language task at hand and the cognitive capacity to perform it.
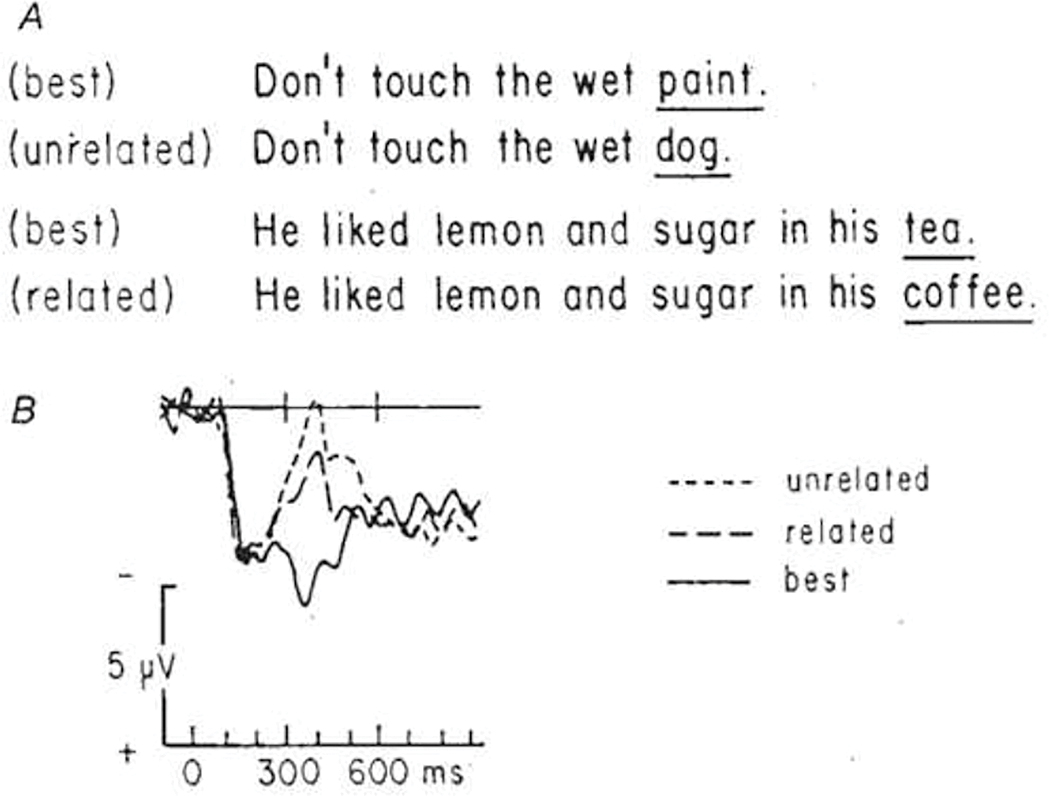
The “N400 congruity effect” is a reduction in N400 amplitude when the eliciting word is a good semantic fit (congruous) to its context (word, sentence, or dialogue) compared to a poor fit (incongruous); the better the fit, the lower the amplitude, and thus the larger the N400 congruity effect.186,187 (Kutas & Hillyard 1984, Berkum 1999) In sentence experiments, there is a high inverse correlation (r = -0.87) between N400 amplitude and the Cloze probability of the eliciting word 186 (Kutas & Hillyard 1984). (Figure 4) Diseases that affect the availability/access of stored conceptual information and/or the ability to integrate the meaning of the word and its context accordingly increase the task difficulty and cognitive costs of semantic processing and are reflected as modulations in N400 amplitude.178 (Kutas 2011)
Figure 4. The N400 Congruity Effect. Adapted from Kutas and Hillyard Nature 1984170.
Two examples of sentences with high contextual constraints completed by low Cloze probability words. Above each experimental sentence is the same sentence terminated by its “best completion”, which was or was not semantically related to the word that was actually presented in the sentence.
B. Grand average ERPs for the best completions (solid waveform), the semantically related (large dashed waveform) and the semantically unrelated (small dashed waveform) low Cloze probability words. There is a high inverse correlation between N400 amplitude and the Cloze probability of the eliciting word.
The separate but related “N400 repetition effect” is modulated by memory functions. Repetition of semantically incongruous words is associated with a reduction in N400 amplitude. This repetition effect is preserved in chronic amnestics despite poor verbal memory performance, implicating an implicit, rather than declarative memory process.*188,189 (Olichney 2000, Olichney 2011) (Figure 5) The N400 repetition effect highlights the link between memory and language processing, as the implicit memory process likely facilitates language comprehension at the sentence level.188 (Olichney 2000) Furthermore, repeated exposure to the same word is thought to ease semantic processing load and integration. The resulting decreased N400 amplitude is therefore usually considered a form of semantic priming.190 (Paller 2007)
Figure 5. The N400 Repetition Effect. Adapted from Olichney et al. Brain 2000172.
Grand Average ERPs from the control and amnesic groups elicited by new (continuous lines) and repeat (dotted lines) words which were semantically incongruous. Negative voltage is plotted in the upward direction. Note the persistent and robust N400 repetition effect in amnestic patients, implicating an implicit, rather than declarative, associated memory process.
*However, in the case of FXTAS, the N400 repetition effect amplitude appears to be reduced in those with relatively worse declarative/explicit memory.191 (Olichney 2010)
3.1.2. Anterior Negativity and P600
The Anterior Negativity (AN) and the Syntactic Positive Shift (SPS)/P600 both have been linked to syntactic processing. AN occurs within the same latency (300–500ms) post-stimulus as the N400 but generally has a more frontal (than the N400) and left hemisphere maximum and is thus sometimes referred to as the lateralized anterior negativity (LAN).192,193 (Friederici 1996, Lau 2006) The AN effect is a relative negativity elicited by mismatches in word-category (e.g. a noun used instead of a verb) or morphosyntactic features (number, case, gender, and tense) that result in syntactic anomalies194 (Friederici 2002) or violations with no acceptable grammatical reading195 (Hagoort 2003). (Figure 6) Diseases that alter AN amplitude or onset latency are presumed to be responsible for initiation of the syntactic structure building process194 (Friederici 2002) or increasing the difficulty in binding a word to an appropriate lexical frame195 (Hagoort 2003).
Figure 6. The Anterior Negativity and SPS/P600 Effects. Adapted from Friederici Trends in Cognitive Sciences 2002177.
A. N400, B. Very Early Left Anterior Negativity (ELAN), C. P600 (Syntactic Positive Shift). Shown are average ERPs for the semantic- and syntactic-violation conditions at selected electrode sites Cz, F7, and Pz. Solid lines represent the correct condition, and dotted lines the incorrect condition. The ELAN and SPS/P600 effects occur in response to syntactic anomalies.
The SPS/P600 is a positive shift occurring at approximately 600 ms after stimulus onset with a centro-posterior maximum.196,197 (Gouvea 2010; Leckey & Federmeier, 2020) The P600, initially referred to as the Syntactic Positive Shift (SPS), is thought by some to be an index of the difficulty of syntactic integration. The SPS/P600 occurs in response to syntactic anomalies, although relative increases in SPS/P600 amplitude can be elicited by complex or ambiguous (but technically correct) syntactic structures as well.198 (Kaan 2000) (Figure 6) The more cognitive resources required for structural re-analysis or to resolve competing interpretations of ambiguous clauses, the larger the SPS/P600 amplitude and duration.195,196 (Gouvea 2010, Hagoort 2003) In the computational lexical-parsing analogy, diseases that alter the SPS/P600 are thought to be responsible for deficits in the ability to unify/arrange lexical components into syntactically coherent phrases.119 (Hagoort 2003)
Another late positive component, variously called the P600 or Late Positive Component (LPC), has a similar time course and scalp topography as the SPS/P600 but is observed in different paradigms, where it is correlated with episodic verbal memory abilities and declarative memory.196,199 (Regel 2014, Gouvea 2010) Exposure to repeated words in word list experiments has revealed larger LPC/P600 amplitudes successfully recognized compared to unrecognized words, and to recalled words compared to un-recalled words during free recall.200 (Paller 1987) (Figure 7) The more intact the source memory (e.g. memory of the original word list), the larger the LPC/P600 amplitude. Thus, the LPC/P600 (sometimes referred to as left parietal P600) is often considered an electrophysiological index of recollection.201 (Rugg 1998)
Figure 7. The LPC/P600 Effect. Adapted from Paller, Kutas, and Mayes Electroencephalography 1987182.
ERPs averaged based on later recognition performance. Words subsequently recognized elicited greater late positive activity than did words subsequently not recognized.
In contrast, when words are repeated in predictable linguistic contexts, the LPC/P600 amplitude is typically smaller in response to repetitions, but the absolute voltage change is larger in individuals with better memory for the item. This is possibly because a different memory retrieval process is engaged for semantically predicable/congruous words.184,188,202 (Olichney Brain 2000, Neurophysiology 2006, Neurology 2008) The LPC/P600 enhancement can persist ≥ 15 minutes;179,203 (Friedman and Johnson 2000, Olichney and Hillert 2004) active forgetting attenuates the LPC 204 (Paller 1990), and in general the LPC/P600 may be broadly associated with non-linguistic, learned structures, e.g. musical chord progression and face recognition205 (Patel 1998).
While the relationship between the SPS/P600 in sentences and the LPC/P600 elicited in memory experiments is unclear (as is the relationship between P600 and P3b more generally)197 (Leckey & Federmeier 2020), an association between memory and language processing is implied in Friederici’s interpretation of the P600: as a marker for syntactic reanalysis and repair during the resolution of syntactic processing. On this view, encoding and recall of various syntactic components would be crucial for successful manipulation and resolution of syntactic ambiguities.194 (Friederici 2002)
3.2. Alzheimer’s Disease
As reviewed by Olichney and Hillert, N400s in AD generally are smaller in amplitude and delayed in peak latency compared to those in normal elderly persons.203 (Olichney 2004) A diminished N400 repetition effect in MCI and AD can be rescued (in the MCI group only) by the removal of lexical ambiguity from the task, facilitating semantic priming.206 (Taler 2009) This suggests that A) MCI patients have a preserved semantic store but impaired processing/access compared to AD patients who have impairments in both the semantic storage and access processes76 (Verma 2012) and B) pathologic N400s in the MCI population can be rescued/restored to age-matched normal values. In mild AD and semantic dementia patients, abnormal N400s are associated with reduced temporal lobe cerebral blood flow, suggesting a close association between the electrophysiological and focal metabolic abnormalities.207 (Grieder 2013)
A longitudinal ERP study of MCI converters to AD demonstrated loss of the N400 congruity effect (and a memory-associated N400 repetition effect (see discussion below)) after a one year of follow-up, prior to most patients’ clinical conversion. This led Olichney et al. to conclude that abnormal N400s in MCI are associated with an increased risk of conversion to AD, with an odds ratio estimated to be in the 4–8 range.184 (Olichney 2008) The N400 repetition and LPC/P600 effects combined have a high sensitivity (100%) and specificity (80%) to AD dementia, with mild AD patients consistently demonstrating reduced or absent P600 and N400 word repetition effects.202 (Olichney 2006) The N400 is also sensitive to neuronal level changes prior to AD symptom onset. Bobes et al. noted alterations in N400 generator topography and amplitude between healthy controls and pre-symptomatic carriers of the familial AD presenilin-1 mutation, and similar topographic and amplitude distributions between pre-symptomatic and symptomatic presenilin-1 mutation carriers.208 (Bobes 2009) Taken together, these studies demonstrate the utility of ERPs for tracking, quantifying, and possibly predicting language dysfunction in MCI as well as for predicting conversion to AD dementia. (Table 4)
Table 4.
ERP Studies**
| N400 Congruity Effect | N400 Repetition Effect | SPS/P600 Effect | AN | LPC/P600 Effect | |
|---|---|---|---|---|---|
|
|
|||||
| Mild Cognitive Impariment (MCI) and Alzheimer’s Disease (AD) | |||||
| MCI | Reduced effect precedes clinical conversion to AD by 1 year.168 | Abnormalities increase risk of conversion to AD. Odds Ratio of 4–8.168 Reduced or absent in mild AD. Topography changes in pre-symptomatic and symptomatic PSEN-1 mutation carriers.190 |
|||
| AD | Reduced or absent in mild AD.184 | ||||
| Post-Stroke Aphasia | |||||
| Broca’s Aphasia | Reduced ampitude and increased latency.115 | Reduced amplitude and increased latency.115 | |||
| Wernicke’s Aphasia | Reduced amplitude.203 | Intact N400 semantic priming.204 | Absent in patients with pSTG lesions.206 | ||
| Subcortical Aphasias | In DBS induced functional VIM lesions, reduction in N400 congruity is associated with impaired word-pair lexical decision tasks.214 | Intact in left BG lesions.210 | Absent in left BG lesions.210 | ||
| Unclassified Aphasias | Reduced in patients with moderate to severe verbal or reading comphrension deficits.207–209 Topographic maximum shifts from the right to left hemisphere after 4 weeks of aphasia therapy.210 | ||||
| Fragile X Tremor/Ataxia Syndrome (FXTAS) | |||||
| FXTAS | Reduced in female FXTAS patients, but with preserved N400 and P600 Repetition Effects.143 | Reduced in patients with poor declarative verbal memory.160 | Intact in female FXTAS patients143 and most male FXTAS patients172. | ||
| Non-Aphasic Patients | Present in thalamic depth recordings. Absent in STN/GPi recordings.211–213 |
Present in thalamic depth recordings. Absent in STN/GPi recordings.211–213 |
Present in thalamic depth recordings. Absent in STN/GPi recordings.211–213 |
||
MMN = Mismatch Negativity (another ERP component), pSTG = Posterior Superior Temporal Gyrus, VIM = Ventral Intermediate Nucleus (of the Thalamus), BG = Basal Ganglia, STN = Subthalamic Nucleus, GPi = Globus Pallidus Interna
Not an Exhaustive List
3.2.1. Memantine in Alzheimer’s Disease
Memantine is a partial NMDAR antagonist that is currently approved in the U.S., Europe, and Japan for the treatment of AD.209 (Matsunaga 2015). Memantine is an open-channel blocker of NMDARs, which weakly binds to the Mg2+ site and preferentially targets excessively open channels, while interfering only minimally with normal synaptic transmission.39 (Lipton 2004) Importantly, memantine purportedly binds preferentially to extrasynaptic over synaptic NMDARs even under pathologic depolarization210 (Xia 2010) and is able to restore LTP impairment and rescue neurons from pro-apoptotic cascades initiated by eNMDAR activation211 (Dau 2014). A meta-analysis by Matsunaga et al. found that memantine monotherapy in AD (9 studies, n=2433) improved cognitive function, AD-associated behavioral disturbances, activities of daily living, global function, and stage of dementia compared to placebo.209 (Matsunaga 2015) These benefits have been consistently shown in moderate to severe stages of AD212,213 (Reisberg 2003, Doody 2004), with less consistent, and more controversial effects in mild AD.214,215 (Peskind 2006, Schneider 2011) Multiple studies have demonstrated that memantine monotherapy216 (Ferris 2009) or the addition of memantine to chronic donepezil use improve language scores on the severe impairment battery (SIB) language subscale in moderate to severe AD patients.216–219 (Tariot 2004, Schmitt 2006, Ferris 2009, Tocco 2014) (Table 5)
Table 5.
Pharmacologic Studies**
| Therapy | Duration | Effect | Interpretation | |
|---|---|---|---|---|
|
|
||||
| Alzheimer’s Disease (Moderate to Severe) | ||||
| Tariot et al. 2004 (n = 322) | Memantine (10 mg BID) + Stable Dose of Donepezil | 24 weeks | Improved SIB, ADCS-ADL19, and CIBIC-Plus. | Addition of Memantine resulted in better outcomes in multiple domains, including language. |
| Schmitt et al. 2006 (n = 290) | Memantine (10 mg BID) + Stable Dose of Donepezil | 24 weeks | Improved SIB Language Domain starting at week 8, Praxis at week 4, and Memory at week 24. | Memantine improves language and memory systems in separate but complementary ways. |
| Ferris et al. 2009 (n = 801) (Analysis of 4 databases including Tariot et al. 2004) | Memantine (10 mg BID) (+ Stable Dose of Donepezil in 403 patients) | 24–28 weeks | More clinically relevant improvement and fewer clinically relevant worsening on SIB-L. | Memantine (with and without donepezil) results in significant benefits for language function for patients with either mild and marked language impairment. |
| Post-Stroke Aphasia | ||||
| Berthier et al. 2009 (n = 27) | Memantine (10 mg BID) + Aphasia Therapy vs. Placebo + Aphasia Therapy | 48 weeks | Persistently improved WAB-AQ after drug washout. Improved WAB-AQ with and without aphasia therapy. |
Memantine associated language improvement persists after drug cessation. |
| Kessler et al. 2000 (n = 24) | Piracetam 2400 mg BID + Speech Therapy | 6 weeks | Improved Aachen Aphasia Test (Spontaneous Speech, Communicative Verbal Behavior, Semantic and Syntactic Structure) | Piracetam improves language function in post-stroke aphasia. |
| Güngör et al. 2011 (n = 30) | Piracetam 24000 mg BID | 6 months | Improved auditory comprehension on the Gülhane Aphasia Test | Long-term Piracetam use improves auditory comprehension in post-stroke aphasia. |
BID = Twice Daily, SIB = Severe Impairment Battery, ADCS-ADL19 = 19-item AD Cooperative Study–Activities of Daily Living Inventory, CIBIC-Plus = Clinician’s Interview-Based Impression of Change Plus Caregiver Input, SIB-L = Severe Impairment Battery - Language Scale, WAB-AQ = Western Aphasia Battery - Aphasia Quotient
Not an Exhaustive List
Kubova et al. reported on the ERP effects of memantine monotherapy (10 mg twice daily (BID)) for 6 months in mild to moderate AD patients. A significant 20 ms decrease in visual-evoked P300 peak latency was noted in 42% of their cohort (n=17, mini-mental state examination (MMSE) score 12–23); this was thought to be associated with improved allocation of attention and working memory updating. N400 was not measured.220 (Kubova 2010) To our knowledge, there are no published studies that evaluate the ERP effects of glutamate modulation (antagonist or agonist) on language dysfunction in AD. (Table 6)
Table 6.
Combined Pharmacologic and ERP Studies*
| Therapy | Duration | Effect | Interpretation | |
|---|---|---|---|---|
|
|
||||
| Alzheimer’s Disease | ||||
| Kubová et al. 2010 (n = 17) | Memantine (10 mg BID) | 6 months | Reduced P300 Peak Latency. | Memantine improved allocation of attention and working memory updating. |
| Post-Stroke Aphasia | ||||
| Barbancho et al. 2015 (n = 27) | Memantine (10 mg BID) + Aphasia Therapy vs. Placebo + Aphasia Therapy | 20 weeks | Reduced N400 amplitude (closer to healthy controls); Increased N400 in the Left Hemisphere | Memantine normalized aberrant glutamate neurotransmi ssion. ERP changes correlated with improved WAB-AQ scores. |
| FXTAS | ||||
| Ortigas et al. 2009 (n = 1) | Memantine (10 mg BID) | 9 months | Improved N400 congruity + word repetition effect. Improved P600 word repetition effect. |
Memantine improved language function. |
| Yang et al. 2014 (n = 41) | Memantine (10 mg BID) vs. Placebo | 12 months | Improved N400 repetition effect amplitude and latency. | Improved verbal memory based on cued recall. |
| Non-Aphasic Patients | ||||
| Grunwald et al. 1999 (n = 39) | Ketamine (0.5 mg/kg) | Once | Attenuated N400 repetition effect. No change in LPC/P600 repetition effect. | Ketamine affects language but not memory predominant systems, which are separate but complementa ry. |
BID = Twice Daily, WAB-AQ = Western Aphasia Battery-Aphasia Quotient, LPC = Late Positive Component
To the best of our knowledge, an exhaustive list of current studies.
3.3. Aphasic Stroke Syndromes
3.3.1. Broca’s Aphasia
Broca’s Aphasia (BA) patients demonstrated reduced amplitude and significantly delayed onset of SPS/P600s during word-category violation tests compared to those in healthy controls and non-aphasic stroke patients with right hemisphere lesions.118 (Wassenaar 2005) In the same study, BA patients also showed a globally delayed and reduced N400 to semantic violations compared to normal controls; although the effect was less pronounced (i.e. absolute onset delay and amplitude change were lower but still statistically significant) compared to the SPS/P600 changes elicited in the syntactic violation task, consistent with greater impairment in syntactic than semantic processing in BA. AN was not elicited.
3.3.2. ernicke’s Aphasia
Wernicke’s Aphasia (WA) patients demonstrated a reduced N400 congruity effect compared to healthy controls in an acoustic word-picture verification test of single word comprehension.221 (Robson 2017) In the same study, WA patients also demonstrated a reduced and inconsistent phonological mismatch negativity, suggesting that the observed comprehension impairment in WA is likely a combination of deficits in phonologic perception and semantic comprehension. Despite the reduced N400 congruity effect, WA patients consistently demonstrate intact N400 semantic priming effects similar to healthy controls, suggesting an intact lexical-semantic store despite semantic comprehension deficits.222 (Hagoort & Kutas 1995)
There is corroborating ERP and functional imaging evidence that the left posterior STG, which is lesioned in classical WA, is involved in the integration of lexical and syntactic information. Patients with pSTG lesions demonstrate a selective absence of SPS/P600*223 (Friederici 2003), and this is consistent with functional imaging evidence of pSTG activity during the processing of syntactic structures of verbs.224 (Grodzinsky 2006) Taken together, these ERP studies suggest that comprehension deficits in WA may be some combination of impairments in sensory/auditory processing, semantic processing, and syntactic integration.
*Further study is needed to establish if AD and MCI patients with reduced or absent P600 effects202 have significant atrophy in the pSTG region as well; such a finding may further substantiate the link between structure (pSTG), function (language), and electrophysiology (P600).
3.3.3. Receptive Aphasias
Independent of stroke classification (i.e. Wernicke’s, Broca’s, Subcortical), aphasic patients with mild comprehension deficits showed an N400 comparable to normal controls while aphasic patients with moderate to severe comprehension deficits had a significant reduction and delay in the N400 to sentence-final semantic anomalies225 (Swaab 1997) and word-pair associations226 (Hagoort 1996). Similar patterns of N400 results were found in mild versus severe reading comprehension-impaired aphasia patients.227 (Chang 2016) This seems to suggest that severely impaired comprehension may be independently associated with an impaired ability to integrate lexical-semantic information into higher order representations, regardless of stroke classification.
3.3.4. Subcortical Aphasias
ERP evidence for BG involvement in language processing is mixed. Patients with left basal ganglia lesions and varying degrees of language comprehension impairment demonstrated intact AN (specifically early left anterior negativity (ELAN)) but absent SPS/P600s compared to healthy controls in a study of verb-specific syntactic violations. The BG lesion group also demonstrated an N400-like negativity with extended duration (300–700 ms) that was absent in healthy controls. Altogether, this was taken to suggest that the BG may be involved in later stages of syntactic processing (hence the affected P600 but not ELAN) and may play a modulatory role in lexical-semantic analysis, perhaps related to the speed of processing.228 (Kotz 2003)
In contrast, in non-aphasic individuals, depth recordings from the STN in a word-pair task demonstrated an ERP component (~318–362 ms) to prime-words only, suggesting that the STN (and the BG, which is closely interconnected via glutamatergic connections between the STN and globus pallidus) play a role in automatic procedural tasks (e.g. readiness inhibition or warning cues in response to a new word-pair task); although this may not be exclusive to language processing.229 (Tiedt 2017) In other studies, depth recordings from the STN and/or GPi in non-aphasic individuals failed to show sensitivity to semantic (N400) or syntactic violations (P600), leading the authors to conclude that the BG was not involved in essential language processing tasks.230,231 (Wahl 2008, Krugel 2014)
A study of functional thalamic lesions induced by deep brain stimulation (DBS) in the ventral intermediate nucleus (VIM) of the thalamus demonstrated reduced N400 congruency amplitudes. This was accompanied by a reduction (slower than normal) response times in a word-pair lexical decision task.232 (Krugel 2014) Thalamic depth recordings of non-aphasic patients also demonstrated N400 congruency effects to semantic phrase violations, and AN and SPS/P600 to syntactic phrase violations. Taken together, these data suggest that the thalamus may gate the flow of language information between cortically distributed language networks and can be involved in both semantic and syntactic processing tasks.229,230 (Wahl 2008, Tiedt 2017) To our knowledge, there are no published studies evaluating the language-related ERP components in thalamic aphasia patients. (Table 4)
3.3.5. Memantine in Aphasic Stroke Syndromes
Barbancho et al. evaluated the ERP effect of memantine monotherapy in a double-blind study involving patients with chronic (> 1-year duration) post-stroke aphasia from left peri-sylvian lesions but heterogeneous presentations of aphasic stroke syndromes. Patients were randomized to treatment with memantine 10 mg BID (n=14) vs placebo (n=14) for a total of 20 weeks, with two weeks of constraint-induced aphasia therapy during weeks 16–18. For the ERP recording phase, participants were asked to silently read words presented on a computer screen, selected from a bank of 300 frequently encountered words.142 (Barbancho 2015)
At baseline evaluation, healthy controls (n=15) demonstrated lower (early sensory) P100 and N400 amplitudes compared to aphasic stroke patients in both the memantine and placebo groups. By week 16, the memantine group demonstrated a larger decrease in P100 and N400 amplitudes (closer to the healthy controls) and a correlated improvement in their Western Aphasia Battery-Aphasia Quotient (WAB-AQ) scores compared to the aphasic stroke patients in the placebo group. The healthy control group did not show a reduction in ERP amplitudes at 16 weeks.
The addition of two weeks of constraint-induced aphasia therapy (CIAT) in conjunction with memantine saw a continued increase in WAB-AQ scores but no subsequent change in the N400. ERP changes were noted over both hemispheres, although treatment with memantine/CIAT resulted in a significant increase in left hemispheric activity compared to the placebo/CIAT group.142 (Barbancho 2015)
This improvement in the N400 was thought to be due to memantine’s effect in normalizing chronic, aberrant glutamate neurotransmission in the perilesional space and in contralateral hemispheric regions deprived of excitatory glutamatergic neuronal input.142 (Barbancho 2015) Based on preclinical data, we would also venture a guess that memantine likely normalized aberrant NMDAR activity downstream of the peri-sylvian lesions, in glutamate-regulated synapses in CSPT circuits, and improved the signal-to-noise ratio in set switching between new words or concepts.131 (Crosson 2013) It is also possible that memantine increased synaptogenesis in compensatory language circuits, as the data in mice of models of FXS suggest that memantine may enhance excitatory synapse formation and rescue delayed dendritic spine maturation.233 (Wei 2012)
The silent reading task used for the ERP recording raises two points of note. One, the test minimizes the effects of working memory and short-term recall, suggesting that the changes in N400 amplitude may be strictly language related. Two, the test lacks the specificity to differentiate between semantic and syntactic associated ERPs. However, the increase in the dominant left hemisphere N400 after memantine therapy is consistent with Wilson et al’s findings, suggesting that memantine-associated glutamate regulation may be involved in, or trigger, a compensatory change, reflected in N400 topography. (Table 6)
An extension of this study also evaluated the effect of memantine on WAB-AQ scores at 24 and 48 weeks (without additional ERP testing). Weeks 20–24 were a memantine washout phase, and weeks 24–48 were an open label phase in which both groups received memantine 20 mg BID. The study group (memantine-memantine) consistently outperformed the control group (placebo-memantine) at both time points whereas both groups demonstrated significant improvements in WAB-AQ scores during the open label phase. 234 (Berthier 2009) The fact that improvements in language performance persisted after the drug washout phase suggests that NMDAR modulation may be associated with long-term remodeling of language networks after stroke, perhaps by altering the local receptor fingerprint (i.e. glutamate receptor density) to better match associated language processing structures. Also, the results of the open label phase demonstrate that memantine can improve language performance in post-stroke aphasia without additional aphasia therapy, although combined therapy leads to better outcomes. (Table 5)
In a study of aphasic stroke patients with heterogeneous lesion sites, an N400 “mismatch effect” was recorded before and after four weeks of non-pharmacologic speech-language therapy.235 (Wilson 2012) Patients were asked to determine if a spoken word was congruent (match) or not congruent (mismatch) with a picture. In the control group, the N400 was maximal over the central-posterior region (with a significant linear trend toward the right hemisphere (likely due to a false localizing dipole178 (Kutas N400 2011)) pre- and post-therapy. In the stroke patients, four weeks of therapy led to a topographic migration of the maximal N400 amplitude from the right to the left hemisphere. Compared to the combined memantine and language therapy study, this hemispheric shift due to language therapy alone was thought to be a compensatory response (possibly from a right hemisphere source), rather than a “normalization” of the N400 toward the language dominant hemisphere, as the control group had a persistent right-hemispheric N400 bias. (Alternatively, the left hemisphere signal could also be due to a false localizing dipole from a right medial temporal source.) Altogether, these studies demonstrate that the N400 is sensitive to interventions in chronic stroke patients, while the different effects on N400 between the two studies suggest that glutamate modulation may play a separate but complementary role to non-pharmacologic language therapy in restoring semantic language processing.
3.3.7. Piracetam in Aphasic Stroke Syndromes
Piracetam and piracetam-related compounds are GABA-derivatives with mixed activity at glutamate receptors, nicotinic-Acetylcholine receptors (nAChR), and GABA receptors.236 (Berthier 2011). Group 1 agents increase AMPA-mediated Ca2+ currents by increasing the receptor binding site density for AMPAR and are considered nootropic drugs due to their potential efficacy in cognitive rehabilitation, including for post-stroke aphasia.237 (Malykh 2010)
One double-blind, placebo-controlled study of 24 patients with post-stroke aphasia combined 2400 mg of piracetam twice daily and intensive speech therapy for 6 weeks. At the end of the study, the piracetam group demonstrated significant improvements in the Aachen Aphasia Test in the subfields of spontaneous speech, especially in communicative verbal behavior, and in the semantic and syntactic structure of their speech compared to the control group. The piracetam-arm also demonstrated increased blood flow in the left transverse temporal gyrus, left triangular part of inferior frontal gyrus, and left posterior superior temporal gyrus on a positron emission tomography (PET) scan during word repetition compared to the control group, although this was thought to be due the drug’s nAchR interactions.238 (Kessler 2000) A 6-month, double blind and placebo-controlled study of 30 patients with post-stroke aphasia on 2400mg of piracetam twice daily demonstrated significant improvement only in auditory comprehension on the Gülhane Aphasia Test compared to the control group239 (Güngör 2011), suggesting that piracetam may exert a (minor) long-term beneficial effect in post-stroke aphasia. (Table 5)
Currently there are no ERP studies evaluating the effect of piracetam-related drugs on language disorders. It is possible that ERP studies may reveal significant long-term changes with piracetam in control groups that are not readily evident with neuropsychologic testing. However, due to piracetam’s mixed mechanisms of action, it is difficult based on clinical evidence alone to determine which effect on language dysfunction can be attributed to its ability to regulate glutamate transmission. Future studies could include evaluation of changes in synaptic AMPAR distribution and density, AMPA-mediated LTP and LTD, and glutamate to GABA receptor ratios particularly in known language-associated brain structures, such as the anterior temporal lobe, in conjunction with ERP measurements in known tasks.
3.4. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS)
A significantly reduced N400 word repetition effect was found in FXTAS patients with poor declarative verbal memory (based on California Verbal Learning Test or CVLT delayed recall and discriminability scores) compared to normal controls, suggesting impaired implicit memory and semantic language processing in the FXTAS group.191 (Olichney 2010) A separate study of exclusively female FXTAS patients demonstrated a reduced N400 congruity effect, but preserved N400 and P600 repetition effects.150 (Yang 2014) This was again suggestive of abnormal semantic processing; the relatively preserved implicit and verbal memory may be due to a less severe phenotype given the female-only study population, who generally have one normal copy of the fragile X gene on their unaffected X chromosome. It is possible that these deficits in semantic processing noted on the N400 congruity task may be related to category B errors on the Hayling Sentence Completion Test in asymptomatic premutation carriers153,161 (Cornish 2011, Hunter 2012), as the latter requires a semantic judgement in addition to an inhibition task (choose an unrelated word to complete the presented sentence) and are demonstrating similar underlying neural vulnerabilities.
3.4.1. Memantine in FXTAS
Ortigas et al. reported that 9 months of memantine 10 mg BID improved the N400 congruity effect and N400 word repetition effect, and the P600 word repetition effect in a 65-year-old female FXTAS patient.240 (Ortigas 2009). (Table 4) Yang et al. expanded upon this observation in a double-blind study of 41 FXTAS patients randomized to treatment with memantine 10 mg BID vs. placebo for one year. The treatment arm (n=21) also demonstrated improvements in the N400 repetition effect amplitude and latency, which correlated with improved verbal memory based on cued recall scores. In contrast, the placebo arm of FXTAS patients had longer N400 latencies compared to their baseline ERP one year earlier. (Figure 8) Although the amplitudes of the P600 word repetition effect correlated well with memory and recall scores, there were no significant changes in the P600 over the course of the study.241 (Yang 2014)
Figure 8. Memantine Effects on FXTAS. Adapted from Yang et al. Neuropsychopharmacology 2014223.
A and B: The N400 repetition effects generated by subtracting ERPs to incongruous old target words from ERPs to incongruous new target words, within each group. Robust treatment effects were demonstrated within the shaded right posterior cluster consisting of five electrodes, with significant decreases in the placebo group and an insignificant trend for N400 repetition effect amplitude increases in the memantine group
C and D: Topographic distribution of the N400 repetition effects across the early (300–400 ms) and late (400–500 ms) time windows, plotted by treatment group and visits, within each group.
Memantine-induced clinical and ERP improvements in these patients underscore the role of defective glutamatergic signaling in memory and language dysfunction in FXTAS. It is likely that NMDAR modulation, particularly antagonism at extrasynaptic NDMARs, rescued at-risk neurons from tonic activation and excitotoxicity and may have fostered recovery of NMDAR-mediated LTP.242 (Hunsaker 2012) Such a mechanism would be in line with findings of sustained Ca2+ currents inducing excitotoxic cell death in FXTAS neurons.167 (Cao 2013) Determining whether NMDAR level dysfunction is downstream of mGluR dysfunction (as in FXS243) will require further study.
Finally, in contrast to MCI and AD data, Yang et al. found that the N400 repetition effect rather than P600 repetition effect was the stronger correlate of verbal memory ability in FXTAS.244 (Yang 2014) This re-enforces the idea that despite similar patterns in cognitive deficits245 (Seritan 2008), different physiological mechanisms lead to the cognitive dysfunction and pathogenesis of FXTAS and AD. (Table 6)
The consistent reduction in the N400 repetition effect across multiple studies suggests that abnormalities in implicit memory and semantic priming are the cause of verbal learning and memory dysfunction in FXTAS. More broadly, these findings support the role of glutamatergic signaling in language processing; the ERP data, in particular, highlight the multiple electrophysiological mechanisms and current disruptions that can produce language and memory dysfunction. In summary, the aforementioned patient studies demonstrate the heterogeneous role of glutamate transmission in the language (and memory) disorders reviewed here.
3.5. Non-Aphasic Patients
We conclude this section with data linking glutamate neurotransmission and language via pharmacological and ERP studies in patients with no language disorders.
Smith et al. demonstrated a clear left lateralized medial temporal lobe (MTL) generator for the N400 repetition effect in depth recording studies of epilepsy patients. The MTL-N400 represented excitation of hippocampal synapses in response to convergent activation from cortical and subcortical projections, and the N400 repetition effect was thought to be associated with synaptic level changes (i.e. LTP induction) from repetitive stimulation of the MTL and the enhanced feedback loop between the MTL and the association cortex.246 (Smith 1986) By virtue of regulating LTP and therefore the N400, NMDAR and AMPAR are implicated in language processing.
Smith et al. also considered the MTL to be a converging point for the posterior association cortex, the dominant peri-sylvian cortex, and the limbic neocortical connections.246 (Smith 1986) This view is similar to Teyler and DiScenna’s ‘hippocampal memory index’ theory which suggests that the hippocampus is functionally designed and anatomically situated to act as an indexing mechanism and amodal hub for integrating sensory information from the neocortex.247 (Teyler and Rudy 2007) Indeed, recent direct hippocampal recordings have demonstrated language-associated changes in increased theta power in response to constrained versus unconstrained phrases.248 (Piai 2016)
These findings are in keeping with topographically distributed models of semantic language formation; with the MTL, rather than the thalamus, binding different aspects of semantic information.
3.5.1. Ketamine in Non-Aphasic Patients
Finally, we review the ERP effects of ketamine — a voltage-dependent open-channel blocker of NMDARs. Ketamine has an intrachannel binding site similar to the NMDAR antagonist memantine, which results in occluded current flow, but has a different pharmacokinetic profile and affects both synaptic and extra-synaptic NMDARs.249 (Kotermanski 2013) Ketamine also has a known rapid anti-depressant effect, which may work through downstream targeting of the calcium/calmodulin-dependent eukaryotic elongation factor 2 kinase (eEF-2K) and psychotropic side effects, as a dissociative anesthetic.250 (Autry 2011)
Grunwald evaluated the effect of ketamine in language processing using a visual word recognition and repetition paradigm and computed ERPs from surface recordings in healthy participants (n=16) and from depth electrodes in temporal lobe epilepsy patients (n=25). A single dose of 0.5 mg/kg IV of ketamine greatly attenuated the N400 repetition effect in both groups, but did not affect the LPC/P600 repetition effect.251 (Grunwald 1999) (Figure 9) That NMDAR modulation preferentially affected the N400 but not the P600 suggests distinct neurotransmitter mechanisms at work in language-predominant and memory-predominant systems, which may act in an independent but complementary fashion. (Table 6) This is in line with the observation that the LPC is memory retrieval dependent whereas N400 repetition has an implicit memory component and is not affected by poor verbal memory recall (i.e. preserved in chronic amnestic patients).188 (Olichney 2000) Although the ‘classic’ posteriorly distributed N400 has been most closely linked to semantic priming and implicit memory, a frontally-distributed N400, sometimes called the F-N400, has been taken to reflect familiarity-based memory processes in some experimental paradigms.201,252 (Rugg 1998, Stróżak 2016)
Figure 9. Ketamine effects on N400. Adapted from Grunwald et al. Proc. Natl. Acad. Sci. U. S. A 1999232.
ERPs averaged across patients. (a) Anterior Medial Temporal Lobe-N400s were significantly affected by ketamine. Recognition effects were significant only without ketamine, not after intravenous administration of this NMDA- receptor antagonist. (b) Ketamine did not affect P600 and LPC/P600 effects.
Focal: recordings from the epileptogenic temporal lobe; Nonfocal: recordings from the contralateral side. Solid line: initial presentations; Dashed line: repetition.
Copyright (1999) National Academy of Sciences, U.S.A.
To summarize the clinical data section, there is ample tangential evidence suggesting an association between glutamate neurotransmission and language disorders, but there are no studies directly addressing this relationship. The glutamate-modulating agent memantine has consistently improved language function in moderate to severe AD219 (Tocco 2014). In the MCI and AD populations, language-related ERP components can be used to evaluate and track language deficits across time. ERP studies have shown sensitivity to effects of memantine on attention in AD patients, suggesting that a language-related ERP study of the effects of memantine in AD could directly address the association between glutamate modulation and language dysfunction.
In stroke patients with cortical or subcortical aphasia, studies using language-related ERP components have been able to parse the language processing deficits into relatively distinct semantic and syntactic components (i.e. N400 and P600, respectively, although there are so-called semantic P600 effects197) (Leckey & Federmier 2020). Memantine use has resulted in mild improvements in N400 amplitude and improvement in WAB-AQ scores suggesting that glutamate modulation is associated with improved language outcomes in the chronic phase of stroke and that the N400 may provide a valid tracking measure over time. However, given the passive word reading task employed by Barbancho et al., it is unclear whether this relation holds for any or all N400s, regardless of task. Finally, in FXTAS, glutamate modulation by memantine was associated with improvements in N400 repetition effect and some measures of verbal memory.
As evidenced by these studies, direct clinical data combining language disorders, ERPs, and glutamate pharmacology are scarce, but the existing literature suggests that they may be interrelated and that glutamate is involved in language processing and performance. This is not surprising, considering that glutamate is the most ubiquitous excitatory neurotransmitter in the cortex, and many aphasias are considered classical examples of disrupted higher cortical function. Cortical structures involved in language processing, despite being topographically distributed, seem to share a similar receptor fingerprint, including a unique density of kainite and NMDA receptors.138 (Zilles 2015)
Moreover, models of widely-distributed functional language networks, such as Quillian’s spreading cortical activation of semantic search3,253 (Quillian 1967, Collins & Loftus 1975) might well be explained by glutamatergic stimulation of neighboring cortical neurons. Further evaluation of language-associated ERPs, particularly N400 congruency and SPS/P600 effects (which are relatively independent of memory function) would help to verify whether these ERP effects are reliable indicators of treatment response. Once the ERP effects have been validated in that setting, we would hope to see more studies aimed at optimizing language processing by modulating glutamate neurotransmission.
4. Discussion
4.1. On Disease Models
All three language disease models discussed in this article are associated with some degree of glutamate dysregulation. We chose AD and FXTAS to represent different neurodegenerative pathologies and Aphasic Stroke Syndromes to represent acquired language disorders. Other promising disease models for studying the association between glutamate and language disorders include developmental disorders such as fragile X-syndrome and autism spectrum disorder. Here we briefly discuss these models as they can provide important insights into the role of glutamate receptors in language development.
The mGluR5 theory of FXS posits that mGluR5 expression is increased in the hippocampus due to loss of translational regulation by FMRP. Increased mGluR5 leads to decreased AMPAR expression, increased LTD, and decreased dendritic and synaptic strength.243 (Pop 2014) Chronic treatment with long-acting mGluR5 antagonist CTEP in mice of FXS restored cognitive deficits, aberrant dendritic spine density, and overactive ERK and mTOR signaling.175 (Michalon 2012) In a clinical study, a subset of FXS patients with full methylation at the FMR1 promotor site reported significant improvements in aberrant social behavior (i.e. stereotypic behavior, hyperactivity, and inappropriate speech) in response to the selective mGluR5 antagonist mavoglurant.254 (Jacquemont 2011) Currently there is a clinical study underway at our institution to determine if metformin, which may inhibit glutamate induced excitotoxicity in neurons255 (Zhou 2016), improves language outcomes in FXS patients.256 (Hagerman 2018)
Autism spectrum disorder is often co-morbid with FXS, and milder social deficits occasionally occur in FXTAS patients257 (Hagerman 2013), and also is associated with glutamate dysregulation258 (Rojas 2014) and language processing deficits259 (Lewis 2006). FMRP levels are reduced in the cerebellar vermis of ASD patients and is associated with increased mGluR5 and reduced GABAA receptor beta 3 expression in the same region.260 (Fatemi 2011) AMPAR receptor density are also reduced in the cerebellum of ASD patients261 (Purcell 2001), whereas NMDAR receptor subunits GluN2A and GluN2B have been shown to be increased in rodent ASD models262 (Rinaldi 2007).
Mutations in the GRIN2B gene which encodes for the GluN2B subunit of NMDAR are associated with autism, intellectual disability,263,264 (Endele 2010, Talkowski 2012) and developmental language disorders265,266 (Ocklenberg 2011, Hu 2016). Ocklenberg et al postulate that variations in NMDAR efficiency could modulate interhemispheric transfer via the corpus callosum (transmission over the corpus collosum is predominantly glutamatergic267 (Kumar 2001)), subsequently influencing language lateralization during development.265 (Ocklenburg 2011) Based on this, interhemispheric communication mediated by glutamate signaling may play a role in the normal development of language. Glutamate dysregulation during development may lead to the onset of language disorders early in life, such as in FXS, or contribute to early language regression as in ASD (or late life regression in FXTAS).
A trial of memantine treatment for 21 months in ASD demonstrated improvements in language function on the Clinical Global Impression Improvement scale.268 (Chez 2007) Studies involving other NMDAR modulators such as acamprosate, amantadine, and minocycline and mGluR5 antagonist AFQ056 are currently underway.258 (Rojas 2014) Combining studies evaluating the efficacy of glutamate modulators with neuropsychologic testing and/or language-related ERP studies, could contribute to our understanding of the role of glutamate neurotransmission in language processing and language development.
4.2. On Pharmacologic Models
As new pharmacologic agents increasingly target specific glutamate receptor subclasses, more options will become available for clinical studies in diseases of glutamate dysregulation. mGluR positive and negative allosteric modulators in particular have shown promise in reversing cognitive impairment, suppressing neuroinflammation, and promoting neuronal survival, possibly independently and via modulation of NMDAR function.269 (Bruno 2017) New AMPAR modulators are also undergoing clinical study and may be beneficial in improving memory and attention via upregulation of BDNF and rescue of dysfunctional synaptic plasticity/LTP.270 (Partin 2015)
It is also possible that targeted therapy at GABA receptors may in part rescue the excitatory-inhibitory imbalance caused by glutamate dysregulation. The effects of piracetam in stroke aphasia patients may in part be due to GABA modulation.238,239 (Kessler 2000, Gungor 2011) There is at least one case report of the GABA-transaminase agonist imipramine improving verbal/language skills in infantile autism, possibly by increasing neuron-to-oligodendrocyte signaling in the corpus callosum.271 (Cohen 2002) Evaluating the efficacy of these agents on language performance would contribute to the further elucidation of the role of glutamate (and GABA) neurotransmission in language disorders.
4.3. On Testing Instruments
As demonstrated in the ERP and Pharmacology section above, there is some variability in ERP results that may lead to seemingly contradictory conclusions. Some possible reasons for this variability include: variability in experimental designs and data recording procedures, artifact rejection or correction, and the highly technical and challenging nature of ERP data interpretation. Inferences and conclusions drawn from ERP experiments require understanding of the paradigms being used: for example, a common ERP paradigm measures the N400 congruity effect to the ERPs to the final word in a sentence or phrase. However, as Hagoort et al. point out, placing the critical word in sentence-final positions results in activation of other global processing factors, and unacceptable completions seem to elicit enhanced N400-like potentials whether the unacceptability is semantic or syntactic in nature; both of which can confound the study interpretation.195 (Hagoort 2003)
In addition to ERP studies, proton spectroscopy, spectral EEG analysis, and transcranial magnetic stimulation (TMS) may be useful in evaluating the association between language and glutamate. Olson et al. serially measured CNS metabolite concentrations in the posterior cingulate gyrus over 12 months in MCI patients using proton spectroscopy and found an association between glutamate/glutamine concentration and declining performance in Boston Naming Tests in a population subset.272 (Olson 2008) Spectral analysis of EEG signals revealed a diffuse slowing in AD but conflicting results in gamma band oscillations.185 (Horvath 2018) As for TMS, Bracco et al. demonstrated that execution of linguistic tasks modified the excitability of the dominant primary motor cortex to TMS in healthy controls but not in patients with amnestic MCI, suggesting alterations in functional connectivity between language-related brain regions and the motor cortex.273 (Bracco 2009)
Finally, deviations from the normal oscillatory pattern of neuronal networks and the functional connectivity between language and motor brain regions support the idea that amnestic MCI, AD, and vascular aphasia each may represent disorders of cognitive networks, either via a network disconnection274 (Hyman 1984) or an “oscillopathy” with abnormal signaling between connected nodes185 (Horvath 2018). The fact that mGluR and KAR are involved in maintaining normal neuronal oscillatory synchronization, again implicates their dysregulation in the pathogenesis of language and cognitive dysfunction. Nevertheless, despite similarities in clinical presentation of vascular dementia and AD, there are significant topographical and oscillatory differences between the two diseases.275 (Neto 2015)
5. Conclusion
In this article, we have proposed that glutamate neurotransmission may play a key role in normal physiological processing of language and in the pathogenesis of several language disorders. We reviewed the mechanisms of action of NMDAR, AMPAR, KAR, and mGluR and the role these glutamate receptors and subtypes may play in semantic and lexical processing and in maintaining widely-distributed functional language comprehension and production networks. We evaluated the preclinical data that glutamate dysregulation contributes to the pathogenesis in three disorders of language processing (AD, FXTAS, and Aphasic Stroke Syndromes). The likely importance of GABA-glutamate balance for normal language processing was also discussed. Finally, we presented clinical ERP and pharmacologic evidence demonstrating improvements in language function in all three as a result of glutamate modulation.
Given the ubiquity of glutamate in the CNS, its role in language processing is likely heterogeneous with overlapping functions of the four receptor subclasses. Maintenance of synaptic plasticity and normal neuronal oscillations appears to be crucial in the access and retrieval of semantic information and the processing and evaluation of syntactically anomalous or ambiguous sentences.
Early language deficits in the neurodegenerative diseases AD and FXTAS may be associated with deficits in implicit and/or explicit memory function(s), whereas the conceptual and lexical stores are relatively unaffected. The NMDAR antagonist memantine appears to improve memory and language dysfunction in these diseases, suggesting that
NMDAR dysregulation may be a part of the disease mechanism. Modulation of NMDAR activity also may improve chronic language deficits in acquired aphasias due to stroke, suggesting continued dysregulation in glutamate transmission one year after the ischemic insult. Based on associated ERP studies, the electrophysiological drivers and the language processing component affected are likely different between the two neurodegenerative disorders, and language deficits in aphasic stroke patients are likely associated with both reduced access to conceptual/semantic information and difficulty achieving syntactic integration.
The extant literature is skewed towards NMDAR function given the limited number of non-NMDA glutamate modulators in clinical and preclinical studies. Preclinical data suggest that AMPAR and mGluR also participate in regulation of synaptic plasticity and neuronal health, while mGluR and KAR may be associated with metaplasticity and maintenance of synchronous neuronal oscillations and distributed language networks. Glutamate to GABA ratios, as well as receptor fingerprints unique to language processing structures, likely also contribute to glutamate’s role in language. The data presented here provide an initial framework for the glutamate-language hypothesis; the advent of novel pharmacologic agents and standardization of language-associated test paradigms (including ERP, spectral EEG, and proton spectroscopy) arguably can help validate the role of glutamate neurotransmission in normal language function and deepen our understanding of how glutamate dysregulation impacts specific language disorders.
Highlights.
L-Glutamate neurotransmission likely plays a physiologic role in the regulation of language access, comprehension, and production.
NMDA, AMPA, and mGlu Receptor dysregulation is implicated in the pathogenesis of Alzheimer’s Disease, FXTAS, and Stroke, and may contribute to language dysfunction in these diseases.
Memantine, a selective NMDA receptor antagonist, improves language function in Alzheimer’s Disease, FXTAS, and Post-Stroke Aphasia patients based on patient report and language-battery tests.
Memantine normalizes language-related ERPs (closer to healthy-controls) in post-stroke aphasia and FXTAS patients.
Ketamine, another NMDAR receptor antagonist, affects language-related ERPs in non-aphasic subjects.
Funding Acknowledgements:
Supported by NIH grants R01-AG048252 and P30-AG10129 to JO, R01-HD22614 to MK, and R01-HD036071 to RH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Traynelis SF et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–96 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quillian MR Word concepts: A theory and simulation of some basic semantic capabilities. Behav. Sci. 12, 410–430 (1967). [DOI] [PubMed] [Google Scholar]
- 3.Collins AM & Loftus EF A spreading-activation theory of semantic processing. Psychol. Rev 82, 407–428 (1975). [Google Scholar]
- 4.Kiefer M. The N400 is modulated by unconsciously perceived masked words: further evidence for an automatic spreading activation account of N400 priming effects. Brain Res. Cogn. Brain Res 13, 27–39 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Meldru BS. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr 130, 1007S–1015S (2000). [DOI] [PubMed] [Google Scholar]
- 6.Makino H. & Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64, 381–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L. et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Banke TG et al. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci 20, 89–102 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin DA et al. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J. Neurosci 30, 11565–75 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Amelio M. et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci 14, 69–76 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Huettner JE Glutamate receptor pores. J. Physiol 593, 49–59 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollmuth LP Ion permeation in ionotropic glutamate receptors: still dynamic after all these years. Curr. Opin. Physiol 2, 36–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henley JM & Wilkinson KA Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci 17, 337–350 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Shi S-H, Hayashi Y, Esteban JA & Malinow R. Subunit-Specific Rules Governing AMPA Receptor Trafficking to Synapses in Hippocampal Pyramidal Neurons. Cell 105, 331–343 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Wang X. -b. & Zhou Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc. Natl. Acad. Sci 107, 11999–12004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park P. et al. The Role of Calcium-Permeable AMPARs in Long-Term Potentiation at Principal Neurons in the Rodent Hippocampus. Front. Synaptic Neurosci 10, 42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torquatto KI, Menegolla AP, Popik B, Casagrande MA & de Oliveira Alvares L. Role of calcium- permeable AMPA receptors in memory consolidation, retrieval and updating. Neuropharmacology 144, 312–318 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Twomey EC, Yelshanskaya MV, Vassilevski AA & Sobolevsky AI Mechanisms of Channel Block in Calcium-Permeable AMPA Receptors. Neuron 99, 956–968.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcomb DJ et al. Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus. Sci. Rep 5, 10934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JW et al. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 72, 1295–303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge MJ Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci 371, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brawek B. & Garaschuk O. Network-wide dysregulation of calcium homeostasis in Alzheimer’s disease. Cell Tissue Res. 357, 427–438 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Noh K-M et al. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. U. S. A 102, 12230–5 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz-Clemente A, Nicoll RA & Roche KW Diversity in NMDA Receptor Composition. Neurosci. 19, 62–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henson MA et al. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb. Cortex 18, 2560–73 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broutman G. & Baudry M. Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J. Neurosci 21, 27–34 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau CG et al. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem. Soc. Trans 37, 1369–74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardingham GE. & Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci 11, 682–96 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panja D. & Bramham CR BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology 76, 664–676 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Zhang S-J et al. Nuclear Calcium Signaling Controls Expression of a Large Gene Pool: Identification of a Gene Program for Acquired Neuroprotection Induced by Synaptic Activity. PLoS Genet. 5, e1000604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Léveillé F. et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J. Neurosci 30, 2623–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dick O. & Bading H. Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors. J. Biol. Chem 285, 19354–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petralia RS et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience 167, 68–87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papouin T. & Oliet SHR Organization, control and function of extrasynaptic NMDA receptors. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369, 20130601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira IL et al. Amyloid beta peptide 1–42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium 51, 95–106 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Yang Q. & Li S. Activation of extrasynaptic NMDA receptors induces LTD in rat hippocampal CA1 neurons. Brain Res. Bull 93, 10–16 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Collingridge GL, Peineau S, Howland JG & Wang YT Long-term depression in the CNS. Nat. Rev. Neurosci 11, 459–473 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Zhou X. et al. Extrasynaptic NMDA Receptor in Excitotoxicity. Neurosci. 21, 337–344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipton SA Failures and Successes of NMDA Receptor Antagonists: Molecular Basis for the Use of Open-Channel Blockers like Memantine in the Treatment of Acute and Chronic Neurologic Insults. NeuroRX 1, 101–110 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleming TM et al. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J. Physiol 589, 3929–3941 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fellin T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–43 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Laycock MV, de Freitas ASW & Wright JLC Glutamate agonists from marine algae. J. Appl. Phycol 1, 113–122 (1989). [Google Scholar]
- 43.Yang EJ, Harris AZ & Pettit DL Synaptic kainate currents reset interneuron firing phase. J. Physiol 578, 259–273 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH & Mulle C. Kainate Receptors Act as Conditional Amplifiers of Spike Transmission at Hippocampal Mossy Fiber Synapses. J. Neurosci 29, 5000–5008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castillo PE, Malenka RC & Nicoll RA Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Carta M, Fièvre S, Gorlewicz A. & Mulle C. Kainate receptors in the hippocampus. Eur. J. Neurosci 39, 1835–1844 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Ruiz A, Sachidhanandam S, Utvik JK, Coussen F. & Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J. Neurosci 25, 11710–8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang L, Xu J, Nedergaard M. & Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron 30, 503–13 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Lourenço J. et al. Synaptic activation of kainate receptors gates presynaptic CB1 signaling at GABAergic synapses. Nat. Neurosci 13, 197–204 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Goldin M. et al. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J. Neurosci 27, 9560–72 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakashiba T, Young JZ, McHugh TJ, Buhl DL & Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–4 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Fisahn A. et al. Distinct Roles for the Kainate Receptor Subunits GluR5 and GluR6 in Kainate-Induced Hippocampal Gamma Oscillations. J. Neurosci 24, 9658–9668 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hart J. et al. Neural substrates of semantic memory. J. Int. Neuropsychol. Soc 13, 865–880 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Niswender CM & Conn PJ Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol 50, 295–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page G. et al. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem. Int 49, 413–421 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Romano C. et al. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol 355, 455–469 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Attucci S, Carlà V, Mannaioni G. & Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br. J. Pharmacol 132, 799–806 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gubellini P, Pisani A, Centonze D, Bernardi G. & Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog. Neurobiol 74, 271–300 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Awad H, Hubert GW, Smith Y, Levey AI & Conn PJ Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J. Neurosci 20, 7871–9 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lüscher C. & Huber KM Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S. et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fellin T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–43 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Whittington MA, Traub RD & Jefferys JGR Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Davidson S. et al. Group II mGluRs suppress hyperexcitability in mouse and human nociceptors. Pain 157, 2081–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hermes MLHJ & Renaud LP Postsynaptic and presynaptic group II metabotropic glutamate receptor activation reduces neuronal excitability in rat midline paraventricular thalamic nucleus. J. Pharmacol. Exp. Ther 336, 840–9 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Nicoletti F. et al. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 60, 1017–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knoflach F. & Kemp JA Metabotropic glutamate group II receptors activate a G protein-coupled inwardly rectifying K + current in neurones of the rat cerebellum. J. Physiol 509, 347–354 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corti C. et al. The Use of Knock-Out Mice Unravels Distinct Roles for mGlu2 and mGlu3 Metabotropic Glutamate Receptors in Mechanisms of Neurodegeneration/Neuroprotection. J. Neurosci 27, 8297–8308 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pelkey KA, Lavezzari G, Racca C, Roche KW & McBain CJ mGluR7 Is a Metaplastic Switch Controlling Bidirectional Plasticity of Feedforward Inhibition. Neuron 46, 89–102 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Martín R. et al. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc- 13–1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J. Biol. Chem 285, 17907–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gogliotti RG et al. mGlu7 potentiation rescues cognitive, social, and respiratory phenotypes in a mouse model of Rett syndrome. Sci. Transl. Med 9, eaai7459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abraham WC Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci 9, 387–387 (2008). [DOI] [PubMed] [Google Scholar]
- 73.McKhann GM et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement 7, 263–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taler V. & Phillips NA Language performance in Alzheimer’s disease and mild cognitive impairment: A comparative review. J. Clin. Exp. Neuropsychol 30, 501–556 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Auriacombe S. et al. A longitudinal study of quantitative and qualitative features of category verbal fluency in incident Alzheimer’s disease subjects: results from the PAQUID study. Dement. Geriatr. Cogn. Disord 21, 260–6 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Verma M. & Howard RJ Semantic memory and language dysfunction in early Alzheimer’s disease: a review. Int. J. Geriatr. Psychiatry 27, 1209–1217 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Chan AS, Salmon DP & De La Pena J. Abnormal Semantic Network for “Animals” But not “Tools” in Patients with Alzheimer’s Disease. Cortex 37, 197–217 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Henry JD, Crawford JR & Phillips LH Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia 42, 1212–1222 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Clark LJ et al. Longitudinal Verbal Fluency in Normal Aging, Preclinical, and Prevalent Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr 24, 461–468 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Auriacombe S. et al. A longitudinal study of quantitative and qualitative features of category verbal fluency in incident Alzheimer’s disease subjects: results from the PAQUID study. Dement. Geriatr. Cogn. Disord 21, 260–6 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Laisney M. et al. When the zebra loses its stripes: Semantic priming in early Alzheimer’s disease and semantic dementia. Cortex 47, 35–46 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Joubert S. et al. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 48, 978–988 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Binder JR, Desai RH, Graves WW & Conant LL Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb. Cortex 19, 2767–2796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lambon Ralph MA Neurocognitive insights on conceptual knowledge and its breakdown. Philos. Trans. R. Soc. B Biol. Sci 369, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olichney JM et al. fMRI responses to words repeated in a congruous semantic context are abnormal in mild Alzheimer’s disease. Neuropsychologia 48, 2476–2487 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peelle JE, Powers J, Cook PA, Smith EE & Grossman M. Frontotemporal neural systems supporting semantic processing in Alzheimer’s disease. Cogn. Affect. Behav. Neurosci 14, 37–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danysz W. & Parsons CG Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine - searching for the connections. Br. J. Pharmacol 167, 324–352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardingham GE, Fukunaga Y. & Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci 5, 405–414 (2002). [DOI] [PubMed] [Google Scholar]
- 89.Li S. et al. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci 31, 6627–38 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bading H. Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J. Exp. Med 214, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Li P, Feng J. & Wu M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci 37, 1039–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rush T. & Buisson A. Reciprocal disruption of neuronal signaling and Aβ production mediated by extrasynaptic NMDA receptors: a downward spiral. Cell Tissue Res. 356, 279–286 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Snyder EM et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci 8, 1051–1058 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Hu R. et al. Overexpression of EphB2 in hippocampus rescues impaired NMDA receptors trafficking and cognitive dysfunction in Alzheimer model. Cell Death Dis. 8, e2717–e2717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li W. & Keifer J. BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol. Learn. Mem 91, 243–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uhász GJ et al. Fibrillar Abeta (1–42) enhances NMDA receptor sensitivity via the integrin signaling pathway. J. Alzheimers. Dis 19, 1055–67 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Minkeviciene R. et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci 29, 3453–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ittner LM et al. Dendritic Function of Tau Mediates Amyloid-β Toxicity in Alzheimer’s Disease Mouse Models. Cell 142, 387–397 (2010). [DOI] [PubMed] [Google Scholar]
- 99.van Dyck CH et al. OC1: Phase 2A Trial of AZD0530 Evaluating 18F-FDG PET, Safety, and Tolerability in Mild Alzheimer’s Dementia. in The Journal of Prevention of Alzheimer’s Disease-JPAD© 5, (2018). [Google Scholar]
- 100.Cerpa W. et al. Wnt-5a occludes Aβ oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol. Neurodegener 5, 3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao W-Q et al. Inhibition of Calcineurin-mediated Endocytosis and α-Amino-3-hydroxy-5-methyl-4- isoxazolepropionic Acid (AMPA) Receptors Prevents Amyloid β Oligomer-induced Synaptic Disruption. J. Biol. Chem 285, 7619–7632 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palop JJ & Mucke L. Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci 13, 812–818 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoover BR et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jo J. et al. Aβ1–42 inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK- 3β. Nat. Neurosci 14, 545–547 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Chamberlain SEL, Sadowski JHLP, Teles-Grilo Ruivo LM, Atherton LA & Mellor JR Long-term depression of synaptic kainate receptors reduces excitability by relieving inhibition of the slow afterhyperpolarization. J. Neurosci 33, 9536–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Evans AJ, Gurung S, Wilkinson KA, Stephens DJ & Henley JM Assembly, Secretory Pathway Trafficking, and Surface Delivery of Kainate Receptors Is Regulated by Neuronal Activity. Cell Rep. 19, 2613–2626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Renner M. et al. Deleterious Effects of Amyloid β Oligomers Acting as an Extracellular Scaffold for mGluR5. Neuron 66, 739–754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim SH et al. Proneurogenic Group II mGluR antagonist improves learning and reduces anxiety in Alzheimer Aβ oligomer mouse. Mol. Psychiatry 19, 1235–1242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caraci F. et al. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: selective activation of mGlu2 receptors amplifies beta-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol. Pharmacol 79, 618–26 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Hu N-W, Ondrejcak T. & Rowan MJ Glutamate receptors in preclinical research on Alzheimer’s disease: Update on recent advances. Pharmacol. Biochem. Behav 100, 855–862 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Deters KD et al. Genome-wide association study of language performance in Alzheimer’s disease. Brain Lang. 172, 22–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piai V. et al. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci 113, 11366–11371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jun JY., William SR., Sanaei Nezha F. & Lambon Ralp MA. GABA concentrations in the anterior temporal lobe predict human semantic processing. Sci. Rep 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de la Vega A. et al. Individual differences in the balance of GABA to glutamate in pFC predict the ability to select among competing options. J. Cogn. Neurosci 26, 2490–2502 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amieva H, Phillips LH, Della Sala S. & Henry JD Inhibitory functioning in Alzheimer’s disease. Brain 127, 949–964 (2004). [DOI] [PubMed] [Google Scholar]
- 116.Berker EA, Berker AH & Smith A. Translation of Broca’s 1865 report. Localization of speech in the third left frontal convolution. Arch. Neurol 43, 1065–72 (1986). [DOI] [PubMed] [Google Scholar]
- 117.Kirshner H. Aphasia and aphasic syndromes. in Bradley’s Neurology in Clinical Practice. (eds. Daroff R, Jankovic J, Mazziotta J. & Pomeroy S) Chap 13 (2016). [Google Scholar]
- 118.Wassenaar M. & Hagoort P. Word-category violations in patients with Broca’s aphasia: An ERP study. Brain Lang. 92, 117–137 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. Neuroimage 20, S18–S29 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Hillis AE Aphasia: Progress in the last quarter of a century. Neurology 69, 200–213 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Robson H, Sage K. & Lambon Ralph MA Wernicke’s aphasia reflects a combination of acoustic- phonological and semantic control deficits: A case-series comparison of Wernicke’s aphasia, semantic dementia and semantic aphasia. Neuropsychologia 50, 266–275 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Robson H, Keidel JL, Lambon Ralph MA & Sage K. Revealing and quantifying the impaired phonological analysis underpinning impaired comprehension in Wernicke’s aphasia. Neuropsychologia (2012). doi: 10.1016/j.neuropsychologia.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 123.Ogar JM et al. Semantic dementia and persisting Wernicke’s aphasia: Linguistic and anatomical profiles. Brain Lang. 117, 28–33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saur D. et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage 49, 3187–3197 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Jefferies E. & Lambon Ralph MA Semantic impairment in stroke aphasia versus semantic dementia: a case- series comparison. Brain 129, 2132–2147 (2006). [DOI] [PubMed] [Google Scholar]
- 126.Robson H. et al. The anterior temporal lobes support residual comprehension in Wernicke’s aphasia. Brain 137, 931–943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thompson HE, Robson H, Lambon Ralph MA & Jefferies E. Varieties of semantic ‘access’ deficit in Wernicke’s aphasia and semantic aphasia. Brain (2015). doi: 10.1093/brain/awv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kraut MA, Calhoun V, Pitcock JA, Cusick C. & Hart J. Neural hybrid model of semantic object memory: Implications from event-related timing using fMRI. J. Int. Neuropsychol. Soc 9, 1031–1040 (2003). [DOI] [PubMed] [Google Scholar]
- 129.Slotnick SD, Moo LR, Kraut MA, Lesser RP & Hart J. Interactions between thalamic and cortical rhythms during semantic memory recall in human. Proc. Natl. Acad. Sci 99, 6440–6443 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nadeau SE & Crosson B. Subcortical Aphasia. Brain Lang. 58, 355–402 (1997). [DOI] [PubMed] [Google Scholar]
- 131.Crosson B. Thalamic mechanisms in language: a reconsideration based on recent findings and concepts. Brain Lang. 126, 73–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carrera E. & Tononi G. Diaschisis: past, present, future. Brain 137, 2408–2422 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Qü M, Mittmann T, Luhmann H. ., Schleicher A. & Zilles K. Long-term changes of ionotropic glutamate and GABA receptors after unilateral permanent focal cerebral ischemia in the mouse brain. Neuroscience 85, 29–43 (1998). [DOI] [PubMed] [Google Scholar]
- 134.Copland DA, Chenery HJ & Murdoch BE Persistent deficits in complex language function following dominant nonthalamic subcortical lesions. J. Med. Speech. Lang. Pathol 8(1), 1–14., (2000). [Google Scholar]
- 135.Nambu A. A new dynamic model of the cortico-basal ganglia loop. Progress in Brain Research 143, 461–466 (2004). [DOI] [PubMed] [Google Scholar]
- 136.Bohsali A. & Crosson B. The Basal Ganglia and Language: A Tale of Two Loops. in 217–242 (Springer, Cham, 2016). doi: 10.1007/978-3-319-42743-0_10 [DOI] [Google Scholar]
- 137.Zilles K. et al. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: Reconciling functional neuroanatomy and neurochemistry. Eur. Neuropsychopharmacol 12, 587–599 (2002). [DOI] [PubMed] [Google Scholar]
- 138.Zilles K, Bacha-Trams M, Palomero-Gallagher N, Amunts K. & Friederici AD Common molecular basis of the sentence comprehension network revealed by neurotransmitter receptor fingerprints. Cortex 63, 79–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pellegrini-Giampietro DE, Zukin RS, Bennett MV, Cho S. & Pulsinelli WA Switch in glutamate receptor subunit gene expression in CA1 subfield of hippocampus following global ischemia in rats. Proc. Natl. Acad. Sci. U. S. A 89, 10499–503 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu SJ & Zukin RS Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 30, 126–134 (2007). [DOI] [PubMed] [Google Scholar]
- 141.López-Valdés HE et al. Memantine enhances recovery from stroke. Stroke 45, 2093–2100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barbancho MA et al. Bilateral brain reorganization with memantine and constraint-induced aphasia therapy in chronic post-stroke aphasia: An ERP study. Brain Lang. 145–146, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 143.Shih AY et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat. Neurosci 16, 55–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattson MP Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci 1144, 97–112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nambu A. et al. Excitatory Cortical Inputs to Pallidal Neurons Via the Subthalamic Nucleus in the Monkey. J. Neurophysiol 84, 289–300 (2000). [DOI] [PubMed] [Google Scholar]
- 146.Slotnick SD, Moo LR, Kraut MA, Lesser RP & Hart J. Interactions between thalamic and cortical rhythms during semantic memory recall in human. Proc. Natl. Acad. Sci 99, 6440–6443 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hamberger MJ et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain 130, 2942–2950 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Salt TE et al. Potentiation of sensory responses in ventrobasal thalamus in vivo via selective modulation of mGlu1 receptors with a positive allosteric modulator. Neuropharmacology 62, 1695–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brouwer JR, Willemsen R. & Oostra BA The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 150B, 782–798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang J-C et al. Abnormal semantic processing in females with fragile X-associated tremor/ataxia syndrome. Genes. Brain. Behav 13, 152–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hagerman RJ & Hagerman P. Fragile X-associated tremor/ataxia syndrome — features, mechanisms and management. Nat. Rev. Neurol 12, 403–412 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Liu J. et al. Signaling defects in iPSC-derived fragile X premutation neurons. Hum. Mol. Genet 21, 3795–805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cornish KM, Hocking DR, Moss SA & Kogan CS Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology 77, 618–622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seritan AL et al. Memantine for fragile X-associated tremor/ataxia syndrome: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 75, 264–71 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Losh M. et al. Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 159B, 660–668 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tassone F. et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 13, 555–62 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tassone F. et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am. J. Med. Genet 91, 144–152 (2000). [DOI] [PubMed] [Google Scholar]
- 158.Kenneson A. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet (2001). doi: 10.1093/hmg/10.14.1449 [DOI] [PubMed] [Google Scholar]
- 159.Kim K. et al. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS One 14, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cornish KM et al. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex 44, 628–636 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hunter JE, Sherman S, Grigsby J, Kogan C. & Cornish K. Capturing the fragile X premutation phenotypes: A collaborative effort across multiple cohorts. Neuropsychology 26, 156–164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goodrich-Hunsaker NJ et al. Young adult female fragile X premutation carriers show age- and genetically- modulated cognitive impairments. Brain Cogn. 75, 255–260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cordeiro L, Abucayan F, Hagerman R, Tassone F. & Hessl D. Anxiety disorders in fragile X premutation carriers: Preliminary characterization of probands and non-probands. Intractable Rare Dis. Res 4, 123–130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Farzin F. et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J. Dev. Behav. Pediatr 27, S137–44 (2006). [DOI] [PubMed] [Google Scholar]
- 165.Chonchaiya W. et al. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum. Genet 131, 581–589 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Berman RF et al. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J. Neurodev. Disord 6, 23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cao Z. et al. Enhanced asynchronous Ca(2+) oscillations associated with impaired glutamate transport in cortical astrocytes expressing Fmr1 gene premutation expansion. J. Biol. Chem 288, 13831–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen Y. et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum. Mol. Genet 19, 196–208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao Z. et al. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum. Mol. Genet 21, 2923–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hunsaker MR, Kim K, Willemsen R. & Berman RF CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus 22, 2260–2275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pretto DI et al. Reduced excitatory amino acid transporter 1 and metabotropic glutamate receptor 5 expression in the cerebellum of fragile X mental retardation gene 1 premutation carriers with fragile X- associated tremor/ataxia syndrome. Neurobiol. Aging 35, 1189–1197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moberget T. & Ivry RB Cerebellar contributions to motor control and language comprehension: Searching for common computational principles. Ann. N. Y. Acad. Sci 1369, 154–171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Burket JA, Herndon AL, Winebarger EE, Jacome LF & Deutsch SI Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: Possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res. Bull 86, 152–158 (2011). [DOI] [PubMed] [Google Scholar]
- 174.Su T. et al. Early continuous inhibition of group 1 mGlu signaling partially rescues dendritic spine abnormalities in the Fmr1 knockout mouse model for fragile X syndrome. Psychopharmacology (Berl). 215, 291–300 (2011). [DOI] [PubMed] [Google Scholar]
- 175.Michalon A. et al. Chronic Pharmacological mGlu5 Inhibition Corrects Fragile X in Adult Mice. Neuron 74, 49–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luck SJ (Steven J. An introduction to the event-related potential technique. [Google Scholar]
- 177.Näätänen R. Implications of ERP data for psychological theories of attention. Biol. Psychol 26, 117–163 (1988). [DOI] [PubMed] [Google Scholar]
- 178.Kutas M. & Federmeier KD Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annu. Rev. Psychol 62, 621–47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Friedman D. & Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc. Res. Tech 51, 6–28 (2000). [DOI] [PubMed] [Google Scholar]
- 180.Kappenman ES & Luck SJ The Oxford Handbook of Event-Related Potential Components. The Oxford Handbook of Event-Related Potential Components (Oxford University Pres, 2012). doi: 10.1093/oxfordhb/9780195374148.001.0001 [DOI] [Google Scholar]
- 181.Duncan CC et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol 120, 1883–908 (2009). [DOI] [PubMed] [Google Scholar]
- 182.Polich J. & Corey-Bloom J. Alzheimer’s disease and P300: review and evaluation of task and modality. Curr. Alzheimer Res 2, 515–25 (2005). [DOI] [PubMed] [Google Scholar]
- 183.Hall M-H et al. Frontal P3 event-related potential is related to brain glutamine/glutamate ratio measured in vivo. Neuroimage 111, 186–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Olichney JM et al. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology 70, 1763–1770 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Horvath A. et al. EEG and ERP biomarkers of Alzheimer’s disease: a critical review. Front. Biosci. (Landmark Ed. 23, 183–220 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Kutas M. & Hillyard SA Brain potentials during reading reflect word expectancy and semantic association. Nature 307, 161–163 (1984). [DOI] [PubMed] [Google Scholar]
- 187.Berkum J. J. A. van, Hagoort P. & Brown CM Semantic Integration in Sentences and Discourse: Evidence from the N400. J. Cogn. Neurosci 11, 657–671 (1999). [DOI] [PubMed] [Google Scholar]
- 188.Olichney JM et al. Word repetition in amnesia: Electrophysiological measures of impaired and spared memory. Brain 123, 1948–1963 (2000). [DOI] [PubMed] [Google Scholar]
- 189.Olichney JM, Yang J-C, Taylor J. & Kutas M. Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer’s disease. J. Alzheimers. Dis 26 Suppl 3, 215–28 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paller KA, Voss JL & Boehm SG Validating neural correlates of familiarity. Trends Cogn. Sci 11, 243–250 (2007). [DOI] [PubMed] [Google Scholar]
- 191.Olichney JM et al. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain 133, 1438–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Friederici AD, Hahne A. & Mecklinger A. Temporal structure of syntactic parsing: Early and late event- related brain potential effects. J. Exp. Psychol. Learn. Mem. Cogn 22, 1219–1248 (1996). [DOI] [PubMed] [Google Scholar]
- 193.Lau E, Stroud C, Plesch S. & Phillips C. The role of structural prediction in rapid syntactic analysis. Brain Lang. 98, 74–88 (2006). [DOI] [PubMed] [Google Scholar]
- 194.Friederici AD Towards a neural basis of auditory sentence processing. Trends Cogn. Sci 6, 78–84 (2002). [DOI] [PubMed] [Google Scholar]
- 195.Hagoort P, Wassenaar M. & Brown CM Syntax-related ERP-effects in Dutch. Cogn. Brain Res 16, 38–50 (2003). [DOI] [PubMed] [Google Scholar]
- 196.Gouvea AC, Phillips C, Kazanina N. & Poeppel D. The linguistic processes underlying the P600. Lang. Cogn. Process 25, 149–188 (2010). [Google Scholar]
- 197.Leckey M. & Federmeier KD The P3b and P600(s): Positive contributions to language comprehension. Psychophysiology 57, 1–15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kaan E, Harris A, Gibson E. & Holcomb P. The P600 as an index of syntactic integration difficulty. Lang. Cogn. Process 15, 159–201 (2000). [Google Scholar]
- 199.Regel S, Meyer L. & Gunter TC Distinguishing neurocognitive processes reflected by P600 effects: evidence from ERPs and neural oscillations. PLoS One 9, e96840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Paller KA, Kutas M. & Mayes AR Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr. Clin. Neurophysiol 67, 360–71 (1987). [DOI] [PubMed] [Google Scholar]
- 201.Rugg MD et al. Dissociation of the neural correlates of implicit and explicit memory. Nature 392, 595–598 [DOI] [PubMed] [Google Scholar]
- 202.Olichney JM et al. Absent event-related potential (ERP) word repetition effects in mild Alzheimer’s disease. Clin. Neurophysiol 117, 1319–1330 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Olichney JM & Hillert DG Clinical applications of cognitive event-related potentials in Alzheimer’s disease. Phys. Med. Rehabil. Clin. N. Am 15, 205–33 (2004). [DOI] [PubMed] [Google Scholar]
- 204.Paller KA Recall and stem-completion priming have different electrophysiological correlates and are modified differentially by directed forgetting. J. Exp. Psychol. Learn. Mem. Cogn 16, 1021–32 (1990). [DOI] [PubMed] [Google Scholar]
- 205.Patel AD, Gibson E, Ratner J, Besson M. & Holcomb PJ Processing Syntactic Relations in Language and Music: An Event-Related Potential Study. J. Cogn. Neurosci 10, 717–733 (1998). [DOI] [PubMed] [Google Scholar]
- 206.Taler V, Klepousniotou E. & Phillips NA Comprehension of lexical ambiguity in healthy aging, mild cognitive impairment, and mild Alzheimer’s disease. Neuropsychologia 47, 1332–1343 (2009). [DOI] [PubMed] [Google Scholar]
- 207.Grieder M. et al. Correlation between Topographic N400 Anomalies and Reduced Cerebral Blood Flow in the Anterior Temporal Lobes of Patients with Dementia. J. Alzheimer’s Dis 36, 711–731 (2013). [DOI] [PubMed] [Google Scholar]
- 208.Bobes MA et al. ERP generator anomalies in presymptomatic carriers of the Alzheimer’s disease E280A PS-1 mutation. Hum. Brain Mapp 31, NA-NA (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Matsunaga S, Kishi T. & Iwata N. Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS One 10, e0123289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xia P, Chen HV, Zhang D. & Lipton SA Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci 30, 11246–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dau A, Gladding CM, Sepers MD & Raymond LA Chronic blockade of extrasynaptic NMDA receptors ameliorates synaptic dysfunction and pro-death signaling in Huntington disease transgenic mice. Neurobiol. Dis 62, 533–542 (2014). [DOI] [PubMed] [Google Scholar]
- 212.Reisberg B. et al. Memantine in Moderate-to-Severe Alzheimer’s Disease. N. Engl. J. Med 348, 1333–1341 [DOI] [PubMed] [Google Scholar]
- 213.Doody R, Wirth Y, Schmitt F. & Möbius HJ Specific functional effects of memantine treatment in patients with moderate to severe Alzheimer’s disease. Dement. Geriatr. Cogn. Disord 18, 227–32 (2004). [DOI] [PubMed] [Google Scholar]
- 214.Peskind ER et al. Memantine Treatment in Mild to Moderate Alzheimer Disease: A 24-Week Randomized, Controlled Trial. Am. J. Geriatr. Psychiatry 14, 704–715 (2006). [DOI] [PubMed] [Google Scholar]
- 215.Schneider LS, Dagerman KS, Higgins JPT & McShane R. Lack of Evidence for the Efficacy of Memantine in Mild Alzheimer Disease. Arch. Neurol 68, 991 (2011). [DOI] [PubMed] [Google Scholar]
- 216.Ferris S. et al. Treatment effects of Memantine on language in moderate to severe Alzheimer’s disease patients. Alzheimer’s Dement. 5, 369–374 (2009). [DOI] [PubMed] [Google Scholar]
- 217.Tariot PN et al. Memantine Treatment in Patients With Moderate to Severe Alzheimer Disease Already Receiving Donepezil. JAMA 291, 317 (2004). [DOI] [PubMed] [Google Scholar]
- 218.Schmitt FA, van Dyck CH, Wichems CH, Olin JT & Memantine MEM-MD-02 Study Group. Cognitive Response to Memantine in Moderate to Severe Alzheimer Disease Patients Already Receiving Donepezil. Alzheimer Dis. Assoc. Disord 20, 255–262 (2006). [DOI] [PubMed] [Google Scholar]
- 219.Tocco M. et al. Effects of memantine treatment on language abilities and functional communication: A review of data. Aphasiology 28, 236–257 (2014). [Google Scholar]
- 220.Kubová Z. et al. Effect of Memantine in Alzheimerʼs Disease Evaluated By Visual-Evoked Potentials to Pattern-Reversal, Motion-Onset, and Cognitive Stimuli. J. Clin. Neurophysiol 27, 334–340 (2010). [DOI] [PubMed] [Google Scholar]
- 221.Robson H, Pilkington E, Evans L, DeLuca V. & Keidel JL Phonological and semantic processing during comprehension in Wernicke’s aphasia: An N400 and Phonological Mapping Negativity Study. Neuropsychologia 100, 144–154 (2017). [DOI] [PubMed] [Google Scholar]
- 222.Hagoort P. & Kutas M. Electrophysiological insights into language deficits. Handbook of Neuropsychology 10, (1995). [Google Scholar]
- 223.Friederici AD & Kotz SA The brain basis of syntactic processes: functional imaging and lesion studies. Neuroimage 20, S8–S17 (2003). [DOI] [PubMed] [Google Scholar]
- 224.Grodzinsky Y. & Friederici AD Neuroimaging of syntax and syntactic processing. Curr. Opin. Neurobiol 16, 240–246 (2006). [DOI] [PubMed] [Google Scholar]
- 225.Swaab T, Brown C. & Hagoort P. Spoken Sentence Comprehension in Aphasia: Event-related Potential Evidence for a Lexical Integration Deficit. J. Cogn. Neurosci 9, 39–66 (1997). [DOI] [PubMed] [Google Scholar]
- 226.Hagoort P, Brown CM & Swaab TY Lexical—semantic event–related potential effects in patients with left hemisphere lesions and aphasia, and patients with right hemisphere lesions without aphasia. Brain 119, 627–649 (1996). [DOI] [PubMed] [Google Scholar]
- 227.Chang C-T, Lee C-Y, Chou C-J, Fuh J-L & Wu H-C Predictability effect on N400 reflects the severity of reading comprehension deficits in aphasia. Neuropsychologia 81, 117–128 (2016). [DOI] [PubMed] [Google Scholar]
- 228.Kotz SA, Frisch S, Von Cramo DV & Friederici AD Syntactic language processing: ERP lesion data on the role of the basal ganglia. J. Int. Neuropsychol. Soc 9, 1053–1060 (2003). [DOI] [PubMed] [Google Scholar]
- 229.Tiedt HO et al. Subcortical Roles in Lexical Task Processing: Inferences from Thalamic and Subthalamic Event-Related Potentials. Hum. Brain Mapp 38, 370–383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wahl M. et al. The Human Thalamus Processes Syntactic and Semantic Language Violations. Neuron 59, 695–707 (2008). [DOI] [PubMed] [Google Scholar]
- 231.Krugel LK, Ehlen F, Tiedt HO, Kühn AA & Klostermann F. Differential impact of thalamic versus subthalamic deep brain stimulation on lexical processing. Neuropsychologia 63, 175–184 (2014). [DOI] [PubMed] [Google Scholar]
- 232.Krugel LK, Ehlen F, Tiedt HO, Kühn AA & Klostermann F. Differential impact of thalamic versus subthalamic deep brain stimulation on lexical processing. Neuropsychologia 63, 175–184 (2014). [DOI] [PubMed] [Google Scholar]
- 233.Wei H. et al. The Therapeutic effect of Memantine through the Stimulation of Synapse Formation and Dendritic Spine Maturation in Autism and Fragile X Syndrome. PLoS One 7, e36981 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Berthier ML et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann. Neurol 65, 577–585 (2009). [DOI] [PubMed] [Google Scholar]
- 235.Wilson KR et al. Changes in N400 topography following intensive speech language therapy for individuals with aphasia. Brain Lang. 123, 94–103 (2012). [DOI] [PubMed] [Google Scholar]
- 236.Berthier ML, Pulvermüller F, Dávila G, Casares NG & Gutiérrez A. Drug Therapy of Post-Stroke Aphasia: A Review of Current Evidence. Neuropsychol. Rev 21, 302–317 (2011). [DOI] [PubMed] [Google Scholar]
- 237.Malykh AG & Sadaie MR Piracetam and Piracetam-Like Drugs. Drugs 70, 287–312 (2010). [DOI] [PubMed] [Google Scholar]
- 238.Kessler J, Thiel A, Karbe H. & Heiss WD Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke 31, 2112–6 (2000). [DOI] [PubMed] [Google Scholar]
- 239.Güngör L, Terzi M. & Onar MK Does long term use of piracetam improve speech disturbances due to ischemic cerebrovascular diseases? Brain Lang. 117, 23–27 (2011). [DOI] [PubMed] [Google Scholar]
- 240.Ortigas MC et al. Improving Fragile X–Associated Tremor/Ataxia Syndrome Symptoms With Memantine and Venlafaxine. J Clin Psychopharmacol 30, 642–644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yang J-C et al. Memantine Effects on Verbal Memory in Fragile X-associated Tremor/Ataxia Syndrome (FXTAS): a Double-Blind Brain Potential Study. Neuropsychopharmacology 39, 2760–2768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hunsaker MR, Kim K, Willemsen R. & Berman RF CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus 22, 2260–2275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pop AS, Gomez-Mancilla B, Neri G, Willemsen R. & Gasparini F. Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology (Berl). 231, 1217–1226 (2014). [DOI] [PubMed] [Google Scholar]
- 244.Yang J-C et al. ERP abnormalities elicited by word repetition in fragile X-associated tremor/ataxia syndrome (FXTAS) and amnestic MCI. Neuropsychologia 63, 34–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Seritan AL et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet 147B, 1138–44 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Smith ME, Stapleton JM & Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr. Clin. Neurophysiol 63, 145–59 (1986). [DOI] [PubMed] [Google Scholar]
- 247.Teyler TJ & Rudy JW The hippocampal indexing theory and episodic memory: Updating the index. Hippocampus 17, 1158–1169 (2007). [DOI] [PubMed] [Google Scholar]
- 248.Piai V. et al. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci 113, 11366–11371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kotermanski SE, Johnson JW & Thiels E. Comparison of behavioral effects of the NMDA receptor channel blockers memantine and ketamine in rats. Pharmacol. Biochem. Behav 109, 67–76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Autry AE et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Grunwald T. et al. Evidence relating human verbal memory to hippocampal N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U. S. A 96, 12085–9 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stróżak P, Abedzadeh D. & Curran T. Separating the FN400 and N400 potentials across recognition memory experiments. Brain Res. 1635, 41–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Quillian MR Word concepts: A theory and simulation of some basic semantic capabilities. Behav. Sci 12, 410–430 (1967). [DOI] [PubMed] [Google Scholar]
- 254.Jacquemont S. et al. Epigenetic Modification of the FMR1 Gene in Fragile X Syndrome Is Associated with Differential Response to the mGluR5 Antagonist AFQ056. Sci. Transl. Med 3, 64ra1–64ra1 (2011). [DOI] [PubMed] [Google Scholar]
- 255.Zhou C. et al. Metformin prevents cerebellar granule neurons against glutamate-induced neurotoxicity. Brain Res. Bull 121, 241–245 (2016). [DOI] [PubMed] [Google Scholar]
- 256.Hagerman RJ A Study of Experimental Treatment With Metformin for Fragile X Syndrome. Available at: https://studypages.com/s/a-study-of-experimental-treatment-with-metformin-for-fragile-x-syndrome-932390/. (Accessed: 9th December 2018)
- 257.Hagerman R. & Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet. Neurol 12, 786–98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rojas DC The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm 121, 891–905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lewis P. et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J. Intellect. Disabil. Res 50, 532–545 (2006). [DOI] [PubMed] [Google Scholar]
- 260.Fatemi SH, Folsom TD, Kneeland RE & Liesch SB Metabotropic glutamate Receptor 5 upregulation in children with autism is associated with underexpression of both fragile X mental retardation protein and GABA A receptor beta 3 in adults with autism. Anat. Rec 294, 1635–1645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Purcell AE, Jeon OH, Zimmerman AW, Blue ME & Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57, 1618–1628 (2001). [DOI] [PubMed] [Google Scholar]
- 262.Rinaldi T, Kulangara K, Antoniello K. & Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Natl. Acad. Sci. U. S. A 104, 13501–13506 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Endele S. et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet 42, 1021–1026 (2010). [DOI] [PubMed] [Google Scholar]
- 264.Talkowski ME et al. Sequencing Chromosomal Abnormalities Reveals Neurodevelopmental Loci that Confer Risk across Diagnostic Boundaries. Cell 149, 525–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ocklenburg S. et al. Variation in the NMDA receptor 2B subunit gene GRIN2B is associated with differential language lateralization. Behav. Brain Res 225, 284–9 (2011). [DOI] [PubMed] [Google Scholar]
- 266.Hu C, Chen W, Myers SJ, Yuan H. & Traynelis SF Human GRIN2B variants in neurodevelopmental disorders. J. Pharmacol. Sci 132, 115–121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kumar SS & Huguenard JR Properties of Excitatory Synaptic Connections Mediated by the Corpus Callosum in the Developing Rat Neocortex. J. Neurophysiol 86, 2973–2985 (2001). [DOI] [PubMed] [Google Scholar]
- 268.Chez MG et al. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: An observation of initial clinical response and maintenance tolerability. J. Child Neurol 22, 574–579 (2007). [DOI] [PubMed] [Google Scholar]
- 269.Bruno V, Caraci F, Copani A, Matrisciano F. & Battaglia G. The impact of metabotropic glutamate receptors into active neurodegenerative processes: A “dark side” in the development of new symptomatic treatments for neurologic and psychiatric disorders. Neuropharmacology 115, 180–192 (2017). [DOI] [PubMed] [Google Scholar]
- 270.Partin KM AMPA receptor potentiators: from drug design to cognitive enhancement. Curr. Opin. Pharmacol 20, 46–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cohen BI Use of a GABA-transaminase agonist for treatment of infantile autism. Med. Hypotheses 59, 115–116 (2002). [DOI] [PubMed] [Google Scholar]
- 272.Bartnik Olson BL et al. Longitudinal Metabolic and Cognitive Changes in Mild Cognitive Impairment Patients. Alzheimer Dis. Assoc. Disord 22, 269–277 (2008). [DOI] [PubMed] [Google Scholar]
- 273.Bracco L. et al. Mild cognitive impairment: loss of linguistic task-induced changes in motor cortex excitability. Neurology 72, 928–34 (2009). [DOI] [PubMed] [Google Scholar]
- 274.Hyman BT, Van Hoesen GW, Damasio AR & Barnes CL Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225, 1168–70 (1984). [DOI] [PubMed] [Google Scholar]
- 275.Neto E, Allen EA, Aurlien H, Nordby H. & Eichele T. EEG Spectral Features Discriminate between Alzheimer’s and Vascular Dementia. Front. Neurol 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]