ABSTRACT
Background
The inflammatory response plays a crucial role in tumor development. Inflammatory markers are recognized prognostic factors in many types of cancer, including gastric cancer. However, the correlation between inflammatory markers and prognosis in remnant gastric cancer (RGC) remains unclear. The aim of this study was to evaluate the importance of inflammatory markers as a prognostic factor in patients who underwent gastrectomy for RGC.
Methods
This multicenter retrospective study involved 107 patients with RGC who underwent curative gastrectomy at 10 institutions in Japan between January 2000 and December 2016. Both overall survival (OS) and relapse-free survival (RFS) were analyzed.
Results
Receiver operating characteristic analyses indicated that the lymphocyte/monocyte ratio (LMR) had a higher area under the curve compared with other potential prognostic factors. Patients were categorized into high- and low LMR groups by the optimal LMR cutoff value. Preoperative LMR was significantly correlated with reconstruction way after the primary surgery (p=0.032) and lymphatic invasion (p=0.046). OS and RFS were significantly worse in the low- vs high LMR groups. Low LMR, T3 or deeper tumor invasion, and low body mass index were independent prognostic factors for OS and RFS.
Conclusion
Preoperative low LMR is associated with poor OS and RFS in patients who undergo gastrectomy for RGC.
Keywords: lymphocyte/monocyte ratio, prognosis, remnant gastric cancer.
The incidence rate of remnant gastric cancer (RGC) is 2.4% among all gastric carcinomas.1 Over time, surgical techniques and chemotherapy protocols for treating gastric cancer have advanced considerably. Consequently, treatment strategies for gastric cancer are clearly outlined in the Japanese guidelines.2 While treatment strategies for primary gastric cancer have been fairly well-established and standardized, there remain numerous unclear points regarding the optimal treatment for RGC, such as the extent of lymph node dissection. Additionally, studies have reported that the 5-year survival rates following gastrectomy are notably worse for patients with stage III RGC compared with those with proximal primary gastric cancer.3 Therefore, the examination of prognostic factors and the development of appropriate treatment strategies for RGC are urgent tasks.
Several studies have reported that inflammatory markers, such as the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and lymphocyte/monocyte ratio (LMR), are significant prognostic factors for numerous gastrointestinal cancers, including gastric cancer, and these factors may influence treatment strategies.4,5,6 The inflammatory response plays a crucial role in the different stages of tumor development, including the promotion, invasion, and metastasis of cancer cells.7 However, there have been no prominent reports on the utility of these inflammatory markers in RGC. This multicenter retrospective study aimed to identify significant inflammatory markers to predict prognosis in patients who undergo curative gastrectomy for RGC.
SUBJECTS AND METHODS
Patients
From January 2000 to December 2016, 171 patients who were diagnosed with RGC underwent gastrectomy in 10 institutions participating in the present study. Among these patients, 8 who underwent procedures other than standard gastrectomy, such as combined resection of other organs, 13 who underwent non-curative gastrectomy, and 43 with missing data on blood sampling results or surgical factors were excluded (Fig. 1). Therefore, we included 107 patients in this analysis. The clinicopathological findings were defined and documented in accordance with the Japanese gastric cancer treatment guidelines.8 Clinical data, namely age, sex, body mass index (BMI), histology, depth of tumor invasion, lymph node metastasis, pathological stage, operation duration, blood loss volume, and surgical procedure for RGC and the primary disease, were collected from the databases of the participating institutions. The Clavien–Dindo classification was used to assess postoperative complications, focusing on events classified as grade ≥ 2.9
Fig. 1.
STROBE diagram. Finally, we included 107 patients in this analysis.
Inflammation and nutritional factors
Peripheral neutrophil, lymphocyte, monocyte, and platelet counts (cells/mm3) were collected from the patients’ medical records. Preoperative blood tests were performed within 30 days before surgery. NLR was calculated by dividing the peripheral neutrophil count by the peripheral lymphocyte count.10 PLR was calculated by dividing the peripheral platelet count by the peripheral lymphocyte count.11 LMR was calculated by dividing the peripheral lymphocyte count by the peripheral monocyte count.12
Statistical analysis
Differences in clinicopathological characteristics between two groups were evaluated using the chi-square test for categorical variables and the Mann–Whitney U test for continuous variables. The Youden index was calculated using receiver operating characteristic analysis for overall survival (OS) to determine optimal cutoffs for NLR, PLR, LMR, age, BMI, and depth of tumor invasion. Survival curves were calculated using the Kaplan–Meier method, and differences between the curves were identified using the log-rank test. Multivariate analyses of factors considered prognostic for OS and relapse-free survival (RFS) were performed using a Cox proportional hazards model. A p value of <0.05 was considered statistically significant. GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) and SPSS for Windows Version 24 (IBM Corp., Armonk, NY) were used for the statistical analyses.
Ethical considerations
The study protocol was approved by the institutional review boards of each participating hospital [Tottori University Hospital ethics committee (19A133); Japanese Red Cross Tottori Hospital ethics committee (82-3); Tottori Prefectural Central Hospital ethics committee (2020-1); Sanin Rosai Hospital ethics committee (2020-13); Matsue City Hospital ethics committee (2A-0005); Hamada Medical Center ethics committee (3072); Yonago Medical Center ethics committee (0204-02); ethics committee of Tottori Prefectural Kousei Hospital (207); Masuda Red Cross Hospital Medical Ethics Committee (75); Saihaku Hospital ethics committee (20072)].
RESULTS
Impact of LMR
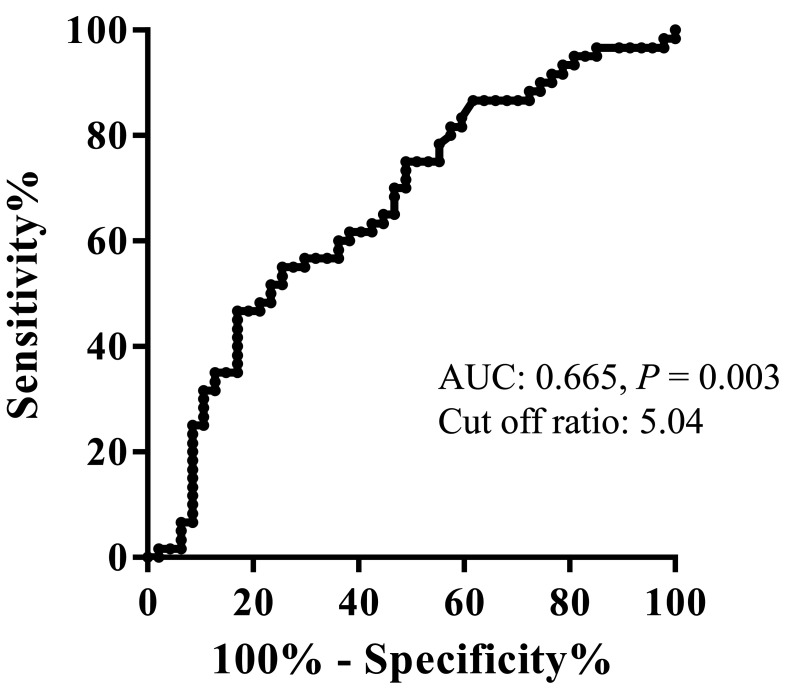
Table 1 shows the areas under the curve (AUC) for several potential prognostic factors that were determined using receiver operating characteristic analysis for the OS of all 107 patients. Among these, LMR had the highest AUC and predictive value. On the basis of this analysis, the optimal LMR cutoff value for OS was determined (Fig. 2), and the patients were divided into a high LMR (LMRHigh, n = 92) and low LMR (LMRLow, n = 159) groups. The patients’ characteristics in both groups are shown in Table 2. Preoperative LMR was significantly correlated with reconstruction way after the primary surgery (P = 0.032) and lymphatic invasion (P = 0.046).
Table 1. Receiver operating characteristic analysis for overall survival (OS).
| Analysis for OS | ||
| Variables | AUC | P-Value |
| NLR | 0.596 | 0.088 |
| PLR | 0.589 | 0.057 |
| LMR | 0.665 | 0.003 |
AUC, area under the curve; LMR, lymphocyte/monocyte ratio; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Fig. 2.
Receiver operating characteristic curves for the lymphocyte/monocyte ratio for overall survival. Lymphocyte/monocyte ratio had the highest area under the curve and predictive value. The optimal lymphocyte/monocyte ratio cutoff value for overall survival was 5.04.
Table 2. Comparison of patient characteristics in the LMRLow and LMRHigh groups.
| Variables | LMRLow | LMRHigh | P-Value |
| (n = 71) | (n = 36) | ||
| Age (years) | 0.35 | ||
| Median (range) | 75 (49–89) | 70 (48–85) | |
| Sex | 0.20 | ||
| Male | 62 (74%) | 28 (73%) | |
| Female | 9 (26%) | 8 (27%) | |
| Histology of the primary disease | 0.28 | ||
| Benign | 27 (35.2%) | 9 (25%) | |
| Malignant | 46 (64.8%) | 27 (75%) | |
| Procedures of primary surgery | 0.71 | ||
| Distal gastrectomy | 64 (90.1%) | 32 (88.9%) | |
| Proximal gastrectomy | 7 (9.9%) | 4 (11.1%) | |
| Reconstruction after primary surgery | 0.032 | ||
| Billroth I | 36 (50.7%) | 28 (77.8%) | |
| Billroth II | 23 (32.4%) | 4 (11.1%) | |
| Roux-en-Y | 3 (4.2%) | 0 (0.0%) | |
| Others | 9 (12.7%) | 4 (11.1%) | |
| Tumor size (cm) | 0.84 | ||
| Median (range) | 3.4 (0.5–14) | 3.8 (0.5–9) | |
| Histology | 0.13 | ||
| differentiated | 50 (70.4%) | 20 (55.6%) | |
| undifferentiated | 21 (29.6%) | 16 (44.4%) | |
| Depth of invasion | 0.28 | ||
| T1/2 | 46 (64.8%) | 27 (75.0%) | |
| T3/4 | 25 (35.2%) | 9 (25.0%) | |
| Lymph node metastasis (present) | 18 (25.4%) | 5 (13.9%) | 0.17 |
| Lymphatic invasion (present) | 44 (62.0%) | 15 (41.7%) | 0.046 |
| Venous invasion (present) | 44 (62.0%) | 17 (47.2%) | 0.15 |
| Stage of disease | 0.34 | ||
| I | 42 (59.2%) | 26 (72.2%) | |
| II | 20 (28.2%) | 8 (22.2%) | |
| III | 9 (12.7%) | 2 (5.6%) | |
| Operative procedure | 0.51 | ||
| Total gastrectomy | 67 (94.4%) | 35 (97.2%) | |
| Distal gastrectomy | 4 (5.6%) | 1 (2.8%) | |
| Approach | 0.80 | ||
| Open | 64 (90.1%) | 33 (91.7%) | |
| Laparoscopic | 7 (9.9%) | 3 (8.3%) | |
| Postoperative complication (present) | 19 (26.8%) | 4 (11.1%) | 0.063 |
| Lymphocytes (cells/mm3) | |||
| Median (range) | 1340 (106–2883) | 1734 (270–3948) | 0.001 |
| Monocytes (cells/mm3) | |||
| Median (range) | 420 (164–3000) | 276 (34–658) | < 0.001 |
TNM staging was in accordance with the 15th edition of the Japanese classification of gastric carcinoma. TNM, tumor, lymph nodes, metastasis.
LMR as a risk factor for OS
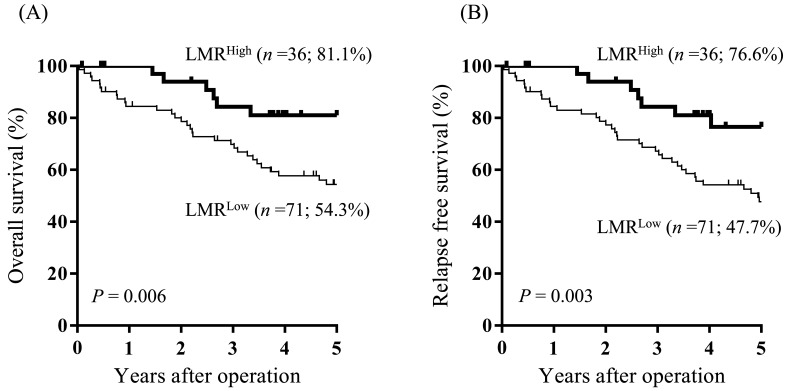
The OS rate in the LMRLow group was significantly worse than that in the LMRHigh group (5-year OS, 54.3% vs 81.1%, respectively; P = 0.006; Fig. 3A).
Fig. 3.
Overall survival- (A) and relapse-free survival (B) curves by the lymphocyte/monocyte ratio. The overall and relapse-free survival rates in the LMRLow group were significantly worse than that in the LMRHigh group.
Univariate analysis revealed that OS was significantly worse in patients with low BMI, positive lymphatic invasion, positive venous invasion, T3 or deeper tumor invasion, positive lymph node metastasis, and low LMR. Multivariate analysis revealed that low BMI, T3 or deeper tumor invasion, and low LMR were independent prognostic factors for OS (Table 3).
Table 3. Univariate and multivariate analyses of overall survival.
| Variables | Univariate analysis | P-Value | Multivariate analysis | P-Value |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Age ≥ 75 | 1.177 (0.6592.102) | 0.581 | ||
| Sex (male) | 0.977 (0.434–2.200) | 0.955 | ||
| BMI < 19.83 | 2.336 (1.255–4.346) | 0.007 | 2.415 (1.270–4.592) | 0.007 |
| Lymphatic invasion | 3.233 (1.639–6.376) | < 0.001 | 1.859 (0.797–4.337) | 0.151 |
| Venous invasion | 2.645 (1.359–5.146) | 0.004 | 1.136 (0.498–2.591) | 0.762 |
| pT ≥ 3 | 3.380 (1.881–6.073) | < 0.001 | 2.372 (1.168–4.818) | 0.017 |
| pN + | 2.432 (1.322–4.472) | 0.004 | 1.128 (0.535–2.377) | 0.752 |
| LMR < 5.04 | 2.764 (1.291–5.920) | 0.009 | 2.868 (1.297–6.344) | 0.009 |
BMI, body mass index; CI, confidence interval; LMR, lymphocyte/monocyte ratio; pN, pathological lymph node metastasis; pT, pathological depth of invasion.
LMR as a risk factor for RFS
The RFS rate in the LMRLow group was significantly worse than that in the LMRHigh group (5-year RFS, 47.7% vs 76.6%, respectively; P = 0.003; Fig. 3B).
Univariate analysis for RFS revealed that the significant risk factors were low BMI, positive lymphatic invasion, positive venous invasion, T3 or deeper tumor invasion, positive lymph node metastasis, and low LMR. Multivariate analysis revealed that low BMI, T3 or deeper tumor invasion, and low LMR were independent prognostic factors for RFS (Table 4).
Table 4. Univariate and multivariate analyses of recurrence-free survival.
| Variables | Univariate analysis | P-Value | Multivariate analysis | P-Value |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Age ≥ 75 | 1.253 (0.730–2.149) | 0.413 | ||
| Sex (male) | 0.992 (0.465–2.118) | 0.984 | ||
| BMI < 19.83 | 2.239 (1.263–3.969) | 0.006 | 2.323 (1.288–4.189) | 0.005 |
| Lymphatic invasion | 2.608 (1.432–4.749) | 0.002 | 1.423 (0.665–3.045) | 0.364 |
| Venous invasion | 2.591 (1.398–4.801) | 0.002 | 1.350 (0.625–2.918) | 0.445 |
| pT ≥ 3 | 3.118 (1.805–5.386) | < 0.001 | 2.220 (1.150–4.285) | 0.017 |
| pN + | 2.158 (1.210–3.849) | 0.009 | 1.084 (0.532–2.207) | 0.824 |
| LMR < 5.04 | 2.824 (1.379–5.780) | 0.005 | 2.990 (1.422–6.288) | 0.004 |
BMI, body mass index; CI, confidence interval; pN, pathological lymph node metastasis; pT, pathological depth of invasion.
DISCUSSION
This multicenter study revealed that LMR was an independent prognostic factor for both OS and RFS in RGC patients. To the best of our knowledge, this is the first time the usefulness of LMR has been reported in patients with RGC.
Initially conceived as a prognostic marker for hematologic malignancies, LMR is a recognized prognostic marker for many types of cancer, reflecting systemic inflammation.13,14,15 However, the precise mechanisms that link LMR to a poor prognosis are unclear. Because LMR is derived from lymphocyte and monocyte counts, a possible explanation for the mechanism is a relationship with tumor-infiltrating immune cells, which play a crucial role against tumor growth. In particular, tumor-infiltrating lymphocytes are important in the cell-mediated anti-tumor immune response. Therefore, low lymphocyte counts can lead to a compromised immune response against cancer cells.16, 17 Furthermore, high numbers of tumor-infiltrating lymphocytes can result in improved clinical outcomes.18, 19 Notably, monocytes have the ability to differentiate into tumor-associated macrophages (TAMs), which play a pivotal role in tumor development. TAMs promote tumor progression by producing a variety of growth factors and cytokines that induce angiogenesis and suppress immune responses.20, 21 Some reports indicate that patients with high- vs low TAMS infiltration experienced poorer OS.22, 23 Thus, LMR is considered to reflect immune status and has notable potential as a valuable prognostic indicator. As with primary gastric cancer,5, 6, 24 LMR is a prognostic factor in RGC. Among the inflammatory markers, LMR had the highest AUC, and LMR remained significant in the multivariate analysis. Our findings revealed that LMR appeared more useful than other commonly studied inflammatory markers as a prognostic marker.
We identified depth of tumor invasion and underweight status as independent prognostic factors in RGC, as with primary gastric cancer,25, 26 but this was not true for lymph node metastasis. Lymph node metastasis is considered a prognostic factor in primary gastric cancer.27 The Japanese treatment guidelines for primary gastric cancer detail the optimal extent of lymph node dissection.8 Although the effectiveness of lymph node dissection has been extensively studied in RGC,28 the debate regarding the extent of dissection persists, possibly owing to changes in lymphatic flow following the initial surgery. There are also reports suggesting that lymph node metastasis is not a prognostic factor. Matsuo et al. reported T stage and venous invasion as important prognostic factors for RGC,29 and Matsunaga et al. reported T stage and geriatric nutritional risk index as significant predictors of both long- and short-term outcomes for older patients with RGC.30 However, there are conflicting reports indicating lymph node metastasis as an important prognostic factor for RGC.31, 32 Therefore, further investigation is needed regarding lymph node metastasis and lymph node dissection. In the present study, LMR was associated with reconstruction way after the primary surgery and lymphatic invasion. Regardless of the LMR value, a high number of patients underwent distal gastrectomy as their initial surgery. However, in the low LMR group, many patients underwent reconstruction with Billroth II. Katai et al. reported that the frequency of metastasis to the jejunal lymph nodes was higher in patients who had undergone Billroth II or Roux-en-Y reconstruction way.28 Therefore, changes in lymphatic flow due to the initial surgery may be related to LMR in some way.
Our study has several limitations. First, as a retrospective study, it inherently carries biases that may affect the interpretation of the results. Second, despite being a multicenter study, the number of patients was small. Third, the study period for eligible cases spanned 16 years owing to the rarity of RGC and the limited number of cases. Consequently, surgeons’ skills and treatment methods have advanced in this period.
In conclusion, LMR, as an inflammatory marker, is considered a useful prognostic factor in patients with RGC.
Acknowledgments
Acknowledgments: We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Katai H,Ishikawa T,Akazawa K,Isobe Y,Miyashiro I,Oda I,et al. ; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018;21:144-54. 10.1007/s10120-017-0716-7 [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Treatment Guidelines. 2021 (6th edition). Gastric Cancer. 2023;26:1-25. PMID: 36342574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada H,Fukagawa T,Haga Y,Oba K. Does remnant gastric cancer really differ from primary gastric cancer? A systematic review of the literature by the Task Force of Japanese Gastric Cancer Association. Gastric Cancer. 2016;19:339-49. 10.1007/s10120-015-0582-0 [DOI] [PubMed] [Google Scholar]
- 4.Karra S,Gurushankari B,Rajalekshmy MR,Elamurugan TP,Mahalakshmy T,Kate V,et al. Diagnostic Utility of NLR, PLR and MLR in Early Diagnosis of Gastric Cancer: an Analytical Cross-Sectional Study. J Gastrointest Cancer. 2023;54:1322-30. 10.1007/s12029-023-00937-0 [DOI] [PubMed] [Google Scholar]
- 5.Liu HL,Feng X,Tang MM,Zhou HY,Peng H,Ge J,et al. Prognostic significance of preoperative lymphocyte to monocyte ratio in patients with signet ring gastric cancer. World J Gastrointest Surg. 2023;15:1673-83. 10.4240/wjgs.v15.i8.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J,Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: A meta-analysis. Int J Surg. 2018;50:67-71. 10.1016/j.ijsu.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI,Greten FR,Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. PMID: 32060757 [DOI] [PMC free article] [PubMed]
- 9.Dindo D,Demartines N,Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita A,Onoda H,Imai N,Iwaku A,Oishi M,Fushiya N,et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988-93. 10.1038/bjc.2012.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirahara T,Arigami T,Yanagita S,Matsushita D,Uchikado Y,Kita Y,et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19:672. 10.1186/s12885-019-5903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibutani M,Maeda K,Nagahara H,Ohtani H,Sakurai K,Yamazoe S,et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21:9966-73. 10.3748/wjg.v21.i34.9966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porrata LF,Ristow K,Colgan JP,Habermann TM,Witzig TE,Inwards DJ,et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica. 2012;97:262-9. 10.3324/haematol.2011.050138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu Z,Wang Q,Wang H,Jiao Y,Li M,Wei W,et al. The Effect of Inflammatory Markers on the Survival of Advanced Gastric Cancer Patients Who Underwent Anti-Programmed Death 1 Therapy. Front Oncol. 2022;12:783197. 10.3389/fonc.2022.783197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishijima TF,Muss HB,Shachar SS,Tamura K,Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:971-8. 10.1016/j.ctrv.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Man Y,Stojadinovic A,Mason J,Avital I,Bilchik A,Bruecher B,et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4:84-95. 10.7150/jca.5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh SY,Kim YB,Suh KW. Prognostic significance of systemic inflammatory response in stage II colorectal cancer. J Surg Res. 2017;208:158-65. 10.1016/j.jss.2016.08.100 [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud SMA,Paish EC,Powe DG,Macmillan RD,Grainge MJ,Lee AHS,et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949-55. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 19.Gooden MJM,de Bock GH,Leffers N,Daemen T,Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93-103. 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanmee T,Ontong P,Konno K,Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6:1670-90. 10.3390/cancers6031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeNardo DG,Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369-82. 10.1038/s41577-019-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komohara Y,Jinushi M,Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1-8. 10.1111/cas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsutsui S,Yasuda K,Suzuki K,Tahara K,Higashi H,Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425-31. 10.3892/or.14.2.425 [DOI] [PubMed] [Google Scholar]
- 24.Lin JP,Lin JX,Cao LL,Zheng CH,Li P,Xie JW,et al. Preoperative lymphocyte-to-monocyte ratio as a strong predictor of survival and recurrence for gastric cancer after radical-intent surgery. Oncotarget. 2017;8:79234-47. 10.18632/oncotarget.17058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Migita K,Takayama T,Matsumoto S,Wakatsuki K,Tanaka T,Ito M,et al. Impact of being underweight on the long-term outcomes of patients with gastric cancer. Gastric Cancer. 2016;19:735-43. 10.1007/s10120-015-0531-y [DOI] [PubMed] [Google Scholar]
- 26.Tokunaga M,Hiki N,Fukunaga T,Ohyama S,Yamaguchi T,Nakajima T. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol. 2009;16:3245-51. 10.1245/s10434-009-0645-8 [DOI] [PubMed] [Google Scholar]
- 27.Yokota T,Ishiyama S,Saito T,Teshima S,Narushima Y,Murata K,et al. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380-4. 10.1080/00365520310008629 [DOI] [PubMed] [Google Scholar]
- 28.Katai H,Ishikawa T,Akazawa K,Fukagawa T,Isobe Y,Miyashiro I,et al. ; Registration Committee of the Japanese Gastric Cancer Association. Optimal extent of lymph node dissection for remnant advanced gastric carcinoma after distal gastrectomy: a retrospective analysis of more than 3000 patients from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer. 2020;23:1091-101. 10.1007/s10120-020-01081-5 [DOI] [PubMed] [Google Scholar]
- 29.Matsuo K,Lee SW,Tanaka R,Imai Y,Honda K,Taniguchi K,et al. T stage and venous invasion are crucial prognostic factors for long-term survival of patients with remnant gastric cancer: a cohort study. World J Surg Oncol. 2021;19:291. 10.1186/s12957-021-02400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsunaga T,Saito H,Osaki T,Fukuda K,Fukumoto Y,Takahashi S,et al. Using the geriatric nutritional risk index to predict outcomes in older patients with remnant gastric cancer after gastrectomy: a retrospective multicenter study in Japan. Surg Today. 2024;54:1360-8. 10.1007/s00595-024-02850-w [DOI] [PubMed] [Google Scholar]
- 31.Zhuo M,Tian L,Han T,Liu TF,Lin XL,Xiao XY. Predictive value of positive lymph node ratio in patients with locally advanced gastric remnant cancer. World J Gastrointest Oncol. 2024;16:833-43. 10.4251/wjgo.v16.i3.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B,Liu T,Cui H,Lu Z,Fang G,Xue X,et al. The value of lymph nodes ratios in the prognosis of resectable remnant gastric cancer through the retrospective propensity score matching analysis. World J Surg Oncol. 2023;21:245. 10.1186/s12957-023-03137-z [DOI] [PMC free article] [PubMed] [Google Scholar]