ABSTRACT
Background
Perioperative hypothermia, a common occurrence in patients undergoing general anesthesia, is defined as a core body temperature below 36°C. The relationship between patient body composition and the incidence of hypothermia remains underreported. This study aims to elucidate the association between body composition and perioperative hypothermia in patients undergoing open gastrectomy.
Methods
Patients undergoing open gastrectomy were enrolled in the study. Patients whose bladder temperature was lower than 36°C were allocated to the hypothermia group, and the other patients were allocated to the control group. The patient’s body composition was evaluated by bioelectrical impedance analysis.
Results
A total of sixty–eight patients participated in this study. Among them, 34 experienced perioperative hypothermia (bladder temperature below 36°C) and were classified into the hypothermia group, while the remaining 34 were placed in the control group. The hypothermia group had a significantly higher body surface area per body weight. Additionally, the hypothermia group exhibited significantly lower total fat mass, skeletal muscle mass index, and basal metabolic rate (P < 0.05). However, body fat percentage and visceral fat mass did not differ significantly between the groups. Multivariate analysis identified total fat mass below 11.2 kg (HR 4.51, 95% CI: 1.35–15.03, P = 0.014) and skeletal muscle mass index below 10.06 kg/m2 (HR 5.61, 95% CI: 1.86–16.93, P = 0.002) as independent risk factors for perioperative hypothermia.
Conclusions
Low total fat mass and a low skeletal muscle mass index are significant risk factors for perioperative hypothermia in patients undergoing open gastrectomy. These risk factors could improve the accuracy of identifying high-risk patients for perioperative hypothermia.
Keywords: body composition, core body temperature, perioperative hypothermia
In patients undergoing surgical procedure under general anaesthesia, redistribution hypothermia is caused by vasodilation due to suppression of thermoregulatory central function and suppression of sympathetic nerves.1
The core body temperature of patients is decreased by exposure of body cavity to ambient air.2 Perioperative hypothermia is defined as a core body temperature of lower than 36 degrees Celsius (°C) at any point in the perioperative period.3 Perioperative hypothermia is reported to occur in 50–90% of surgical cases4, 5 and to be a risk factor for shivering,6 blood coagulation disorder,7, 8 surgical site infection9 and myocardial ischemia10 Intraoperative heating is reported to prevent perioperative hypothermia and shorten the wound healing period, the fasting management period, and the hospitalization period.11 Accordingly, preventing perioperative hypothermia is essential to improve a patient’s postoperative outcome.
Several reports suggested that a high Body Mass Index (BMI) may contribute to the suppression of perioperative hypothermia.12, 13 In these reports, the target population was non-Asian. It was difficult to use the data from these results due to the difference in average BMI levels between Asians and the non-Asians in those reports. A few studies targeting the Japanese suggested that low visceral fat mass is a risk factor for hypothermia.14 However, there were few reports that have cross-sectionally examined the relationship between body composition and incidence of hypothermia.
Therefore, this study was aimed to clarify the relationship between body composition and hypothermia in Japanese patients undergoing gastrectomy.
MATERIALS AND METHODS
Trial design and patients
This study was an observational, single-center retrospective cohort study. Japanese male patients aged 20–80 years who underwent open radical gastrectomy with epidural anesthesia for primary gastric cancer at Cancer Institute Hospital of JFCR from January 1, 2018, to March 31, 2020, were collected in this study. The following exclusion criteria were applied in this study; patients that received preoperative enteral nutrition, American Society of Anesthesiologists Physical Status classification (ASA-PS) > 2, continuous systemic administration of steroids, intraoperative intravenous amino acid administration, patients with multiple cancer, patients with respiratory complications requiring continuous oxygen administration, and a history of abdominal surgery prior to gastrectomy. Patients whose bladder temperature was lower than 36°C were allocated to the hypothermia group (Group H), and the other patients were allocated to the control group (Group C).
Induction and maintenance of anesthesia
None of the study participants received preanesthetic medication. Patients took 500–750 mL of oral rehydration solution 2 hours before being admitted to the operating room. After entering the operating room, ECG monitoring, non-invasive blood pressure monitoring, and pulse oximetry were applied, and a peripheral venous catheter was inserted. An epidural catheter was inserted between T8 and T10 vertebrae. The patients were preoxygenated with 100% oxygen via a facemask. Anesthesia was induced with propofol and fentanyl. Rocuronium was administered to facilitate tracheal intubation. Anesthesia was maintained with remifentanil and desflurane or sevoflurane. Anesthetic management including hemodynamic and respiratory stabilization, and fluid management were at the discretion of the anesthesiologist.
Temperature management
None of the study participants received prewarming. All patients had their axillary temperature measured prior to entering the operating room, which was used as the baseline body temperature. The core temperature was measured using a Foley catheter with a BARD© (Medicon, Tokyo, Japan) temperature sensor placed in the bladder. All patients had a Foley catheter inserted immediately after the induction of anesthesia, and temperature measurements were started at approximately the same time. A heat and moisture exchanger (Ecotherm®, GVS Japan, Tokyo, Japan) was placed in the ventilator circuit. A forced-air warming device with an underbody disposable blanket (3M Bair Hugger® Model 750) was used to prevent hypothermia. Body warming by the forced-air warmer was started at the time of the surgical incision; the set temperature was 38–42°C. The operating room environment was maintained at 40% of humidity and 26–26.5°C of temperature when the patient entered the operation room, and at 24–25°C during the surgery. The infusion solution administered to the patient was taken from a warming cabinet with a controlled internal temperature of 40°C.
Body composition measurement
Body composition was measured by bioelectrical impedance analysis using InBody S10®. Of the measured values, the following items were selected as independent factors of body composition: body mass index (BMI, kg/m2), body surface area/body weight (BSA/BW, m2/kg), total fat mass (TFM, kg), percent body fat (PBF, %), visceral fat area (VFA, cm2), skeletal muscle mass index (SMI, kg/m2), soft lean mass (SLM, kg), basal metabolic rate (BMR, kcal)
Ethics approval and consent to participate
This study was conducted in accordance with the “Declaration of Helsinki” and the “Ethical Guidelines for Medical Research Involving Human Subjects”. This study was approved by the International University of Health and Welfare Research Ethics Review Committee (approval number 20-Ig-93) and the Ethics Review Committee of Cancer Institute Hospital of Japanese Foundation for Cancer Research (JFCR) (approval number 2020-GA-1307). Consent was obtained by disclosing information about the study to the subjects (through notices posted in the Cancer Institute Hospital and on the hospital website) and by ensuring that the subjects had the opportunity to refuse participation in the study. If a subject was deceased, a surrogate was allowed to express refusal on their behalf.
Data collection
Information on age and gender, weight, height, serum total protein, total lymphocyte, total cholesterol, serum albumin, serum prealbumin, Carlson Comorbidity Index (myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, diabetes, hemiplegia, moderate or several renal disease, diabetes with end organ damage, any tumor, leukemia, lymphoma, moderate or severe liver disease, metastatic solid tumor and AIDS), ASA-PS, reconstruction method, operation time, estimated intraoperative blood loss, and postoperative complications of each patient were extracted from the database and electronic patient records of Cancer Institute Hospital of JFCR from 15/12/2020 to 31/3/2022.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney U test for continuous variables and χ2 test for nominal variables. Among the body composition factors, Receiver operating characteristic (ROC) curves were created for factors that were significantly different by the Mann-Whitney U test. The Youden index was calculated based on the ROC curve, and the relationship between each factor with the maximum value and the occurrence of hypothermia was examined by logistic regression analysis using the stepwise method. SPSS 28 was used for statistical processing, and a P value less than 0.05 was considered statistically significant.
RESULTS
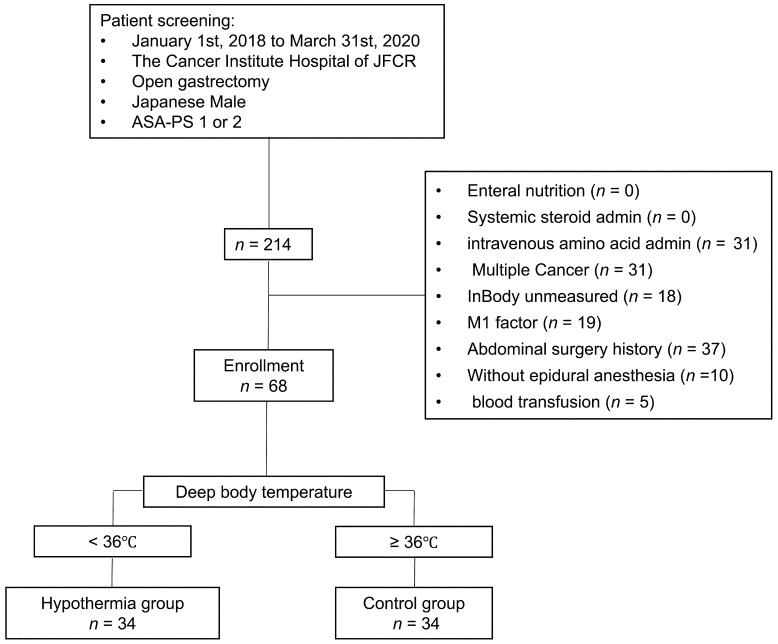
Two hundred fourteen patients were selected from the electronic medical recording system in the present study. Of the 214 patients, 146 patients met the exclusion criteria of this study. The remaining 68 patients were the subjects to be analyzed, thirty-four patients in Group H and thirty-four patients in Group C (Fig. 1).
Fig. 1.
Patient screening flowchart. Sixty-eight of 214 patients collected from the electronic medical recording system were enrolled in the present study. Of 68 patients, thirty-four patients were divided into the hypothermia group (Group H) and the remaining patients were divided into the control group (Group C).
Patient age was significantly older in Group H than in Group C, 69 (36–83) vs. 64.5 (25–87) years old, P = 0.049. There were no significant differences in other patients’ background factors. The duration of hypothermia was 91 (2–342) minutes. The duration of hypothermia as a percentage of the total anesthesia time (hypothermia duration rate) was 14.6 (5.0–81.0) % (Table 1).
Table 1. Patients' characteristics.
| Group H | Group C | P value | ||
| n = 34 | n = 34 | |||
| Age (years’ old) | 69 (36–83) | 64.5 (25–87) | 0.049† | |
| Charlson Comorbidity Index ≥ 3 | 1 | 3 | 0.30‡ | |
| Controlling Nutrition Status score ≥ 5 | 2 | 1 | 0.56‡ | |
| Time to incision (min) | 32.5 (22–45) | 32.5 (22–65) | 0.62† | |
| Operation time (min) | 266.5 (147–489) | 279 (140–481) | 0.87† | |
| Baseline body temperature (degrees Celsius) | 36.5 (35.8–37.2) | 36.4 (35.8–36.9) | 0.57† | |
| Blood loss (g) | 325 (40–1180) | 245 (20–1400) | 0.36† | |
| Urine output (mL) | 280 (30–1300) | 290 (60–1240) | 0.16† | |
| Procedure | Distal gastrectomy | 16 | 19 | 0.47‡ |
| Total gastrectomy | 18 | 15 | ||
| Hypothermia duration (min) | 91 (2–342) | |||
| Percentage of hypothermia duration in total operation time (%) | 14.6 (5.0–81.0) | |||
†Mann-Whitney’s U test, ‡Chi-square test.
The BSA/BW(m2/kg) was significantly higher in Group H than Group C (P = 0.002). The SMI was significantly lower in Group H than Group C (P = 0.003). The BMR was significantly lower in Group H than Group C (P = 0.001). The serum prealbumin level was no significant difference between the two groups (P = 0.641) (Table 2).
Table 2. Body composition analysis.
| Group H | Group C | P value | |
| n = 34 | n = 34 | ||
| Height (cm) | 162 (152–179) | 167 (154–181) | 0.001† |
| Body weight (kg) | 56.6 (43.3–98.1) | 66.6 (51.3–89.8) | < 0.001† |
| Body mass index (kg/m2) | 22.2 (16.5–31) | 23.6 (19.7–31) | 0.005† |
| Body surface area§ (m2) | 1.59 (1.16–2.16) | 1.76 (1.48–2.07) | < 0.001† |
| Body surface area§ per body weight (m2/kg) | 0.028 (0.019–0.033) | 0.026 (0.022–0.029) | 0.002† |
| Total fat mass (kg) | 11.2 (3.6–21.2) | 13.2 (5.6–28.4) | 0.037† |
| Percent body fat (%) | 19.6 (5.9–39) | 22.85 (8.7–40.1) | 0.185† |
| Visceral fat area (cm2) | 48.8 (11.4–111) | 58.2 (15.6–124.8) | 0.050† |
| Soft lean mass (kg) | 43.5 (33.2–71.7) | 49.7 (38.4–69.6) | 0.001† |
| Fat free mass (kg) | 46.3 (35.2–75.9) | 52.6 (40.8–74.0) | 0.001† |
| Skeletal muscle mass index (kg/m2) | 9.417 (7.397–13.918) | 10.154 (7.091–13.445) | 0.003† |
| Basal metabolic rate (kcal) | 1329 (1055–2010) | 1472 (971–1969) | 0.001† |
| Prealbumin (mg/dL) | 27.3 (11.6–36.7) | 24.7 (11.6–43.8) | 0.641† |
§Du Bois Method, †Mann-Whitney’s U test. Continuous variables were described as Median (Min–Max).
Body composition analysis. BSA/BW was significantly higher in the Group H than in the Group C. TFM, SMI, and BMR were significantly lower in the Group H than in the Group C.
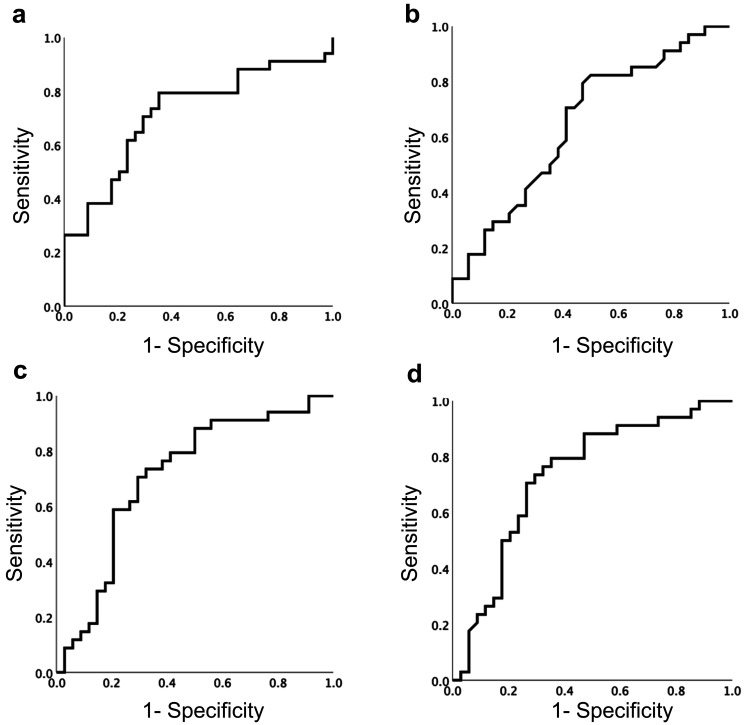
Receiver operating characteristic (ROC) curves were constructed between BSA/BW, TFM, SMI, and BMR and the occurrence of hypothermia. The area under the curve of each ROC curve was 0.718 for BSA/BW, 0.647 for TFM, 0.709 for SMI, and 0.731 for BMR (Fig. 2).
Fig. 2.
Receiver operating characteristic curves for hypothermia occurrence and each body composition factor. a) Body surface area/ body weight, b) Total fat mass, c) Skeletal muscle mass index, d) Basal metabolic rate. Cut off value was determined by maximum degree of Youden index.
The Youden index was calculated based on the ROC curve, and the maximum value of each factor was used as the cutoff value for each factor. BSA/BW was 0.027 m2/kg (sensitivity 70.6%, specificity 70.6%), TFM was 11.2 kg (sensitivity 82.4%, specificity 50%), SMI was 10.06 kg/m2 (sensitivity 70.6%, specificity 70.6%), and BMR was 1460 kcal (sensitivity 70.6%, specificity 73.5%).
The relationship between BSA/BW larger than 0.027 m2/kg, TFM less than 11.2 kg, SMI less than 10.06 kg/m2, and BMR less than 1460 kcal as independent factors and the occurrence of hypothermia were demonstrated by logistic regression analysis using the stepwise method. Moreover, the multivariate analysis highlighted that TFM less than 11.2 kg and SMI less than 10.06 kg/m2 were independent risk factors for the occurrence of hypothermia (TFM less than 11.2 kg; Hazard ratio 4.51, 95% confidence interval 1.35–15.03, P = 0.014; SMI less than 10.06 kg/m2; Hazard ratio 5.61, 95% confidence interval 1.86-16.93, P = 0.002) (Table 3).
Table 3. Logistic regression analysis of hypothermia and each body composition.
| Univariate | Multivariate || | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age > 85 year’s old | 1.40 (0.19–1.87) | 0.21 | ||
| BMI < 22.4 kg/m2 | 3.43 (1.26–9.37) | 0.016 | 0.300 | |
| BSA per BW ≥ 0.027 m2/kg | 5.02 (1.79–14.05) | 0.002 | 0.930 | |
| Total fat mass < 11.2 kg | 4.67 (1.54–14.143) | 0.006 | 4.51 (1.35–15.03) | 0.014 |
| SMI < 10.06 kg/m2 | 5.76 (2.02–16.359) | 0.001 | 5.61 (1.86–16.93) | 0.002 |
| BMR < 1460 kcal | 5.76 (2.02–16.35) | 0.001 | 0.120 | |
BMI, Body Mass Index; BSA, Body Surface Area; SMI, Skeletal Muscle Mass Index; BMR, Basal metabolic rate. ||Forward-backward stepwise selection method.
DISCUSSION
This study clarified that total fat mass and skeletal muscle mass index were risk factors for perioperative hypothermia in Japanese men undergoing open gastrectomy. Previous reports suggested that risk factors for hypothermia were blood transfusion,15 BMI ≤ 35 kg/m2, 12, 13 operation time of exceeding 2 hours,16 abdominal surgery,17 combined epidural anesthesia,17 administration of normothermic fluids,18 operating room temperature set below 20°C,19 absence of using active body surface warming systems,20 and patients over 65 years of age.21 However, despite the common understanding that perioperative hypothermia is defined as a temperature below 36°C in all previous reports, it remains unclear what duration below 36°C or what proportion of the anesthesia time spent below 36°C qualifies as perioperative hypothermia. In this study, we defined Group H as the group in which the temperature fell below the cutoff value during anesthesia, resulting in an incidence rate of 50%. This result is considered to be a reasonable ratio compared with previous reports.
In this study, to homogenize the background of the patient population, data collection and analysis were conducted exclusively on male patients, excluding gender differences. In this study, the difference in surgical procedure could be neglected because the surgical technique was standardized, and operation time was not significantly different between the two groups. With regard to the use of epidural anesthesia, ten of 214 patients (3.8%) did not receive epidural anesthesia. Those patients without epidural anesthesia were excluded from this study in order to homogenize the background of the patient population.
At our institution, the routine anesthetic protocol involves warming intravenous fluids at 40°C. As for blood transfusion, we excluded it from the analysis in this study because only 5 of 214 patients (1.9%) underwent blood transfusion. The temperature of operating room is maintained at 24–26°C for all patients in the daily anesthetic management at our institution. Additionally, a forced air warming system was consistently employed from the outset of each surgical procedure. Accordingly, we analyzed the data obtained from the patients undergoing open gastrectomy in a standardized environment as possible as we could.
Although there were some reports that BMI ≤ 35 kg/m2, 12, 13 was a risk factor for hypothermia, we did not find any cases that exceeded this cutoff value. It is difficult to apply these results on Asians because those studies were conducted among Europeans and Americans, and it is necessary to consider differences in body shape due to racial differences. Therefore, our Asian population or patients with BMI ≤ 35 kg/m2 needs new body composition indices other than BMI to predict the occurrence of perioperative hypothermia.
The prevalence of patients with a BSA/BW ratio greater than 0.027 m2/kg was notably higher in Group H. This suggests that a larger ratio of body surface area to body volume may increase susceptibility to external temperature. The primary mechanisms of intraoperative heat loss are radiation, convection, conduction, and evaporation, with radiation playing the most significant role.22 The amount of heat lost via radiation is proportional to the fourth power of the temperature difference between two adjacent surfaces and the radiative heat transfer between them. While a forced-air warming device heats the dorsal trunk of open gastrectomy patients, the ventral trunk and upper extremities remain covered by surgical drapes, which can be influenced by the operating room temperature via conduction. Consequently, a larger body surface area per unit of body weight might amplify the risk of hypothermia due to increased contact with the covering fabric.
As a method to assess skeletal muscle mass index (SMI), total psoas major muscle area (TPA) is a widely used. However, TPA is measured from specific axial image of computer tomography, by using dedicated processing software. In contrast, bioelectric impedance analysis (BIA) measures body composition by analyzing resistance to weak currents at multiple frequencies. There is a positive correlation between TPA and BIA values, indicating that BIA is non-inferior to TPA as an assessment method.23 Total fat mass (TFM) can be measured by dual-energy X-ray absorptiometry (DEXA). Patients would be forced to undergo additional radiation exposure to obtain the information. Because BIA can measure multiple body composition factors simultaneously and has been reported to be non-inferior to other methods,24 patients undergoing radical surgery for gastric cancer at our hospital are routinely assessed using BIA before surgery.
At our institution, body composition is measured at 12:00 PM, with patients fasting from 6:00 AM. The measurement is performed by specialized rehabilitation staff trained in body composition measurement using InBody, to minimize examination bias.
The BMR equation used in InBody is Cunningham’s equation (BMR=Fat Free Mass× 21.6+370).25 This formula is not affected by age or gender and is obtained by excluding fat mass from body composition. Prior research has demonstrated a strong positive correlation between fat free mass and basal metabolic rate, as well as between fat free mass and skeletal muscle mass.26, 27 This implies a positive relationship between skeletal muscle mass and basal metabolic rate.
Thus, we propose that skeletal muscle mass could be viewed as a hypothermia risk factor, with potential risk arising when the BMR calculated via Cunningham’s equation falls below 1460 kcal.
Our multivariate analysis has affirmed that TFM < 11.2 kg and SMI < 10.06 kg/m2 independently correlate with hypothermia risk. However, body fat percentage and visceral fat mass were not significantly different in the two groups. These results suggest that subcutaneous fat mass may be related to the occurrence of hypothermia. Miyazaki et al. found visceral fat mass, rather than subcutaneous fat, affected hypothermia during laparoscopic surgery.14 Notably, abdominal cavity was exposed to insufflation gas during laparoscopic surgery, whereas the present study was conducted in the open surgery that the abdominal cavity was exposed to the ambient environment. This divergence in surgical techniques likely accounts for the disparate outcomes, suggesting subcutaneous fat might mitigate heat loss during laparotomy.
Another independent risk factor for hypothermia was SMI < 10.06 kg/m2. In regulating body temperature, humans engage in heat production through cutaneous vasoconstriction, non-shivering thermogenesis in brown adipose tissue and skeletal muscle, and shivering thermogenesis in skeletal muscle.28 This implies that sarcolipin-mediated non-shivering thermogenesis, may contribute to the suppression of hypothermia. Bal et al. reported that sarcolipin was involved in non-shivering thermogenesis in skeletal muscle in mice.29 They observed that the mice treated with curare (a neuromuscular junction inhibitor) inhibited from shivering to maintain their temperature in the cold environments. Shivering was suppressed in the patients received muscle relaxants during general anesthesia. Therefore, sarcolipin in skeletal muscle may be involved in non-shivering thermogenesis in humans and may suppress hypothermia during laparotomy. Thus, the total amount of sarcolipin in skeletal muscle was likely to play a significant role in maintaining body temperature under general anesthesia using a muscle relaxant. This may in part explain that the incidence of perioperative hypothermia was lower with an SMI ≥ 10.06 kg/m2 in patients undergoing open gastrectomy.
The limitation of this study was as follows; 1) a single institution, 2) a single sex, 3) a small sample size, 4) a retrospective cohort study, 5) a combination of epidural anesthesia with general anesthesia, and 6) one surgical procedure. Additionally, it is necessary to examine confounding between each of the standardized background factors and the risk factors obtained in this study. The sample size of this study was small to examine the confounding, therefore, further large–scale research is considered necessary. However, we aimed to accentuate the effect of body composition by limiting variables to overcome these limitations. Further prospective multicenter study would be needed to validate the results obtained in this study.
The present study clarified that TFM < 11.2 kg and SMI < 10.06 kg/m2 were likely to become new independent risk factors for hypothermia in male patients undergoing open gastrectomy. New risk factors for hypothermia revealed in this study may allow for improving the precision of screening in a potential high–risk patient of perioperative hypothermia, thereby being able to prevent postoperative complications such as shivering, blood coagulation disorders increased surgical site infection, and myocardial ischemia induced by perioperative hypothermia.
AVAILABILITY OF DATA AND MATERIALS
Since the datasets generated and/or analyzed during the current study were sourced from electronic medical record information exclusive to the Cancer Hospital of JFCR, they are not publicly accessible. However, they can be obtained from the corresponding author upon reasonable request.
AUTHORS’ CONTRIBUTIONS
AA designed the study, collected the data, interpreted the data and drafted the manuscript. EA analyzed the data and participated in drafting the manuscript. YM and SN participated in the designing of the study and coordination of the study. YI reviewed the manuscript and participated in the coordination of the study and interpretation of data. All authors have approved the final version of the manuscript and are accountable for all aspects of this study.
Acknowledgments
Acknowledgements: We are grateful to Aya Fujihara for helpful corrected the data.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387:2655-64. 10.1016/S0140-6736(15)00981-2 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen N,Fleming NW,Singh A,Lee SJ,Goldman CD,Wolfe BM. Evaluation of core temperature during laparoscopic and open gastric bypass. Obes Surg. 2001;11:570-5. 10.1381/09608920160557039 [DOI] [PubMed] [Google Scholar]
- 3.Forbes SS,Eskicioglu C,Nathens AB,Fenech DS,Laflamme C,McLean RF,et al. ; Best Practice in General Surgery Committee, University of Toronto. Evidence-based guidelines for prevention of perioperative hypothermia. J Am Coll Surg. 2009;209:492-503e1. 10.1016/j.jamcollsurg.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Lynch S,Dixon J,Leary D. Reducing the risk of unplanned perioperative hypothermia. AORN J. 2010;92:553-65. 10.1016/j.aorn.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 5.Roberson MC,Dieckmann LS,Rodriguez RE,Austin PN. A review of the evidence for active preoperative warming of adults undergoing general anesthesia. AANA J. 2013;81:351-6. [PubMed] [Google Scholar]
- 6.Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531-43. 10.1097/00000542-200108000-00040 [DOI] [PubMed] [Google Scholar]
- 7.Schmied H,Reiter A,Kurz A,Sessler DI,Kozek S. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289-92. 10.1016/S0140-6736(96)90466-3 [DOI] [PubMed] [Google Scholar]
- 8.Rajagopalan S,Mascha E,Na J,Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71-7. 10.1097/01.anes.0000296719.73450.52 [DOI] [PubMed] [Google Scholar]
- 9.Bu N,Zhao E,Gao Y,Zhao S,Bo W,Kong Z,et al. Association between perioperative hypothermia and surgical site infection. Medicine (Baltimore). 2019;98:e14392. 10.1097/MD.0000000000014392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank SM,Fleisher LA,Breslow MJ,Higgins MS,Olson KF,Kelly S,et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277:1127-34. 10.1001/jama.1997.03540380041029 [DOI] [PubMed] [Google Scholar]
- 11.Kurz A,Sessler DI,Lenhardt R; Study of Wound Infection and Temperature Group. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-16. 10.1056/NEJM199605093341901 [DOI] [PubMed] [Google Scholar]
- 12.Groene P,Zeuzem C,Baasner S,Hofmann-Kiefer K. The influence of body mass index on temperature management during general anaesthesia— A prospective observational study. J Eval Clin Pract. 2019;25:340-5. 10.1111/jep.13064 [DOI] [PubMed] [Google Scholar]
- 13.Özer AB,Yildiz Altun A,Erhan ÖL,Çatak T,Karatepe U,Demirel I,et al. The effect of body mass index on perioperative thermoregulation. Ther Clin Risk Manag. 2016;12:1717-20. 10.2147/TCRM.S122700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki R,Hoka S,Yamaura K. Visceral fat, but not subcutaneous fat, is associated with lower core temperature during laparoscopic surgery. PLoS One. 2019;14:e0218281. 10.1371/journal.pone.0218281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sari S,Aksoy SM,But A. The incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia and an examination of risk factors. Int J Clin Pract. 2021;75:e14103. 10.1111/ijcp.14103 [DOI] [PubMed] [Google Scholar]
- 16.Paulikas CA. Prevention of unplanned perioperative hypothermia. AORN J. 2008;88:358-68. 10.1016/j.aorn.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 17.Radauceanu DS,Dragnea D,Craig J. NICE guidelines for inadvertent peri‐operative hypothermia. Anaesthesia. 2009;64:1381-2. 10.1111/j.1365-2044.2009.06141_18.x [DOI] [PubMed] [Google Scholar]
- 18.Campbell G,Alderson P,Smith AF,Warttig S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Libr. 2015;2015:CD009891. 10.1002/14651858.CD009891.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank SM,Beattie C,Christopherson R,Norris EJ,Rock P,Parker S,et al. Epidural versus general anesthesia, ambient operating room temperature, and patient age as predictors of inadvertent hypothermia. Anesthesiology. 1992;77:252-7. 10.1097/00000542-199208000-00005 [DOI] [PubMed] [Google Scholar]
- 20.Madrid E,Urrútia G,Roqué i Figuls M,Pardo-Hernandez H,Campos JM,Paniagua P,et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Libr. 2016;2016:CD009016. 10.1002/14651858.CD009016.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billeter AT,Hohmann SF,Druen D,Cannon R,Polk HC Jr. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery. 2014;156:1245-52. 10.1016/j.surg.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 22.Sessler DI,Todd MM. Perioperative heat balance. Anesthesiology. 2000;92:578-96. 10.1097/00000542-200002000-00042 [DOI] [PubMed] [Google Scholar]
- 23.Saito M,Seshimo A,Miyake K,Yamaguchi R,Okamoto T. Efficiency of Bioelectric Impedance Analysis as an Evaluation Method of Skeletal Muscle Mass After Gastrectomy. Int Surg. 2018;102:422-6. 10.9738/INTSURG-D-17-00035.1 [DOI] [Google Scholar]
- 24.Kim M,Shinkai S,Murayama H,Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual‐energy X ‐ray absorptiometry for the assessment of body composition in a community‐dwelling older population. Geriatr Gerontol Int. 2015;15:1013-22. 10.1111/ggi.12384 [DOI] [PubMed] [Google Scholar]
- 25.Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963-9. 10.1093/ajcn/54.6.963 [DOI] [PubMed] [Google Scholar]
- 26.Heymsfield SB,Gallagher D,Kotler DP,Wang Z,Allison DB,Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132-8. 10.1152/ajpendo.2002.282.1.E132 [DOI] [PubMed] [Google Scholar]
- 27.Müller MJ,Bosy-Westphal A,Kutzner D,Heller M. Metabolically active components of fat free mass (FFM) and resting energy expenditure (REE) in humans. Forum Nutr. 2003;56:301-3. [PubMed] [Google Scholar]
- 28.Blondin DP,Haman F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb Clin Neurol. 2018;156:153-73. 10.1016/B978-0-444-63912-7.00010-2 [DOI] [PubMed] [Google Scholar]
- 29.Bal NC,Maurya SK,Sopariwala DH,Sahoo SK,Gupta SC,Shaikh SA,et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575-9. 10.1038/nm.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Since the datasets generated and/or analyzed during the current study were sourced from electronic medical record information exclusive to the Cancer Hospital of JFCR, they are not publicly accessible. However, they can be obtained from the corresponding author upon reasonable request.