Abstract
Objectives This study aimed to test whether early oxygenation failure severity categories (absent/mild/moderate/severe) were associated with health-related quality of life (HRQL) deterioration among children who survived sepsis-related acute respiratory failure.
Methods We performed a secondary analysis of a study of community-acquired pediatric septic shock, Life After Pediatric Sepsis Evaluation. The primary outcome was an adjusted decline in HRQL ≥ 25% below baseline as assessed 3 months following admission. Logistic regression models were built to test the association of early oxygenation failure including covariates of age and nonrespiratory Pediatric Logistic Organ Dysfunction-2 score. Secondarily, we tested if there was an adjusted decline in HRQL at 6 and 12 months and functional status at 28 days.
Results We identified 291 children who survived to discharge and underwent invasive ventilation. Of those, that 21% (61/291) had mild oxygenation failure, 20% (58/291) had moderate, and 17% (50/291) had severe oxygenation failure. Fifteen percent of children exhibited a decline in HRQL of at least 25% from their baseline at the 3-month follow-up time point. We did not identify an association between the adjusted severity of oxygenation failure and decline in HRQL ≥ 25% at 3-, 6-, or 12-month follow-up. Children with oxygenation failure were more likely to exhibit a decline in functional status from baseline to hospital discharge, but results were similar across severity categories.
Conclusion Our findings that children of all oxygenation categories are at risk of HRQL decline suggest that those with mild lung injury should not be excluded from comprehensive follow-up, but more work is needed to identify those at the highest risk.
Keywords: shock, septic; health-related quality of life; acute hypoxemic respiratory failure; respiratory distress syndrome, pediatric; postintensive care syndrome; functional status
Introduction
In 2015, the Pediatric Acute Lung Injury Consensus Conference (PALICC) created a definition for pediatric acute respiratory distress syndrome (PARDS), which stratifies disease severity based on oxygenation index (OI) or oxygenation saturation index (OSI). 1 These severity indices measured early in the disease are associated with in-hospital mortality and ventilator-free days. 1 2 3 The PALICC authors also recommended that “physical, neurocognitive, emotional, family, and social function be evaluated within 3 months of hospital discharge for children who survive moderate to severe PARDS.” 4 However, no studies have evaluated the association between these severity categories and long-term health-related quality of life (HRQL) in survivors after PARDS or respiratory failure.
Acute respiratory failure and PARDS are often caused by sepsis—accounting for 19% of all PARDS cases and 34% of PARDS deaths. 5 The multicenter, longitudinal clinical and outcome data from Life After Pediatric Sepsis Evaluation (LAPSE) investigation provides a rigorously collected data set of children with septic shock-associated acute respiratory failure. We sought to identify whether the severity of oxygenation failure as measured by oxygenation indices is associated with a child's HRQL and functional status after hospitalization in children with respiratory failure associated with sepsis. Specifically, we hypothesized that more severe early oxygenation failure would be associated with a ≥ 25% decline in HRQL. If these early indices are associated with long-term functional and HRQL outcomes, they may be useful in risk stratification and targeting resources during and after the hospitalization toward those most at risk for impaired recovery.
Materials and Methods
Data Source
This study was determined to be exempt from human subjects review by the University of Michigan Institutional Review Board (HUM00179778). We conducted a secondary analysis of the LAPSE study: a prospective cohort study conducted at 12 U.S. academic pediatric intensive care units (PICUs) that enrolled children with community-acquired septic shock from 2013 to 2017 (NCT01415180; R01HD073362). LAPSE defined septic shock as two or more systemic inflammatory response syndrome criteria, documented or suspected infection, and need for fluid resuscitation and vasoactive-inotropic support within 72 hours. 6
Study Population
Children were included in this secondary analysis if they had community-acquired septic shock and required invasive mechanical ventilation. We are unable to determine if the children enrolled in LAPSE met all criteria for PARDS due to the absence of chest radiograph data. We chose to only evaluate the outcomes of children who survived to hospital discharge to understand the association of early oxygenation severity with after-hospital recovery, since it is established that oxygenation severity is associated with mortality. Only patients with a baseline assessment and at least one follow-up assessment were included in the HRQL outcome models. We excluded patients whose highest level of respiratory support was noninvasive ventilation. The presence of chronic comorbid conditions were assessed according to the Pediatric Medical Complexity Algorithm. 7
Exposures
Our primary exposure of interest was the oxygenation failure severity category encountered on the day of PICU admission (day 0) or the following day. LAPSE study sites recorded respiratory parameters including OI and/or OSI twice daily (0800, 2000). OI is defined as
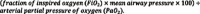 . OSI is defined as
. OSI is defined as
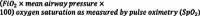 ) for SpO
2
values ≤ 97%. A four-category variable indicating the severity of oxygenation failure (absent/mild/moderate/severe) was created. We stratified severity consistent with the PALICC recommendations for PARDS (mild: 4 ≤ OI < 8 or 5 ≤ OSI < 7.5, moderate: 8 ≤ OI < 16 or 7.5 ≤ OSI < 12.3, severe: OI ≥ 16 or OSI ≥ 12.3).
1
We used the highest OI, or if the OI was unavailable, the highest OSI during the first two study days.
) for SpO
2
values ≤ 97%. A four-category variable indicating the severity of oxygenation failure (absent/mild/moderate/severe) was created. We stratified severity consistent with the PALICC recommendations for PARDS (mild: 4 ≤ OI < 8 or 5 ≤ OSI < 7.5, moderate: 8 ≤ OI < 16 or 7.5 ≤ OSI < 12.3, severe: OI ≥ 16 or OSI ≥ 12.3).
1
We used the highest OI, or if the OI was unavailable, the highest OSI during the first two study days.
Outcomes
The primary outcome was a persistent, serious deterioration in HRQL, defined as a decline ≥ 25% below baseline as assessed 3 months following admission for the sepsis event—consistent with the primary study. 6 LAPSE investigators evaluated HRQL at baseline, 7 days, 28 days, 3 months, 6 months, and 12 months. 6 8 Our primary endpoint of interest was the change in HRQL at 3 months because this was felt to represent a patient-centered, clinically significant outcome. 9 The HRQL instruments used were the Pediatric Quality of Life Inventory 4.0 Generic Scale, infant and pediatric versions (PedsQL), 10 or the Stein-Jessup Functional Status II-Revised (FS II-R) instruments. 11 LAPSE investigators cited prior experience that PedsQL may not accurately assess children with a severe developmental disability, so they offered FS II-R as an alternative if parents preferred this instrument. 6 Surveys were completed standardly by parent-proxy through a Web-based system but could also be completed by text message or telephone interview. Both outcome measures utilize a scale of 0 to 100, with 0 being the worst outcome.
Secondarily, we assessed the change in HRQL at 6 and 12 months relative to the premorbid baseline. We were also interested in reporting the proportion of patients who failed to return to their premorbid baseline HRQL. Since the accepted minimally important clinical difference in PedsQL is 4.5 points, 10 we created a binary outcome of whether or not each patient returned to within 4.5 points of their baseline HRQL measure to identify return to baseline. There is no accepted minimally important clinical difference for FS II-R, so we used a return to within 4.5 points of baseline measure as baseline for consistency across patients as previously done. 8
Another secondary outcome was the development of a new functional morbidity assessed at discharge or hospital day 28. Previous work has defined this as a decline in the Functional Status Scale (FSS) ≥ 3 from baseline. 12 13 The FSS evaluates six domains of functioning from 1 (normal) to 5 (very severe dysfunction) for a total score of 6 to 30, with higher scores indicating more dysfunction. 8 LAPSE research staff assessed FSS at baseline, day 7, and day 28 or hospital discharge, whichever came first. 8
Statistical Analyses
Descriptive statistics of all demographic variables (including frequency distributions or medians and interquartile ranges [IQRs]) were conducted. Bivariate analyses of cohort characteristics were conducted with the Kruskal–Wallis test for continuous variables or Pearson's chi-squared for categorical variables.
We assessed whether early oxygenation severity was associated with ≥ 25% decline in HRQL from baseline at 3 months by building a logistic regression model. We included four-category oxygenation severity (absent/mild/moderate/severe) as an independent variable. A priori, we decided to adjust for patient age and the first collected nonrespiratory Pediatric Logistic Organ Dysfunction-2 score (PELOD-2). 14 15 Nonrespiratory PELOD-2 was chosen to evaluate whether oxygenation severity was reflective of overall illness severity and extrapulmonary organ dysfunction or an independent risk factor.
Secondarily, we built separate logistic regression models to test whether early oxygenation severity was associated with ≥ 25% decline in HRQL from baseline for the 6- and 12-month follow-up time points. To test whether oxygenation severity was associated with functional status decline, we created a logistic regression model including a covariate of nonrespiratory PELOD-2 and outcome of FSS increase ≥ 3. 9 16 We also performed a post hoc exploratory analysis testing the association of the highest oxygenation failure severity category with the primary outcome at 3, 6, and 12 months.
Analyses were completed using Stata 16 (StataCorp, College Station, Texas, United States).
Results
Participants and Participant Characteristics
Of the 389 unique patients in the LAPSE cohort, 89% (348/389) received invasive mechanical ventilation. Hospital mortality was 7.0% for those without oxygenation failure, 8.7% for mild, 10.1% for moderate, and 16.4% for severe oxygenation failure, respectively ( p = 0.19). A total of 291 children received invasive mechanical ventilation, completed a baseline HRQL assessment, and survived to hospital discharge. Of those 291, 122 (42%) did not meet oxygenation failure criteria, 61 (21%) had mild, 58 (20%) had moderate, and 50 (17%) had severe oxygenation failure ( Table 1 ) in the first two study days. Full cohort characteristics (including those who did not survive to discharge and did not complete baseline assessment) are shown in Supplementary Table S1 (online only). Among survivors, the median PICU length of stay was 9 days (IQR 5–15 days). The median first day of mechanical ventilation in all oxygenation failure severity categories was day 0 (IQR 0–0 days). The median duration of mechanical ventilation was 8 days (IQR 4–12 days). Of the 291, 178 had a parent or guardian who completed HRQL assessment at the 3-month time point (108 PedsQL and 70 FS II-R), 154 had a 6-month completed assessment (91 PedsQL and 63 FS II-R), and 145 patients had a 12-month completed assessment (90 PedsQL and 55 FS II-R).
Table 1. Characteristics of children with septic shock and respiratory failure who survived to hospital discharge.
| Characteristics | Oxygenation failure absent ( n = 122) |
Mild oxygenation failure ( n = 61) | Moderate oxygenation failure ( n = 58) | Severe oxygenation failure ( n = 50) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 63 (52) | 32 (52) | 37 (64) | 28 (56) |
| Female | 59 (48) | 29 (48) | 21 (36) | 22 (44) |
| Age in years, median (IQR) | 6.7 (2.5–13.3) | 4.4 (1.4–11.5) | 4.6 (1.2–11.8) | 7.2 (1.6–12.4) |
| Race/Ethnicity | ||||
| American Indian/Alaskan Native | 4 (3%) | 2 (3%) | 2 (3%) | 0 (0%) |
| Asian | 7 (6%) | 2 (3%) | 3 (5%) | 3 (6%) |
| Black or African-American | 29 (24%) | 6 (10%) | 10 (17%) | 14 (28%) |
| White | 73 (60%) | 48 (79%) | 40 (69%) | 32 (64%) |
| Native Hawaiian or Other Pacific Islander | 2 (2%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Unknown/Not reported | 10 (8%) | 6 (9%) | 4 (7%) | 2 (4%) |
| Baseline HRQL measure, median (IQR) | ||||
| PedsQL ( n = 180) | 82.6 (68.8–94.0) | 79.7 (63.0–90.7) | 82.7 (72.8–92.3) | 80.6 (68.8–97.2) |
| FS II-R ( n = 111) | 67.6 (60.7–85.7) | 64.3 (53.6–85.7) | 64.3 (57.1–78.6) | 75.0 (60.7–85.7) |
| Baseline FSS, median (IQR) | 7 (6–12) | 7 (6–11) | 6 (6–8) | 6 (7–13) |
| Arterial lactate (mmol/L), median (IQR) | ||||
| Day of PICU admission ( n = 155) | 3.2 (2–5.1) | 1.6 (1.1–3.1) | 2.3 (1.4–4.1) | 3.1 (1.6–5.5) |
| Highest value ( n = 233) | 3.2 (2–5.3) | 2.2 (1.4–3.4) | 2.6 (1.8–4.4) | 3.1 (2.3–7.6) |
| Vasoactive-inotropic score, median (IQR) | ||||
| Day of PICU admission ( n = 283) | 5 (0–12) | 2 (0–12) | 2.5 (0–10) | 4 (0–15) |
| Highest value | 10 (5–20) | 10 (5–18) | 10 (5–20) | 15 (8–28) |
| Medical history, n (%) | ||||
| Immunocompromised | 22 (18) | 4 (7) | 6 (10) | 9 (18) |
| Congenital immunodeficiency | 3 (6) | 0 (0) | 0 (0) | 1 (4) |
| Bone marrow/SCT | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Malignancy | 3 (6) | 0 (0) | 2 (8) | 4 (17) |
| Rheumatologic disease | 2 (4) | 0 (0) | 0 (0) | 1 (4) |
| Sickle cell disease | 2 (4) | 0 (0) | 0 (0) | 0 (0) |
| Medical complexity algorithm category | ||||
| No chronic comorbid conditions | 59 (48%) | 27 (44%) | 37 (63%) | 22 (44%) |
| Chronic comorbid conditions (noncomplex) | 4 (3%) | 5 (8%) | 4 (7%) | 4 (8%) |
| Chronic comorbid conditions (complex) | 59 (48%) | 28 (46%) | 17 (29%) | 24 (48%) |
| Baseline tracheostomy, n (%) | 12 (10) | 3 (3) | 3 (5) | 5 (10) |
| Adjunct respiratory support | ||||
| Neuromuscular blockade | 88 (72%) | 51 (84%) | 48 (83%) | 45 (90%) |
| Days of neuromuscular blockade | 1 (0–3) | 2 (1–3) | 3 (1–6) | 5 (2–7) |
| HFV | 6 (5%) | 2 (3%) | 3 (5%) | 11 (22%) |
| ECLS | 2 (2%) | 2 (3%) | 3 (5%) | 7 (14%) |
| Mechanical ventilation days, median (IQR) | 6 (4–10) | 7 (5–12) | 9.5 (6–15) | 16 (10–27) |
| PICU LOS (d), median (IQR) | 8 (5–12) | 8 (6–15) | 10 (8–14) | 17.5 (11–28) |
| Hospital LOS (d), median (IQR) | 15 (8–24) | 15 (10–23) | 16.5 (12–25) | 28 (16–44) |
Abbreviations: ECLS, extracorporeal life support or ventricular assist device; FS II-R, Functional Status II-Revised; FSS, Functional Status Scale; HFV, high frequency ventilation; HRQL, health-related quality of life; IQR, interquartile range; LOS, length of stay; PedsQL, Pediatric Quality of Life Inventory 4.0; PICU, pediatric intensive care unit.
Health-Related Quality of Life
At 3 months, 15% (27/178) of patients with acute respiratory failure who survived to discharge exhibited a persistent, serious deterioration in HRQL of at least 25% from their baseline (the primary endpoint). When we stratify by early oxygenation severity, 16% (12/73) of patients without oxygenation failure demonstrated ≥ 25% decline in HRQL at the 3-month time point, compared with 8% (3/36) of patients with mild oxygenation failure, 13% (5/38) of children with moderate respiratory failure, and 23% (7/31) of patients with severe oxygenation failure. However, in logistic regression models including nonrespiratory PELOD-2 and patient age, we did not find an association between the severity category (absent/mild/moderate/severe) and ≥ 25% decline from baseline at 3 months ( Table 2 ).
Table 2. Multivariable logistic regression models for > 25% decline in HRQL from baseline at 3, 6, and 12 months.
| Follow-up time point | |||
|---|---|---|---|
| 3 mo ( n = 178) | 6 mo ( n = 154) | 12 mo ( n = 145) | |
| Adjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |
| Oxygenation failure severity | |||
| Absent | Ref | Ref | Ref |
| Mild | 0.65 (0.16–2.61) | 0.85 (0.16–4.56) | 0.50 (0.10–2.55) |
| Moderate | 0.85 (0.26–2.77) | 0.76 (0.14–4.01) | 0.69 (0.17–2.80) |
| Severe | 1.59 (0.54–4.71) | 2.17 (0.64–7.28) | 1.09 (0.30–3.96) |
Abbreviations: CI, confidence interval; HRQL, health-related quality of life.
Note: Models adjusted for patient age and Pediatric Logistic Organ Dysfunction-2 score.
The proportion of patients with at least 25% decline at the 3-month time point was similar to the proportion at the 6- and 12-month time points (11 and 12%, respectively, p = 0.52) ( Fig. 1A ). In a secondary analysis, we did not find an association between the severity of oxygenation failure and ≥ 25% decline in HRQL at the 6- and 12-month time points.
Fig. 1.
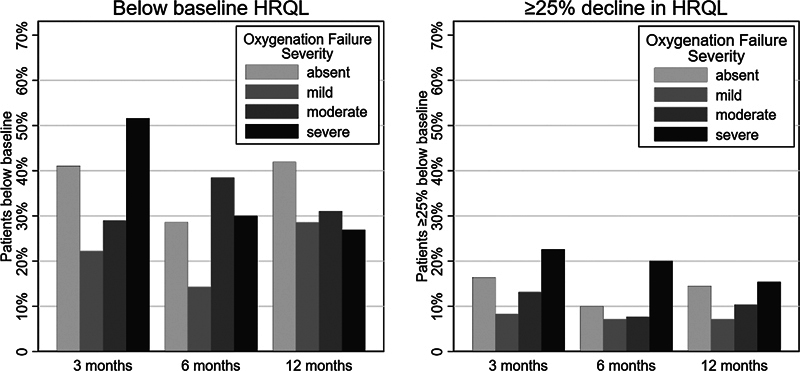
The percent of children ≥ 4.5 points or ≥ 25% below baseline health-related quality of life measure at each follow-up time point. The denominator for the percentage shown is the number of children completing assessments at each time point: 3-month, n = 178; 6-month, n = 154; 12-month, n = 145.
Thirty-seven percent (65/178) of patients with acute respiratory failure had not returned to their baseline HRQL at 3 months. In a logistic regression model including age and nonrespiratory PELOD-2, we did not observe an association between oxygenation severity categories and failure to return to the baseline HRQL at the 3-, 6-, or 12-month time points ( Supplementary Table S2 , online only) ( Fig. 1B ). When stratifying the 291 survivors with respiratory failure by the highest severity category achieved in a post hoc exploratory analysis, there was no association with severity category at any time point ( Supplementary Table S3 , online only).
Functional Status
The baseline FSS was similar across severity categories ( Table 1 ). At hospital discharge or day 28 (whichever came first), the percentage of patients with a new functional morbidity (a functional status decline ≥ 3 points from baseline) was 15% for those without oxygenation failure, 28% for those with mild, 28% for those with moderate, and 31% with severe oxygenation failure ( Fig. 2 ). In a logistic regression model including age and nonrespiratory PELOD-2, mild, moderate, and severe categories were associated with new functional morbidity when compared with those without oxygenation failure; however, there were no differences between mild, moderate, or severe categories ( Table 3 ).
Fig. 2.
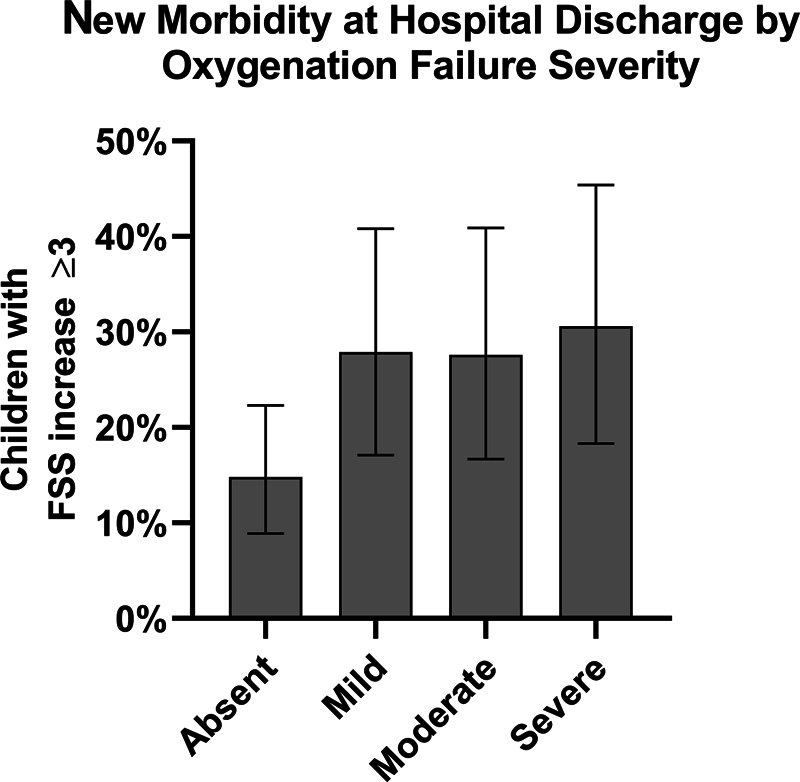
The percent of children with new functional morbidity (≥ 3 point increase in Functional Status Scale) at hospital discharge or day 28; absent n = 122, mild n = 61, moderate n = 58, severe n = 49.
Table 3. Logistic regression models for new morbidity at discharge (Functional Status Scale increase greater than 3 points from baseline).
| FSS decline ≥ 3 | ||
|---|---|---|
| Unadjusted | Adjusted for age and nonrespiratory PELOD-2 | |
| Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Oxygenation failure severity | ||
| Absent | Ref | Ref |
| Mild | 2.23 a (1.05, 4.73) | 2.21 a (1.04, 4.73) |
| Moderate | 2.20 a (1.03, 4.72) | 2.14 (0.99, 4.62) |
| Severe | 2.55 a (1.16, 5.60) | 2.42 a (1.09, 5.36) |
Abbreviations: CI, confidence interval; FSS, Functional Status Scale; PELOD-2, Pediatric Logistic Organ Dysfunction-2 score.
p < 0.05, n = 290.
Discussion
In this analysis of survivors of septic shock with respiratory failure, we found that impairment in HRQL is common, with at least one in five children not returning to their premorbid HRQL. We failed to show an association between early oxygenation failure severity categories and a decline in 3-, 6-, or 12-month posthospitalization HRQL. The presence of hypoxemic respiratory failure was also associated with new functional morbidity at hospital discharge, but this was not significantly different between the oxygenation failure severity categories. This analysis of children with septic shock and acute respiratory failure suggests that children with any degree of oxygenation severity (mild, moderate, or severe) may have an enduring risk of HRQL decline.
The proportion of children with a > 25% decline in HRQL and failure to return to baseline HRQL was notably similar at the 3-, 6-, and 12-month follow-up time points. This finding is consistent with previous studies of long-term outcomes demonstrating a strong correlation between 3- and 12-month outcomes. 17 Additionally, in all severity categories, even among those with absent or mild hypoxemia, at least one in five children failed to return to their prehospital baseline HRQL by 3 months after their illness, which is also consistent with previous studies in pediatric respiratory failure and community-acquired sepsis. 18 19 While this finding is limited by the loss to follow-up in the study, it suggests that the window for screening and intervention may be sooner than 3 months after discharge and so it is essential to establish reliable early postdischarge follow-up.
We hypothesized that the degree of early oxygenation failure would be associated with a decline in HRQL in part because the degree of oxygenation failure as determined by oxygenation indices has been associated with mortality. 1 The recommendation from PALICC authors to follow the physical, neurocognitive, and emotional development in those with moderate or severe PARDS had strong consensus agreement. Similarly, other analyses of this cohort have shown that markers of disease severity, such as severe acute kidney injury, are associated with impaired HRQL after a hospitalization. 9 However, our study failed to show that oxygenation severity categories were reliably associated with a persistent, serious decline in HRQL. When stratifying patients by the highest severity level achieved, we still failed to find an association between oxygenation failure severity. This suggests that those with mild disease should not be excluded from screening for impairments in HRQL based on oxygenation severity alone.
At present, pediatric intensivists do not have reliable early ways of identifying which patients are at risk for impaired recovery in physical, cognitive, emotional, and social health, but recognize impairment is common. 20 Importantly, this study evaluated children requiring mechanical ventilation who all met criteria for community-acquired septic shock. There are many potential etiologies and pathways that can lead to acute lung injury and it is possible that other etiologies of respiratory failure would demonstrate an association between oxygenation failure severity and HRQL outcomes. 21 Given the heterogeneity of patients who develop respiratory failure and lung disease, predicting which children are at risk of morbidity after respiratory failure likely should involve a comprehensive evaluation of baseline risk factors, in-hospital practices, and after-discharge supports.
The presence of oxygenation indices meeting criteria for PARDS was associated with new functional morbidity at hospital discharge or day 28 in this cohort. Overall, 29% of patients in the severe oxygenation failure category revealed significant deterioration in functional status at 28 days compared with 11% of patients without hypoxemia. However, the rate of new morbidity between severity categories was not significantly different. Interestingly, duration of ventilation and intensive care unit (ICU) length of stay varied predictably across the severity categories—those with severe hypoxemia required the longest duration of ventilation and PICU stay. However, the lack of difference between oxygenation failure groups in the acquisition of new morbidity suggests that the relationship is more complex than severe lung injury having longer ICU courses and therefore higher morbidity. As knowledge of the long-term impact of a critical illness grows, it becomes increasingly important to identify whether there are modifiable aspects of intensive care delivery that can mitigate this impact.
This study has several limitations. The ability to interpret HRQL outcomes is limited by the children lost to long-term follow-up. It is plausible that these patients have important differences (patients who have worse outcomes or difficult recovery may be less likely to respond to follow-up). 22 23 It is possible that our inability to find an association in this study is due to the small number of patients in each cohort. Long-term follow-up studies in pediatric critical illness should continue to look for ways to engage families in the research process and minimize patient attrition. 24 25 Importantly, because chest radiographs were not collected in the original study, it is unknown whether these patients would meet full diagnostic criteria for PARDS. While we are unable to determine whether these children would have the radiographic findings consistent with PARDS, most patients appear to have met the other PARDS criteria (timing is acute in onset and degree of oxygenation failure measured by OI or OSI). Because of the high numbers of patients with sepsis who have respiratory failure, future studies would benefit from understanding how the complete PALICC definition for PARDS predicts long-term outcomes in sepsis patients.
Conclusion
Among children with community-acquired septic shock and respiratory failure, impairment in HRQL is common. We did not demonstrate a difference among those with mild, moderate, or severe early oxygenation failure severity, suggesting that follow-up efforts should include even those with mild disease. Identifying those at risk should evaluate baseline risk factors, in-hospital practices, and postdischarge support and not rely solely on disease severity. Future studies and post-ICU follow-up programs should screen for HRQL and new morbidity after critical illness and monitor the impact on longer-term child and family well-being.
Acknowledgments
We would like to thank all the patients and guardians of LAPSE patients for their participation in the study. We would also like to thank Dr. Lisa Prosser, PhD for her guidance and the LAPSE investigators and research staff at each clinical performance site.
Funding Statement
Funding This research was conducted without external funding.
Footnotes
Conflict of Interest R.P.B. reports grants from the National Institutes of Health (R01 HL153519-ASCEND; K12 HL138039-TACTICAL; R01 HD01543-Pediatric Implantable Artificial Lung) outside the submitted work; R.P.B. also discloses that he is a member of the Extracorporeal Life Support Organization (ELSO) Registry Chair.
Supplementary Material
References
- 1.Pediatric Acute Lung Injury Consensus Conference Group Khemani R G, Smith L S, Zimmerman J J, Erickson S.Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference Pediatr Crit Care Med 201516(05, Suppl 1):S23–S40. [DOI] [PubMed] [Google Scholar]
- 2.Yehya N, Thomas N J, Khemani R G. Risk stratification using oxygenation in the first 24 hours of pediatric acute respiratory distress syndrome. Crit Care Med. 2018;46(04):619–624. doi: 10.1097/CCM.0000000000002958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flori H R, Glidden D V, Rutherford G W, Matthay M A. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(09):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 4.Pediatric Acute Lung Injury Consensus Conference Group Quasney M W, López-Fernández Y M, Santschi M, Watson R S.The outcomes of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference Pediatr Crit Care Med 201516(05, Suppl 1):S118–S131. [DOI] [PubMed] [Google Scholar]
- 5.Pediatric Acute Respiratory Distress syndrome Incidence and Epidemiology (PARDIE) Investigators ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Khemani R G, Smith L, Lopez-Fernandez Y M et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2018;2600(18):1–14. [Google Scholar]
- 6.Life After Pediatric Sepsis Evaluation (LAPSE) Investigators . Zimmerman J J, Banks R, Berg R A et al. Critical illness factors associated with long-term mortality and health-related quality of life morbidity following community-acquired pediatric septic shock. Crit Care Med. 2020;48(03):319–328. doi: 10.1097/CCM.0000000000004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group . Simon T D, Cawthon M L, Stanford S et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(06):e1647–e1654. doi: 10.1542/peds.2013-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Life After Pediatric Sepsis Evaluation (LAPSE) Investigators . Zimmerman J J, Banks R, Berg R A et al. Trajectory of mortality and health-related quality of life morbidity following community-acquired pediatric septic shock. Crit Care Med. 2020;48(03):329–337. doi: 10.1097/CCM.0000000000004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Life After Pediatric Sepsis Evaluation (LAPSE) Investigators . Starr M C, Banks R, Reeder R W et al. Severe acute kidney injury is associated with increased risk of death and new morbidity after pediatric septic shock. Pediatr Crit Care Med. 2020;21(09):e686–e695. doi: 10.1097/PCC.0000000000002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varni J W, Burwinkle T M, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(06):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Stein R EK, Jessop D J. Functional status II(R). A measure of child health status. Med Care. 1990;28(11):1041–1055. doi: 10.1097/00005650-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network . Pollack M M, Holubkov R, Funai T et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15(09):821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network . Pollack M M, Holubkov R, Glass P et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(01):e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groupe Francophone de Réanimation et d'Urgences Pédiatriques . Leclerc F, Duhamel A, Deken V, Le Reun C, Lacroix J, Leteurtre S. Nonrespiratory Pediatric Logistic Organ Dysfunction-2 score is a good predictor of mortality in children with acute respiratory failure. Pediatr Crit Care Med. 2014;15(07):590–593. doi: 10.1097/PCC.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 15.Groupe Francophone de Réanimation et d'Urgences Pédiatriques (GFRUP) . Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: an update of the Pediatric Logistic Organ Dysfunction score. Crit Care Med. 2013;41(07):1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 16.Pinto N P, Rhinesmith E W, Kim T Y, Ladner P H, Pollack M M. Long-term function after pediatric critical illness: results from the survivor outcomes study. Pediatr Crit Care Med. 2017;18(03):e122–e130. doi: 10.1097/PCC.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 17.Therapeutic Hypothermia after Pediatric Cardiac Arrest (THAPCA) Trial Investigators . Slomine B S, Silverstein F S, Page K et al. Relationships between three and twelve month outcomes in children enrolled in the therapeutic hypothermia after pediatric cardiac arrest trials. Resuscitation. 2019;139:329–336. doi: 10.1016/j.resuscitation.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Watson R S, Asaro L A, Hutchins L et al. Risk factors for functional decline and impaired quality of life after pediatric respiratory failure. Am J Respir Crit Care Med. 2019;200(07):900–909. doi: 10.1164/rccm.201810-1881OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killien E Y, Farris R WD, Watson R S, Dervan L A, Zimmerman J J. Health-related quality of life among survivors of pediatric sepsis. Pediatr Crit Care Med. 2019;20(06):501–509. doi: 10.1097/PCC.0000000000001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning J C, Pinto N P, Rennick J E, Colville G, Curley M AQ. Conceptualizing post intensive care syndrome in children-the PICS-p Framework. Pediatr Crit Care Med. 2018;19(04):298–300. doi: 10.1097/PCC.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 21.Kohne J G, Flori H R. Switzerland AG: Springer; 2020. Risk factors and etiologies of pediatric acute respiratory distress syndrome; pp. 33–46. [Google Scholar]
- 22.Howe C J, Cole S R, Lau B, Napravnik S, Eron J J., Jr Selection bias due to loss to follow up in cohort studies. Epidemiology. 2016;27(01):91–97. doi: 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim J G, Molenberghs G. Missing data methods in longitudinal studies: a review. Test. 2009;18(01):1–43. doi: 10.1007/s11749-009-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunna K, Al-Ani A, Nikooie R et al. Participant retention in follow-up studies of acute respiratory failure survivors. Respir Care. 2020;65(09):1382–1391. doi: 10.4187/respcare.07461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox M E, Ely E W. Challenges in conducting long-term outcomes studies in critical care. Curr Opin Crit Care. 2019;25(05):473–488. doi: 10.1097/MCC.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


