Abstract
Dermal substitutes have been introduced in burn care to improve wound healing outcomes; however, their use remains limited in standard treatments. This systematic review and meta‐analysis aimed to evaluate the outcomes of dermal substitutes in patients with burns and patients requiring burn scar reconstruction and subsequently contribute to optimising the integration of dermal substitutes into clinical practice and reducing the knowledge gap. A comprehensive search across various databases included human studies from peer‐reviewed journals on dermal substitutes for deep dermal and full‐thickness burns, and scar reconstruction across all ages. Data from comparative trials were extracted, focusing on patient and wound characteristics, treatment specifics, and outcomes related to wound healing and scar quality. Meta‐analysis was performed on trials reporting similar post‐burn measures, with statistical heterogeneity assessed. Outcomes were presented using mean differences or odds ratios with 95% confidence intervals. A total of 31 comparative trials were included. The overall quality of the studies was considered moderate. The meta‐analysis indicated delayed re‐epithelialization 4–7 days after treatment with a collagen‐elastin matrix compared to split‐thickness skin graft in acute burns (−7.30%, p = 0.02). Significant improvement in subjective scar quality was observed with acellular dermal matrix compared to split‐thickness skin graft in acute burn wounds 6 months post‐operative (−1.95, p <0.01). While acknowledging the initially delayed wound healing, incorporating dermal substitutes into the surgical treatment of burn patients holds promise for enhancing scar quality. However, future research must prioritise outcome measure uniformity, address variations in dermal substitute application, and standardise indications for consistent and effective practices.
Keywords: burn reconstruction, burns, dermal substitutes, meta‐analysis, surgery, systematic review, tissue engineering
Abbreviations
- PRISMA
preferred reporting Items for systematic reviews and meta‐analyses
- PROSPERO
register of systematic reviews
- Rob2
cochrane risk of bias 2 tool
- STSG
split‐thickness skin graft
- TBSA
total body surface area
- VSS
Vancouver scar scale
1. INTRODUCTION
Despite significant progress made in recent decades, professionals involved in the care of patients with severe burns continue to face significant challenges. One of these challenges concerns the management and treatment of patients suffering from deep dermal and full‐thickness burns. Despite numerous available treatment modalities, uncertainties remain regarding the optimal management of burns of this depth, even though a significant amount of scientific research focused on this topic. Management of the burn wound in the acute phase and subsequent interventions, such as surgical procedures, becomes especially important in the context of wound healing and scar quality. 1 , 2 Several studies have investigated the optimal timing for surgical interventions in these burns. 3 , 4 , 5 In addition, extensive research has been conducted into promoting wound healing and therefore improving scar quality in burn patients. 1 , 6 , 7
The choice of conservative treatment for deep dermal and full‐thickness burns usually results in cosmetically unfavourable scarring and often compromises functionality. Consequently, the treatment approach for these burns requires a different strategy, often resulting in surgical intervention in the form of excision and skin grafting. This surgical approach plays a crucial role in providing wound coverage, minimising infection risk, and promoting re‐epithelialization. 6 , 7 , 8 , 9
The standard treatment method for deep dermal and full‐thickness burns is autologous split‐thickness skin graft (STSG). Despite the technique's proven effectiveness on burns for decades, it is associated with several negative effects, including donor site morbidity and poor aesthetic outcomes such as hypertrophic scarring and pigmentation abnormalities. To address these issues and improve patient outcomes, research has been conducted to reduce these negative effects. Since 1980, efforts have focused on the development of skin substitutes with the goal of improving scar quality and patient satisfaction. 10
Skin substitutes have been developed to address the complex challenges of wound healing in contexts such as burn care and reconstructive surgery, making them components of advanced medical interventions. 11 These skin constructs are intended to mimic the structure and function of the dermal layer of human skin and are typically composed of bio‐engineered materials or biomimetic constructions. 12 Due to the combination of different components such as elastin, collagen, and synthetic polymers, these products can serve as a basis for tissue repair and regeneration. When applied to wounds, dermal substitutes facilitate cell migration and new tissue formation, thereby promoting wound healing. 13 Due to their unique composition, skin substitutes offer several benefits, including improvements in scar quality, and potentially improving outcomes for patients with burns and those undergoing reconstructive procedures. 8 , 11 , 14 , 15 , 16 , 17
Dermal substitutes can be broadly classified into two types. First, there are single‐stage dermal substitutes, which are applied to a debrided wound bed and covered with an STSG during a single surgical procedure. The second variant, two‐stage dermal substitutes, involves two separate surgical procedures. In the first stage, the dermal substitute is applied to the wound, but the wound is not closed during this procedure. Instead, a temporary sealing membrane made from silicone, or another material is used. A second surgical procedure is then necessary to close the wound permanently with an STSG. This procedure is mostly performed 3–4 weeks later when the dermal substitute has integrated and neo‐vascularization has taken place in the wound bed. 11 , 14 , 15 , 16
In burn and burn scar reconstruction procedures, the integration of dermal substitutes may potentially negatively influence wound healing rates due to the longer time required for the skin to grow through a dermal template compared to an STSG. Despite this potential negative impact on wound healing, we hypothesize that the use of dermal substitutes will positively influence scar formation in the treatment of burns and burn scar reconstruction. Although dermal substitutes have demonstrated favourable outcomes compared to the traditional gold standard of STSGs, their integration into standard burn treatment protocols remains limited. Dermal substitutes could allow for patient‐specific application depending on specific requirements of the burn wound. However, it is worth noting that we may not have fully reached this point yet, and further progress may be needed. Challenges include the lack of clear indications for the use of these substitutes and the lack of a comprehensive review of all available evidence on dermal substitutes in burn patients.
A recent international survey conducted by our group found that most professionals in the global community recognise substantial evidence supporting the effectiveness of dermal substitutes. However, only 63% of the professionals who have experience using dermal substitutes considered the body of evidence sufficient (van den Bosch et al.). 18 To address this knowledge gap, the objective of the current systematic review with meta‐analysis was to provide a comprehensive overview of all existing evidence concerning the outcomes of dermal substitutes in burn patients.
2. METHODS AND ANALYSIS
This systematic review adhered to the guidelines and principles outlined in the 2009 Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement. The research protocol was registered to the International Prospective Register of Systematic Reviews on 25 February 2023 (ID: CRD42023399544).
2.1. Search strategy
On 25 July 2022, a comprehensive search was conducted across several databases, including PubMed, EMBASE, Web of Science, and the Cochrane Library. The search strategy was developed in collaboration with a medical information specialist (GB). This search focused on synonyms for ‘dermal substitutes’ in combination with synonyms for ‘burns’ or ‘reconstructive surgical procedures’. The search did not impose any restrictions based on methodology or publication date but did exclude studies involving animals. References and citations were analysed to identify potential articles for inclusion, in cases where they had not already been found through the electronic search (Supplementary Table 1). On 19 October 2023, this search was repeated to include the most recent studies before publication (Supplementary Table 2).
2.2. Study selection
This systematic review included studies using dermal substitutes as an intervention for deep dermal and full‐thickness burn treatment, as well as the reconstruction of burn‐related scars in patients across all age groups and total body surface areas. Studies using ‘off‐the‐shelf’ dermal substitutes as permanent replacements for lost dermis were included. Various study types, including randomised controlled trials, cohort studies, case series, and case reports were included, with publication in peer‐reviewed journals as a requirement for eligibility. Excluded were studies that were not conducted on humans, and publications not in English or Dutch. The screening process involved two independent investigators (ASB and RAFV), who performed both title and abstract screening and full‐text evaluation. Any discrepancies in the assessment of an article's eligibility were resolved through consultation with a third investigator (EM). The screening procedures in both stages were performed using the web‐based platform RAYYAN (https://www.rayyan.ai).
2.3. Data extraction
The data extraction process focussed on all comparative studies, inter‐ or intrapatient, intended for meta‐analysis. A standardised data extraction method adapted from the Cochrane Collaboration Model was used. 19 Data were collected by two independent researchers (ASB and RAFV), covering various aspects of the studies including the objective and design of the study, control treatment, and types of dermal substitutes used. Additionally, data regarding patient demographics, wound characteristics, treatment specifics, and various aspects of wound healing and scar quality were collected. Specifically, data collection emphasised outcomes such as graft take, re‐epithelialization rates, and scar quality evaluation by both subjective and objective measurement instruments. Patient‐reported outcomes, functional parameters, and complications were also documented. Any discrepancies between ASB and RAFV were resolved through discussions, with EM serving as a final mediator if necessary.
2.4. Risk of bias assessment
Two independent investigators (ASB and RAFV) conducted a risk of bias assessment for all included comparative trials, using the Cochrane Risk of Bias 2 (Rob2) tool.
2.5. Dealing with missing data
When data was missing, the authors were contacted by email to request the missing information. If the data were not provided by the authors, it led to exclusion of those studies from the meta‐analysis.
2.6. Data synthesis
The meta‐analysis of this systematic review included multiple sub‐analyses for each outcome measurement. A sub‐analysis was conducted when two or more clinically homogeneous studies reported outcome measures at the same postoperative time point using the same measurement methods. This meta‐analysis was conducted using Cochrane Collaboration's RevMan 5.4 (Oxford, UK) in a non‐Cochrane environment. The interventions were divided into two categories: (1) the use of dermal substitutes in the acute treatment of burns and (2) the use of dermal substitutes in scar reconstruction resulting from burns. Statistical heterogeneity was evaluated using the I2 and p‐value statistics. A fixed‐effect model was used in cases where no significant heterogeneity was detected between studies (I2 ≤50% or p ≥0.1), while a random‐effect model was used when substantial heterogeneity was evident (I2 >50% or p <0.1). When reporting continuous outcome measures, the intervention effect was presented as the mean difference, accompanied by the associated 95% confidence interval (95% CI). For dichotomous or categorical outcome measures, the intervention effect was presented as the odds ratio, together with the associated 95% CI. An intervention effect was considered statistically significant if the p‐value was less than 0.05.
3. RESULTS
3.1. Study selection
The initial search identified a total of 14,837 initial records from PubMed, Embase, Web of Science, and Cochrane Library, reducing this number to 8180 unique records following deduplication (Figure 1). Subsequently, 240 records were sought for retrieval, with reports not retrieved due to studies involving full skin equivalents (n = 26) or inaccessible full texts (n = 14). About a year later, an updated search was conducted. A total of 2360 new records were identified from PubMed, Embase, Web of Science, and Cochrane Library, reducing this number to 1780 unique records after deduplication. Subsequently, 123 records were sought for retrieval, with exclusions mainly relating to studies retrieved during previous searches (n = 40) or inaccessible full texts (n = 4). Eligibility criteria were applied during full‐text assessment of the remaining 200 reports from the first search and 79 records from the updated search. This led to 89 exclusions. There were no additional records that met the inclusion criteria via the citation and reference search in either search.
FIGURE 1.
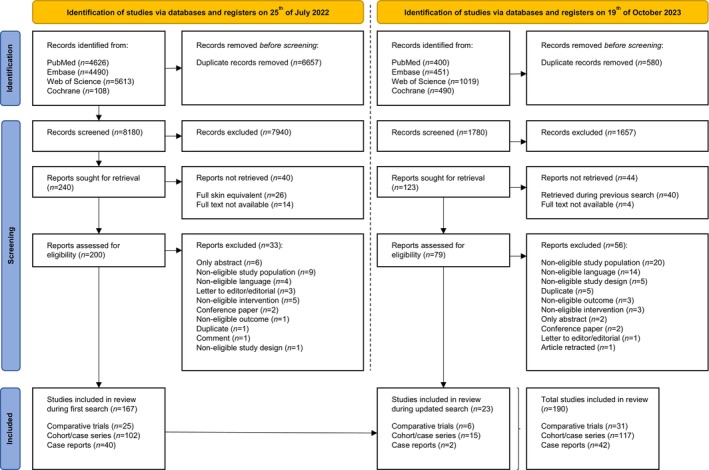
PRISMA flow chart. Identification of studies via databases and registers.
3.2. Description of studies
3.2.1. Results of the search
A total of 190 studies were ultimately included in this systematic review, comprising 31 comparative studies, 117 cohort and case series, and 42 case reports. Among these, the 31 comparative studies were deemed to represent the highest level of evidence for evaluating the efficacy of dermal substitutes. Consequently, they were selected for consideration in the meta‐analysis to assess the outcomes of dermal substitutes in both burn patients and patients requiring reconstructive procedures following burn scars.
3.2.2. Included studies
A total of 31 comparative studies were included, comprising 14 randomised controlled trials, one non‐randomised controlled trial, 12 intra‐individual comparison studies, two observational comparative studies, and two matched control studies. These studies resulted in nine possible comparisons, as two or more studies reported on the same comparison. When two or more trials, sharing clinical homogeneity, reported outcome measures at the same time point post‐burn or postoperative, a sub‐analysis could be performed. This resulted in meta‐analysis for four different comparisons: (1) Matriderm® (MedSkin Solutions Dr. Suwelack AG, Billerbeck, Germany) compared to STSG; (2) acellular dermal matrix (Jieya Matrix; Beijing Jieya Laifu Biotechnology Company, Ltd., Beijing, China) compared to STSG; (3) Glyaderm® (Euro Skin Bank, Beverwijk, The Netherlands) compared to STSG and (4) Matriderm® (Dr. Otto Suwelack Skin&Health Care AG, Billerbeck, Germany) compared to Integra® (Integra Life Sciences, Plains boro, NJ) in acute burns and reconstructive surgery of burn sequelae.
A total of 14 sub‐analyses were conducted for four different comparisons for acute burns (n = 9), and reconstruction of burn scars (n = 5). Thirteen out of 31 comparative trials were included in these sub‐analyses. 17 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 These sub‐analyses reported eight different outcome variables, including graft take (n = 2); re‐epithelialization (n = 1); regrafting (n = 1); scar elasticity by Cutometer (n = 2); scar assessment by (adapted) Vancouver scar scale (VSS) (n = 5); scar contraction by planimetry (n = 2); wound healing rate in days (n = 1) (Supplementary Table 3).
3.3. Risk of bias in included studies
The risk of bias was assessed for all 31 comparative studies by two independent researchers (ASB and RAFV). The risk of bias summaries is shown in Figure 2A (acute burn wounds) and Figure 2B (reconstruction of burn scars). The overall quality of the included studies in both categories was considered moderate. An important contributing factor for this rating was the lack of a double‐blind design. Although the nature of these studies makes it almost impossible to implement a double‐blind design, this limitation could still introduce bias.
FIGURE 2.
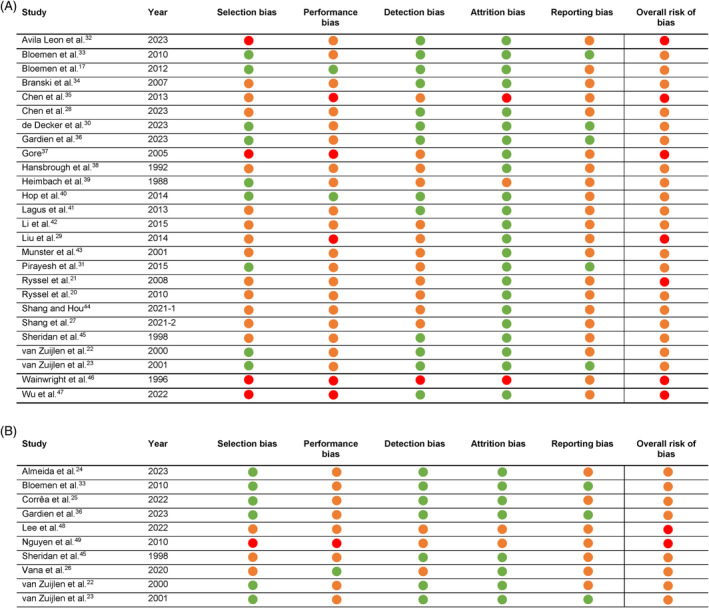
(A) Assessment of risk of bias using the Cochrane Collaboration's risk of bias tool 2—acute burn wounds  Low risk;
Low risk;  Some risk;
Some risk;  High risk. (B) Assessment of risk of bias using the Cochrane Collaboration's risk of bias tool 2—reconstruction of burn scars.
High risk. (B) Assessment of risk of bias using the Cochrane Collaboration's risk of bias tool 2—reconstruction of burn scars.  Low risk;
Low risk;  Some risk;
Some risk;  High risk.
High risk.
3.4. Outcomes of dermal substitutes in acute burn wounds
Out of 31 records that met the eligibility criteria, 26 studies reported on the use of dermal substitutes in the surgical treatment of patients with acute burns. 17 , 20 , 21 , 22 , 23 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 The study characteristics, interventions, and type of dermal substitute of each of these studies are described in Table 1. These records were published in English between 1988 and 2023, mostly conducted in the USA (n = 8) and the Netherlands (n = 7). Sample size varied between 5 and 1208 patients. Most records represented randomised controlled trials. 17 , 27 , 28 , 29 , 33 , 34 , 35 , 39 , 40 , 42 , 44 The majority of the studies studied the dermal substitute Matriderm® (n = 7).
TABLE 1.
Characteristics of included studies—Acute burn wounds.
| Author | Year | Country | Design | Study population | Sample size | Intervention | Control | |
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Avila Leon et al. 32 | 2023 | Colombia | Observational comparative study | Patients admitted to the burn unit who had secondary skin defects from burns | 33 grafted body areas (29 patients) | 35 grafted body areas (29 patients) | Glycerolized acellular dermal matrix (GADM) + autograft | Autograft |
| Bloemen et al. 33 | 2010 | The Netherlands | RCT | Patients admitted and needed surgical treatment for acute burn wounds or reconstruction of burn scars | 35 scar pairs in 26 patients | 35 scar pairs in 26 patients | Matriderm® + split‐skin graft | Split‐skin graft |
| Bloemen et al. 17 | 2012 | The Netherlands | RCT | Patients with: 1. Deep dermal or full‐thickness burn wounds requiring skin transplantation; 2. ≥18 years; 3. TBSA third‐degree burns ≤15%; 4. Study wound surface area min. 10 cm2; 5. Study wound surface area max. 300 cm2; 6. Informed consent |
23 patients (Intervention + TNP: 21 patients; only TNP: 22 patients) |
20 patients | Matriderm® + split‐skin graft | Split‐skin graft |
| Branski et al. 34 | 2007 | USA | RCT | Patients with: 1. Burn size ≥50% TBSA and ≥40% TBSA full‐thickness burn; 2. Patients admitted within 72 h of injury; 3. Patients not septic at admission | 10 patients | 10 patients | Integra® + split‐skin graft | Split‐skin graft |
| Chen et al. 35 | 2013 | China | RCT | Admitted patients with deep burns who needed surgical treatment 1. Burn area ≤60%; 2. Third‐degree burn area ≤40%; 3. Thermal burns; 4. No exposed bones/joints/nerves or tendons; 5. No serious heart/liver/kidney and blood system complications; 6. no systemic infection | 30 patients | 30 patients | Porcine acellular dermal xenograft + autologous split‐thickness skin | Autologous split‐thickness skin grafting |
| Chen et al. 28 | 2023 | China | RCT | Patients: 1. Admitted ≥24 h; 2. Clear consciousness, ability to communicate and answer questions normally; 3. Complete medical record data |
1208 patients (Burn group (n = 158), Trauma group (n = 105)) |
1208 patients (Burn group (n = 602), Trauma group (n = 343)) |
Acellular dermis matrix + autologous ultra‐thin split‐thickness skin composite transplantation | Traditional skin graft repair |
| de Decker et al. 30 | 2023 | Belgium | Intra‐individual comparison | Patients with: 1. Deep partial thickness and full‐thickness burns as shown by Laser Doppler Imaging (LDI) and/or clinically evaluated by two plastic surgeons or a burn care coordinator; 2. Other full‐thickness skin defects besides burns (e.g., necrotizing fasciitis, deglovements or phalloplasty donor sites after free flap harvest; 3. Possibility to follow complete treatment schedule; 4. Informed consent; 5. Age between 18 and 80 years |
82 wounds in 66 patients (Burn injuries (n = 29); Phalloplasty donor site (n = 29); Other full‐thickness skin defects (n = 8)) |
82 wounds in 66 patients (Burn injuries (n = 29); Phalloplasty donor site (n = 29); Other full‐thickness skin defects (n = 8)) |
Glyaderm® + split‐thickness skin graft | Split‐thickness skin graft |
| Gardien et al. 36 | 2023 | The Netherlands | Intra‐individual comparison | 1. Informed consent; 2. Age ≥18 years with full‐thickness skin defects that require skin grafting; 3. ≤ 50% Total body surface area burned with full‐thickness skin defects at time of intervention; 4. Full‐thickness skin defects configured in such a way that two comparable and measurable areas can be grafted, both a minimum of 3 × 3 cm |
24 patients (Reconstructive wound (n = 5), acute burn wound (n = 19)) |
24 patients (Reconstructive wound (n = 5), acute burn wound (n = 19)) |
Novomaix® + split‐thickness skin graft | Split‐thickness skin graft |
| Gore 37 | 2005 | USA | Matched control | Patients of ≥70 years admitted to the Burn Center following thermal injury | 10 patients | 18 patients | AlloDerm® + thin autograft (depth 0.005 inches) | Autografting (depth 0.014 inches) |
| Hansbrough et al. 38 | 1992 | USA | Clinical trial | Patients in whom burn wounds would be surgically excised within 2 weeks of injury | 17 patients | 17 patients | Dermagraft® + thin MESTSG | Standard thickness MESTSGs |
| Heimbach et al. 39 | 1988 | USA | RCT | Patients who were hospitalised with extensive flame or scald burns that were considered to be life‐threatening and, in the opinion of the investigator, would not heal within 3 weeks and were amenable to early excision and grafting | 136 sites in 106 patients | 136 sites in 106 patients | Artificial dermis + meshed autograft | Meshed autograft |
| Hop et al. 40 | 2014 | The Netherlands | RCT | Patients with: 1. Deep dermal or full thickness burn wounds requiring skin transplantation; 2. Age ≥18 years; 3. TBSA full thickness burns 15%; 4. Study wound surface area min. 10 cm2 and max. 300 cm2; 5. Informed consent |
23 patients (Intervention + TNP: 21 patients; only TNP: 22 patients) |
20 patients | Matriderm® + split‐skin graft | Split‐skin graft |
| Lagus et al. 41 | 2013 | Finland | Intra‐individual comparison | Patients with: 1. Age 17–70 years; 2. TBSA >20%; 3. Burns located on the anterior side of the body | 10 patients | 10 patients | Integra® + split thickness skin graft | Split thickness skin graft |
| Li et al. 42 | 2015 | China | RCT | Patients who sustained burns | 30 patients | 30 patients | Acellular dermis matrix + autologous split‐thickness | Autologous split‐thickness skin |
| Liu et al. 29 | 2013 | China | RCT | Patients with deeply burned hands where eschar is excised | 27 patients | 26 patients | Acellular dermis matrix + autologous split‐thickness skin | Autologous medium‐thickness skin |
| Munster et al. 43 | 2001 | USA | Intra‐individual comparison | Patients with a burn size and third degree component large enough to plan a two‐stage procedure for complete wound coverage | 17 patients | 17 patients | AlloDerm® + immediately covered with thin (6/000 inch) split thickness autograft | Split thickness autograft with standard thickness (10–12/000 inch) |
| Pirayesh et al. 31 | 2015 | The Netherlands | Intra‐individual comparison | Patients ≤80 years with: 1. Full‐thickness burns or lower arm defects after free flap harvesting; 2. The possibility to follow the complete treatment schedule |
32 sites in 28 patients (13 burn wound sites in 9 patients; 19 radial forearm flap sites in 19 patients) |
32 sites in 28 patients (13 burn wound sites in 9 patients; 19 radial forearm flap sites in 19 patients) |
Glyaderm® + split‐thickness skin graft | Split thickness skin graft |
| Ryssel et al. 21 | 2008 | Germany | Intra‐individual comparison | Admitted patients who needed surgical treatment for acute burns | 28 treated areas in 10 patients | 28 treated areas in 10 patients | Matriderm® + split‐thickness autograft | Split‐thickness autograft |
| Ryssel et al. 20 | 2010 | Germany | Intra‐individual comparison | Patients: 1. Who required surgical treatment for acute burns on the dorsal surface of both hands; 2. Age 18–70 years; 3. Sufficient knowledge of German to complete the self‐report questionnaire; 4. no other serious hand injuries that might affect principle outcomes | 18 treated areas in 18 patients | 18 treated areas in 18 patients | Matriderm® + split‐thickness skin graft | Split‐thickness skin graft |
| Shang and Hou 44 | 2021–1 | China | RCT | Patients with: 1. Integrated clinical data; 2. TBSA >85% with III degree TBSA >50% and scar area >50% TBSA; 3. Normally function in major organs including heart, lung, liver, and kidney; 4. Informed consent | 28 patients | 28 patients | Acellular dermis matrix + autologous split‐thickness skin graft | Intermediate‐thickness skin graft |
| Shang et al. 27 | 2021‐2 | China | RCT | Patients with: 1. Flame burns; 2. TBSA 85%–95%, in which the sum of deep second‐ and third‐degree wounds exceeded 50% of TBSA, and the scalp was normal; 3. Informed consent; 4. Normal mental status and good compliance. | 32 patients |
32 patients |
Acellular dermis matrix + autologous razor‐thin graft | Autologous scar tissue + autologous razor‐thin graft |
| Sheridan et al. 45 | 1998 | USA | Matched control | Patients with limited (<25%) areas of the body surface available for donor harvest who needed either acute resurfacing or reconstructive procedures, both of which required split‐thickness auto grafting. |
10 sites on 6 children (9 reconstructive sites and 1 acute burn site) |
10 sites on 6 children (9 reconstructive sites and 1 acute burn site) |
AlloDerm® + thin autograft | Autograft |
| van Zuijlen et al. 22 | 2000 | The Netherlands | Intra‐individual comparison | Admitted patients who needed surgical treatment for acute burn wounds or reconstruction of scar tissue that remained after a burn injury | 42 paired wound areas in 31 patients | 42 paired wound areas in 31 patients | Matriderm® + split‐thickness autograft | Split‐thickness autograft |
| van Zuijlen et al. 23 | 2001 | The Netherlands | Intra‐individual comparison | Admitted patients who needed excision and skin grafting for acute burn wounds or reconstruction of scar tissue that remained after a burn injury. | 42 paired wound areas in 31 patients | 42 paired wound areas in 31 patients | Matriderm® + split skin graft | Split skin graft |
| Wainwright et al. 46 | 1996 | USA | Intra‐individual comparison | Presence of contiguous or mirror‐image sites (measuring between 6 cm × 6 cm and 7.5 cm × 15.5 cm) of full‐thickness or deep partial‐thickness burn injury, the patient's expected survival, the need for two or more operative procedures during hospitalisation, and the patient's informed consent | 43 patients | 43 patients | AlloDerm® + split‐thickness skin graft | Split‐thickness skin graft |
| Wu et al. 47 | 2022 | USA | Observational comparative study | Adults who underwent wound reconstruction of the head and/or neck, with either BTM or CCS bilayer |
5 patients (burn (n = 2), trauma (n = 1), surgical wounds (n = 1), and skin cancer (n = 1)) |
10 patients (burn (n = 6), trauma (n = 2), surgical wounds (n = 2)) |
NovoSorb biodegradable temporising matrix (BTM) + split‐thickness skin graft | Integra collagen‐chondroitin silicone (CCS) + split‐thickness skin graft |
The studies represented 2129 patients consisting of 1358 acute burn patients (Table 1). The average age of all study patients, including other groups than acute burn patients (e.g., trauma‐ or oncology patients), varied between 5.2 and 78 years old, and most of the patients were male. The average burn size ranged from 7.7% to 95%, and the highest average full‐thickness burn size was 70% (±3%) 34 (Table 2).
TABLE 2.
Characteristics of study participants—Acute burn wounds.
| Study | Year | Male n (%) | Age mean (SD or range) | Burn size mean (SD or range) | Full‐thickness burn mean (SD or range) | Aetiology (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||
| Avila Leon et al. 32 | 2023 | 16 (48.5) | 20 (57.1) | 19.7 | 20.1 | 11.8 | 14.6 | 9 | 9 | Flame (47); Scald (31); Solid (9); Electricity (4); Chemical (3); Other (6) | |
| Bloemen et al. 33 | 2010 | 15 (58) | 41.3 (18.7) | 24.3 (14.7) | 11.8 (14.1) | ‐ | |||||
| Bloemen et al. 17 | 2012 | 12 (52) | 14 (70) | 48 (19.4) | 53 (18.3) | 9.6 (8.1) | 7.7 (7.4) | ‐ | ‐ | Flame (60.9); Scald (13); Other (26.1) | Flame (70); Other (30) |
| Branski et al. 34 | 2007 | 7 (70) | 9 (90) | 7.4 (−) | 6.2 (−) | 70 (5) | 74 (4) | 65 (6) | 70 (3) | ‐ | ‐ |
| Chen et al. 35 | 2013 | 20 (66.7) | 18–60 years | 25%–60% | ≤40% | Thermal (100) | |||||
| Chen et al. 28 | 2023 | 997 (82.5) | 39 (2–71) | ‐ | ‐ | ‐ | |||||
| de Decker et al. 30 | 2023 | 44 (66.7) | 39.5 (18) | 12.3 (7.5) | ‐ | ‐ | |||||
| Gardien et al. 36 | 2023 | 13 (54.2) | 53.5 (19–87) | 8.0 (2–67) | 5.0 (1–52) | Flame (63); Fat (16); Bioethanol (11); Contact (5); Scald (5) | |||||
| Gore 37 | 2005 | ‐ | ‐ | 78 (2) | 79 (2) | 17 (2) | 20 (3) | 15 (1) | 19 (3) | Thermal (100) | Thermal (100) |
| Hansbrough et al. 38 | 1992 | ‐ | 31.1 (15.1) | 23.8 (21.4) | 23.8 (21.4) | ‐ | |||||
| Heimbach et al. 39 | 1988 | 80 (75.5) |
< 15 years: 27 (25.5) >60 years: 20 (19) |
46 (19) | 32 (20) | Thermal (100) | |||||
| Hop et al. 40 | 2014 | 12 (52) | 14 (70) | 48 (19.4) | 53 (18.3) | 9.6 (8.1) | 7.7 (7.4) | ‐ | ‐ |
Flame (60.9) Scald (13) Other (26.1) |
Flame (70) Other (30) |
| Lagus et al. 41 | 2013 | 9 (90) | 36.8 (−) | 35.8 (18.6) | ‐ | Thermal (90); Electrical (10) | |||||
| Li et al. 42 | 2015 | 25 (83.3) | 24 (100) | 30.5 (10.7) | 25.3 (15.3) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu et al. 29 | 2013 | 16 (57.1) | 17 (56.7) | 28.4 (3.6) | 26.8 (4.6) | 88.4 (4.3) | 87.8 (3.7) | ‐ | ‐ | ‐ | ‐ |
| Munster et al. 43 | 2001 | 13 (76.5) | 48.4 (4.4) | 28.7 (3.6) | ‐ | ‐ | |||||
| Pirayesh et al. 31 | 2015 | ‐ | 33.1 (10.4) | ‐ | ‐ | ‐ | |||||
| Ryssel et al. 21 | 2008 | 7 (70) | 49.5 (16.2) | 45.6 (14.5) | ‐ | Flame (80); Scald (10); Contact (10) | |||||
| Ryssel et al. 20 | 2010 | 13 (72.2) | 45.1 (17.4) | 43.3 (11.8) | ‐ |
Flame (88.9) Scald (11.1) |
|||||
| Shang and Hou 44 | 2021–02 | 20 (62.5) | 19 (59.4) | 37.6 (3.8) | 37.8 (3.9) | 85%–95% | 50% of TBSA | Flame (100) | |||
| Shang et al. 27 | 2021–06 | 16 (57.1) | 15 (53.6) | 36.5 (3.5) | 36.4 (3.5) | >85% | >50% | ‐ | |||
| Sheridan et al. 45 | 1998 | ‐ | 5.2 (0.9) | 68.7 (6.7) | ‐ | ‐ | |||||
| van Zuijlen et al. 22 | 2000 | 18 (58.1) | 32.9 (19.3) | 19.8 (14.5) | 9.0 (11.2) |
Flame (80.6) Scald (16.1) Steam injury (3.2) |
|||||
| van Zuijlen et al. 23 | 2001 | ‐ | 32.9 (13.3) | 19.8 (14.5) | 9.0 (11.2) | ‐ | |||||
| Wainwright et al. 46 | 1996 | 25 (58.1) | 45.1 (16–89) | 30.5 (3–87) | 20.1 (0–81) | ‐ | |||||
| Wu et al. 47 | 2022 | 3 (60) | 4 (40) | 66 (12.8) | 49.5 (11.7) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
Nine sub‐analyses were feasible to synthesise the relevant data from these 26 studies. First, seven out of 26 studies compared transplantation of full‐thickness wounds with Matriderm® and STSG with transplantation of only STSG. Between 5 and 7 days post‐operative, the graft take was higher in the control group compared to the experimental group (−3.13%; 95% CI [−9.15, 2.90]; I2 = 59%; p = 0.31), but this difference in result did not reach statistical significance (Figure 3A). 17 , 20 , 21 , 22 On the contrary, there was a statistically significant difference in re‐epithelialization rate at 4–7 days post‐surgery, with the rate being lower in the experimental group (−7.30%; 95% CI [−13.54, −1.05]; I2 = 0%; p = 0.02) (Figure 3B). 17 , 22 The number of regrafting procedures was higher in the experimental group (1.99; 95% CI [0.56, 7.03]; I2 = 0%; p = 0.29), but did not reach statistical significance (Figure 3C). 17 , 20 , 21 , 22 At 12 months post‐surgery, a slight difference in elasticity (Uf‐ratio) in the scar was observed when measured by the Cutometer (−0.05; 95% CI [−0.28, 0.18]; I2 = 48%; p = 0.67). However, this difference was not statistically significant (Figure 3D). 17 , 23 Finally, the clinical scar assessment by VSS at 12 months post‐surgery was lower, thus more comparable to normal skin, in the experimental group (−1.59; 95% CI [−5.09, 1.91]; I2 = 84%; p = 0.37), but the difference was not statistically significant (Figure 3E). 20 , 23 Within the first comparison, studies were not clinically homogenic enough to allow for sub‐analysis for the following outcomes: contamination; patient‐reported outcomes; scar erythema and melanin; scar roughness; and complications. Thereby, there was insufficient data to perform a sub‐analysis on the outcome measures: tissue hardness (n = 1); costs (n = 1); histopathology (n = 1); and mobility (n = 1).
FIGURE 3.
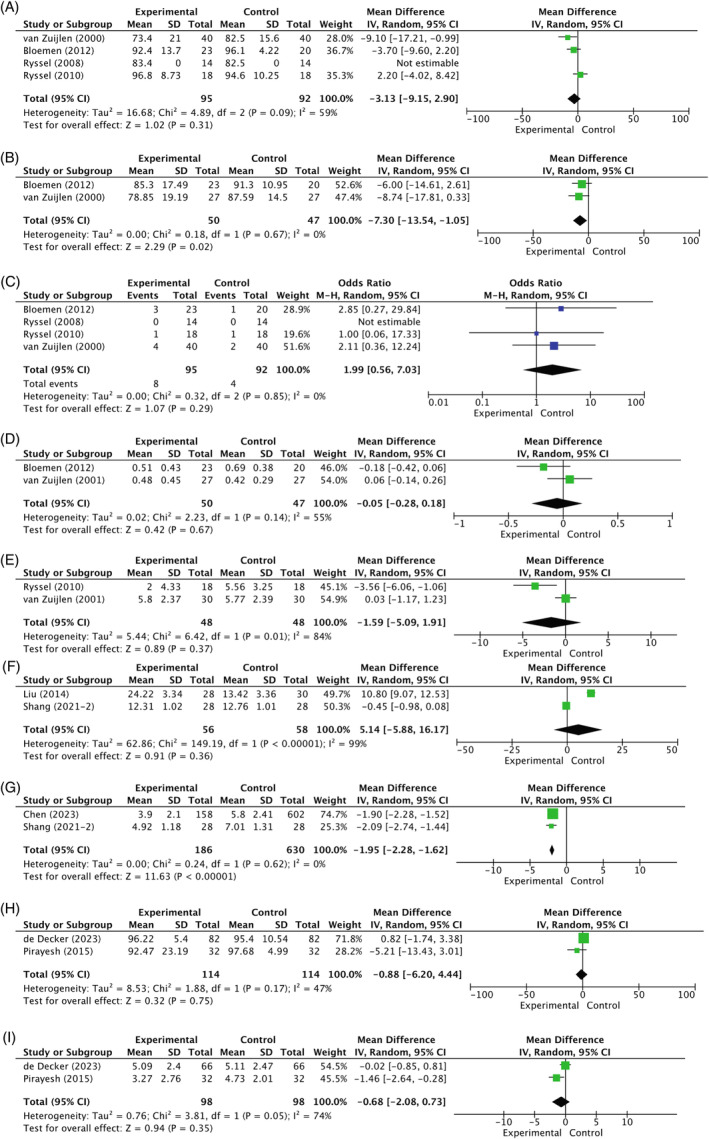
(A) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in % graft take (5–7 days post‐surgery) in acute burns. (Ryssel et al.: We did not receive information on standard deviation, therefore this study was not included in the definitive meta‐analysis. In this study among 10 patients, the graft take in the dermal substitute group and the control group was 83.4% and 82.5%, respectively (p = 0.25). We did not expect that this would have changed the overall results. 21 ) (B) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in % re‐epithelialization (4–7 days post‐surgery) in acute burns. (C) Comparison 1. Matriderm® versus split‐thickness skin graft: Odds ratio of regrafting procedures during admission in acute burns. (Definition regrafting: The number of grafted wounds that required regrafting during the period of admission. Ryssel et al.: There were no complications in both groups. This was statistically not possible, but we do not expect with a odds ratio of 1.00 this would have a difference on the overall results in this meta‐analysis. 21 ) (D) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in scar elasticity (Uf‐ratio) measured by Cutometer (12 months post‐surgery) in acute burns. (E) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in scar assessment score by Vancouver scar scale (12 months post‐surgery) in acute burns. (F) Comparison 3. Acellular dermis matrix versus split‐thickness skin graft: Mean difference in healing time (days) in acute burns. (G) Comparison 3. Acellular dermis matrix versus split‐thickness skin graft: Mean difference in scar assessment score by Vancouver Scar Scale (6 months post‐surgery) in acute burns. (H) Comparison 4. Glyaderm® versus split‐thickness skin graft: Mean difference in % graft take (7 days post‐surgery) in acute burns. (I) Comparison 4. Glyaderm® versus split‐thickness skin graft: Mean difference in scar assessment score by Adapted Vancouver Scar Scale (12 months post‐surgery) in acute burns.
Five out of 26 studies on acute burns compared the application of acellular dermal matrix with STSG to STSG alone. 27 , 28 , 29 , 42 , 44 The wound healing time in days was higher in the experimental group (5.14; 95% CI [−5.88, 16.17]; I2 = 99%; p = 0.36), but did not reach statistical significance (Figure 3F). 27 , 29 On the contrary, the clinical scar assessment by VSS at 6 months post‐operative was statistically significantly different between both groups (−1.95; 95% CI [−2.28, −1.62]; I2 = 0%; p = <0.01) (Figure 3G). 27 , 28 The VSS was lower and therefore closer to normal skin in the experimental group. Within this comparison, there was insufficient data to perform a sub‐analysis on the outcome measures: graft take (n = 1); scar contraction (n = 1); costs (n = 1); histopathology (n = 1); infection (n = 1); donor site quality (n = 1); healing rate (n = 1); quality of life (n = 1); survival rate (n = 1); length of hospital stay (LOS) (n = 1); and scar appearance (n = 1). Due to clinical heterogeneity, no sub‐analysis for the outcome mobility could be performed.
Another comparison in the acute burn group concerned Glyaderm® plus STSG to STSG alone. 30 , 31 At 7 days post‐surgery, there was a trend towards a higher graft take in the control group. However, this difference was not statistically significant (−0.88; 95% CI [−6.20, 4.44]; I2 = 47%; p = 0.75) (Figure 3H). 30 , 31 The clinical scar assessment by the Adapted VSS at 12 months post‐operative was lower in the experimental group. The scars in the experimental group were closer to normal skin according to the clinicians, but this result did not reach statistical significance (−0.68; 95% CI [−2.08, 0.73]; I2 = 74%; p = 0.35) (Figure 3I). 30 , 31 Within this comparison, there was limited data to perform a sub‐analysis on the following outcome measures: re‐epithelialization (n = 1); patient‐reported outcomes (n = 1); pain (n = 1); histopathology (n = 1); scar hydration (n = 1); and scar appearance (n = 1). Scar elasticity parameters were measured by different devices in the two studies, namely DermaLab and Cutometer dual MPA 580 (Courage + Khazaka electronic GmbH, Köln, Germany). Therefore, a sub‐analysis could not be performed for this outcome.
3.5. Outcomes of dermal substitutes in burn scar reconstructive surgery
Out of 31 records that met the eligibility criteria, 10 studies reported on the use of dermal substitutes in the reconstruction of burn scars or contractures resulting from burns. 22 , 23 , 24 , 25 , 26 , 33 , 36 , 45 , 48 , 49 The study characteristics, interventions, and type of dermal substitute used in each of these studies are described in Table 3. The records were published in English between 1998 and 2023, conducted in the Netherlands (n = 4 (2 cohorts)), Brazil (n = 3), Republic of Korea (n = 1), the UK (n = 1), the USA (n = 1). Sample size varied between six and 31 patients. Five records were intra‐individual comparison studies, 22 , 23 , 25 , 36 , 49 four studies were randomised controlled trials, 24 , 26 , 33 , 48 and one matched control study. 45 Most studied dermal substitutes in reconstructive patients were Matriderm® (n = 7) and Integra® (n = 3).
TABLE 3.
Characteristics of included studies—reconstruction of burn scars.
| Author | Year | Country | Design | Study population | Sample size | Intervention | Control | |
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Almeida et al. 24 | 2023 | Brazil | RCT | Patients >18 years old with chronic burn contractures that resulted in functional impairments, with ≥1 year of post‐burn follow‐up, in which surgical treatment was performed with STSG |
10 patients a 9 patients b 10 patients c |
10 patients | Integra® + STSG, Matriderm® + STSG and Pelnac® + STSG | STSG |
| Bloemen et al. 33 | 2010 | The Netherlands | RCT | Patients admitted and needed surgical treatment for acute burn wounds or reconstruction of burn scars | 34 scar pairs in 26 patients | 34 scar pairs in 26 patients | Matriderm® + split‐skin graft | Split‐skin graft |
| Corrêa et al. 25 | 2022 | Brazil | Intra‐individual comparison | Patients with burn contractures treated using autologous skin grafts and dermal matrix |
10 patients a 10 patients c 9 patients b |
10 patients | Integra® + STSG, Pelnac® + STGS and Matriderm® + STSG | Skin graft |
| Gardien et al. 36 | 2023 | The Netherlands | Intra‐individual comparison | 1. Informed consent; 2. Age ≥18 y with full‐thickness skin defects that require skin grafting; 3. ≤ 50% Total body surface area burned with full‐thickness skin defects at time of intervention; 4. Full‐thickness skin defects configured in such a way that two comparable and measurable areas can be grafted, both a minimum of 3 × 3 cm |
24 patients (Reconstructive wound (n = 5), acute burn wound (n = 19)) |
24 patients (Reconstructive wound (n = 5), acute burn wound (n = 19)) |
Novomaix® + split‐thickness skin graft | Split‐thickness skin graft |
| Lee et al. 48 | 2022 | Republic of Korea | RCT | Patients who experienced a burn injury in the past years to least 6 months before the biopsy and had received treatment for at least 6 months before the biopsy with a deep second‐ or third‐degree burn and who had developed a hypertrophic scar |
6 patients d 11 patients b 18 patients e |
28 patients | FTSG, Matriderm® + STSG and AlloDerm® + STSG | STSG |
| Nguyen et al. 49 | 2010 | UK | Intra‐individual comparison | All adults ≥18 years who had been treated using Integra and SSG >1 year |
6 patients (Burn reconstruction (n = 4), primary burn (n = 2), congenital naevus (n = 1)) |
6 patients (Burn reconstruction (n = 4), primary burn (n = 2), congenital naevus (n = 1)) |
Integra® + split‐skin graft | Split‐skin graft |
| Sheridan et al. 45 | 1998 | USA | Matched control | Patients with limited (<25%) areas of the body surface available for donor harvest who needed either acute resurfacing or reconstructive procedures, both of which required split‐thickness autografting |
10 sites on 6 children (9 reconstructive sites and 1 acute burn site) |
10 sites on 6 children (9 reconstructive sites and 1 acute burn site) |
Acellular allogenic dermis + thin autograft | Autograft alone |
| Vana et al. 26 | 2020 | Brazil | RCT | Patients with impaired mobility resulting from burn sequelae who needed surgery in a two‐step procedure | 12 patients | 12 patients | Matriderm® (2 mm) + split‐thickness skin graft | Integra® + split‐thickness skin graft |
| van Zuijlen et al. 22 | 2000 | The Netherlands | Intra‐individual comparison | Admitted patients who needed surgical treatment for acute burn wounds or reconstruction of scar tissue that remained after a burn injury | 44 paired wound areas in 31 patients | 44 paired wound areas in 31 patients | Matriderm® + split‐thickness autograft | Split‐thickness autograft |
| van Zuijlen et al. 23 | 2001 | The Netherlands | Intra‐individual comparison | Admitted patients who needed excision and skin grafting for acute burn wounds or reconstruction of scar tissue that remained after a burn injury | 44 paired wound areas in 31 patients | 44 paired wound areas in 31 patients | Matriderm® + split skin graft | Split skin graft |
Integra®.
Matriderm®.
Pelnac®.
Full‐thickness skin graft (FTSG).
AlloDerm®.
A total of 289 reconstructive patients of which 266 reconstructive patients after hypertrophic burn scars or contractures were presented in the included studies (Table 3). Their mean age varied from 5.2 to 53.5 years old. Most of the patients were female (58.5%), and the most presented contracture sites were upper extremities (n = 91) (Table 4).
TABLE 4.
Characteristics of study participants—reconstruction of burn scars.
| Study | Year | Male n (%) | Age mean (SD) | Type of patients | Contracture site (%) | |||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |||
| Almeida et al. 24 | 2023 |
5 (50) a 4 (44.4) b 2 (20) c |
7 (70) |
33 (16.8) a 35.1 (19.5) b 28.4 (16.6) c |
37.1 (17.3) | Burn contractures |
Axilla (10) a ; (22.2) b ; (10) c Cervical region (20) a ; (33.3) b ; (20) c Inframammary region (20) a ; (30) c Trunk (10) a |
Axilla (20) Cervical region (20) UE (40) Trunk (20) |
| Bloemen et al. 33 | 2010 | 16 (62) | 42.3 (18.2) | Burn scars | ‐ | ‐ | ||
| Corrêa et al. 25 | 2022 | 18 (46.2) | 33.1 (−) | Burn contractures | UE (28.2); Cervical region (28.2); Axilla (17.9); Inframammary region (12.8); Trunk (7.7); LE (5.1) | |||
| Gardien et al. 36 | 2023 | 13 (54.2) | 53.5 (19–87) | Burn reconstruction, acute burn wound | Arm (29); Trunk (25); Leg (25); Hand (8); Neck (8); Foot (4) | |||
| Lee et al. 48 | 2022 |
5 (83.3) d 9 (81.8) b 11 (61.1) e |
22 (78.6%) |
42 (12.7) d 44.6 (15.3) b 26.4 (11.2) e |
27.3 (12.1) | Hypertrophic burn scars |
Trunk (9.1) b UE (66.7) d ; (45.5) b ; (61.1) e Inguinal (5.6) e |
UE (35.7); LE (28.6); Trunk (21.4); Face/neck (14.3) |
| Nguyen et al. 49 | 2010 | 2 (50) | 35 (−) | Burn contractures | Trunk (50); LE (25); Hand (25) | Trunk (50); LE (25); N/A (25) | ||
| Sheridan et al. 45 | 1998 | 3 (50) | 5.2 (0.9) | Burn contractures | ‐ | ‐ | ||
| Vana et al. 26 | 2020 | 5 (41.7) b | 3 (25) a | 33 (15.7) | 35.8 (13.3) | Impaired mobility resulting from burn sequelae |
UE (33.3); Neck (25); Axilla (25); Trunk (16.7) |
UE (50); Neck (25); Axilla (16.7); Trunk (8.3) |
| van Zuijlen et al. 22 | 2000 | 20 (64.5) | 33.9 (17.5) | Burn contractures | Neck (34.1); UE (25); LE (15.9); Trunk (13.6); Axilla (11.4) | |||
| van Zuijlen et al. 23 | 2001 | ‐ | 33.9 (17.5) | Burn contractures | ‐ | |||
Abbreviations: LE, lower extremity; UE, upper extremity.
Integra®.
Matriderm®.
Pelnac®.
Full‐thickness Skin Graft (FTSG).
AlloDerm®.
Two comparisons were suitable for meta‐analysis within these 10 studies: Matriderm® versus STSG; and Matriderm® versus Integra®. A total of five sub‐analyses were conducted. First, six out of 10 studies compared Matriderm® plus STSG to STSG alone. 22 , 23 , 24 , 25 , 33 , 48 Note that some studies investigated more than one type of dermal substitute; however, only the data from the Matriderm® and the control group were utilised for this meta‐analysis. At 12 months post‐operative, the scar elasticity (Uf) measured by Cutometer was higher in the experimental group. However, this difference was not statistically significant (0.06; 95% CI [−0.01, 0.12]; I2 = 0%; p = 0.10) (Figure 4A). 23 , 24 In addition, while the clinical scar assessment using the VSS 12 months post‐surgery was lower in the experimental group, suggesting a scar appearance more comparable to normal skin than the control group, this difference was not statistically significant (−0.47; 95% CI [−1.44, 0.51]; I2 = 0%; p = 0.35) (Figure 4B). 23 , 24 Finally, percentage contraction at 12 months post‐operative compared to the area of the wound directly after excision showed more contraction in the experimental group (10.18; 95% CI [−4.82, 25.19]; I2 = 0%; p = 0.18) (Figure 4C). 23 , 25 However, none of these results reached statistical significance. In the acute burn group, it was previously explained why certain outcomes were excluded from the meta‐analysis within this comparison. This was the same for the reconstructive group.
FIGURE 4.
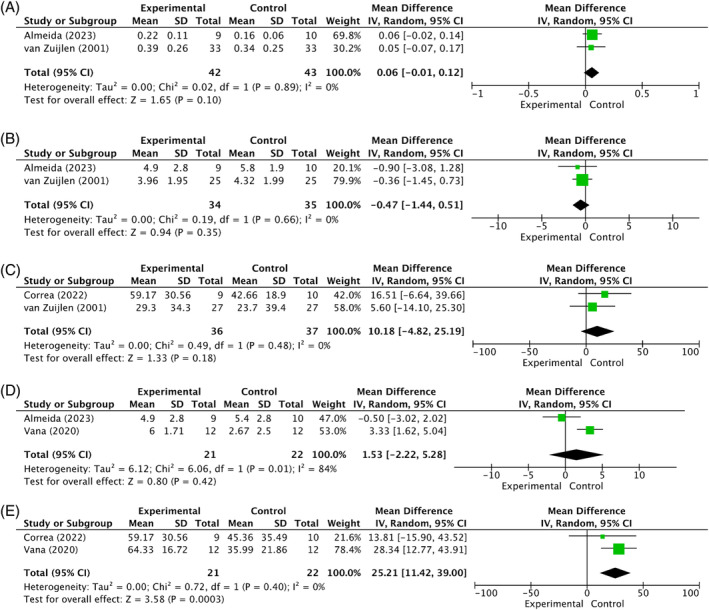
(A) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in scar elasticity (Uf) measured by Cutometer (12 months post‐surgery) in reconstructed scars after burns. (B) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in scar assessment score by Vancouver Scar Scale (12 months post‐surgery) in reconstructed scars after burns. (C) Comparison 1. Matriderm® versus split‐thickness skin graft: Mean difference in % contraction measured by Planimetry (12 months post‐surgery) in reconstructed scars after burns. (D) Comparison 2. Matriderm® versus Integra®: Mean difference in scar assessment score by Vancouver Scar Scale (12 months post‐surgery) in reconstructed scars after burns. (The experimental group is Matriderm®, and the control group is Integra®. Almeida et al. 24 compare Matriderm® 1‐mm Flex in a one‐stage procedure with Integra® Double Layer in a two stage procedure, while Vana et al. 26 compare Matriderm® 2‐mm in a two‐stage procedure with Integra® Double Layer in a two‐stage procedure). (E) Comparison 2. Matriderm® versus Integra®: Mean difference in % scar contraction measured by Planimetry (12 months post‐surgery) in reconstructed scars after burns. (The experimental group is Matriderm®, and the control group is Integra®. Correa et al. 25 compare Matriderm® 1‐mm Flex in a one‐stage procedure with Integra® Double Layer in a two stage procedure, while Vana et al. 26 compare Matriderm® 2‐mm in a two‐stage procedure with Integra® Double Layer in a two‐stage procedure).
In addition, three out of 10 studies compared Matriderm® plus STSG to Integra® plus STSG. 24 , 25 , 26 In the following sections of this article, Matriderm® will be referred to as the experimental group and Integra® as the control group. Twelve months post‐operative, a scar assessment using VSS showed higher scores for Matriderm® compared to Integra® (1.53; 95% CI [−2.22, 5.28]; I2 = 84%; p = 0.42) (Figure 4D). 24 , 26 This suggests that Integra® resulted in scars more comparable to normal skin. However, the observed difference was not statistically significant, and clinical heterogeneity was seen between the included studies. Namely, there was a difference in the application method of Matriderm® between the studies. Matriderm® was applied in a one‐stage procedure in the studies of Correa et al. 25 and Almeida et al., 24 whereas it was applied in a two‐stage procedure in the study by Vana et al. 26 The same clinical variability applies to this sub‐analysis, where Matriderm® showed significantly higher mean percentage contraction rates compared to Integra® (25.21%; 95% CI [11.42, 39.00]; I2 = 0%; p = 0.0003) (Figure 4E). 25 , 26 Within this comparison, there was insufficient data to perform a sub‐analysis on outcomes: graft take (n = 1); tissue hardness (n = 1); histopathology (n = 1); and mobility (n = 1). A sub‐analysis on complications could not be performed as all included studies that reported on this outcome, presented no complications in both groups. All results are shown in the summary of findings in Supplementary Table 4.
4. DISCUSSION
This systematic review investigated the outcomes of dermal substitutes in acute burns and the reconstruction of burn scars. Meta‐analysis was conducted to comprehensively examine comparative studies within the current literature on this subject. Based on the findings of this systematic review and meta‐analysis, it can be concluded that the use of dermal substitutes in burns and the reconstruction of burn scars may offer benefits in enhancing scar quality. However, initially, the rate of wound healing appeared to be somewhat slower in the one‐step procedures. Nevertheless, both data on rate of wound healing and scar quality outcomes showed minimal differences between the two groups. It is important to note that study design heterogeneity, differences in application methods, and small sample sizes contributed to few significant differences in the results between the outcomes between patients treated with a dermal substitute and those receiving a standard treatment such as STSG.
A statistically significant difference was observed in the comparison of re‐epithelialization between Matriderm® and STSG (p = 0.02). The findings indicated that epithelialization 4–7 days post‐surgery for acute burns was lower in the Matriderm® group compared to the control group. 17 , 22 Across the spectrum of wound healing parameters such as graft take, regrafting during admission, and healing time in days, most included studies reported lower values for wound healing in the dermal substitute groups compared to the control groups. This is in line with previous findings in the literature. 39 In the sub‐analysis regarding healing time in days, it was noted that, on average, wounds took 5 days longer to close in the acellular dermal matrix group. 27 , 29
When comparing acellular dermal matrix with STSG, a significant difference was found in the VSS six months post‐acute burn surgery (p <0.01). 27 , 28 In this case, a significantly lower VSS score was seen in the acellular dermal matrix group compared to the control group, indicating that the resulting scar approached normal skin characteristics across various factors such as vascularity, pigmentation, pliability, and height. Furthermore, across most sub‐analyses concerning scar quality, a trend towards improved outcomes, especially in the clinical scar assessment, was observed for the dermal substitutes group. However, only the scar assessment by VSS 6 months after surgery in acute burns in the comparison of acellular dermal matrix compared to STSG showed a statistically significant difference. In the comparisons Matriderm® compared to STSG, and Glyaderm® compared to STSG, the scar assessment by VSS showed better results for the experimental group, but these results were not statistically different. A possible reason for this lack of statistical significance in these comparisons, as well as the lack of statistical significance in the results of objectively measured scar parameters (such as scar elasticity by Cutometer), could be attributed to several factors. These may include the relatively small sample sizes and/or the inherent variability in scar maturation processes among different patients. Additionally, variations in study methodologies, application techniques, and patient characteristics across the different studies could have contributed to the observed outcomes.
In addition, another notable difference was found in the comparison between Matriderm® and Integra® regarding the mean percentage of scar contraction measured by planimetry in reconstructed post‐burn scars (p <0.01). 25 , 26 Due to the heterogeneity between the two studies, namely Almeida et al. 24 comparing Matriderm® 1‐mm Flex in a one‐stage procedure with Integra® Double Layer in two stages, while Vana et al. 26 compare Matriderm® 2‐mm in a two‐stage procedure with Integra® Double Layer in a two‐stage procedure, the interpretation of this result should be made with caution. The results suggest that Matriderm® induces more contraction in a two‐stage procedure than Integra® does. However, given the heterogeneity between these two studies and the results of van Zuijlen et al. 23 and Correa et al. 25 showing lower mean percentage contraction, this significant difference can be questioned. It could be possible that the application of Matriderm® and STSG in one procedure provides less contraction.
To the best of our knowledge, there is insufficient research to determine the optimal use of Matriderm® in a one‐step versus a two‐step procedure, or whether Integra® is more effective in a two‐step procedure while Matriderm® may be more advantageous in a one‐stage application. This underscores the necessity for further research to address these concerns and to establish clearer indications for the use of various dermal substitutes, taking into account procedural variations and their effects on clinical outcomes. Facilitating these actions could potentially provide for a more precise differentiation between wounds that may benefit from direct combined application of a substitute and STSG and those that may exhibit improved scar quality through a two‐step dermal substitute procedure.
The findings of this systematic review with meta‐analysis partly align with our initial expectations. As anticipated, we observed a slight negative trend towards dermal substitutes in rate of wound healing, evidenced by prolonged graft take, re‐epithelialization, and wound healing duration in days. In addition, we expected a positive trend towards improved scar quality and a positive trend towards improved clinical scar assessment with the use of dermal substitutes, which was indeed seen in this analysis. However, while our analysis revealed some positive trends in scar quality improvement with the use of dermal substitutes, it is important to acknowledge potential limitations surrounding these findings.
One such limitation is that we focused solely on burns and burn scar reconstruction, excluding other purposes for dermal substitute use. Additionally, it is worth noting that we only included studies that evaluate ‘off‐the‐shelf’ and permanent dermal substitutes as an intervention. The observed lack of statistically significant differences in many parameters may be partly due to the heterogeneous nature of the included studies and the relatively small sample sizes.
The overall completeness and comparability of evidence were hindered by limited uniformity in outcome variables, variations in the timing of application of dermal substitutes, and differences in the indications for their use. Additionally, this review was constrained by the available literature, which notably lacks well‐controlled studies with objective outcome measures in older studies, potentially affecting the generalizability and reliability of the findings. The overall quality of the included studies was considered moderate, which could have potentially impacted the robustness of the conclusions drawn from this analysis. To properly investigate the effect of dermal substitutes, several changes in this research field are recommended.
First, larger study population groups are necessary to increase the statistical power and generalizability of the findings. Furthermore, standardising outcome measures in intra‐patient trials would allow more meaningful comparisons between study groups. Addressing these concerns through concerted efforts in research methodology and trial design will not only strengthen the evidence base but also pave the way for more effective and targeted interventions in the treatment of burns and patients requiring reconstruction of burn scars.
This systematic review has been conducted as part of the project ‘Optimizing Top Specialized Burn Care in the Netherlands’. Within this overarching project, our focus has been on developing personalised treatment strategies using dermal substitutes in the treatment of burn patients. A significant aspect of this project has involved comprehensive data collection to gather all available evidence in the field. Our goal is to utilise this gathered evidence to refine and optimise the care provided to burn patients, tailoring treatment strategies to individual needs and circumstances. The results of this systematic review will help to optimise the integration of dermal substitutes into clinical practice and facilitate the development of an evidence‐based guideline for their use in burn care, ultimately striving to improve patient outcomes.
5. CONCLUSIONS
In conclusion, while acknowledging the initially delayed wound healing, the integration of dermal substitutes into the surgical treatment of burn patients shows promise for enhancing scar quality. However, several implications for both practice and future research are crucial. Future studies should prioritise greater uniformity in outcome measures to enable meaningful comparisons between studies. Additionally, addressing disparities in the application of skin substitutes and standardising indications for their use are important steps towards establishing consistent and effective practices in clinical settings. Furthermore, additional research into cost‐effectiveness of dermal substitutes is warranted, focusing on whether their use reduces the need for subsequent surgeries for burn scar reconstruction and if the costs incurred are justified by their benefits. If these considerations are addressed, the field could progress towards a more standardised and Evidence‐based approach. This evolution is vital for enhancing the reliability and effectiveness of skin substitutes in the challenging contexts of burns and burn scar reconstruction, ultimately contributing to improved clinical outcomes.
AUTHOR CONTRIBUTIONS
Designing study: ASB, RAFV, AP, EB, CHV, YL, PPMZ, and EM. Designing and providing search: ASB, RAFV, AP, EB, CHV, YL, GB, PPMZ, and EM. Selection of studies: ASB and RAFV. Data extraction of comparative trials (data for meta‐analysis): ASB and RAFV. Analysis and interpretation of data: ASB and RAFV. Writing original draft: ASB. Revising the article: ASB, RAFV, AP, EB, CHV, YL, PPMZ, and EM. Supervision: RAFV, AP, EB, CHV, YL, PPMZ, and EM. Project administration: ASB, RAFV, AP, EB, CHV, YL, PPMZ, and EM. Funding acquisition: AP, EB, CHV, PPMZ, and EM. Every author has approved the final manuscript in its submitted form and is willing to take responsibility for all aspects of the research.
FUNDING INFORMATION
The research has been conducted as part of the project ‘Optimizing top specialized burn care in the Netherlands’ funded by ZonMW, grant number: 10070022010003. The study sponsors were not involved in the search strategy, data collection, analysis, and interpretation of data, writing of the manuscript, or in the decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest in this research.
Supporting information
Supplementary Table 1: Search strategy (first search on 25th July, 2022 and updated search on 19 October, 2023).
Supplementary Table 2: Final number of references of both searches.
Supplementary Table 3: Sub‐analyses per comparison.
Supplementary Table 4: Summary of findings—Acute burn wounds.
ACKNOWLEDGEMENTS
We would like to thank all authors who we asked to provide their missing data when this was not reflected in their articles. We would also like to thank ZonMW for funding this research, and the Alliance of Dutch Burn Care (ADBC), Red Cross Hospital Beverwijk, Martini Hospital Groningen, and Maasstad Hospital Rotterdam for their support.
van den Bosch AS, Verwilligen RAF, Pijpe A, et al. Outcomes of dermal substitutes in burns and burn scar reconstruction: A systematic review and meta‐analysis. Wound Rep Reg. 2024;32(6):960‐978. doi: 10.1111/wrr.13226
The National Burn Care, Education & Research group, The Netherlands consist of: M.E. van Baar: Alliance of Dutch Burn Care (ADBC) and Burn Centre, Maasstad Hospital, Rotterdam; Erasmus MC, University Medical Centre Rotterdam, Department of Public Health, Rotterdam, The Netherlands; K.C. Baran: Burn Centre, Red Cross Hospital, Beverwijk, The Netherlands; S. Blok: Burn Centre, Martini Hospital, Groningen, The Netherlands, Research Group Healthy Ageing, Allied Health Care and Nursing, Hanze University of Applied Sciences, Groningen, The Netherlands, University of Groningen, University Medical Center Groningen, Department for Human Movement Sciences, Groningen, The Netherlands; L. van Dammen: Alliance of Dutch Burn Care (ADBC) and Burn Centre, Maasstad Hospital, Rotterdam; Erasmus MC, University Medical Centre Rotterdam, Burn Centre, Martini Hospital, Groningen, The Netherlands, Burn Centre, Martini Hospital, Groningen, The Netherlands; M.E. van Eck: Burn Centre, Martini Hospital, Groningen, The Netherlands; S.J.G. Geelen: Burn Centre, Martini Hospital, Groningen, The Netherlands, Research Group Healthy Ageing, Allied Health Care and Nursing, Hanze University of Applied Sciences, Groningen, The Netherlands, University of Groningen, University Medical Center Groningen, Department for Human Movement Sciences, Groningen, The Netherlands. R. van Gemert: Dutch Association of Burn Survivors; M.K. Nieuwenhuis: Research Group Healthy Ageing, Allied Health Care and Nursing, Hanze University of Applied Sciences, Groningen, The Netherlands, University of Groningen, University Medical Center Groningen, Department for Human Movement Sciences, Groningen, The Netherlands; R.F.C. Salemans: Alliance of Dutch Burn Care (ADBC) and Burn Centre, Maasstad Hospital, Rotterdam, Erasmus MC, University Medical Centre Rotterdam, Trauma Research Unit, Department of Surgery, The Netherlands; C.M.H. van Schie: Dutch Burns Foundation, Beverwijk, The Netherlands; S.M.H.J. Scholten‐Jaegers: Burn Centre, Martini Hospital, Groningen, The Netherlands; R.S., M. Thambithurai: Alliance of Dutch Burn Care (ADBC) and Burn Centre, Maasstad Hospital, Rotterdam, Erasmus School of Health Policy and Management, Erasmus University Rotterdam, Rotterdam, The Netherlands; D. van Uden: Alliance of Dutch Burn Care (ADBC) and Burn Centre, Maasstad Hospital, Rotterdam; I. Visser: Dutch Association of Burn Survivors; M. van der Vlegel: Alliance of Dutch Burn Care (ADBC) and Burn Centre, Maasstad Hospital, Rotterdam, Burn Centre, Red Cross Hospital, Beverwijk, The Netherlands; G. Versluis: Dutch Association of Burn Survivors, Burn Centre, Maasstad Hospital, Rotterdam, The Netherlands; A. Meij‐de Vries: Burn Centre and Department of Surgery, Red Cross Hospital, Beverwijk, The Netherlands, Amsterdam UMC location University of Amsterdam, Paediatric Surgical Centre, Emma Children's Hospital, Amsterdam, The Netherlands; H. Wanders: Dutch Association of Burn Survivors.
Contributor Information
Anna S. van den Bosch, Email: avandenbosch@rkz.nl.
Anouk Pijpe, Email: apijpe@rkz.nl.
The National Burn Care, Education & Research group, The Netherlands:
M.E. van Baar, K.C. Baran, S. Blok, L. van Dammen, M.E. van Eck, S.J.G. Geelen, R. van Gemert, M.K. Nieuwenhuis, R.F.C. Salemans, C.M.H. van Schie, S.M.H.J. Scholten‐Jaegers, R.S., M. Thambithurai, D. van Uden, I. Visser, M. van der Vlegel, G. Versluis, A. Meij‐de Vries, and H. Wanders
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Yoon D, Cho YS, Joo SY, Seo CH, Cho YS. A clinical trial with a novel collagen dermal substitute for wound healing in burn patients. Biomater Sci. 2020;8(3):823‐829. doi: 10.1039/C9BM01209E [DOI] [PubMed] [Google Scholar]
- 2. van Zuijlen PPM, Angeles AP, Kreis RW, Bos KE, Middelkoop E. Scar assessment tools: implications for current research. Plast Reconstr Surg. 2002;109(3):1108‐1122. doi: 10.1097/00006534-200203000-00052 [DOI] [PubMed] [Google Scholar]
- 3. Hop MJ, Hoogewerf CJ, van Baar ME, van der Vlies CH, Middelkoop E. A call for evidence: timing of surgery in burns. Burns. 2012;38(4):617‐618. doi: 10.1016/j.burns.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 4. Saaiq M, Zaib S, Ahmad S. Early excision and grafting versus delayed excision and grafting of deep thermal burns up to 40% total body surface area: a comparison of outcome. Ann Burns Fire Disasters. 2012;25(3):143‐147. [PMC free article] [PubMed] [Google Scholar]
- 5. van Zuijlen PPM, Vloemans AFPM, Tempelman FRH, Kreis RW. The timing of surgery for deep burns of the hands: early versus delayed surgery. Burns. 2007;33(6):807. doi: 10.1016/j.burns.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3‐17. doi: 10.1016/j.addr.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 7. Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19(1):243. doi: 10.1186/s13054-015-0961-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6:1. doi: 10.1038/s41572-020-0145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goei H, van der Vlies CH, Hop MJ, et al. Long‐term scar quality in burns with three distinct healing potentials: a multicenter prospective cohort study. Wound Repair Regen. 2016;24(4):721‐730. doi: 10.1111/wrr.12438 [DOI] [PubMed] [Google Scholar]
- 10. Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive Burn injury. Ann Surg. 1981;194(4):413‐428. doi: 10.1097/00000658-198110000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shahrokhi S, Arno A, Jeschke MG. The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen. 2014;22(1):14‐22. doi: 10.1111/wrr.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodgkinson T, Bayat A. Dermal substitute‐assisted healing: enhancing stem cell therapy with novel biomaterial design. Arch Dermatol Res. 2011;303(5):301‐315. doi: 10.1007/s00403-011-1131-2 [DOI] [PubMed] [Google Scholar]
- 13. van der Veen VC, van der Wal MBA, van Leeuwen MCE, Ulrich MMW, Middelkoop E. Biological background of dermal substitutes. Burns. 2010;36(3):305‐321. doi: 10.1016/j.burns.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 14. Wardhana A, Valeria M. Efficacy of skin substitutes for management of acute Burn cases: a systematic review. Ann Burns Fire Disasters. 2022;35(3):227‐236. [PMC free article] [PubMed] [Google Scholar]
- 15. Widjaja W, Tan J, Maitz PKM. Efficacy of dermal substitute on deep dermal to full thickness burn injury: a systematic review. ANZ J Surg. 2017;87(6):446‐452. doi: 10.1111/ans.13920 [DOI] [PubMed] [Google Scholar]
- 16. Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33(8):946‐957. doi: 10.1016/j.burns.2007.03.020 [DOI] [PubMed] [Google Scholar]
- 17. Bloemen MCT, van der Wal MBA, Verhaegen PDHM, et al. Clinical effectiveness of dermal substitution in burns by topical negative pressure: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(6):797‐805. doi: 10.1111/j.1524-475X.2012.00845.x [DOI] [PubMed] [Google Scholar]
- 18. van den Bosch AS, Verwilligen RAF, Pijpe A, et al. Application of dermal substitutes in the surgical treatment of full‐thickness wounds: outcomes of an international survey. Int Wound J. 21(7):e14932. doi: 10.1111/iwj.14932 [DOI] [Google Scholar]
- 19. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryssel H, Germann G, Kloeters O, Gazyakan E, Radu CA. Dermal substitution with Matriderm® in burns on the dorsum of the hand. Burns. 2010;36(8):1248‐1253. doi: 10.1016/j.burns.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 21. Ryssel H, Gazyakan E, Germann G, Ohlbauer M. The use of MatriDerm in early excision and simultaneous autologous skin grafting in burns—a pilot study. Burns. 2008;34(1):93‐97. doi: 10.1016/j.burns.2007.01.018 [DOI] [PubMed] [Google Scholar]
- 22. van Zuijlen PPM, van Trier AJM, Vloemans JFPM, Groenevelt F, Kreis RW, Middelkoop E. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one‐stage grafting model. Plast Reconstr Surg. 2000;106(3):615‐623. doi: 10.1097/00006534-200009030-00014 [DOI] [PubMed] [Google Scholar]
- 23. van Zuijlen PPM, Vloemans JFPM, van Trier AJM, et al. Dermal substitution in acute burns and reconstructive surgery: a subjective and objective long‐term follow‐up. Plast Reconstr Surg. 2001;108(7):1938‐1946. doi: 10.1097/00006534-200112000-00014 [DOI] [PubMed] [Google Scholar]
- 24. Almeida IR, Gonqalves AC, Corrêa FB, et al. Evaluation of clinical and biomechanical features of scars resulting from the treatment of Burn contractures comparing acellular dermal matrices. Ann Surg. 2023;277(2):198‐205. doi: 10.1097/SLA.0000000000005371 [DOI] [PubMed] [Google Scholar]
- 25. Corrêa FB, Castro JCD, Almeida IR, Farina‐Junior JA, Coltro PS. Evaluation of contraction of the split‐thickness skin graft using three dermal matrices in the treatment of burn contractures: a randomised clinical trial. Wound Repair Regen. 2022;30(2):222‐231. doi: 10.1111/wrr.13002 [DOI] [PubMed] [Google Scholar]
- 26. Vana LPM, Battlehner CN, Ferreira MA, Caldini EG, Gemperli R, Alonso N. Comparative long‐term study between two dermal regeneration templates for the reconstruction of burn scar contractures in humans: clinical and histological results. Burns. 2020;46(3):596‐608. doi: 10.1016/j.burns.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 27. Shang F, Lu Y, Gao J, Hou Q. Comparison of therapeutic effects between artificial dermis combined with autologous split‐thickness skin grafting and autologous intermediate‐thickness skin grafting alone in severely burned patients: a prospective randomised study. Int Wound J. 2021;18(1):24‐31. doi: 10.1111/iwj.13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen L, Yang J, Wang DY, et al. Multicenter effect analysis of one‐step acellular dermis combined with autologous ultra‐thin split thickness skin composite transplantation in treating burn and traumatic wounds. Int Wound J. 2023;21:e14341. doi: 10.1111/iwj.14341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Li Y, Sha D. Cografts of artificial dermis matrix and autogenetic split‐thickness of repaired skin in severe hand wounds in patients with deep burns. Arch Biol Sci. 2014;66(1):173‐178. doi: 10.2298/ABS1401175L [DOI] [Google Scholar]
- 30. De Decker I, Hoeksema H, Verbelen J, et al. A single‐stage bilayered skin reconstruction using Glyaderm® as an acellular dermal regeneration template results in improved scar quality: an intra‐individual randomized controlled trial. Burns Trauma. 2023;11:tkad015. doi: 10.1093/burnst/tkad015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pirayesh A, Hoeksema H, Richters C, Verbelen J, Monstrey S. Glyaderm(®) dermal substitute: clinical application and long‐term results in 55 patients. Burns. 2015;41(1):132‐144. doi: 10.1016/j.burns.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 32. Avila Leon JL, Rivero CR, Guerrero L, et al. Immediate results of the use of split‐thickness skin autografts with and without acellular dermal matrix in patients with burns, a comparative study in a Colombian population. J Burn Care Res. 2023;45:348‐355. doi: 10.1093/jbcr/irad131 [DOI] [PubMed] [Google Scholar]
- 33. Bloemen MCT, van Leeuwen MCE, van Vucht NE, van Zuijlen PPM, Middelkoop E. Dermal substitution in acute burns and reconstructive surgery: a 12‐year follow‐up. Plast Reconstr Surg. 2010;125(5):1450‐1459. doi: 10.1097/PRS.0b013e3181d62b08 [DOI] [PubMed] [Google Scholar]
- 34. Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of integra in primary burn management: a randomized pediatric clinical trial*. Crit Care Med. 2007;35(11):2615‐2623. doi: 10.1097/01.CCM.0000285991.36698.E2 [DOI] [PubMed] [Google Scholar]
- 35. Chen X, Feng X, Xie J, et al. Application of acellular dermal xenografts in full‐thickness skin burns. Exp Ther Med. 2013;6(1):194‐198. doi: 10.3892/etm.2013.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gardien KLM, Pijpe A, Brouwer KM, et al. Short‐ and long‐term outcomes of an acellular dermal substitute versus standard of care in Burns and Reconstructions: a phase I/II Intrapatient randomized controlled trial. Adv Skin Wound Care. 2023;36(10):540‐548. doi: 10.1097/asw.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gore DC. Utility of acellular allograft dermis in the Care of Elderly Burn Patients. J Surg Res. 2005;125(1):37‐41. doi: 10.1016/j.jss.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 38. Hansbrough JF, Doré C, Hansbrough WB. Clinical trials of a living dermal tissue replacement placed beneath meshed, split‐thickness skin grafts on excised Burn wounds. J Burn Care Rehabil. 1992;13(5):519‐529. doi: 10.1097/00004630-199209000-00004 [DOI] [PubMed] [Google Scholar]
- 39. Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi‐center randomized clinical trial. Ann Surg. 1988;208(3):313‐320. doi: 10.1097/00000658-198809000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hop MJ, Bloemen MCT, van Baar ME, et al. Cost study of dermal substitutes and topical negative pressure in the surgical treatment of burns. Burns. 2014;40(3):388‐396. doi: 10.1016/j.burns.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 41. Lagus H, Sarlomo‐Rikala M, Böhling T, Vuola J. Prospective study on burns treated with integra®, a cellulose sponge and split thickness skin graft. Burns. 2013;39(8):1577‐1587. doi: 10.1016/j.burns.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 42. Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long‐term evaluation of patients with extensive burns. Burns. 2015;41(4):689‐699. doi: 10.1016/j.burns.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 43. Munster AM, Smith‐Meek M, Shalom A. Acellular allograft dermal matrix: immediate or delayed epidermal coverage? Burns. 2001;27(2):150‐153. doi: 10.1016/S0305-4179(00)00096-6 [DOI] [PubMed] [Google Scholar]
- 44. Shang F, Hou Q. Effects of allogenic acellular dermal matrix combined with autologous razor‐thin graft on hand appearance and function of patients with extensive burn combined with deep hand burn. Int Wound J. 2021;18(3):279‐286. doi: 10.1111/iwj.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheridan R, Choucair R, Donelan M, Lydon M, Petras L, Tompkins R. Acellular allodermis in burns surgery: 1‐year results of a pilot trial. J Burn Care Rehabil. 1998;19(6):528‐530. doi: 10.1097/00004630-199811000-00012 [DOI] [PubMed] [Google Scholar]
- 46. Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full‐thickness burns. J Burn Care Rehabil. 1996;17(2):124‐136. doi: 10.1097/00004630-199603000-00006 [DOI] [PubMed] [Google Scholar]
- 47. Wu SS, Wells M, Ascha M, Duggal R, Gatherwright J, Chepla K. Head and neck wound reconstruction using biodegradable temporizing matrix versus collagen‐chondroitin silicone bilayer. Eplasty. 2022;22:e31. [PMC free article] [PubMed] [Google Scholar]
- 48. Lee MY, Kim H, Kwak IS, Jang Y, Choi Y. Immunohistochemical analysis of postburn scars following treatment using dermal substitutes. Anal Cell Pathol. 2022;2022:1‐13. doi: 10.1155/2022/3686863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nguyen DQA, Potokar TS, Price P. An objective long‐term evaluation of integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns. 2010;36(1):23‐28. doi: 10.1016/j.burns.2009.07.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Search strategy (first search on 25th July, 2022 and updated search on 19 October, 2023).
Supplementary Table 2: Final number of references of both searches.
Supplementary Table 3: Sub‐analyses per comparison.
Supplementary Table 4: Summary of findings—Acute burn wounds.
Data Availability Statement
Data available on request from the authors.


