Abstract
The aim of this meta‐analysis is to compare the clinical outcomes in patients with and without residual osteomyelitis (ROM) after surgical bone resection for diabetic foot osteomyelitis (DFO). We completed a systematic literature search using PubMed, Scopus, and Embase using keywords DFO, Residual OM (ROM), and positive bone margins. The study outcomes included wound healing, antibiotic duration, amputation, and re‐infection. Five hundred and thirty patients were included in the analysis; 319 had no residual osteomyelitis (NROM), and 211 had ROM. There was not a significant difference in the proportion of wounds that healed 0.6 (p = 0.1, 95% confidence intervals [95% CI] 0.3–1.3). The risk of infection was 2.0 times higher (OR = 2.0, p = 0.02, 95% CI 1.1–3.4), and the risk of amputation was 4.3 times higher (OR = 4.3, p = 0.0001, 95% CI 2.4–7.6) in patients with ROM. Patients with ROM received antibiotics significantly longer. The mean difference was 16.3 days (p = 0.02, 95% CI 11.1–21.1).
Keywords: amputation, diabetes, foot ulcer, infection, neuropathy, osteomyelitis
Abbreviations
- 95% CI
95% confidence intervals
- DFO
diabetic foot osteomyelitis
- IDSA
Infectious Disease Society of America
- IWGDF
International Working Group on the Diabetic Foot
- NOS
Newcastle‐Ottawa Scale
- NROM
no residual osteomyelitis
- PAD
peripheral arterial disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis
- ROM
residual osteomyelitis
1. INTRODUCTION
Diabetic foot osteomyelitis (DFO) is a challenging pathology, comprising 60% of patients with diabetic foot infections admitted to hospital. 1 , 2 , 3 The treatment of DFO consists of medical management, surgical resection/amputation, or a combination of the two treatment approaches. Patients with DFO are more likely to require surgery and have higher rates of re‐infection, hospitalisation, amputation, and prolonged antibiotic exposure compared with people with soft tissue infections. 3 , 4 The 2012 Infectious Disease Society of America (IDSA) 5 and the 2023 the International Working Group on the Diabetic Foot (IWGDF) and IDSA guidelines 6 recommend that when surgery was required for osteomyelitis, proximal bone margins should be obtained, and if the margins do not show residual osteomyelitis (ROM), the duration of post‐surgery antibiotic treatment could be reduced to 2–5 days. There have been several retrospective studies that have compared clinical outcomes among diabetic patients with and without ROM after surgery. The aim of this meta‐analysis is to compare the clinical outcomes in patients with and without ROM after surgical treatment for DFO.
2. METHODS
2.1. Data sources and searches
This systematic review and meta‐analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement. 7 A literature search of PubMed, Scopus, and Embase, using Medical Subject (MeSH), and Boolean operations were employed in the search strategy and the final search was conducted on 17 August 2023. A combination and variation of the terms (diabetic foot osteomyelitis) AND (diabetic foot osteomyelitis AND residual osteomyelitis OR positive bone margins). For each database, a specific search was generated and converted accordingly. We included only studies published in the English language. No institutional review board approval was required for the current study. This systematic review and meta‐analysis is registered with the Research Registry (reviewregistry1689).
2.2. Inclusion and exclusion criteria
We included only studies that compared surgical patients with and without ROM in patients with diabetic foot infection. ROM was defined as a pathology‐positive bone biopsy or a positive culture after surgical resection of infected bone. A clean bone margin (surgical sites with no residual bone infection) was defined as a pathology‐negative or culture negative bone biopsy. Negative pathology was defined as no report of osteomyelitis in the pathology report. Negative cultures were defined as a culture from the proximal resected margin with no bacterial growth at the time of final surgery. Antibiotic duration included oral and parenteral antibiotics as reported in respective studies. Local antibiotic use was not included in the duration. Studies with <10 participants were excluded. We did not impose a restriction on the year of publication. We imposed no restrictions on publication status. Excluded studies included case reports, basic science research, non‐human populations, non‐English translatable studies, systematic reviews, and meta‐analyses. Two independent reviewers (T.L.C. and M.C.R.) selected relevant studies with any disagreements resolved by a third reviewer A.T. A further reference list was also created to capture articles that were not found during the initial database searches but were relevant to this study. Where a consensus was not reached, disagreements were resolved through discussion.
2.3. Data extraction and critical appraisal
M.C.R. independently extracted data, which T.L.C. compared with the original citation. Disagreements were resolved through discussion. Extracted data from eligible studies included: first author, publication year, country, peripheral arterial disease (PAD), number of wounds healed, amputation, study size, mean age, percentage of males, re‐infections, and antibiotic duration (Tables 1 and 2). Re‐ulceration (n = 1) and time to heal (n = 2) data were collected but there were not enough studies for analysis. The papers were assessed for quality using the Newcastle‐Ottawa Quality Assessment Scales. M.C.R. and T.S. independently scored each study and discussed reported differences.
TABLE 1.
Summary of studies that report surgical bone resection with and without residual osteomyelitis.
| Study | Year | Study design | Country | Average F/U (months) | N | Age (years) | Male % | No. of PAD |
|---|---|---|---|---|---|---|---|---|
| Aragon‐Sanchez | 2023 | Prospective | Costa Rica | 20.8 | 93 | 59.5 ± 12.6 | 77.4 | 19 |
| Weng | 2023 | Retrospective | United States | 12.0 | 92 | 53.38 ± 10.5 | 58.6 | 34 |
| Aragon‐Sanchez | 2021 | Retrospective | Costa Rica | 7.2 | 28 | 58.1 ± 11.8 | 78.6 | 9 |
| Johnson | 2019 | Retrospective | United States | 12.0 | 66 | NR | 93.9 | 27 |
| Schmidt | 2019 | Prospective | United States | 12.0 | 72 | 56.9 ± 12.8 | 81.9 | NR |
| Beieler | 2012 | Retrospective | United States | 26.2 | 50 | NR | 88 | 10 |
| Atway | 2012 | Retrospective | United States | 9.1 | 27 | 56.74 ± 11.42 | 66.7 | 10 |
| Kowalski | 2011 | Retrospective | United States | NR | 111 | 63.0 ± 14 | 75 | NR |
Abbreviation: PAD, peripheral arterial disease.
TABLE 2.
Outcomes from published papers in patients with and without ROM.
| Study | Year | Number of subjects | Wounds healed | Amputation | Re‐infection | Antibiotic duration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ROM | No ROM | ROM | No ROM | ROM | No ROM | ROM | No ROM | ROM | No ROM | ||
| Aragon‐Sanchez | 2023 | 61 | 31 | 59 | 30 | 10 | 0 | 13 | 6 | NR | NR |
| Weng | 2023 | 35 | 57 | NR | NR | 12 | 8 | NR | NR | 30 ± 15.0 | 18 ± 15.0 |
| Aragon‐Sanchez | 2021 | 7 | 21 | 7 | 21 | 0 | 0 | 1 | 2 | NR | NR |
| Johnson | 2019 | 18 | 48 | 10 | 29 | NR | NR | 8 | 19 | 37.6 ± 24.1 | 17.7 ± 29.6 |
| Schmidt | 2019 | 9 | 63 | NR | NR | 0 | 0 | NR | NR | 32 ± 36.0 | 23.69 ± 18.5 |
| Beieler | 2012 | 31 | 11 | NR | NR | 12 | 3 | 12 | 3 | 43 | 19 |
| Atway | 2012 | 11 | 16 | 8 | 13 | 3 | 0 | NR | NR | 24.81 ± 15.48 | 10.44 ± 11.64 |
| Kowalski | 2011 | 39 | 72 | NR | NR | 17 | 11 | 11 | 6 | 19 | 14 |
Abbreviations: NROM, no residual osteomyelitis; ROM, residual osteomyelitis.
2.4. Data analysis
The primary outcome (wound healing rate) and secondary outcomes (amputation, re‐ulceration, re‐infection, and antibiotic duration) were reported with 95% confidence intervals (95% CI). For variables with low heterogeneity a fixed‐effects model was used. For variables with moderate or high heterogeneity a random‐effects model was chosen to account for the variability among studies. To quantify heterogeneity, we utilised Higgin's & Tompson's I 2 statistic. 8 Low heterogeneity was defined as I 2 < 50%, moderate heterogeneity as I 2 = 50%–75%, and high heterogeneity as I 2 > 75%. Data analysis was done using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), using the meta and metafor package. Funnel plots and Egger's test were used to evaluate publication bias. Egger's test revealed no publication bias (Figure 1).
FIGURE 1.
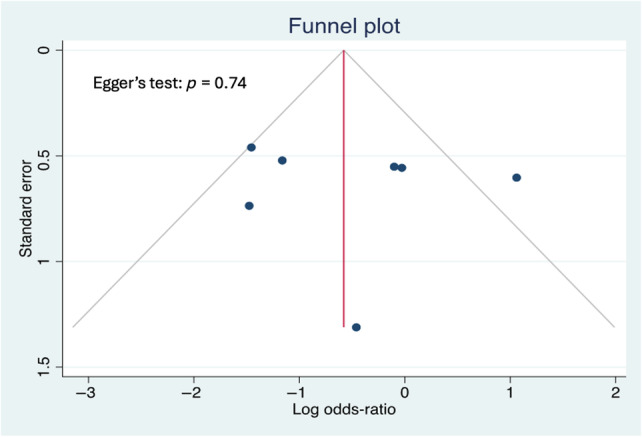
Funnel Plot measuring publication bias Egger's test indicates that there is no strong evidence of publication bias in the meta‐analysis. The high p‐value (0.74) suggests that the observed results are likely not significantly skewed by a tendency to publish studies with positive or statistically significant findings.
3. RESULTS
3.1. Study characteristics and quality
Eighty‐six citations were identified after the removal of duplicates. Following the title and abstract screening, 11 citations were assessed for full‐text eligibility. Of these, three studies were excluded which did not include histopathology or microbiological culture of bone margins in patients with diabetic foot infection. This resulted in eight studies included in the final analysis. See Figure 2 for PRISMA diagram. Five hundred and thirty patients were included in the analysis. Patient characteristics are summarised in Table 1. Of these, 319 had no residual osteomyelitis (NROM) and 211 had ROM at the surgical margin. There was a mean follow‐up time of 14.1 months (95% CI 9.4–18.8). The mean age was 57.9 years (95% CI 55.3–60.5). The mean percentage of males was 77.5% (95% CI 69.7–85.3). The reported NOS scores range was 4–7 out of 9 as given in Table 3.
FIGURE 2.
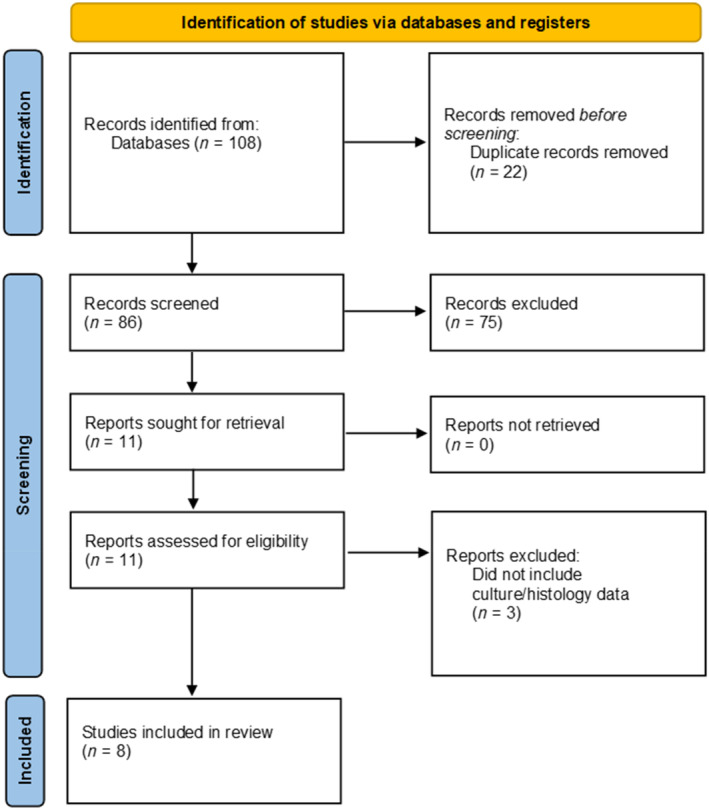
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRSIMA) flow chart of search process. The figure presents the records extracted from three different scientific databases and registrars: PubMed, Scopus, and Embase. All articles were screened based on the inclusion/exclusion criteria and 11 studies were evaluated in full review. Three studies were excluded from this list, yielding eight papers for meta‐analysis.
TABLE 3.
Newcastle‐Ottawa scoring table.
3.2. Antibiotic duration
Only four studies 9 , 10 , 11 , 12 reported means and standard deviations for antibiotic duration which allowed for meta‐analysis. Four studies included 257 pooled participants. Seventy‐three had ROM and 184 had NROM (Figure 3). Patients with ROM were treated with antibiotics significantly longer than people with no residual infection. The mean difference was 16.3 days (p = 0.02, 95% CI 11.1–21.1). There was no evidence of heterogeneity, Cochrane Q = 4.5 (p = 0.2) and I 2 = 33.2 (95% CI 0–76.3).
FIGURE 3.
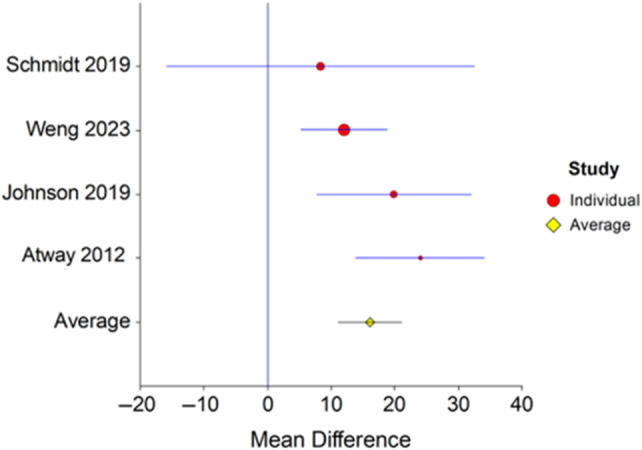
Forest plot of antibiotic duration in patients with and without residual osteomyelitis. Forest plot depicting the mean difference in antibiotic duration in patients with and without residual osteomyelitis following surgical resection. Patients with residual osteomyelitis had longer antibiotic treatment with an average mean difference across all studies of 16.3 days (p = 0.02, 95% CI 11.1–21.1). There was no evidence of heterogeneity, Cochrane Q = 4.5 (p = 0.2) and I 2 = 33.2 (95% CI 0–76.3).
3.3. Amputation
Six studies 10 , 11 , 12 , 13 , 14 , 15 , 16 with 193 patients with residual bone infection and 271 patients with NROM reported amputation rates (Figure 4). Using a fixed‐effects model, the risk of amputation was 4.3 times higher when there was ROM (OR = 4.3, p = 0.0001, 95% CI 2.4–7.6). There was no evidence of heterogeneity, Cochrane Q = 1.4 (p = 0.96) and I 2 = 0.0 (95% CI 0.0–0.0).
FIGURE 4.
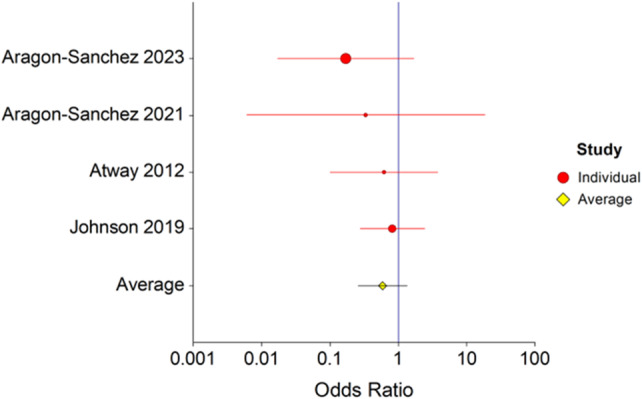
Forest plot of re‐amputation in patients with and without residual osteomyelitis. Forest plot depicting re‐amputation in patients with and without residual osteomyelitis following surgical resection. The risk of amputation was 4.3 times higher when there was residual osteomyelitis, (OR = 4.3, p = 0.0001, 95% CI 2.4–7.6). There was no evidence of heterogeneity, Cochrane Q = 1.4 (p = 0.96) and I 2 = 0.0 (95% CI 0.0–0.0).
3.4. Wound healing
Four studies 9 , 12 , 13 , 14 with 97 patients with ROM and 116 patients with no residual bone infection reported healing rates (Figure 5). There was not a significant difference in the proportion of wounds that healed. The fixed effects odds ratio analysis was 0.6 (p = 0.1, 95% CI 0.3–1.3). There was no evidence of heterogeneity, Cochrane Q = 1.5 (p = 0.7) and I 2 = 0.0 (95% CI 0.0–70.4).
FIGURE 5.
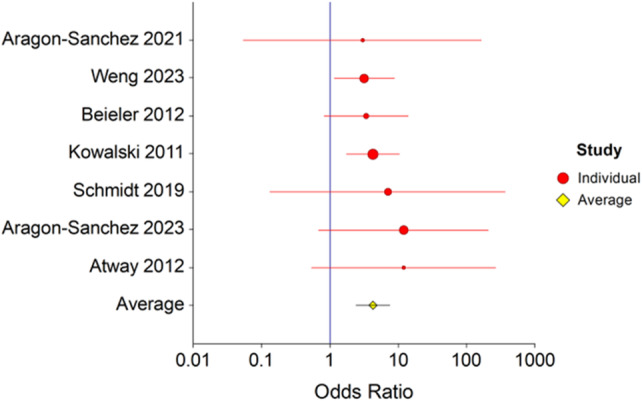
Forest plot of wound healing rates in patients with and without residual osteomyelitis. Forest plot depicting the association of healing in patients with and without residual osteomyelitis following surgical resection. The average OR across studies was calculated as 0.6 (p = 0.1, 95% CI 0.3–1.3), indicating that residual osteomyelitis does not have a deleterious effect on wound healing. There was no evidence of heterogeneity, Cochrane Q = 1.5 (p = 0.7) and I 2 = 0.0 (95% CI 0.0–70.4).
3.5. Re‐infection
Five studies 9 , 13 , 14 , 15 , 16 with 156 patients with ROM and 183 patients with clean margins reported re‐infection rates (Figure 6). There was a significant difference in re‐infection rates between patients with clean bone margins and ROM. The fixed effects odds ratio analysis was 2.0 (p = 0.02, 95% CI 1.1–3.4). There was no evidence of heterogeneity, Cochrane Q = 4.4 (p = 0.35) and I 2 = 9.11 (95% CI 0.0–81.1).
FIGURE 6.
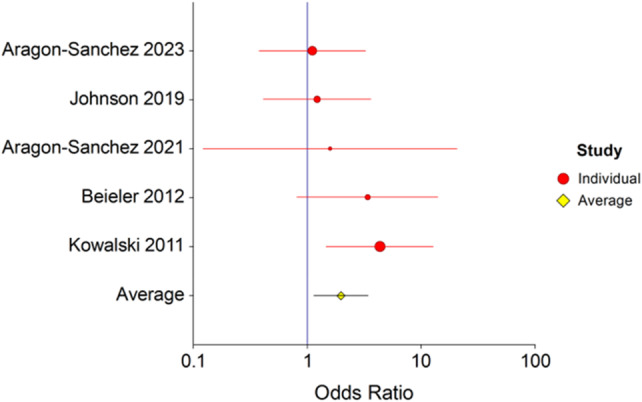
Forest plot of re‐infection in patients with and without residual osteomyelitis. Forest plot depicting re‐infection in patients with residual osteomyelitis following surgical resection. The risk of re‐infection was 2.0 times higher in patients with residual osteomyelitis, (OR = 2.0, p = 0.02, 95% CI 1.1–3.4). There was no evidence of heterogeneity, Cochrane Q = 4.4 (p = 0.35) and I 2 = 9.11 (95% CI 0.0–81.1).
4. DISCUSSION
The results of this meta‐analysis demonstrate that patients with ROM have a two‐fold increased risk of re‐infection, and a four‐fold increased risk of amputation compared with patients that had all infected bone surgically resected. In addition, patients with ROM have longer antibiotic treatment. This is the first meta‐analysis to evaluate the results of ROM at the proximal surgical margin in patients with DFO. Conventional wisdom would suggest that ROM would lead to increased risk of re‐infection, lower rates of wound healing, longer duration of antibiotics and more amputations.
Amputation, wound healing, and re‐infection are common outcomes used to define success for the treatment of osteomyelitis. 6 , 17 In the studies included in this meta‐analysis, the proportion of wounds that healed in patients with ROM ranged from 55% to 95%. In patients with NROM, the rate of healing ranged from 60% to 100%. 6 As given in Table 2, only three studies used wound healing as an outcome in evaluating ROM. Most studies that report outcomes in DFO do not report if there is ROM. In these studies, the rate of wound healing is highly variable, reported as high as 86%. 18 , 19 Wound healing is a poor surrogate marker for the successful treatment of osteomyelitis because there are many diabetes‐related disease processes and treatments that impact wound healing that are completely unrelated to osteomyelitis. 20 , 21 Amputation after the initial treatment for DFO is also used as a measure of success. Patients with ROM were 4.1 times more likely to have an amputation. In the six studies available for analysis, amputation rates for patients with positive bone margins ranged from 16% to 40%. There are many underlying factors that contribute to lower extremity amputation beside osteomyelitis, such as PAD, gangrene, soft tissue infection, chronic kidney disease, socioeconomic status, and access to specialty medical care. 22 , 23 , 24 , 25 These variables are usually not reported. In DFO studies that were excluded from this meta‐analysis, the incidence of amputation ranged from 36% to 60% (Table 4). 26 , 27 , 28
TABLE 4.
Summary of outcomes in patients with and without residual osteomyelitis
| Variable | Number of studies | Odds ratio | 95% CI | p‐Value | Cochrane Q |
|---|---|---|---|---|---|
| Amputation | 7 | 4.3 | 2.4–7.6 | <0.01 | 1.4 |
| Re‐infection | 5 | 2.0 | 1.1–3.4 | 0.02 | 4.4 |
| Wound healing | 4 | 0.6 | 0.3–1.3 | 0.10 | 1.5 |
| Variable | Number of studies | Mean difference(days) | |||
| Antibiotic duration | 4 | 16.3 | 11.1–21.1 | 0.02 | 4.5 |
Re‐infection is perhaps the most important outcome to evaluate osteomyelitis treatment. Re‐infection in the five available studies included in the meta‐analysis varied widely and ranged from 14% to 44%. Our results show that re‐infection was two times higher in patients with ROM after bone resection. The importance of evaluating re‐infection is that it can drive re‐hospitalisation which subsequently may lead to more antibiotic administration, and more surgery. In one study, osteomyelitis had a 56% re‐infection rate with nearly all patients requiring re‐admission. 1
The longer duration of antibiotics may be related to re‐infection or based on the training and belief system of the person prescribing antibiotics. The most common dogma for treating DFO is that it requires 6 weeks of parental antibiotics. However, recent research has compared outcomes based on 3 versus 6 weeks 29 and 6 versus 12 weeks for DFO. 30 If the physician making antibiotic decisions embraces the IDSA/IWGDF recommendations, 5 , 17 shortening the duration of antibiotic treatment may be due to the treating physician simply follow published recommendations, since there are no clear stopping rules for antibiotics in osteomyelitis. 17 Aragon‐Sanchez et al. 13 , 14 treated patients for the shortest duration whether there was ROM or not.
The strengths of this study include evaluating relevant outcomes important in clinical practice. However, there are some limitations to this meta‐analysis. As in all meta‐analyses, this study was limited by the quality of the original studies. The majority of the studies included were nonrandomized retrospective studies with different inclusion/exclusion criteria. Selection bias may have been introduced through the study design of several studies as patients were only included if they had complete pathology reports or had not deceased within 1 year. Additionally, not all outcomes of interest were available from each publication included in our analysis, which required a pooled analysis from fewer patients thus the full effect may not be fully quantified. Another limitation we identified was that wound healing follow‐up was not clearly defined in most studies. For instance, Aragon‐Sanchez and colleagues reported that the surgical site was assessed two to three times a week until the wound healed.
In addition, there were inconsistent operational definitions for important variables such as how osteomyelitis was initially defined and then how ROM was defined. As given in Table 5, the reference standards varied among studies. Re‐infection was reported in five studies 9 , 13 , 14 , 15 , 16 with different definitions between respective studies. Aragon‐Sanchez reported recurrence of infection, which was defined as clinically and/or radiologically diagnosed infection and/or needing antibiotic therapy and/or surgical treatment. 14 In another study, Aragon‐Sanchez also included wound dehiscence and re‐ulceration as recurrences. 13 Johnson et al. 9 and Kowalski et al. 15 reported re‐infection as relapse which was defined as a confirmed infection of the proximal amputation site via pathological or microbiological examination. Bieler et al. 16 did not explicitly define infection recurrence. However, all the studies used bone culture and/or histology to define ROM. Some studies have reported poor concordance with bone cultures and histology and poor agreement among pathologists examining the same bone specimens. The outcomes of DFO are difficult to evaluate because there are many other factors in addition to residual bone infection that could have impacted clinical outcomes, such as healing, re‐infection, and amputation. We have not been able to identify a study that identified osteomyelitis as a risk factor for healing, but other co‐morbidities and treatment variables are associated with healing such as PAD, glucose control, and end‐stage renal disease. 31 , 32
TABLE 5.
Summary of the reference standard for studies of residual osteomyelitis.
| Reference | Study | No. | OM diagnosis reference standard | Treatment success reference standard |
|---|---|---|---|---|
| Aragon‐Sanchez et al. 13 | Prospective | 93 |
Clinical signs, probe to bone test, and 2 view radiographs Bone specimen for microbiology and histological analysis |
No recurrence within 1 year after the last surgical procedure. (Recurrence defined as clinical signs of infection, requiring antibiotic administration, and/or surgery, and/or re‐admission.) Wound dehiscence and/or re‐ulceration also were considered recurrences |
| Weng et al. 11 | Retrospective | 92 | Histopathological report | No same site amputation within 12‐month of index surgery |
| Aragon‐Sanchez et al. 14 | Retrospective | 28 |
Clinical signs, probe to bone test, and 2 view radiographs Bone specimen for microbiology and histological analysis |
No recurrence of infection within 6‐month study period, (recurrence defined as clinically and/or radiologically diagnosed infection and/or needing antibiotic therapy and/or surgical treatment) |
| Johnson et al. 9 | Retrospective | 66 | Bone histology report | Complete epithelialization of soft tissue defect and absence of repeat amputation of the index foot |
| Schmidt et al. 10 | Prospective | 72 |
Clinical symptoms, probe to bone, imaging, laboratory values Bone specimen for microbiology and histological analysis |
Not defined |
| Beieler et al. 16 | Retrospective | 50 | Histopathologic findings, exposed bone, radiographs or Magnetic Resonance Imaging (MRI) | Not requiring further treatment for DFO |
| Kowalski et al. 15 | Retrospective | 111 | Bone culture; bone histology | No infection relapse of proximal amputation site via histopathology or culture |
| Atway et al. 12 | Retrospective | 27 | Bone culture, MRI, bone scan, radiography | Amputation site healed without wound dehiscence and full weightbearing |
There are several treatments and prevention strategies that impact outcomes that are not addressed in osteomyelitis studies in this meta‐analysis. The first is the type of treatments that are provided after hospital discharge such as off‐loading, 33 wound debridement and advanced therapies like negative pressure wound therapy or hyperbaric oxygen. 34 For instance, ulcers treated with total contact casts heal faster with fewer infections compared with other methods to off‐load the foot like shoes or sandals. 35 , 36 Multispecialty teams have been shown to improve healing and reduce complications. 37 , 38 Second, prevention care after the wound is healed such as bespoke shoes and insoles, regular foot care, and foot‐specific education is never addressed, but has been shown to reduce the incidence of ulcers. For instance, bespoke shoes and insoles have been shown to reduce the incidence of re‐ulceration by half. 39 Ulceration provides an opening for bacterial infection. It is distinctly uncommon for adults to have a foot infection without a wound. Patients treated without multispecialty care will have fewer wounds that heal and double the incidence of re‐ulceration than patients treated in a more comprehensive group. 37 , 38 The cascade of re‐infection, hospitalisation, and amputation would also be significantly higher. Since most patients are not treated in a comprehensive programme, we expect outcomes could be significantly better than reported.
In conclusion, our analysis shows that residual bone infection carries an increased risk of re‐infection and an increased risk of amputation and prolonged antibiotic treatment, but it does not affect wound healing. Future prospective studies should address the limitations of defining residual bone infection and margins with no infection.
AUTHOR CONTRIBUTIONS
M.C.R. performed data acquisition and initial draft preparation. T.S. and M.A.S. performed data collection. T.L.C. and A.N.T. interpreted and analysed the data. M.J.S. critically reviewed article. L.A.L. formulated study design and wrote the article. All authors discussed the results and contributed to the final article.
FUNDING INFORMATION
This work was supported by the institutional and departmental funds.
CONFLICT OF INTEREST STATEMENT
No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Reyes MC, Suludere MA, Tarricone AN, et al. Residual diabetic foot osteomyelitis after surgery leads to poor clinical outcomes: A systematic review and meta‐analysis. Wound Rep Reg. 2024;32(6):872‐879. doi: 10.1111/wrr.13215
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lavery LA, Ryan EC, Ahn J, et al. The infected diabetic foot: re‐evaluating the Infectious Diseases Society of America diabetic foot infection classification. Clinost Infect Dis. 2020;70(8):1573‐1579. doi: 10.1093/cid/ciz489 [DOI] [PubMed] [Google Scholar]
- 2. Wukich DK, Hobizal KB, Sambenedetto TL, Kirby K, Rosario BL. Outcomes of osteomyelitis in patients hospitalized with diabetic foot infections. Foot Ankle Int. 2016;37(12):1285‐1291. doi: 10.1177/1071100716664364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int. 2013;34(3):351‐358. doi: 10.1177/1071100712467980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Senneville E, Lipsky BA, Abbas ZG, et al. Diagnosis of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3281. doi: 10.1002/dmrr.3281 [DOI] [PubMed] [Google Scholar]
- 5. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132‐e173. doi: 10.1093/cid/cis346 [DOI] [PubMed] [Google Scholar]
- 6. Senneville E, Albalawi Z, van Asten SA, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes‐related foot infections (IWGDF/IDSA 2023). Diabetes Metab Res Rev. 2024;40(3):e3687. doi: 10.1002/dmrr.3687 [DOI] [PubMed] [Google Scholar]
- 7. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 9. Johnson MJ, Shumway N, Bivins M, Bessesen MT. Outcomes of limb‐sparing surgery for osteomyelitis in the diabetic foot: importance of the histopathologic margin. Open Forum Infect Dis. 2019;6(10):ofz382. doi: 10.1093/ofid/ofz382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt BM, McHugh JB, Patel RM, Wrobel JS. Prospective analysis of surgical bone margins after partial foot amputation in diabetic patients admitted with moderate to severe foot infections. Foot Ankle Spec. 2019;12(2):131‐137. doi: 10.1177/1938640018770285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weng B, Oskooilar Y, Zakhary B, et al. Evaluating predictive value of surgical resected proximal bone margins in diabetic foot osteomyelitis with clinical outcomes at 1 year. Open Forum Infect Dis. 2023;10(1):ofac689. doi: 10.1093/ofid/ofac689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: a standardized method for evaluating bone margins with intraoperative culture. J Foot Ankle Surg. 2012;51(6):749‐752. doi: 10.1053/j.jfas.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 13. Aragon‐Sanchez J, Viquez‐Molina G, Lopez‐Valverde ME, Rojas‐Bonilla JM, Segura‐Retana E. Residual osteomyelitis at the resection margin after conservative surgery is not associated with the recurrence of diabetic foot infection and may successfully be treated without postoperative antibiotic therapy. Diabet Med. 2023;40(10):e15162. doi: 10.1111/dme.15162 [DOI] [PubMed] [Google Scholar]
- 14. Aragon‐Sanchez J, Viquez‐Molina G, Lopez‐Valverde ME. Controversial issues regarding positive bone margins in surgery for diabetic foot osteomyelitis: a pilot study. Int J Low Extrem Wounds. 2024;23(1):109‐115. doi: 10.1177/15347346211041267 [DOI] [PubMed] [Google Scholar]
- 15. Kowalski TJ, Matsuda M, Sorenson MD, Gundrum JD, Agger WA. The effect of residual osteomyelitis at the resection margin in patients with surgically treated diabetic foot infection. J Foot Ankle Surg. 2011;50(2):171‐175. doi: 10.1053/j.jfas.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 16. Beieler AM, Jenkins TC, Price CS, Saveli CC, Bruntz M, Belknap RW. Successful limb‐sparing treatment strategy for diabetic foot osteomyelitis. J Am Podiatr Med Assoc. 2012;102(4):273‐277. doi: 10.7547/1020273 [DOI] [PubMed] [Google Scholar]
- 17. Senneville E, Albalawi Z, van Asten SA, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes‐related foot infections (IWGDF/IDSA 2023). Clin Infect Dis. 2023;79(1):286. ciad527. doi: 10.1093/cid/ciad527 [DOI] [PubMed] [Google Scholar]
- 18. Lazaro‐Martinez JL, Aragon‐Sanchez J, Garcia‐Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care. 2014;37(3):789‐795. doi: 10.2337/dc13-1526 [DOI] [PubMed] [Google Scholar]
- 19. Peters EJG, Albalawi Z, van Asten SA, et al. Interventions in the management of diabetes‐related foot infections: a systematic review. Diabetes Metab Res Rev. 2024;40(3):e3730. doi: 10.1002/dmrr.3730 [DOI] [PubMed] [Google Scholar]
- 20. Lavery LA, Crisologo PA, La Fontaine J, Bhavan K, Oz OK, Davis KE. Are we misdiagnosing diabetic foot osteomyelitis? Is the gold standard gold? J Foot Ankle Surg. 2019;58(4):713‐716. doi: 10.1053/j.jfas.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarricone A, De La Mata K, Chen S, Krishnan P, Landau S, Soave R. Relationship between pH shifts and rate of healing in chronic nonhealing venous stasis lower‐extremity wounds. J Foot Ankle Surg. 2020;59(4):748‐752. doi: 10.1053/j.jfas.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 22. Tarricone A, Gee A, De La Mata K, et al. Health disparities in nontraumatic lower extremity amputations. A systematic review and meta‐analysis. Ann Vasc Surg. 2023;88:410‐417. doi: 10.1016/j.avsg.2022.09.033 [DOI] [PubMed] [Google Scholar]
- 23. Dugravot A, Fayosse A, Dumurgier J, et al. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: a 24‐year follow‐up of the Whitehall II cohort study. Lancet Public Health. 2020;5(1):e42‐e50. doi: 10.1016/S2468-2667(19)30226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yammine K, Hayek F, Assi C. A meta‐analysis of mortality after minor amputation among patients with diabetes and/or peripheral vascular disease. J Vasc Surg. 2020;72(6):2197‐2207. doi: 10.1016/j.jvs.2020.07.086 [DOI] [PubMed] [Google Scholar]
- 25. Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg. 2016;55(3):591‐599. doi: 10.1053/j.jfas.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 26. Peters EJ, Lavery LA, Armstrong DG. Diabetic lower extremity infection: influence of physical, psychological, and social factors. J Diabetes Complications. 2005;19(2):107‐112. doi: 10.1016/j.jdiacomp.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 27. Mutluoglu M, Sivrioglu AK, Eroglu M, et al. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand J Infect Dis. 2013;45(7):497‐503. doi: 10.3109/00365548.2013.765589 [DOI] [PubMed] [Google Scholar]
- 28. Gurlek A, Bayraktar M, Savas C, Gedik O. Amputation rate in 147 Turkish patients with diabetic foot: the Hacettepe University Hospital experience. Exp Clin Endocrinol Diabetes. 1998;106(5):404‐409. doi: 10.1055/s-0029-1212006 [DOI] [PubMed] [Google Scholar]
- 29. Gariani K, Pham TT, Kressmann B, et al. Three weeks versus six weeks of antibiotic therapy for diabetic foot osteomyelitis: a prospective, randomized, noninferiority pilot trial. Clin Infect Dis. 2021;73(7):e1539‐e1545. doi: 10.1093/cid/ciaa1758 [DOI] [PubMed] [Google Scholar]
- 30. Tone A, Nguyen S, Devemy F, et al. Six‐week versus twelve‐week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open‐label controlled randomized study. Diabetes Care. 2015;38(2):302‐307. doi: 10.2337/dc14-1514 [DOI] [PubMed] [Google Scholar]
- 31. Lavery LA, Lavery DC, Hunt NA, La Fontaine J, Ndip A, Boulton AJ. Amputations and foot‐related hospitalisations disproportionately affect dialysis patients. Int Wound J. 2015;12(5):523‐526. doi: 10.1111/iwj.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo Y, Wang Y, Liu H, Jiang X, Lei S. High glucose environment induces NEDD4 deficiency that impairs angiogenesis and diabetic wound healing. J Dermatol Sci. 2023;112(3):148‐157. doi: 10.1016/j.jdermsci.2023.09.007 [DOI] [PubMed] [Google Scholar]
- 33. Armstrong DG, Lavery LA. Evidence‐based options for off‐loading diabetic wounds. Clin Podiatr Med Surg. 1998;15(1):95‐104. [PubMed] [Google Scholar]
- 34. Sansom CE, Laughton CA, Neidle S, Schwalbe CH, Stevens MF. Structural studies on bio‐active compounds. Part XIV. Molecular modelling of the interactions between pentamidine and DNA. Anticancer Drug des. 1990;5(3):243‐248. [PubMed] [Google Scholar]
- 35. Lavery LA, Higgins KR, La Fontaine J, Zamorano RG, Constantinides GP, Kim PJ. Randomised clinical trial to compare total contact casts, healing sandals and a shear‐reducing removable boot to heal diabetic foot ulcers. Int Wound J. 2015;12(6):710‐715. doi: 10.1111/iwj.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It's not what you put on, but what you take off: techniques for debriding and off‐loading the diabetic foot wound. Clin Infect Dis. 2004;39(Suppl 2):S92‐S99. doi: 10.1086/383269 [DOI] [PubMed] [Google Scholar]
- 37. Chung J, Modrall JG, Ahn C, Lavery LA, Valentine RJ. Multidisciplinary care improves amputation‐free survival in patients with chronic critical limb ischemia. J Vasc Surg. 2015;61(1):162‐169. doi: 10.1016/j.jvs.2014.05.101 [DOI] [PubMed] [Google Scholar]
- 38. Sumpio BE, Armstrong DG, Lavery LA, Andros G; group SAw . The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the Society for Vascular Surgery and the American Podiatric Medical Association. J Vasc Surg. 2010;51(6):1504‐1506. doi: 10.1016/j.jvs.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 39. van Netten JJ, Raspovic A, Lavery LA, et al. Prevention of foot ulcers in persons with diabetes at risk of ulceration: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2024;40(3):e3652. doi: 10.1002/dmrr.3652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


