Abstract
Introduction
Difficult coronary sinus (CS) anatomy may lead to difficulty in optimal left ventricular (LV) lead placement and lead displacements leading to nonresponse to cardiac resynchronization therapy (CRT).
Methods
In this retrospective study, we studied the CRT parameters of devices implanted by single operator during the time period from January 2014 till December 2021, where different off-label techniques were used to place/stabilize LV lead. The technique used to stabilize LV lead, CRT parameters at baseline and follow up were noted for each patient.
Results
Out of 133 CRTs implanted during the study period, 23 patients (17.29 %) required off-label techniques. Stylet and guidewire retaining techniques were used in 11/23 (47.82 %) and 7/23 (30.43 %) patients respectively. In two patients, LV lead was jailed using coronary stent to prevent displacement. Two patients had CS stenosis and required balloon dilation while one patient had tortuous posterolateral vein which was straightened using a coronary stent. There was technical failure of 6/23 LV leads (26.08 %) with loss of capture, at a median follow up of 44 months (Range: 06–114 months). Out of these 6 patients, stylet and guidewire retaining techniques were used in 4 and 2 patients respectively.
Conclusion
Despite having acceptable parameters at implantation, these techniques particularly stylet and guidewire retention, may lead to non-capture of LV lead on long term follow ups. Better LV leads like active fixation leads and conduction system pacing (His Bundle/left bundle branch pacing) should be preferred in difficult CS anatomy.
Keywords: Coronary sinus stenting, CRT, Difficult coronary sinus anatomy, Guidewire retaining technique, Stylet retaining technique
1. Introduction
Cardiac resynchronization therapy (CRT) is a class 1 recommendation for symptomatic patients of heart failure with reduced ejection fraction (HFrEF) and ventricular dyssynchrony.1,2 By improving the inter and intra ventricular dyssynchrony, CRT improves the functional status, exercise capacity, quality of life and reduces the heart failure related hospitalizations and mortality in selected patients of HFrEF.1, 2, 3, 4 However even in this subgroup, 30 % of patients fail to respond to CRT.5,6 Out of all factors responsible for non-response to CRT, optimal left ventricular (LV) lead placement and stability remain critical technical factor,7,8 so that the myocardial areas with delayed activation are recruited, synchrony is restored and mechanical function of heart improves. Due to varied coronary sinus (CS) anatomy, the endovascular placement and optimal positioning of LV lead through epicardial veins may not always be possible using traditional technique. The alternative is to place the LV lead surgically, but it places the patient under additional general anaesthesia risk, makes the procedure lengthy and is associated with higher rates of infections and acute kidney injury.9 For these reasons, various lead stabilizing methods like “retained guidewire/stylet” techniques and using coronary hardware, have been described to get optimal endovascular LV lead positioning.10, 11, 12, 13, 14, 15, 16, 17. Though acute outcomes have been encouraging, the long-term results of such techniques are under-reported.
In this retrospective study, we studied CRT parameters of the patients done by single operator during the time period from January 2014 till December 2021, where variety of techniques to stabilize the LV lead were used in complex CS anatomy.
2. Material and methods
We assessed the records of patients who underwent CRT during the time period January 2014 till December 2021 under single operator at a tertiary care centre. All patients in whom LV lead were placed using standard technique and any other off-label techniques were included in the analysis. The technique used to stabilize the LV lead and their baseline parameters were noted. All these patients were followed up and their current LV lead parameters were recorded.
3. Results
A total of 133 CRT procedures were performed between January 2014 till December 2021 under single operator (author PB). All patients in whom CRT was implanted were in New York Heart Association (NYHA) class II-III. The mean LV ejection fraction (LVEF) was 29.5 % (±4.5 %). Mean QRS was 154 ms (±24 ms) and 120/133 (90.22 %) patients had left bundle branch block (LBBB). Ten patients had atrial fibrillation. Out of 133 CRT patients, 23 patients (17.29 %) required off-label techniques for placement of LV lead due to difficult CS anatomy. All these 23 patients had LBBB at baseline with a mean QRS duration of 160 ± 4 ms and had a mean LVEF of 26.3 % ± 4.7 % at time of CRT implantation.
3.1. Procedural details
Following CS cannulation using subclavian vein puncture, CS venogram was performed with occlusion balloon. In 20/23 patients (86.95 %) posterolateral branch was used to place the LV lead, in rest a mid-lateral vein was chosen. In all patients, a coronary guidewire 0.014 inch wire was introduced into the selected branch as far as possible and the LV lead was introduced over wire into selected branch. In patients who had an unstable LV lead position or who had intra-procedural dislodgements, we used stylet/wire retaining technique or a stent to stabilize the LV lead. In guidewire retaining technique, the wire over which the LV lead was advanced was left in situ; while in stylet retaining technique, a stiff stylet was introduced into the LV lead and the end of stylet was cut. The stylet and guidewire retaining techniques were used in 11/23 (47.82 %) and 7/23 (30.43 %) patients respectively. In two patients, a coronary stent was inflated in the CS to stabilize the LV lead (Fig. 1A and B). Two patients had stenosis of CS, which was balloon dilated while one patient had tortuous postero-lateral vein which was straightened with stent (Fig. 2A, B, C). In all three patients LV lead could be placed in desired branch. The mean LV lead threshold, impedance and R wave at installation in these 23 patients was 1.67 ± 0.37 V @ 0.5 ms, 761.78 ± 82.70 Ω and 7.47 ± 1.45 V respectively. None of these patients had any peri-procedural pericardial effusion or any other complication. The baseline characteristics, parameters of these patients at time of LV lead implantation and individual follow up duration are documented in Table 1, Table 2.
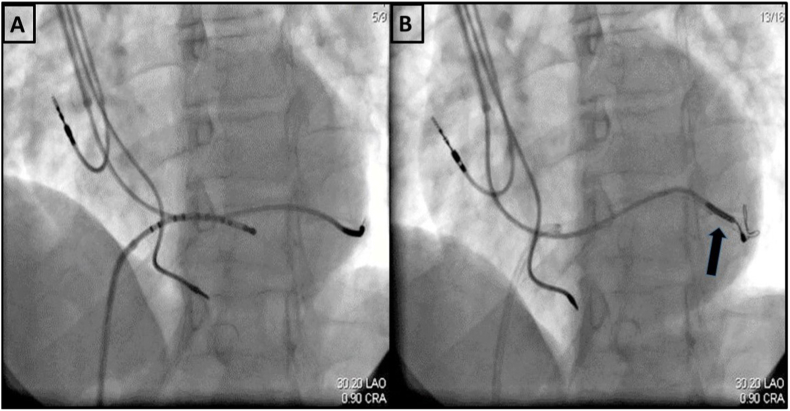
Fig. 1.
A - Unstable position of Left ventricular (LV) lead; Decapolar catheter being used to negotiate guiding catheter into the coronary sinus; 1B - stent placed to jail the already placed LV lead (between the stent and venous wall) to stabilize its position.
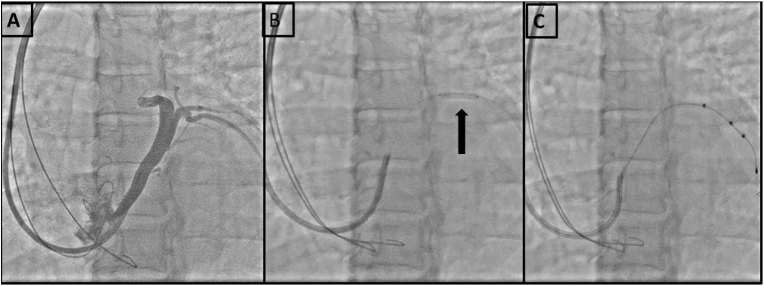
Fig. 2.
A-shows a S shaped bend in the proximal region of lateral vein; 2B -shows the bend being stented with a bare metal stent; 2C - shows successful placement of Left ventricular lead through the stent.
Table 1.
Baseline characteristics of the patients requiring alternative technique to stabilize LV lead (n = 23).
| Mean Age in years (±SD) | 57.3 (8.36) |
| Males/females (n) | 15/8 |
| Mean LVEDD in mm (±SD) | 59.21 (2.43) |
| Mean LVESD in mm (±SD) | 43.56 (2.69) |
| Mean LVEF % (±SD) | 26.35 (4.70) |
| Mean QRS (±SD) | 160.82 (4.37) |
| Mean threshold in Volts @0.5 ms (±SD) | 1.67 (0.37) |
| Mean Impedance in Ω (±SD) | 761.78 (82.70) |
| Mean R wave in millivolts (±SD) | 7.47 (1.45) |
| Technique used to stabilize/place the LV lead (n = 23) | |
| Stylet retaining technique | 11 (47.82 %) |
| Guidewire retaining technique | 7 (30.43 %) |
| Stent outside the LV lead | 2 (8.69 %) |
| POBA to CS | 2 (8.69 %) |
| Stent to straighten the tortuous CS vein | 1 (4.34 %) |
CS, Coronary sinus; LV, Left Ventricle; LVEDD, Left ventricular end diastolic diameter; LVEF, Left ventricular ejection fraction; LVESD, Left ventricular end systolic diameter; POBA, Plain old balloon angioplasty.
Table 2.
Details of all patients in whom alternative technique was used to stabilize LV lead.
| Patient Number | Technique used for stabilization of LV lead | Lead parameter at implantation | Lead status (preserved or failed) | Total duration of follow up (months) | Lead parameter at last follow up visit |
|---|---|---|---|---|---|
| 1 | Stylet Retaining | T: 2.1 | Preserved | 30 months (Patient died of RTA) | T: 1.9 |
| I:645 | I:567 | ||||
| R: 9.0 | R: 8.0 | ||||
| 2 | Wire Retaining | T: 1.90 | Preserved | 114 months | T: 2.1 |
| I:760 | I:687 | ||||
| R:10.0 | R:8.0 | ||||
| 3 | Wire Retaining | T: 1.8 | Failed at 41 months | 41 months | No capture |
| I:780 | I:> 2000 | ||||
| R:7.0 | |||||
| 4 | Stent outside the LV lead | T: 1.2 | Preserved | 98 months | T: 1.8 |
| I:848 | I:689 | ||||
| R:6.0 | R:8.5 | ||||
| 5 | Wire Retaining | T: 0.9 | Preserved | 90 months | T: 1.2 |
| I:765 | I: 840 | ||||
| R:7.0 | R:6.0 | ||||
| 6 | Stylet Retaining | T: 1.9 | Failed at 36 months | 36 months | No capture |
| I:730 | |||||
| R:6.0 | |||||
| 7 | POBA to CS | T: 1.5 | Preserved | 10 months Sudden cardiac death at home (CRT-P) |
T: 1.8 |
| I:590 | I:670 | ||||
| R:7 | R:8 | ||||
| 8 | Stylet Retaining | T: 1.7 | Failed at 12 months | 12 months | No capture |
| I:846 | Variable impedance | ||||
| R:5.5 | |||||
| 9 | Wire Retaining | T:1.1 | Preserved | 78 months | T:1.5 |
| I:809 | I:689 | ||||
| R:9 | R:8.5 | ||||
| 10 | Stent to straighten the tortuous CS vein | T:1.4 | Preserved | 76 months | T:1.8 |
| I:734 | I:541 | ||||
| R:5.5 | R:6.0 | ||||
| 11 | Stylet Retaining | T: 1.6 | Preserved | 72 months | T: 2.0 |
| I:798 | I:840 | ||||
| R:7.5 | R:8 | ||||
| 12 | Stylet Retaining | T:1.9 | Failed at 15 months | 15 months | No capture |
| I:874 | I > 2000 | ||||
| R:6.5 | |||||
| 13 | Wire Retaining | T: 2.3 | Preserved | 6 months (Died because of progressive heart failure) 70 months | T: 2.5 |
| I:678 | I:560 | ||||
| R: 8.5 | R: 7 | ||||
| 14 | Stylet Retaining | T:2.1 | Preserved | 70 months | T:2.2 |
| I:857 | I:789 | ||||
| R: 8.0 | R: 7.0 | ||||
| 15 | Wire Retaining | T:1.8 | Preserved | 62 months | T:1.6 |
| I: 844 | I: 920 | ||||
| R:9.0 | R:8.0 | ||||
| 16 | Stylet Retaining | T:1.9 | Preserved | 60 months | T:2.0 |
| I:698 | I:760 | ||||
| R:7.5 | R:7 | ||||
| 17 | Stent outside the LV lead | T:1.0 | Preserved | 52 months | T:1.0 |
| I:587 | I:690 | ||||
| R:8.5 | R:8 | ||||
| 18 | Stylet Retaining | T:1.8 | Failed at 08 months | 8 months | Non capture |
| I:760 | |||||
| R:8.0 | |||||
| 19 | POBA to CS | T:1.3 | Preserved | 48 months | T:1.5 |
| I:845 | I:740 | ||||
| R:10.0 | R:8.0 | ||||
| 20 | Stylet Retaining | T:1.9 | Preserved | 44 months | T:2.0 |
| I:745 | I:807 | ||||
| R:5.5 | R:5.0 | ||||
| 21 | Wire Retaining | T: 2.1 | Failed at 26 months | 26 months | Non capture |
| I:807 | I > 2000 | ||||
| R:6.0 | |||||
| 22 | Stylet Retaining | T:1.8 | Preserved | 36months | T:2.0 |
| I:698 | I:567 | ||||
| R:6.0 | R:5.5 | ||||
| 23 | Stylet Retaining | T: 1.5 | Preserved | 30 months | T: 1.8 |
| I: 823 | I: 956 | ||||
| R:9.0 | R:8.5 |
CS, Coronary Sinus; I, Impedance in Ω; LV, Left ventricle; POBA, Percutaneous Old Balloon Angioplasty; R, R wave in millivolts; T, threshold in Volts @0.5 ms.
3.2. Long term follow-up
There was technical failure of LV leads in 6/23 patients (26.08 %) at a median follow up of 44 months (Range: 06 months–114 months). Out of these 6 patients, stylet and guidewire retaining techniques were used in 4 and 2 patients respectively. The patients in whom coronary guidewire was used had a lead fracture at 26 and 41 months of CRT implantation (Fig. 3A and B). One of the patients who had lead fracture at 26 months of implantation was managed with left bundle branch pacing and the LVEF improved to 50 % in 3 months (Fig. 4A and B). Out of four stylet stabilized leads, one lead was displaced within 6 months and in rest three there was rise in lead impedance with failure to capture over duration of 8–36 months (median: 13.5 months). However no obvious mechanical deformity could be seen on fluoroscopy. Table 3 provides the details of patients with LV lead failure. At a median follow up of 44 months, 03 patients died: 01 in a road traffic accident, 01 had a sudden cardiac arrest and 01 patient died of progressive LV failure as illustrated in Table 2. The mean LV lead threshold, impedance and R wave for 17/23 leads at median follow up of 44 months were 1.80 ± 0.36 V @ 0.5 ms, 724.23 ± 124.85 Ω and 7.35 ± 1.11 V respectively. The mean LVEF and QRS of at follow up were 44.02 % (±5.54 %) and 116.66 ms (±11.16 ms).
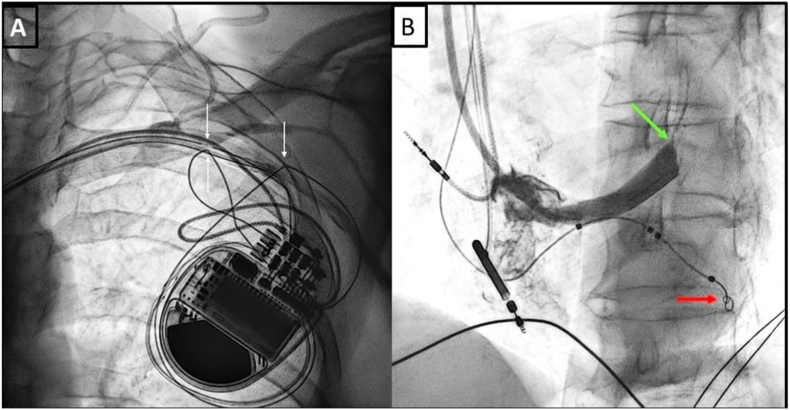
Fig. 3.
A- Lead fracture in the pocket; 3B- Left ventricular lead stabilized by guidewire retention technique.
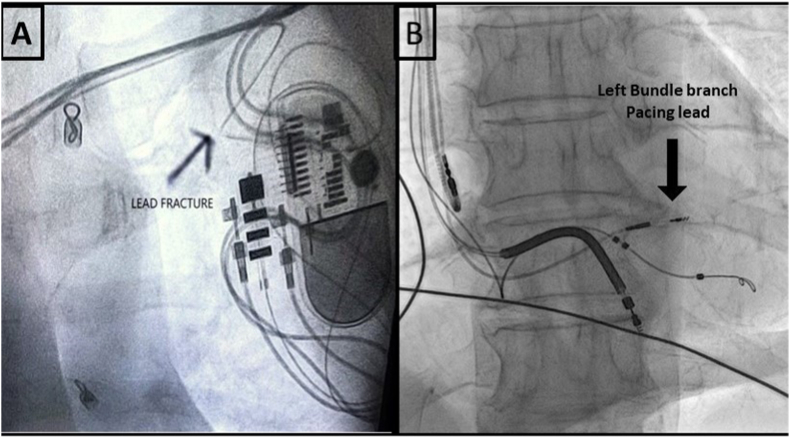
Fig. 4.
A- LV lead fracture in the pocket (acute bend). 4B – A new left bundle lead is implanted.
Table 3.
Details of patients with LV lead failure.
| S.No | Technique used for lead stabilization | Time after CRT implantation when LV lead failure was detected | Deranged parameters on interrogation (LV lead) | Fluoroscopy finding | Measures taken to address the situation |
|---|---|---|---|---|---|
| 1 | Stylet retaining | 8 months | Non capture @ maximum output | Lead displaced (retracted to main stem of CS) | Lead re-implantation attempted but not successful |
| 2 | Stylet retaining | 12 months | 9 months: Alarm for variable LV lead impedance and non-capture @ maximum output | No abnormality detected | Re-implantation of self-retaining screwing lead |
| 3 | Stylet retaining | 15 months | Abrupt rise of impedance to > 2000 Ω and non- capture @ maximum output | No abnormality detected | Patient not willing for redo procedure and placed on medical management |
| 4 | Wire retaining | 26 months | Abrupt rise of impedance >2000 Ω and non-capture @ maximum output | Acute bend in the lead suggesting lead fracture | Implantation of Left Bundle lead and LVEF improved to >50 % in 3 months after Left bundle branch pacing. |
| 5 | Stylet retaining | 36 months | Non-capture @ maximum output | No abnormality detected | Re-implantation of another LV lead in different vein with suboptimal results |
| 6 | Wire retaining | 41 months | Abrupt rise of impedance to >2000 Ω and non- capture @ maximum output | Acute bend in the lead suggesting fracture. | Escalation of medical therapy |
CRT, Cardiac Resynchronization therapy; LV, Left Ventricle.
4. Discussion
LV lead placement into appropriate CS branch and its stability remain important determinants of optimal response to CRT. Due to anatomical limitations, LV lead cannot be placed in ideal CS branch in one third of the cases.10 Even after initial success in ideal placement of LV lead in difficult CS anatomy, the rate of subsequent lead dislodgement remains as high as 5 %–9 %.11, 12, 13 This happens, most often, due to unstable position of LV lead.14,15 CS stenting to stabilize the LV lead after it has been introduced into ideal venous branch has been well described.16, 17, 18, 19 While Cesario et al17 and Kowalski et al18 have reported a maximum follow up of 2 and 5 months respectively, Szilagyi et al19 have shown acceptable LV lead threshold and impedance at a follow up of 12 months (11.5 ± 5.5, 2–23 months). The risks involved with this procedure are CS dissection, perforation, stenosis and lead insulation failure. Further the concerns about anticipated difficulty in removing LV lead endovascularly if it fails or gets infected remain valid.20,21 Fortunately, two of our patients, where we have used stents for stabilization of LV lead have remained stable and the LV lead parameters have been acceptable at a follow up of 98 and 52 months respectively.
The use of coronary guidewire to stabilize the LV lead was first described by De Cock et al.22 The coronary guidewires are not designed to be permanently placed in the vessels; so the concern remains that they may get fatigued and eventually get fractured,23,24 as was reported by Nagele et al.25 In a study by Arbelo et al, there was increase in impedance of all 3 leads at 6 months to 01 year follow up which were stabilized using retained guidewire technique.26 There was insulation failure along with deformation and fracture of electrode coils.26 In the index study, two patients (out of 7, 28.57 %) in whom guidewire was used to stabilize the LV lead had mechanical failure in form of lead fracture at a follow up of 26 and 41 months. Sharifkazemi et al reported promising data on stylet retaining technique at a mean follow up of 12.5 + 2.5 months.27 However in our study, 4/11 patients (36.36 %) where stylet was used for LV lead stabilization had loss of capture. Though stylet is stiffer than guidewire, it lacks the flexibility and is not designed to bear the torque permanently. Apart from insulation failure, as reported by Osztheimer, the stiff stylet may also fracture the lead and can result in serious complication like lung penetration by retained stylet.28 For stenosed CS, balloon dilatation and venous stenting have been used earlier also.29,30 In one patient, we used CS stenting to straighten the tortuous branch.
Though stylet and guidewire retention technique can give acceptable short-term results, neither stylet nor guidewire has been developed to bear the permanent torque and can get fatigued on long term use. When stent is used to stabilize the LV lead, not only can it damage the lead & its insulation but, would also make it impossible to remove the lead in case of mechanical failure or infection. With the availability of newer active fixation leads like Attain Stability Quad MRI SureScan™, using such innovative techniques should be the last resort. Though, long term data on CRT is more robust, the available evidence on other physiological methods of pacing to decrease the ventricular dyssynchrony like His bundle pacing and left bundle branch pacing is promising.31, 32, 33 Hence in the absence of ideal CS anatomy, His bundle/left bundle pacing may be used as an alternative or may be used upfront and CRT can be considered as back-up if anatomy is not suitable for conduction system pacing.
Limitations: The study reports data predominantly of the cases performed in the past where in the advances of LV lead (active fixation) and physiological pacing was not prevalent. It is also a single centre and sigle operator study and may be subjected to bias related to it. The use of surgical implanatation of LV lead was not considered as all cases could be performed percutaneously albiet by using alternative techniques.
5. Conclusion
Amongst different techniques used to stabilize LV lead in difficult CS anatomy, guidewire or stylet retaining technique were the commonest. Despite having acceptable parameters at implantation, these techniques may lead to non-capture of LV lead on long term follow ups. The retained stylet may be associated with potentially life threatening complications like heart chamber/lung penetration. Use of stents to stabilize the LV lead may lead to difficulty in future extraction of lead in case of infection or non-capture. Better LV leads like active fixation leads, conduction system pacing like His Bundle/left bundle branch pacing and epicardial pacing should be preferred in difficult CS anatomy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 2.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 3.Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 4.Cleland J.G., Daubert J.C., Erdmann E., et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 5.Daubert C., Behar N., Martins R.P., Mabo P., Leclercq C. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. 2017;38(19):1463–1472. doi: 10.1093/eurheartj/ehw270. [DOI] [PubMed] [Google Scholar]
- 6.Rickard J., Michtalik H., Sharma R., et al. Predictors of response to cardiac resynchronization therapy: a systematic review. Int J Cardiol. 2016;225:345–352. doi: 10.1016/j.ijcard.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 7.Mullens W., Grimm R.A., Verga T., et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53(9):765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Pothineni N.V.K., Supple G.E. Navigating challenging left ventricular lead placements for cardiac resynchronization therapy. J Innov Card Rhythm Manag. 2020;11(5):4107–4117. doi: 10.19102/icrm.2020.110505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ailawadi G., Lapar D.J., Swenson B.R., et al. Surgically placed left ventricular leads provide similar outcomes to percutaneous leads in patients with failed coronary sinus lead placement. Heart Rhythm. 2010;7(5):619–625. doi: 10.1016/j.hrthm.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu C.M., Wing-Hong Fung J., Zhang Q., Sanderson J.E. Understanding nonresponders of cardiac resynchronization therapy--current and future perspectives. J Cardiovasc Electrophysiol. 2005;16(10):1117–1124. doi: 10.1111/j.1540-8167.2005.40829.x. [DOI] [PubMed] [Google Scholar]
- 11.McAlister F.A., Ezekowitz J.A., Wiebe N., et al. Systematic review: cardiac resynchronization in patients with symptomatic heart failure [published correction appears in Ann Intern Med. 2005 Feb 15;142(4):311] Ann Intern Med. 2004;141(5):381–390. doi: 10.7326/0003-4819-141-5-200409070-00101. [DOI] [PubMed] [Google Scholar]
- 12.Strickberger S.A., Conti J., Daoud E.G., et al. Patient selection for cardiac resynchronization therapy: from the Council on Clinical Cardiology Subcommittee on electrocardiography and arrhythmias and the quality of care and outcomes research interdisciplinary working group, in collaboration with the Heart Rhythm Society. Circulation. 2005;111(16):2146–2150. doi: 10.1161/01.CIR.0000161276.09685.4A. [DOI] [PubMed] [Google Scholar]
- 13.Koos R., Sinha A.M., Markus K., et al. Comparison of left ventricular lead placement via the coronary venous approach versus lateral thoracotomy in patients receiving cardiac resynchronization therapy. Am J Cardiol. 2004;94(1):59–63. doi: 10.1016/j.amjcard.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 15.Abraham W.T., Fisher W.G., Smith A.L., et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 16.Kamalzadeh H., Yazdani S., Jalali M. Coronary sinus stenting for the management of left ventricular lead displacement during resynchronization therapy: a report of two cases. J Tehran Heart Cent. 2018;13(1):27–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Cesario D.A., Shenoda M., Brar R., Shivkumar K. Left ventricular lead stabilization utilizing a coronary stent. Pacing Clin Electrophysiol. 2006;29(4):427–428. doi: 10.1111/j.1540-8159.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski O., Prokopczuk J., Lenarczyk R., Pruszkowska-Skrzep P., Polonski L., Kalarus Z. Coronary sinus stenting for the stabilization of left ventricular lead during resynchronization therapy. Europace. 2006;8(5):367–370. doi: 10.1093/europace/eul022. [DOI] [PubMed] [Google Scholar]
- 19.Szilagyi S., Merkely B., Roka A., et al. Stabilization of the coronary sinus electrode position with coronary stent implantation to prevent and treat dislocation. J Cardiovasc Electrophysiol. 2007;18(3):303–307. doi: 10.1111/j.1540-8167.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 20.Hansky B. Stenting of coronary veins: a critical comment. Europace. 2008;10(12):1363. doi: 10.1093/europace/eun305. [DOI] [PubMed] [Google Scholar]
- 21.Ermis C., Benditt D.G. Stent-stabilization of left ventricular pacing leads for cardiac resynchronization therapy: a promising concept? J Cardiovasc Electrophysiol. 2007;18(3):308–309. doi: 10.1111/j.1540-8167.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- 22.DeCock C.C., Jessurun E.R., Allaart C.A., Visser C.A. Repetitive intraoperative dislocation during transvenous left ventricular lead implantation: usefulness of the retained guidewire technique. Pacing Clin Electrophysiol. 2004;27(12):1589–1593. doi: 10.1111/j.1540-8159.2004.00690.x. [DOI] [PubMed] [Google Scholar]
- 23.Furman S. Repetitive left ventricular lead dislocation. Pacing Clin Electrophysiol. 2004;27(12):1588. doi: 10.1111/j.1540-8159.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- 24.Love C.C. Retention wire: déjà vu all over again? Pacing Clin Electrophysiol. 2004;27(12):1587. doi: 10.1111/j.1540-8159.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- 25.Nägele H., Hashagen S., Ergin M., Azizi M., Behrens S. Coronary sinus lead fragmentation 2 years after implantation with a retained guidewire. Pacing Clin Electrophysiol. 2007;30(3):438–439. doi: 10.1111/j.1540-8159.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 26.Arbelo E., García-Quintana A., Caballero E., et al. Deterioro tardío del electrodo venoso ventricular izquierdo estabilizado con la técnica de la guía retenida para la terapia de resincronización cardiaca [Late failure of left ventricular leads stabilized using the retained guidewire technique in patients undergoing cardiac resynchronization therapy] Rev Esp Cardiol. 2008;61(1):91–94. [PubMed] [Google Scholar]
- 27.Sharifkazemi M.B., Aslani A. Stabilization of the coronary sinus lead position with permanent stylet to prevent and treat dislocation. Europace. 2007;9(10):875–877. doi: 10.1093/europace/eum151. [DOI] [PubMed] [Google Scholar]
- 28.Osztheimer I., Duray G., Hüttl K., Merkely B. Fracture and lung penetration of a left ventricular lead stabilized by retained stylet. Can J Cardiol. 2016;32(12):1576.e19–1576.e20. doi: 10.1016/j.cjca.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Van Gelder B.M., Meijer A., Basting P., Hendrix G., Bracke F.A. Successful implantation of a coronary sinus lead after stenting of a coronary vein stenosis. Pacing Clin Electrophysiol. 2003;26(9):1904–1906. doi: 10.1046/j.1460-9592.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandler D.A., Feigenblum D.Y., Bernstein N.E., Holmes D.S., Chinitz L.A. Cardiac vein angioplasty for biventricular pacing. Pacing Clin Electrophysiol. 2002;25(12):1788–1789. doi: 10.1046/j.1460-9592.2002.01788.x. [DOI] [PubMed] [Google Scholar]
- 31.Ali N., Keene D., Arnold A., Shun-Shin M., Whinnett Z.I., Afzal Sohaib S.M. His bundle pacing: a new frontier in the treatment of heart failure. Arrhythmia Electrophysiol Rev. 2018;7(2):103–110. doi: 10.15420/aer.2018.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strocchi M., Lee A.W.C., Neic A., et al. His-bundle and left bundle pacing with optimized atrioventricular delay achieve superior electrical synchrony over endocardial and epicardial pacing in left bundle branch block patients. Heart Rhythm. 2020;17(11):1922–1929. doi: 10.1016/j.hrthm.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Upadhyay G.A., Razminia P., Tung R. His-bundle pacing is the best approach to physiological pacing. Heart Rhythm O2. 2020;1(1):68–75. doi: 10.1016/j.hroo.2020.03.001. Published 2020 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]