Abstract
Background/Aims
Although increased mortality has been reported among people with Mycobacterium avium complex pulmonary disease (MAC-PD), data are limited on survival associated with various antibiotic regimens used to treat MAC-PD. We conducted a comparative analysis of 3-year mortality in Medicare beneficiaries with bronchiectasis using various MAC-PD regimens.
Methods
We included Medicare beneficiaries aged ≥65 years with bronchiectasis (01/2006–12/2014). We limited our cohort to new MAC-PD therapy users. MAC-PD therapy was defined as ≥60-day prescriptions for a macrolide plus ≥1 other MAC-PD antibiotic. Guideline-based therapy (GBT) included a macrolide, ethambutol, and/or rifamycin. Using Cox proportional hazard models, we calculated adjusted hazard ratios (aHR) for death up to 3 years after therapy start between the following groups: (1) 2007 GBT versus non-GBT; (2) 2020 GBT versus non-GBT; and (3) macrolide-ethambutol-rifamycin (3-drug) versus macrolide-ethambutol (2-drug).
Results
We identified 4820 new MAC-PD therapy users, of whom 866 (17.9%) were deceased within 3 years of therapy initiation. Of 3040 (63.1%) beneficiaries prescribed 2007 GBT, 472 (15.5%) were deceased by 3 years, compared to 394 (22.1%) of 1780 (36.9%) prescribed non-GBT (aHR 0.82; 95% confidence interval [CI], .72–.94). We observed a similar trend for 2020 GBT versus non-GBT (aHR 0.81; 95% CI, .70–.94]). Three-year-mortality was similar between those starting 3-drug versus 2-drug regimens (aHR 0.89; 95% CI, .74–1.08]).
Conclusions
Among Medicare new MAC-PD therapy users, 3-year-mortality was higher in those prescribed non-GBT regimens compared to GBT regimens. Whether this finding suggests improved efficacy of GBT and/or differential characteristic of those using non-GBT regimens deserves further study.
Keywords: bronchiectasis, evidence based medicine, infectious disease guidelines, mortality, Mycobacterium avium complex, Nontuberculous mycobacteria, pulmonary infections
BACKGROUND
Nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms that can cause pulmonary disease in individuals with underlying lung conditions, including bronchiectasis, emphysema, cystic fibrosis, or significant scarring from prior infections [1]. Pulmonary disease resulting from Mycobacterium avium complex (MAC), the most commonly isolated NTM, is a chronic inflammatory airway disease often associated with chronic cough, fatigue, night sweats, weight loss, and depression, with frequent relapses and recurrence [1–5]. Increasing prevalence and incidence of MAC pulmonary disease (MAC-PD) have been observed in various settings worldwide [1, 6–11]. In addition, more recent epidemiologic data have demonstrated increased mortality among individuals with MAC-PD compared to those without; however, limited data are available regarding a survival benefit with antibiotic regimens [12–16].
Treatment for MAC-PD typically requires 3–4 antibiotics for more than a year and is often complicated by frequent interruptions and early discontinuation because of drug interactions, side effects, adverse events, and development of resistance, which is thought to be more frequent in the setting of non-guideline-based therapy (non-GBT). In particular, macrolide resistance is of serious concern because it is associated with poor outcomes [2, 4, 17–21]. Although American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) GBT is recognized as the most effective regimen with improved clinical and economic outcomes, adherence to guidelines has been poor [22–26]. Given variations in both underlying disease and MAC-PD severity, the long duration of treatment, frequent complications, and changes in therapy over a treatment course, assessing the effect of treatment regimen on mortality is challenging [27]. We compared 3-year mortality following prescription for GBT and alternative antibiotic regimens in U.S. Medicare beneficiaries with bronchiectasis.
METHODS
We used a cohort of first-time MAC therapy users previously identified [21]. We used U.S. Medicare data from the Centers for Medicare and Medicaid Services (parts A and B plus D, but not part C) between 1 January 2006 and 31 December 2014 to identify beneficiaries aged ≥65 years with bronchiectasis, identified by the International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes 494.0 or 494.1 (bronchiectasis with or without acute exacerbation). We identified beneficiaries who were prescribed a first-time multidrug regimen for MAC-PD. We excluded those with a diagnosis for cystic fibrosis, HIV, or previous organ transplantation any time before initiation of antibiotic therapy for MAC-PD.
First-time MAC-PD therapy was defined as a ≥60-day supply of an antibiotic regimen containing a macrolide plus ≥1 of the following: rifamycin, ethambutol, fluoroquinolone, or intravenous/inhaled amikacin, requiring a minimum of 12 months of Medicare coverage without evidence of prior MAC-PD antibiotic regimen prescription. These regimens are not used for any other infections other than a few rare NTM species and are thus likely to be highly specific to MAC-PD [28, 29]. We did not use diagnosis codes for identification of cases of MAC-PD given poor sensitivity and specificity of code-based case finding for MAC-PD [28, 29]. We defined GBT as a macrolide and ethambutol with or without a rifamycin based on 2007 or 2020 ATS/IDSA guidelines [4]. Baseline period was defined as the time between data start (01 January 2006) or beneficiaries’ Medicare enrollment and their MAC-PD therapy start date.
Treatment start date was defined as the start date of the first regimen meeting the treatment definition (ie, the first overlapping prescription of the initial multidrug regimen). Treatment end date was estimated by adding the number of days of supply of the initial regimen to the treatment start date and was determined by the date a macrolide-based multidrug antibiotic no longer met the MAC therapy definition or switched to another regimen, allowing for a 30-day gap between medication refills.
Using descriptive statistics, we examined beneficiaries’ characteristics by comparison groups. Baseline demographic (eg, age, sex) and clinical (eg, comorbidities, concomitant medications) characteristics were identified by International Classification of Diseases, Ninth Revision, Clinical Modification, codes-based Medicare claims made during the baseline period. We used Cox proportional hazard regression to evaluate time to all-cause mortality (death) up to 3 years after therapy start between the following initial MAC-PD therapy regimens in an intention-to-treat analysis: (1) 2007 GBT (macrolide-ethambutol-rifamycin) versus non-GBT; (2) 2020 GBT (macrolide-ethambutol ± rifamycin) versus non-GBT; and (3) macrolide-ethambutol-rifamycin (3-drug) versus macrolide-ethambutol (2-drug) and we calculated adjusted hazard ratios (aHR). Comparisons 2 and 3 were selected based on the discussion regarding the potential use for macrolide-ethambutol in the 2020 ATS/IDSA guidelines and comparisons in an ongoing randomized clinical trial [2, 21, 30]. In multivariable modeling, we controlled for covariates selected a priori based on a causal model and associated directed acyclic graphs; selected covariates comprised race, ethnicity, region of residence, age at therapy start, year at therapy start, sex, duration of MAC-PD therapy, Charlson comorbidity index, chronic obstructive pulmonary disease (COPD)/emphysema, lung cancer, rheumatoid arthritis, asthma, and baseline use of digoxin, anticoagulants, antihypertensives/β-blockers, narcotics, and oral steroids [31]. Beneficiaries were censored at: (1) death (outcome of interest) or (2) end of study period (31/12/2014), whichever occurred earlier. Covariate-adjusted Kaplan-Meier curves were estimated to illustrate the time-to-death outcome in the comparison groups. All analyses were performed using SAS statistical software 9.4 (SAS Institute; https://www.sas.com). This study was reviewed and approved by the institutional review board at Oregon Health & Science University (IRB00015347).
RESULTS
We identified 4820 beneficiaries who were first-time MAC therapy users, requiring a minimum of 60 days on the initial therapy; 3040 (63.1%) were prescribed 2007 GBT and 1780 (36.9%) were prescribed non-GBT; 3713 (77.2%) were prescribed 2020 GBT and 1096 (22.8%) non-GBT. Most were female (3669, 76.1%) and White (4223, 87.6%) with a median age of 77.8 years (standard deviation [SD] 6.1) and mean time enrolled in Medicare of 7.0 years (SD 2.2) (Table 1). In addition to bronchiectasis, 3614 (75.0%) also had a diagnosis of COPD, 2666 (55.3%) gastroesophageal reflux disease, and an additional 1805 (58.2%) had oral corticosteroid exposure within 1 year before initiating therapy for MAC-PD. Median duration of initial MAC-PD therapy regimen was 176 days (interquartile range [IQR] 95, 333.0). Median time of follow-up was 950 days (IQR 440, 1659).
Table 1.
Baseline Characteristics of U.S. Medicare Beneficiaries Prescribed MAC Antibiotic Regimen, 2006–2014
| N (%) or Mean (SD) | N (%) or Mean (SD) | N (%) or Mean (SD) | P Value | P Value | P Value | |
|---|---|---|---|---|---|---|
| 2007 non-GBT (N = 1780) | 2007 GBT (N = 3040) | Macrolide + Ethambutol (2-drug) (N = 682) |
2007 GBT versus non-GBT |
2020 GBT versus non-GBT |
3-drug versus 2-drug |
|
| Demographic characteristics | ||||||
| Age at treatment start (y) | 78.1 (SD 6.4) | 77.6 (SD 6.0) | 78.5 (SD 6.4) | <.01 | .74 | <.01 |
| Female | 1318 (74.0%) | 2351 (77.3%) | 516 (75.7%) | <.01 | <.01 | .34 |
| Race, ethnicity | .27 | .94 | .10 | |||
| White | 1537 (86.4%) | 2686 (88.4%) | 581 (85.2%) | |||
| Asian | 109 (6.1%) | 155 (5.1%) | 45 (6.6%) | |||
| Hispanic | 73 (4.1%) | 102 (3.4%) | 31 (4.6%) | |||
| Black | 40 (2.3%) | 58 (1.9%) | 19 (2.8%) | |||
| Combined Groupa | 21 (1.2%) | 30 (1.3%) | <10 (<0.1%) | |||
| Number of years in Medicare since age 65 | 6.9 (SD 2.3) | 7.1 (SD 2.3) | 7.1 (SD 2.2) | .02 | <.01 | .87 |
| Clinical characteristics b | ||||||
| Chronic obstructive pulmonary disease/emphysema | 1407 (79.0%) | 2207 (72.6%) | 505 (74.1%) | <.01 | <.01 | .45 |
| Oral corticosteroid usec | 1119 (62.9%) | 1686 (55.5%) | 391 (57.3%) | <.01 | <.01 | .40 |
| Gastroesophageal reflux | 994 (55.8%) | 1672 (55.0%) | 344 (50.4%) | .57 | <.01 | .03 |
| Asthma | 564 (31.7%) | 877 (28.9%) | 199 (29.2%) | .04 | <.01 | .88 |
| Diabetes mellitus | 541 (30.4%) | 807 (26.6%) | 206 (30.2%) | <.01 | .03 | .05 |
| Rheumatologic disease | 97 (5.5%) | 110 (3.6%) | 39 (5.7%) | <.01 | .07 | .01 |
| Pseudomonas infection | 191 (10.7%) | 190 (6.3%) | 53 (7.8%) | <.01 | <.01 | .14 |
| Lung cancer | 172 (9.7%) | 266 (8.8%) | 51 (7.5%) | .29 | .01 | .27 |
| Primary immune deficiency | 132 (7.4%) | 144 (4.7%) | 40 (5.9%) | <.01 | <.01 | .23 |
| Allergic bronchopulmonary aspergillosis | 20 (1.1%) | 26 (0.9%) | 9 (1.3%) | .36 | .86 | .26 |
| Alpha-1 antitrypsin deficiency | 7 (0.4%) | 13 (0.4%) | <10 (<0.1%) | .86 | .40 | .58 |
| Primary ciliary dyskinesia | 0 | <10 (<0.1%) | 0 | .13 | .58 | .34 |
| Silicosis | <10 (<0.1%) | <10 (<0.1%) | 0 | .90 | .54 | .50 |
| Chronic kidney disease | 0 | <10 (<0.1%) | 0 | .44 | .78 | .63 |
| Charlson comorbidity index score | 2.4 (SD 1.7) | 2.0 (SD 1.5) | 2.3 (SD 1.6) | <.01 | <.01 | <.01 |
| Healthcare utilization | ||||||
| Number of clinician office visits per year | 5.9 (SD 5.0) | 5.1 (SD 4.4) | 5.6 (SD 4.9) | <.01 | <.01 | <.01 |
| Number of visits to pulmonologist per year | 1.6 (SD 1.6) | 1.4 (SD 1.9) | 1.5 (SD 1.6) | <.01 | <.01 | .13 |
| Number of visits to infectious disease specialists per year | 0.7 (SD 1.1) | 0.5 (SD 0.8) | 0.6 (SD 0.8) | <.01 | <.01 | .15 |
| Number of any hospitalizations per year | 1.2 (SD 1.8) | 0.9 (SD 1.4) | 1.1 (SD 1.5) | <.01 | <.01 | .07 |
| Number of hospitalizations from respiratory illness per year | 0.7 (SD 0.8) | 0.6 (SD 0.7) | 0.6 (SD 0.8) | <.01 | <.01 | .20 |
| Number of acute respiratory exacerbations per yeard | 0.6 (SD 0.9) | 0.8 (SD 0.6) | 0.6 (SD 0.6) | <.001 | <.01 | .62 |
Abbreviations: GBT, guideline-based therapy; MAC, Mycobacterium avium complex; SD, standard deviation.
aBeneficiaries who identify as American Indian, Alaska Native, more than 1 race and ethnicity, or who did identify with any of the provided categories were combined due to low numbers.
bIdentified by International Classification of Diseases, Ninth Revision, Clinical Modification, codes-based Medicare claims made during the baseline period.
cNational Drug Codes for oral corticosteroids were used to identify use of oral corticosteroid during the baseline period (eg, 1 or more oral corticosteroid dispensed within 12 mo before starting MAC-pulmonary disease therapy).
dPrescriptions of antibiotics typically used for acute respiratory exacerbation (erythromycin, azithromycin, clarithromycin, inhaled tobramycin, levofloxacin, moxifloxacin, ciprofloxacin, amoxicillin, amoxicillin/clavulanate, or doxycycline) for ≥7 d but ≤28 d.
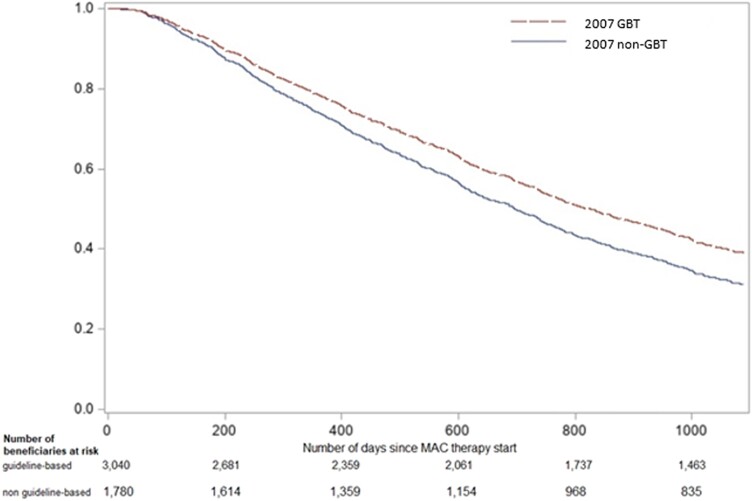
Compared to beneficiaries prescribed GBT per 2007 or 2020 ATS/IDSA guidelines, those prescribed non-GBT had higher health care utilization, including a higher number of office visits and hospitalizations (P < .01). In those receiving non-GBT, Charlson comorbidity index was higher and a greater proportion had primary immune deficiency, COPD/emphysema, or had at least 1 oral corticosteroid prescribed in the 12 months before MAC-PD therapy initiation (Table 1). Median duration of therapy was 198.0 (IQR 108.5, 354.0) days and 145.0 (IQR 88.0, 274.5) days for 2007 GBT and non-GBT, respectively. Fewer regimens included amikacin in the 2007 GBT group compared to the non-GBT group; 8 (0.3%) GBT and 50 (2.9%) non-GBT, P <.01. Among the 3040 beneficiaries prescribed 2007 GBT, 472 (15.5%) were deceased by 3 years since therapy start, compared to 394 (22.1%) of those prescribed non-GBT. The aHR for death in 2007 GBT versus non-GBT group was 0.82 (95% confidence interval [CI], .72–.94) (Figure 1, Table 2).
Figure 1.
Covariate-adjusted Kaplan-Meier for 3-year-mortality in U.S. Medicare beneficiaries prescribed MAC 2007 GBT versus non-GBT*.
*Adjusted for race, ethnicity, region of residence, age at therapy start, year at therapy start, sex, duration of MAC-PD therapy, Charlson comorbidity index, COPD/emphysema, lung cancer, rheumatoid arthritis, asthma, digoxin, anticoagulants, antihypertensives/β-blockers, narcotics, and oral steroids.
Table 2.
Covariate-adjusted Hazard Ratios for 3-year Mortality in U.S. Medicare Beneficiaries Prescribed MAC Regimensa
| All-cause Mortality | |||
|---|---|---|---|
| Reference | Adjusted Hazard Ratio (95% CI) | ||
| Comparison 1b | 2007 Non-GBT 394 (22.1%) |
2007 GBT 472 (15.5%) |
0.82 (.72–.94) |
| Comparison 2c | 2020 Non-GBT 244 (22.2%) |
2020 GBT 622 (16.8%) |
0.81 (.70–.94) |
| Comparison 3d | 2-drug 150 (22.0%) |
3-drug 472 (15.6%) |
0.89 (.74–1.08) |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; GBT, guideline-based therapy; MAC, Mycobacterium avium complex; PD, pulmonary disease.
aAdjusted for race, ethnicity, region of residence, age at therapy start, sex, year at therapy start, duration of MAC-PD therapy, Charlson comorbidity index, COPD/emphysema, lung cancer, rheumatoid arthritis, asthma, digoxin, anticoagulants, antihypertensives/β-blockers, narcotics, and oral steroids.
b2007 GBT (macrolide-ethambutol-rifamycin) versus non-GBT.
c2020 GBT (macrolide-ethambutol ± rifamycin) versus non-GBT.
dMacrolide-ethambutol-rifamycin (3-drug) versus macrolide-ethambutol (2-drug).
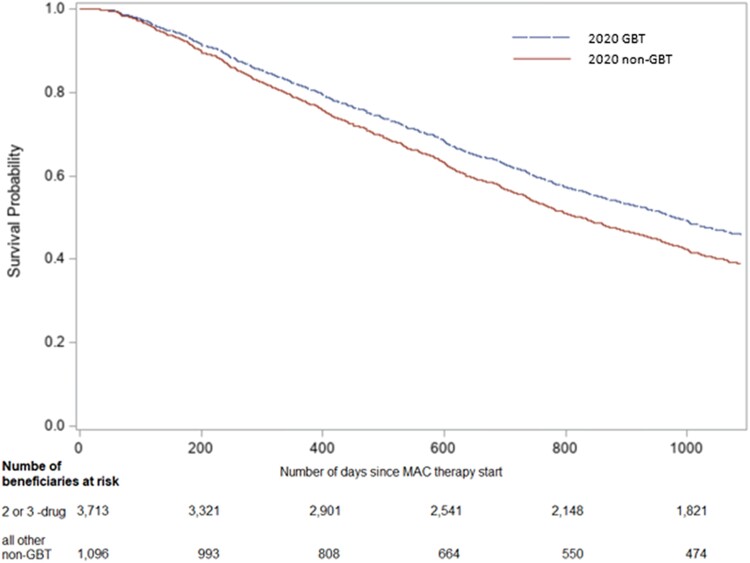
Median duration of therapy was 195.0 (IQR 109.0, 354.0) days and 118.0 (IQR 82.0, 220.0) days for 2020 GBT and non-GBT, respectively. Of 3713 (77.2%) beneficiaries prescribed 2020 GBT, 622 (16.8%) were deceased by 3 years, compared to 244 (22.2%) of 1096 (22.8%) prescribed non-GBT (aHR 0.81 [95% CI, .70–.94]) (Figure 2).
Figure 2.
Covariate-adjusted Kaplan-Meier for 3-year mortality in U.S. Medicare beneficiaries prescribed MAC 2020 GBT versus non-GBT*.
*Adjusted for race, ethnicity, region of residence, age at therapy start, year at therapy start, sex, duration of MAC-PD therapy, Charlson comorbidity index, COPD/emphysema, lung cancer, rheumatoid arthritis, asthma, digoxin, anticoagulants, antihypertensives/β-blockers, narcotics, and oral steroids.
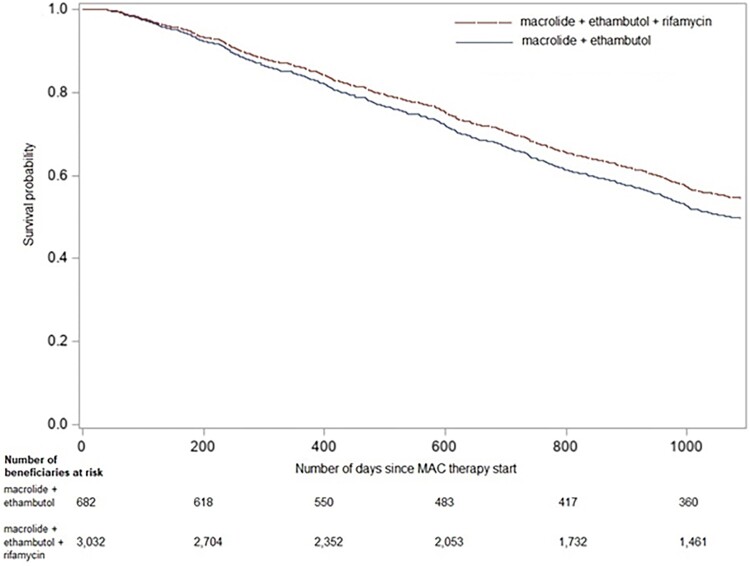
Median duration of therapy for the those prescribed 3-drug regimens was 198.0 (IQR 109.0, 198.0) days compared to 182 (IQR 108.0, 350.0) days for those prescribed 2-drug regimens. Of 1780 (36.9%) beneficiaries prescribed 3-drug regimens, 472 (15.6%) were deceased at 3 years compared to 150 (22.0%) of those prescribed 2-drug regimens, with an aHR of 0.89 (95% CI, .74–1.08) (Figure 3).
Figure 3.
Covariate-adjusted Kaplan-Meier for 3-year-mortality in U.S. Medicare beneficiaries prescribed macrolide-ethambutol-rifamycin (3-drug) versus macrolide-ethambutol (2-drug)*.
*Adjusted for race, ethnicity, region of residence, age at therapy start, year at therapy start, sex, duration of MAC-PD therapy, Charlson comorbidity index, COPD/emphysema, lung cancer, rheumatoid arthritis, asthma, digoxin, anticoagulants, antihypertensives/β-blockers, narcotics, and oral steroids.
DISCUSSION
Among Medicare beneficiaries with bronchiectasis prescribed an initial MAC-PD antibiotic regimen, 3-year mortality was higher in those prescribed non-GBT regimens compared to the 2007 or 2020 GBT antibiotic regimens. There were no statistically significant differences in mortality between 3-drug versus 2-drug regimens. However, there is separation in the Kaplan-Meier curves with more deaths in the 2-drug versus 3-drug regimen; this requires further investigation given the discussion in the 2020 ATS/IDSA guidelines listing a macrolide and ethambutol as a potential regimen as drug interactions and side effects frequently preclude rifamycin use [2, 4]. Ongoing clinical trials will provide additional information on differences in the 2- versus 3-drug regimens [30]. More data are needed regarding differential mortality by antibiotic regimen in MAC-PD; however, this analysis provides ongoing support for use of GBT.
Our study results are comparable to previous studies reporting on MAC-PD mortality and antibiotic regimens. Brode et al. performed a similar evaluation of 3148 individuals in Ontario with incident MAC-PD and found no statistically significant difference in mortality between regimens; however, only 16% of their population was prescribed at least 2 antibiotics, potentially limiting statistical power [27]. Similar to our study, hazard ratios favored GBT and crude death rates were 106.3/1000 person-years for 2007 GBT and 112.4/1000 person-years for non-GBT [27]. In 170 people with MAC-PD treated in a randomized trial comparing ethambutol and rifamycin with either ciprofloxacin or clarithromycin, all-cause mortality was higher in the clarithromycin group (48% vs 29%); however, no difference was observed in mortality attributable to MAC-PD [32].
Our work has several notable strengths. First, US population-based data on MAC-PD outcomes including mortality are severely limited. Results from this study add important, population-based data on mortality of individuals treated for MAC-PD with GBT or alternative regimens. Second, Medicare claims data are representative of the older US population. Given the age distribution of MAC-PD, which primarily affects older individuals, Medicare data provide a readily available and efficient source of data to conduct epidemiologic studies on MAC-PD. Third, Medicare data capture the entirety of beneficiaries’ health care encounters, including pharmacy prescriptions in those with Part D coverage. Therefore, our study data have minimal missingness.
Our study has some limitations. In addition to drug regimen, multiple clinical factors have been associated with mortality and poor outcomes in MAC-PD, including cavitary disease, presence of macrolide resistance, lack of or timing of culture conversion, and elevated erythrocyte sedimentation rate [2, 4, 19, 20, 23, 33–35]. Because our study evaluated claims data only, we were not able to assess features of MAC-PD severity or presence of macrolide resistance. Limited population-level data are available evaluating macrolide resistance in MAC in the United States. Available data are from a specialty referral laboratory, which results in limited generalizability, preventing our ability to estimate the potential distribution or impact of macrolide resistance on mortality in our cohort [36]. Amikacin use was low overall but is frequently not initiated in all cavitary MAC-PD because of toxicity [37, 38]. Reasons for such a low proportion of amikacin may include provider hesitancy given toxicity or incomplete capture resulting from intravenous antibiotics being billed outside of Medicare Parts A, B, and D, as home infusion of antibiotics are not covered or incompletely covered, or are sometimes billed through Medicare part C Medadvantage plans [39]. Without additional clinical data, it is unknown if amikacin use in this setting was associated with more severe MAC-PD given it is part of GBT for those with cavitary or macrolide-resistant disease. However, we hypothesize people with more severe disease (ie, cavitary, increased area of lung impacted) may be more likely to be referred to providers with expertise in managing MAC-PD, thus they would be more likely to be initiated on a GBT regimen, which would bias our results towards the null hypothesis and lead to an underestimation of the effect [40]. Given lack of culture data, pulmonary infections caused by other NTM species may be misclassified as MAC-PD, especially in the non-GBT group, because treatment regimens depend on species; however, we suspect this misclassification to be low [28, 29]. Given the slow progression of MAC-PD, along with slow response to therapy, the ideal timing to evaluate impact of the MAC-PD regimen on mortality is unknown, which may obscure potential associations. We also did not capture mortality in those treated for fewer than 60 days, which is unlikely to be significantly impacted by MAC-PD regimen given the indolent nature of this disease. In addition, the age of the population with MAC-PD indicates a higher burden of comorbidities and an opportunity for new diagnoses, such as malignancy, which may influence mortality in our population. We evaluated and adjusted for potential confounding comorbidities at baseline for our study; however, new diagnoses were not captured. Additionally, confounding by indication is an important consideration for this work because non-GBT users may be sicker and have more comorbidities than GBT users. We considered propensity scores, which led to similar results and characteristics of comparison groups were fairly well balanced. Therefore, we used multivariable adjustment in our modeling approach for easier interpretation. Our model controlled for comorbidities and the use of drugs with interactions against rifamycins; however, we also cannot completely rule out the possibility of residual confounding because of the possibility that patients with more severe disease or worsened underlying comorbidities were more likely given non-GBT regimens.
We evaluated 3-year mortality in first-time MAC-PD therapy users by initial MAC antibiotic regimens. The comparison groups were based on initial prescription for MAC-PD regimens, which does not include the impact of regimen changes or discontinuation, both of which occur frequently in the treatment of MAC-PD, and our results must be interpreted with that in mind [21]. The increased survival observed with GBT in our evaluation, may also be impacted by provider experience in treating MAC-PD, as Marras et al. found that mycobacterial experts were more likely to use GBT regimens for both initial and recurrent disease, and estimated more people with positive cultures had MAC-PD compared to those without expertise mycobacterial management [40]. The ATS/IDSA guidelines also stress the need for ongoing monitoring of chest imaging, symptoms, and sputum cultures, which may be preferentially done by providers with more experience and expertise in managing MAC-PD and result in differential mortality [2]. More data are needed regarding the impact of individual regimens on mortality, which is challenging in the setting of prolonged treatment duration associated with frequent side effects, adverse events, regimen changes, and new diagnoses of additional comorbidities that may alter MAC-PD management and overall survival.
Given the survival benefit of both 2007 and 2020 GBT observed in our analysis, it is also important to reflect on prior observations of many people with MAC-PD being prescribed non-GBT, suggesting a potential negative impact on a substantial proportion of this population. Despite ATS/IDSA guidelines being published in 2007, Adjemian et al. identified only 13% of people received GBT in the United States, with 30% receiving a regimen with increased risk for macrolide resistance [22]. An analysis of the Veterans Health Administration found only 41.9% of veterans with MAC-PD were prescribed GBT [41]. Similarly, an evaluation of Optum Clinformatics Data Mart identified only 49.7% of those treated were initiated on GBT [24]. In our cohort, nearly one quarter were prescribed a macrolide and a fluoroquinolone, a regimen associated with increased risk of macrolide resistance [17, 19, 21, 22]. The potential difference in mortality may be more impactful when you consider that those started on macrolide monotherapy are not represented in many of these non-GBT evaluations, including this one, that use a MAC antibiotic regimen prescription for inclusion because 2 or more drugs are required [21, 27, 41]. However, transition to macrolide monotherapy is common, putting people with MAC-PD at risk for macrolide resistance [2, 4, 17, 19–22, 41]. Given this, we suspect our aHRs for mortality with non-GBT regimens are likely underestimated. Based on these studies, we think in some settings, well over 50% of the population with MAC-PD are receiving non-GBT with potential for a higher risk of mortality 3 years after treatment initiation.
CONCLUSION
In a large population of Medicare beneficiaries with bronchiectasis and MAC-PD, we identified a lower aHR for 3-year mortality among those initiated on a GBT regimen compared to a non-GBT regimen for their initial MAC-PD treatment. Though impact of specific regimens on the long and winding road leading to mortality is challenging to evaluate in MAC-PD given the complexities and duration of therapy, variation in disease severity, frequent antibiotic changes, comorbidities, and indolent nature of this infection, this work provides further support for use of GBT in this population. Further research is needed to identify factors associated with mortality following MAC-PD antibiotic regimen initiation, including optimal duration of follow-up, impact of disease severity, and impact of subsequent antibiotic regimen changes over time.
Contributor Information
Cara D Varley, Division of Infectious Diseases, Department of Medicine, Oregon Health & Science University, Portland, Oregon, USA; Center for Infectious Disease Studies, Oregon Health & Science University-Portland State University School of Public Health, Portland, Oregon, USA.
Jennifer H Ku, Center for Infectious Disease Studies, Oregon Health & Science University-Portland State University School of Public Health, Portland, Oregon, USA; Department of Research & Evaluation, Kaiser Permanente Southern California Medical Group, Pasadena, California, USA.
Emily Henkle, Center for Infectious Disease Studies, Oregon Health & Science University-Portland State University School of Public Health, Portland, Oregon, USA.
Luke Strnad, Division of Infectious Diseases, Department of Medicine, Oregon Health & Science University, Portland, Oregon, USA; Center for Infectious Disease Studies, Oregon Health & Science University-Portland State University School of Public Health, Portland, Oregon, USA.
Kevin L Winthrop, Division of Infectious Diseases, Department of Medicine, Oregon Health & Science University, Portland, Oregon, USA; Center for Infectious Disease Studies, Oregon Health & Science University-Portland State University School of Public Health, Portland, Oregon, USA.
Notes
Acknowledgments. Lennon & McCartney for the title inspiration. Dr. Amber Streifel for her assistance with the intricacies of Medicare reimbursement.
Financial support . None.
References
- 1. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 2010;182:977–82. [DOI] [PubMed] [Google Scholar]
- 2. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020; 71:e1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falkinham JO 3rd. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 2009; 107:356–67. [DOI] [PubMed] [Google Scholar]
- 4. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 5. Boyle DP, Zembower TR, Qi C. Relapse versus reinfection of Mycobacterium avium complex pulmonary disease. Patient characteristics and macrolide susceptibility. Ann Am Thorac Soc 2016; 13:1956–61. [DOI] [PubMed] [Google Scholar]
- 6. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc 2015; 12:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner D, van Ingen J, Adjemian J, et al. Annual prevalence and treatment estimates of nontuberculous mycobacterial pulmonary disease in Europe: a NTM-NET collaborative study. Eur Respir J 2014; 44:P1067. [Google Scholar]
- 9. Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis 2016; 22:1116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis 2016; 16:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H, Myung W, Koh WJ, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg Infect Dis 2019; 25:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis 2018; 18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marras TK, Campitelli MA, Lu H, et al. Pulmonary nontuberculous mycobacteria-associated deaths, Ontario, Canada, 2001–2013. Emerg Infect Dis 2017; 23:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc 2014; 11:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Novosad SA, Henkle E, Schafer S, et al. Mortality after respiratory isolation of nontuberculous mycobacteria. A comparison of patients who did and did not meet disease criteria. Ann Am Thorac Soc 2017; 14:1112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006; 174:928–34. [DOI] [PubMed] [Google Scholar]
- 18. Griffith DE, Brown-Elliott BA, Shepherd S, McLarty J, Griffith L, Wallace RJ Jr. Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2005; 172:250–3. [DOI] [PubMed] [Google Scholar]
- 19. Grosset J, Ji B. Prevention of the selection of clarithromycin-resistant Mycobacterium avium-intracellulare complex. Drugs 1997; 54 Suppl 2:23–7; discussion 28–9. [DOI] [PubMed] [Google Scholar]
- 20. Morimoto K, Namkoong H, Hasegawa N, et al. Macrolide-resistant Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc 2016; 13:1904–11. [DOI] [PubMed] [Google Scholar]
- 21. Ku JH, Henkle E, Carlson KF, et al. Evaluation of Mycobacterium avium complex pulmonary disease treatment completion and adherence to ATS/IDSA guidelines. Clin Infect Dis 2022; 76:e1408–15. [DOI] [PubMed] [Google Scholar]
- 22. Adjemian J, Prevots DR, Gallagher J, Heap K, Gupta R, Griffith D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc 2014; 11:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukushima K, Kitada S, Komukai S, et al. First line treatment selection modifies disease course and long-term clinical outcomes in Mycobacterium avium complex pulmonary disease. Sci Rep 2021; 11:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marras TK, Mirsaeidi M, Vinnard C, et al. Guidelines-based treatment associated with improved economic outcomes in nontuberculous mycobacterial lung disease. J Med Econ 2019; 22:1126–33. [DOI] [PubMed] [Google Scholar]
- 25. van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017; 49:1601855. [DOI] [PubMed] [Google Scholar]
- 26. Brode SK, Chung H, Campitelli MA, et al. Prescribing patterns for treatment of Mycobacterium avium complex and M. xenopi pulmonary disease in Ontario, Canada, 2001–2013. Emerg Infect Dis 2019; 25:1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brode SK, Chung H, Campitelli MA, et al. The impact of different antibiotic treatment regimens on mortality in Mycobacterium avium complex pulmonary disease: a population-based cohort study. Eur Respir J 2020; 56:1901875. [DOI] [PubMed] [Google Scholar]
- 28. Ku JH, Henkle EM, Carlson KF, Marino M, Winthrop KL. Validity of diagnosis code-based claims to identify pulmonary NTM disease in bronchiectasis patients. Emerg Infect Dis 2021; 27:982–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winthrop KL, Baxter R, Liu L, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf 2011; 20:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winthrop K. ClinicalTrials.gov: Comparison of two- versus three-antibiotic therapy for pulmonary Mycobacterium avium complex disease (MAC2v3). Available at: https://clinicaltrials.gov/ct2/show/NCT03672630. Accessed 28 December 2020.
- 31. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10:37–48. [PubMed] [Google Scholar]
- 32. Jenkins PA, Campbell IA, Banks J, Gelder CM, Prescott RJ, Smith AP. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax 2008; 63:627–34. [DOI] [PubMed] [Google Scholar]
- 33. Im Y, Hwang NY, Kim K, Kim H, Kwon OJ, Jhun BW. Impact of time between diagnosis and treatment for nontuberculous mycobacterial pulmonary disease on culture conversion and all-cause mortality. Chest 2022; 161:1192–200. [DOI] [PubMed] [Google Scholar]
- 34. Kim JY, Park J, Choi Y, Kim TS, Kwak N, Yim JJ. Microbiological cure at treatment completion is associated with longer survival in patients with Mycobacterium avium complex pulmonary disease. Chest 2023;164:1108–14. [DOI] [PubMed] [Google Scholar]
- 35. Fleshner M, Olivier KN, Shaw PA, et al. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int J Tuberc Lung Dis 2016; 20:582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calado Nogueira de Moura V, Nguyen M-VH, Hunkins JJ, Daley CL, Khare R. In vitro susceptibility patterns for slowly growing non-tuberculous mycobacteria in the USA from 2018 to 2022. J Antimicrob Chemother 2023; 78:2849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kobashi Y, Matsushima T, Oka M. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med 2007; 101:130–8. [DOI] [PubMed] [Google Scholar]
- 38. Aznar ML, Marras TK, Elshal AS, Mehrabi M, Brode SK. Safety and effectiveness of low-dose amikacin in nontuberculous mycobacterial pulmonary disease treated in Toronto, Canada. BMC Pharmacol Toxicol 2019; 20:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Streifel AC, Sikka MK. The urgent need for Medicare reimbursement for home infusion antibiotics amidst a pandemic. Clin Infect Dis 2020; 71:3250–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group . Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc 2014; 11:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Varley CD, Smith J, Ku JH, Strnad LC. Factors associated with guideline-based therapy for mycobacterium avium complex pulmonary disease in rural and urban US veterans (abstract). Am J Respir Crit Care Med 2023; 207:A4247.