Abstract
Background
Surgery for giant pituitary adenomas (GPAs) poses significant challenges, particularly when the tumor cannot be fully addressed using either the endoscopic endonasal approach (EEA) or the microscopic transcranial approach (MTCA) alone.
Method
We utilized two combined approaches for complex GPAs: 1) expanded endoscopic endonasal approach (EEEA) combined with microscopic pterional approach (MPA), and 2) EEEA combined with endoscopic transventricular approach (ETVA).
Conclusion
The combined approach is an effective surgical technique for complex GPAs. The EEEA-MPA approach is suitable for GPAs with superior and lateral extension, while the EEEA-ETVA approach is beneficial for tumors with lateral ventricular invasion.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00701-024-06371-w.
Keywords: Giant pituitary adenoma, Microscopic pterional approach, Expanded endoscopic endonasal approach, Endoscopic transventricular approach, Combined approach
Relevant surgical anatomy
The endoscopic endonasal approach (EEA) has become widely accepted for treating pituitary adenomas (PAs) [4, 5]. However, for lesions extending to the suprasellar region, parasellar areas, retrosellar space, and clival regions, its effectiveness is limited by poor visibility through a narrow corridor. The expanded endoscopic endonasal approach (EEEA) offers clear exposure to deep-seated supradiaphragmatic, retrosellar, and clival lesions, which are challenging to reach via traditional transcranial approaches (TCA) [6, 8], which typically use pterional and interhemispheric approaches, remain crucial for managing PAs with extensive extrasellar invasion but a nearly normal-sized sella turcica. However, it may limit visualization of the intrasellar region and the anterior third ventricle, often requiring excessive bone removal and brain retraction. For complex giant PAs, an I-stage combined approach should be considered to achieve safe gross total resection (GTR) [1, 2, 10].
Description of the technique
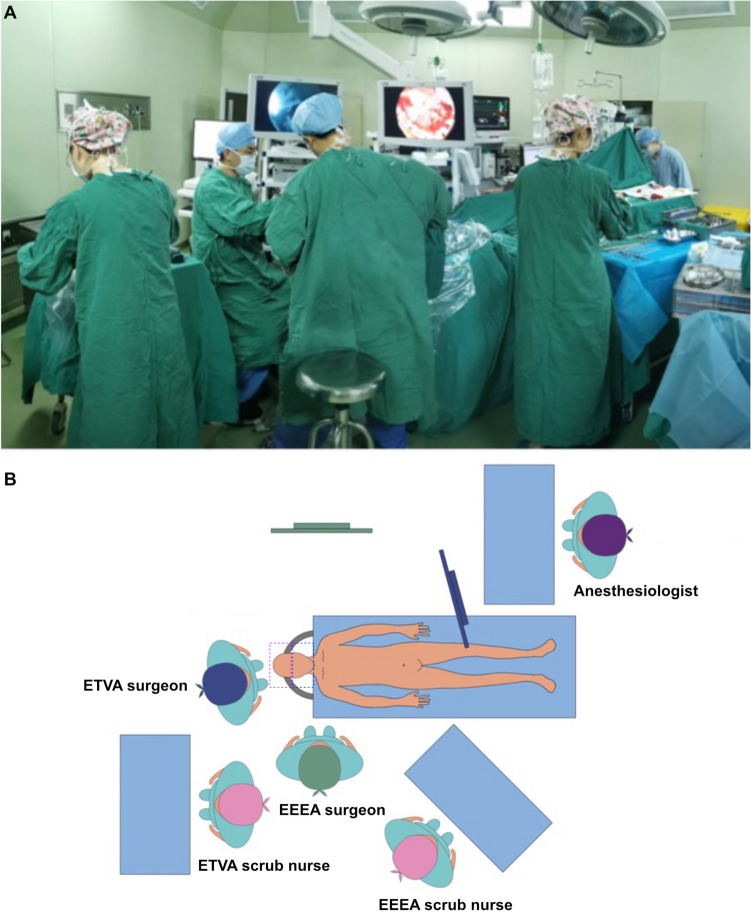
The patient was placed in the supine position with the head fixed using a Mayfield clamp. The fascia lata donor site (the right thigh) was prepared for cranial base reconstruction. Since the surgical wound in the MPA or ETVA is classified as Class I, and that in the EEEA is classified as Class II, each phase had separate surgical teams, tables, endoscopic systems, and surgical instruments to avoid possible contamination (Fig. 1A and B).
Fig. 1.
A: Actual photograph of the operating room setup during an EEEA-ETVA combined approach surgery; B: Schematic diagram of the operating room setup for the EEEA-ETVA combined approach: The operating room setup for the EEEA-ETVA procedure includes two operative fields, two neurosurgical teams, and two neuroendoscopic systems, each with its own monitor set up around the patient. The EEEA and ETVA are performed simultaneously within a shared surgical field without interference between the teams. The purple dashed box represents the range of the Class I surgical wound, while the blue box indicates the range of the Class II surgical wound.
The procedure began with the endonasal stage, up until the sellar dura was opened. The sellar dura area was then packed with iodine-soaked pledgets. The transcranial team subsequently performed the craniotomy, exposing the dura in the corresponding area but keeping it intact. The endonasal team then opened the sellar dura and resected the tumor from below. Once the lower compartment was mostly resected, the transcranial team opened the upper dura and continued resection from above. Starting with the endonasal stage cuts off most of the tumor’s blood supply, reducing bleeding risk during the transcranial stage. Furthermore, exposing the transcranial dura first allows for immediate intervention in the event of unexpected bleeding.
EEEA
The middle turbinate was removed from one side, and the nasoseptal flap was mobilized using blunt dissection. The mucosal side of the vascularized septal flap was then pushed further into the nasopharynx. After identifying the sphenoid ostia, an extended sphenoidotomy was performed, removing substantial parts of the anterior wall and septum of the sphenoid sinus, which is crucial in EEEA to obtain sufficient exposure. The bone covering the sella turcica was removed and the opening was extended laterally to the cavernous sinus and anteriorly to the sphenoidal planum. Adequate exposure of the deep lateral side of the cavernous sinus was achieved by exposing the bilateral pterygoid processes, particularly the pterygopalatine fossa. After removing the sellar floor and opening the bone covering the anterior walls of the bilateral cavernous sinuses, the tumor around the cavernous sinus segments of the bilateral internal carotid arteries (ICA) was resected. Dissection continued along the interface between the tumor and the pituitary gland and the tumor tissue involving the upper compartment was further resected through the roof wall of the cavernous sinus lateral to the ICA.
Combined MPA
Since the inferior part of the tumor had already been removed in the EEEA phase, dissection between the tumor and attached structures was easier because the superior compartment of the tumor had descended. The roof of the cavernous sinus was opened longitudinally, and the surrounding brain parenchyma was dissected away from the tumor. The resection continued in the cavernous sinus until the hemostatic gelatin packing from the nasal cavity was observed. The tumor was carefully dissected from the arteries, including the middle cerebral artery (MCA) and its branches (Fig. 2, Video 1).
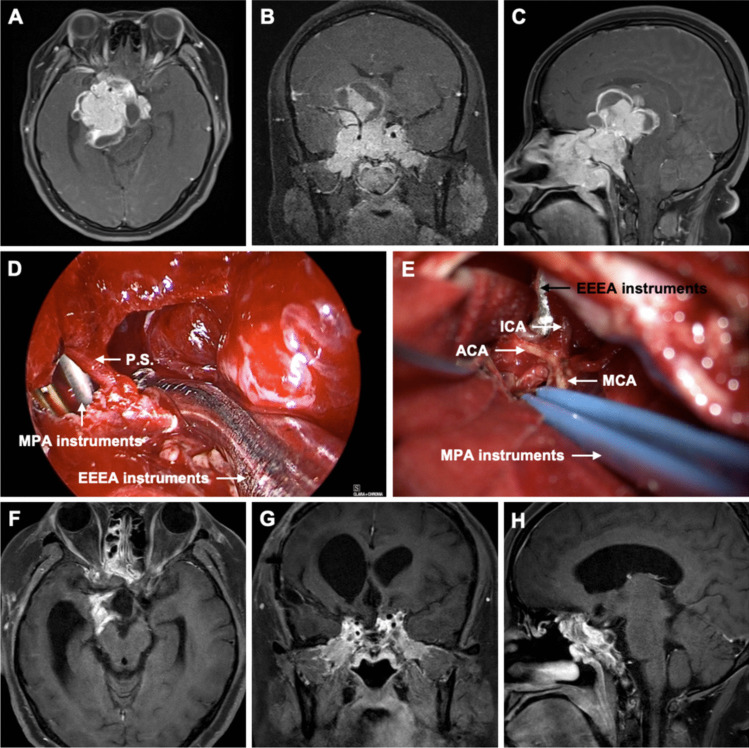
Fig. 2.
Preoperative axial (A), coronal (B), and sagittal (C) gadolinium-enhanced T1-weighted MR images showed an irregular mass in the middle cranial fossa measuring 7.3 × 5.9 × 5.8 cm. Superiorly, it protruded into the suprasellar cistern. Laterally, it invaded the bilateral cavernous sinuses, encasing the C2-C7 segments of the bilateral internal carotid arteries and the M1 segment of the right middle cerebral artery. Anteriorly, it invaded the bilateral optic canals, superior orbital fissure, ethmoid sinus, sphenoid sinus, and nasopharynx. Posteriorly, it protruded into the interpeduncular cistern and pontine cistern, compressing the right pons and right cerebral peduncle. D Intraoperative image from the EEEA, showing the tumor debulking delivered through the transcranial route. E Intraoperative image from the MPA, showing the internal carotid artery (ICA), anterior cerebral artery (ACA), and middle cerebral artery (MCA). Postoperative axial (F), coronal (G), and sagittal (H) gadolinium-enhanced T1-weighted MR images obtained 3 months after surgery showed gross total resection (GTR) of the tumor. P.S.: pituitary stalk, EEEA: expanded endoscopic endonasal approach, MPA: microscopic pterional approach
The role of the transcranial team was mainly supportive: they dissected the upper part of the tumor away from blood vessels, manipulated the diaphragm and tumor capsule inferiorly, and pushed the lesion down so that the EEEA team could resect the tumor safely, directly, and completely.
Combined ETVA
The side of the ETVA depended on the predominant side of tumor extension. A circular bone flap approximately 3 cm in diameter in the frontal skin was made after exposing the skull and dura. The middle frontal gyrus cortex was identified and excised. The Endoport was then gradually inserted into the lateral ventricle until the tumor tissue was visible. After ensuring adequate CSF drainage, tumor resection was performed endoscopically. The suprasellar part of the tumor was dissected from adjacent critical neurovascular structures by visual monitoring from the EEEA side (Fig. 3, Video 2). Subsequently, intratumoral decompression was performed, and the adhesions between the tumor and ventricle wall were dissected.
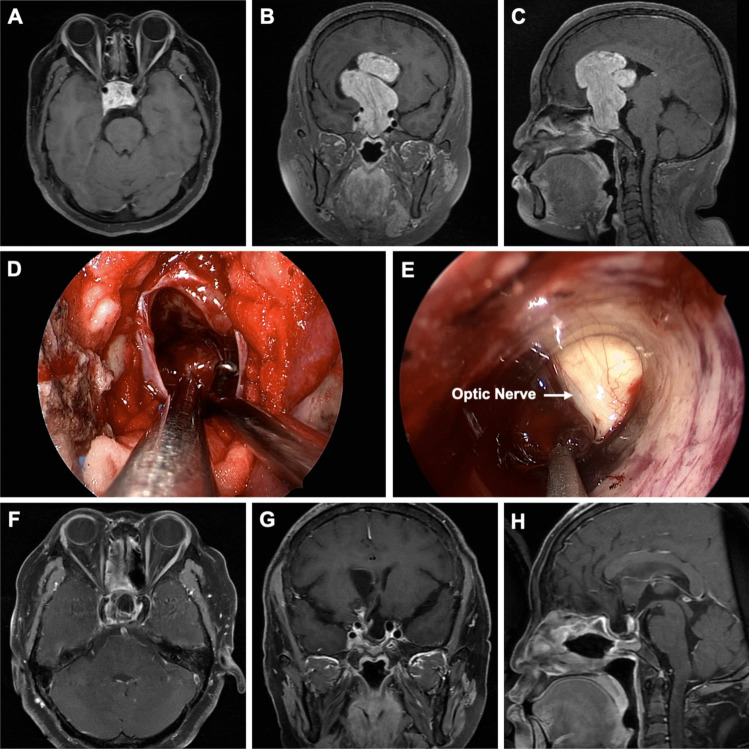
Fig. 3.
Preoperative axial gadolinium-enhanced T1-weighted (A), coronal (B), and sagittal (C) MR images revealed a giant mass measuring 7.0 × 5.4 × 4.1 cm. The lesion protruded upward into the suprasellar region and extended into the lateral ventricle. The right frontal lobe was compressed, and the lesion was located close to the bilateral cavernous sinuses. D Intraoperative image from the EEEA side. E Intraoperative image from the ETVA side. Postoperative axial gadolinium-enhanced T1-weighted (F), coronal (G), and sagittal (H) MR images obtained 3 months after surgery showed gross total resection (GTR) of the tumor
Collaboration between surgical teams
During both stages, each team resected the tumor in their assigned area while providing support to the other as needed. In the EEEA-MPA approach, the transcranial team carefully dissected tumor tissue from critical blood vessels and gently pushed it downward, allowing for safer resection by the EEEA team. After resection, the endonasal view was essential for assessing the integrity of ventral structures which were not fully visible transcranially.
Reconstruction
Tumor resection was confirmed by superior and inferior views. Saline irrigation fluid was directed from the intracranial side toward the nasal cavity to wash the surgical corridor. Skull base reconstruction was performed using an artificial dura mater and pedicled nasoseptal flap. In this stage, both the transcranial and endonasal closures are performed simultaneously, reducing overall surgery time and infection risk.
Early postoperative management
Early anesthesia recovery allows prompt assessment of vision and visual fields. Monitoring for intracranial infection is essential, including regular checks of temperature, meningeal signs, and blood infection markers, with lumbar puncture promptly performed if infection is suspected. A loading dose of hormone replacement therapy is given immediately post-op, then gradually tapered to physiological levels. For patients with ventricular involvement, multiple follow-up CT scans are performed early post-op to confirm the absence of hydrocephalus.
Indications
The main part of the tumor is located above the tuberculum sellar;
Extends upward, compressing the third ventricle floor, hypothalamus, or lateral ventricles; anteriorly to the sphenoidal planum; posteriorly to the interpeduncular cistern; laterally beyond cavernous sinus cranial nerves, compressing the temporal lobe. Particular consideration should be given to cases with lateral extension when the tumor cannot be accessed by standard transnasal approach.
The tumor has a multilobulated irregular shape, encasing critical vessels and nerves.
Limitations
The combined approach surgery requires additional equipment and surgical team, and its complexity also contributes to longer operative times. Its widespread adoption has been limited by skepticism about safety and effectiveness [3, 7, 9]. Moreover, differing wound classifications from the combined approach increase the risk of infection, and complications from both EEEA and TCA may arise simultaneously.
How to avoid complications
An organized operating room layout enhances surgical efficiency and safety. Separate instruments, scrub nurses, endoscopic systems, and surgical materials are essential to prevent cross-contamination. Mastery of each individual approach is crucial for surgical success, and proficiency in executing each technique independently is vital for optimal outcomes.
Specific information for the patient
Patients should be informed that the combined approach may result in longer surgery times, a higher risk of postoperative infection, increased surgical costs, and the possibility of requiring immediate postoperative care in the Neuro Intensive Care Unit (NICU).
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1 The general steps of the combined endoscopic endonasal approach - microscopic pterional approach procedure (EEEA-MPA) include the resection of the middle turbinate, the creation of a pedicled nasoseptal flap in the nasal cavity, exposure of the bilateral pterygopalatine fossae (especially the right side), resection of the tumor in the sphenoid sinus, ethmoid sinus, and sellar region, and removal of the tumor in the cavernous sinus area. The MPA mainly involves the dissection of the adhesions between the tumor and the internal carotid artery and its branches, decompression of the bilateral optic nerves, and coordination with the EEEA to remove the residual tumor tissue (MP4 160021 KB)
Video 2 The general steps of the combined endoscopic endonasal approach - endoscopic transventricular approach (EEEA-ETVA) procedure include the resection of the middle turbinate, the creation of a pedicled nasoseptal flap, the removal of the sphenoid sinus bone to expose the sellar floor, and the opening of the sellar dura to resect the tumor. The ETVA primarily involves endoscopic intratumoral decompression, the dissection of adhesions between the tumor and the ventricular wall, the removal of the suprasellar tumor tissue, and the decompression of the optic nerves (MP4 152796 KB)
Authors' contributions
Ao Shen wrote the main manuscript, contributed to the acquisition and analysis of data, and prepared Figs. 1–3 and Videos 1–2. Dongjie Zhou contributed to the acquisition and analysis of data and prepared Figs. 2–3. Yue Min contributed to the acquisition and analysis of data and critically revised the manuscript for important intellectual content. Liang Lyu critically revised the manuscript for important intellectual content. Peizhi Zhou made substantial contributions to the conception and design of the work and prepared Videos 1–2. All authors reviewed and approved the manuscript.
Funding
This study was funded by Foundation of Science and Technology Department of Sichuan Province, No. 2024YFFK0058.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, approved the ethical review of the study (No.453).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. The article explores the use of two combined approaches, EEEA-MPA and EEEA-ETVA, for treating complex giant pituitary adenomas (GPAs), providing insights into their effectiveness depending on tumor extension and invasion.
2. The indications for combined approach surgery need to take into account the tumor’s maximum size, the location of the main part of the tumor, the tumor’s shape, its anterior, posterior, and lateral extensions, as well as its encasement of surrounding blood vessels and nerves.
3. To avoid cross-contamination, separate surgical instruments, scrub nurses, and endoscopic systems must be used for each phase of the combined approach, given the different classifications of surgical wounds (Class I for MPA/ETVA and Class II for EEEA).
4. EEEA is particularly useful for accessing deep-seated suprasellar, parasellar, retrosellar, and clival lesions.
5. In the combined EEEA-MPA approach, the transcranial team assists by manipulating and dissecting tumor tissue away from critical blood vessels and pushing the tumor down to allow for safer resection by the EEEA team.
6. In cases where the tumor extends into the lateral ventricles, the ETVA provides direct access, allowing endoscopic tumor resection. Combined EEEA-ETVA is more useful in GPAs that invade the lateral ventricle.
7. Continuous neuro-electrophysiological monitoring during surgery aids in the identification and preservation of cranial nerves, such as the oculomotor nerve, reducing the risk of postoperative deficits.
8. The combined approach increases surgery complexity and risks, such as longer surgical times, higher infection risks, and the need for postoperative care in the NICU, informing both surgeons and patients.
9. Although the combined approach has advantages, mastering each individual approach is crucial for surgical success. Proficiency in executing each technique independently is essential for optimal outcomes.
10. Choosing the type of combined approach based on the tumor’s extension is crucial. An appropriate simultaneous combined approach can decrease the risk of and bleeding from the residual tumor.
References
- 1.Elshazly K, Kshettry VR, Farrell CJ, Nyquist G, Rosen M, Evans JJ (2018) Clinical outcomes after endoscopic endonasal resection of giant pituitary adenomas. World Neurosurg 114:e447–e456 [DOI] [PubMed] [Google Scholar]
- 2.Gaillard S, Adeniran S, Villa C et al (2022) Outcome of giant pituitary tumors requiring surgery. Front Endocrinol (Lausanne) 13:975560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han S, Gao W, Jing Z, Wang Y, Wu A (2017) How to deal with giant pituitary adenomas: transsphenoidal or transcranial, simultaneous or two-staged? J Neurooncol 132(2):313–321 [DOI] [PubMed] [Google Scholar]
- 4.Makarenko S, Alzahrani I, Karsy M, Deopujari C, Couldwell WT (2022) Outcomes and surgical nuances in management of giant pituitary adenomas: a review of 108 cases in the endoscopic era. J Neurosurg 137(3):635–646 [DOI] [PubMed] [Google Scholar]
- 5.Micko A, Agam MS, Brunswick A, Strickland BA, Rutkowski MJ, Carmichael JD, Shiroishi MS, Zada G, Knosp E, Wolfsberger S (2022) Treatment strategies for giant pituitary adenomas in the era of endoscopic transsphenoidal surgery: a multicenter series. J Neurosurg 136(3):776–785 [DOI] [PubMed] [Google Scholar]
- 6.Özer Mİ, Kutlay AM, Durmaz MO et al (2020) Extended endonasal endoscopic approach for anterior midline skull base lesions. Clin Neurol Neurosurg 196:106024 [DOI] [PubMed] [Google Scholar]
- 7.Qiao N, Gao W, Deng X et al (2024) Combined simultaneous transsphenoidal and transcranial regimen improves surgical outcomes in complex giant pituitary adenomas: a longitudinal retrospective cohort study. Int J Surg 110(7):4043–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silveira-Bertazzo G, Manjila S, Carrau RL, Prevedello DM (2020) Expanded endoscopic endonasal approach for extending suprasellar and third ventricular lesions. Acta Neurochir (Wien) 162(10):2403–2408 [DOI] [PubMed] [Google Scholar]
- 9.Tosaka M, Shimizu T, Miyagishima T, Tanaka Y, Osawa T, Aihara M, Yamaguchi R, Yoshimoto Y (2019) Combined supra-infrasellar approach to pituitary macroadenoma with oculomotor cistern extension: surgical strategy and experience. Acta Neurochir (Wien) 161(5):1025–1031 [DOI] [PubMed] [Google Scholar]
- 10.Zada G, Du R, Laws ER (2011) Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J Neurosurg 114(2):286–300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 The general steps of the combined endoscopic endonasal approach - microscopic pterional approach procedure (EEEA-MPA) include the resection of the middle turbinate, the creation of a pedicled nasoseptal flap in the nasal cavity, exposure of the bilateral pterygopalatine fossae (especially the right side), resection of the tumor in the sphenoid sinus, ethmoid sinus, and sellar region, and removal of the tumor in the cavernous sinus area. The MPA mainly involves the dissection of the adhesions between the tumor and the internal carotid artery and its branches, decompression of the bilateral optic nerves, and coordination with the EEEA to remove the residual tumor tissue (MP4 160021 KB)
Video 2 The general steps of the combined endoscopic endonasal approach - endoscopic transventricular approach (EEEA-ETVA) procedure include the resection of the middle turbinate, the creation of a pedicled nasoseptal flap, the removal of the sphenoid sinus bone to expose the sellar floor, and the opening of the sellar dura to resect the tumor. The ETVA primarily involves endoscopic intratumoral decompression, the dissection of adhesions between the tumor and the ventricular wall, the removal of the suprasellar tumor tissue, and the decompression of the optic nerves (MP4 152796 KB)
Data Availability Statement
No datasets were generated or analysed during the current study.