Abstract
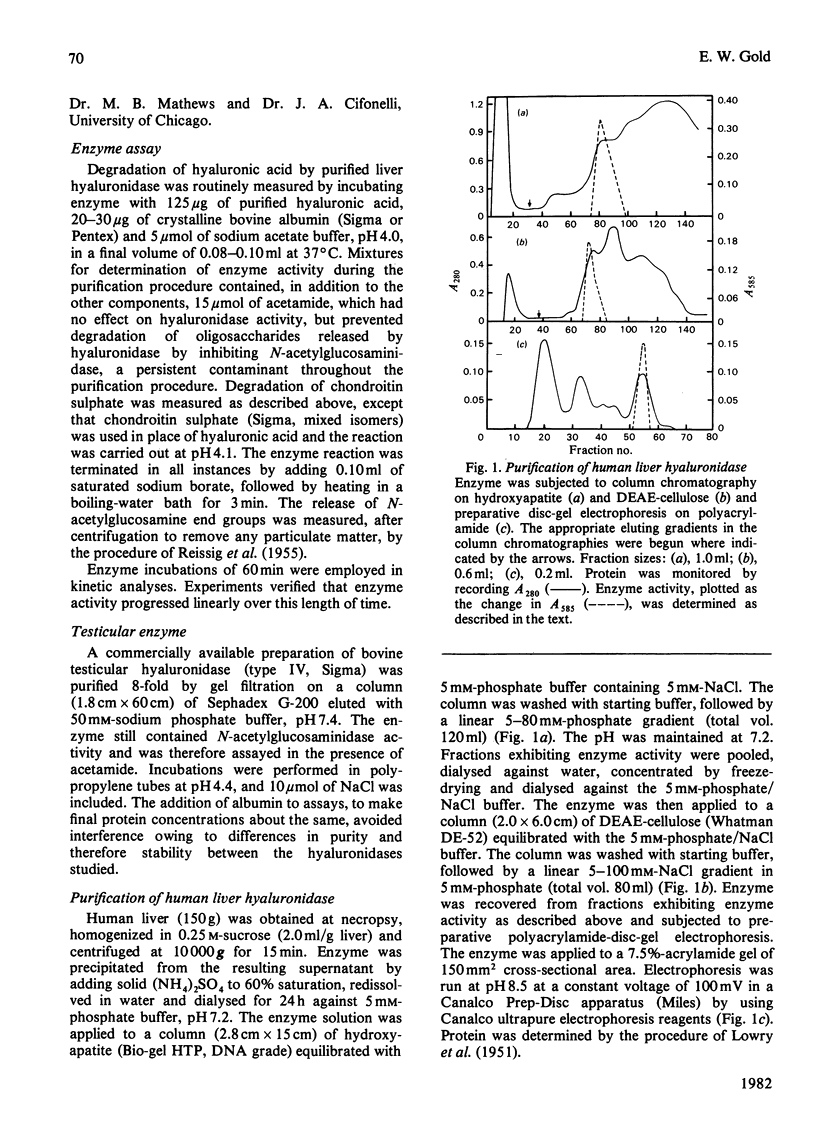
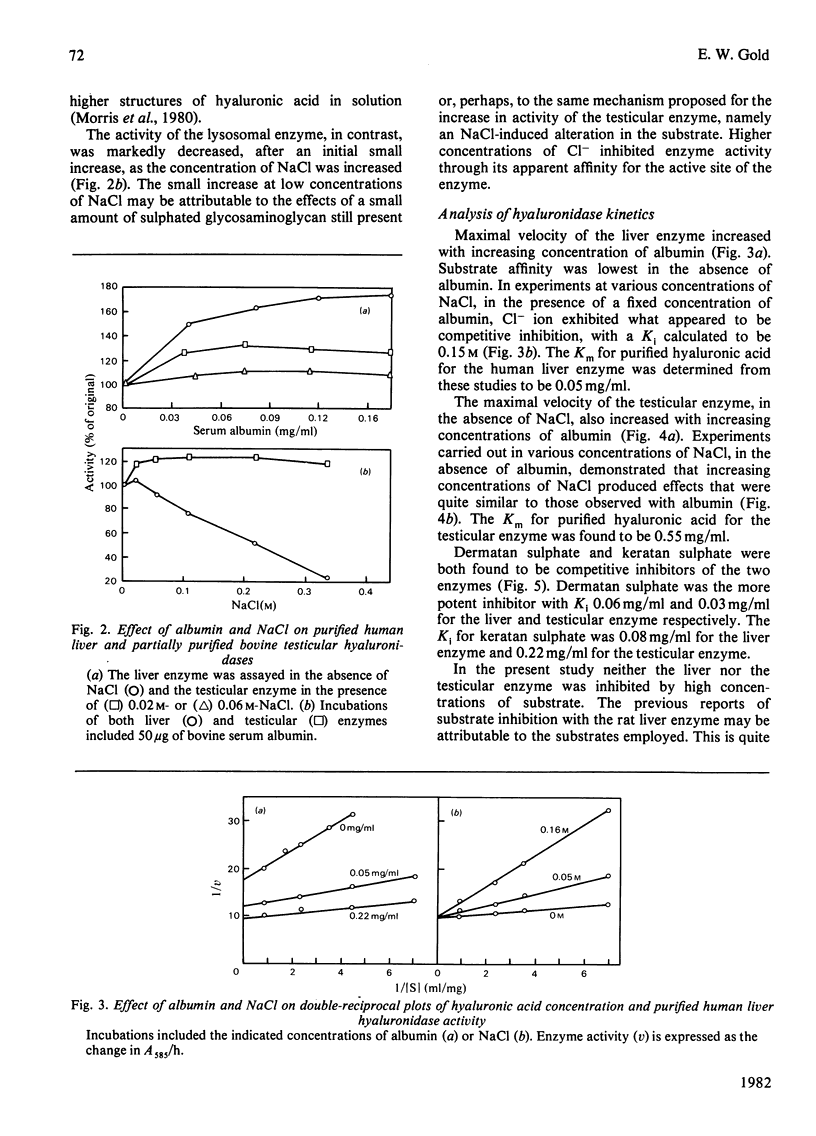
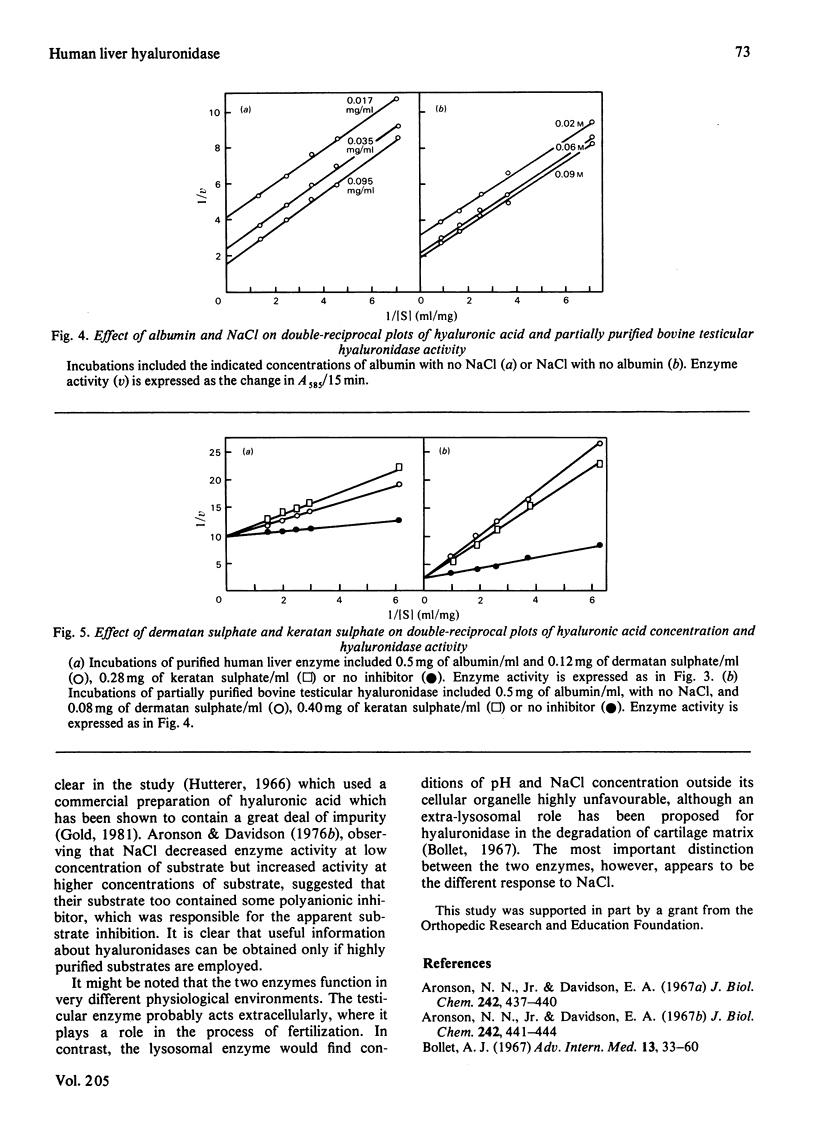
Human liver hyaluronidase was purified to homogeneity by (NH4)2SO4 fractionation, chromatography on hydroxyapatite and DEAE-cellulose, and preparative disc polyacrylamide-gel electrophoresis. The enzyme had a pH optimum of 3.8-4.0, a molecular weight (determined by gel filtration) of 76000, and a Km of 0.05 mg/ml for purified human umbilical-cord hyaluronic acid. It generally resembled hyaluronidases studied in other tissues which are believed to be lysosomal, but shared a number of characteristics with a partially purified bovine testicular hyaluronidase. Neither enzyme exhibited inhibition by high concentrations of substrate, but both were competitively inhibited by dermatan sulphate and keratan sulphate. Both enzymes exhibited increased activity in the presence of albumin, probably owing to an increased susceptibility of substrate to enzyme action. The liver enzyme was inhibited by NaCl, but the testicular enzyme exhibited an increase in activity in the presence of the salt which was similar to the effect observed with albumin. The different response toward Cl- ion appeared to be the most significant difference between the two enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Davidson E. A. Lysosomal hyaluronidase from rat liver. I. Preparation. J Biol Chem. 1967 Feb 10;242(3):437–440. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Davidson E. A. Lysosomal hyaluronidase from rat liver. II. Properties. J Biol Chem. 1967 Feb 10;242(3):441–444. [PubMed] [Google Scholar]
- BOLLET A. J., BONNER W. M., Jr, NANCE J. L. THE PRESENCE OF HYALURONIDASE IN VARIOUS MAMMALIAN TISSUES. J Biol Chem. 1963 Nov;238:3522–3527. [PubMed] [Google Scholar]
- Bollet A. J. Connective tissue polysaccharide metabolism and the pathogenesis of osteoarthritis. Adv Intern Med. 1967;13:33–60. [PubMed] [Google Scholar]
- Gold E. W. The quantitative spectrophotometric estimation of total sulfated glycosaminoglycan levels. Formation of soluble alcian blue complexes. Biochim Biophys Acta. 1981 Apr 3;673(4):408–415. doi: 10.1016/0304-4165(81)90472-4. [DOI] [PubMed] [Google Scholar]
- Hutterer F. Degradation of mucopolysaccharides by hepatic lysosomes. Biochim Biophys Acta. 1966 Feb 28;115(2):312–319. doi: 10.1016/0304-4165(66)90430-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morris E. R., Rees D. A., Welsh E. J. Conformation and dynamic interactions in hyaluronate solutions. J Mol Biol. 1980 Apr;138(2):383–400. doi: 10.1016/0022-2836(80)90294-6. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Stephens R. W., Ghosh P., Taylor T. K. The characterisation and function of the polysaccharidases of human synovial fluid in rheumatoid and osteoarthritis. Biochim Biophys Acta. 1975 Jul 14;399(1):101–112. doi: 10.1016/0304-4165(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Welsh E. J., Rees D. A., Morris E. R., Madden J. K. Competitive inhibition evidence for specific intermolecular interactions in hyaluronate solutions. J Mol Biol. 1980 Apr;138(2):375–382. doi: 10.1016/0022-2836(80)90293-4. [DOI] [PubMed] [Google Scholar]
- Yamada M., Hasegawa E., Kanamori M. Purification of hyaluronidase from human placenta. J Biochem. 1977 Feb;81(2):485–494. doi: 10.1093/oxfordjournals.jbchem.a131482. [DOI] [PubMed] [Google Scholar]


