Abstract
1. Ox liver glutamate dehydrogenase is activated by bovine pancreatic alpha-chymotrypsin, but the extent of activation is dependent on the age of the dehydrogenase preparation. 2. The degree of activation is constant and the pseudo-first-order rate constant of activation is directly proportional to the concentration of proteinase used. 3. Commercial preparations of alpha-chymotrypsin differ in their ability to produce a secondary inactivation phase, and this was shown to be due to low tryptic contamination. The 'superactive' form of glutamate dehydrogenase has an increased sensitivity to tryptic inactivation as compared with the native enzyme. 4. Analysis of the activation by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis revealed that the subunit molecular weight of 'superactive' glutamate dehydrogenase differs by less than 5% from that of the native subunit.
Full text
PDF



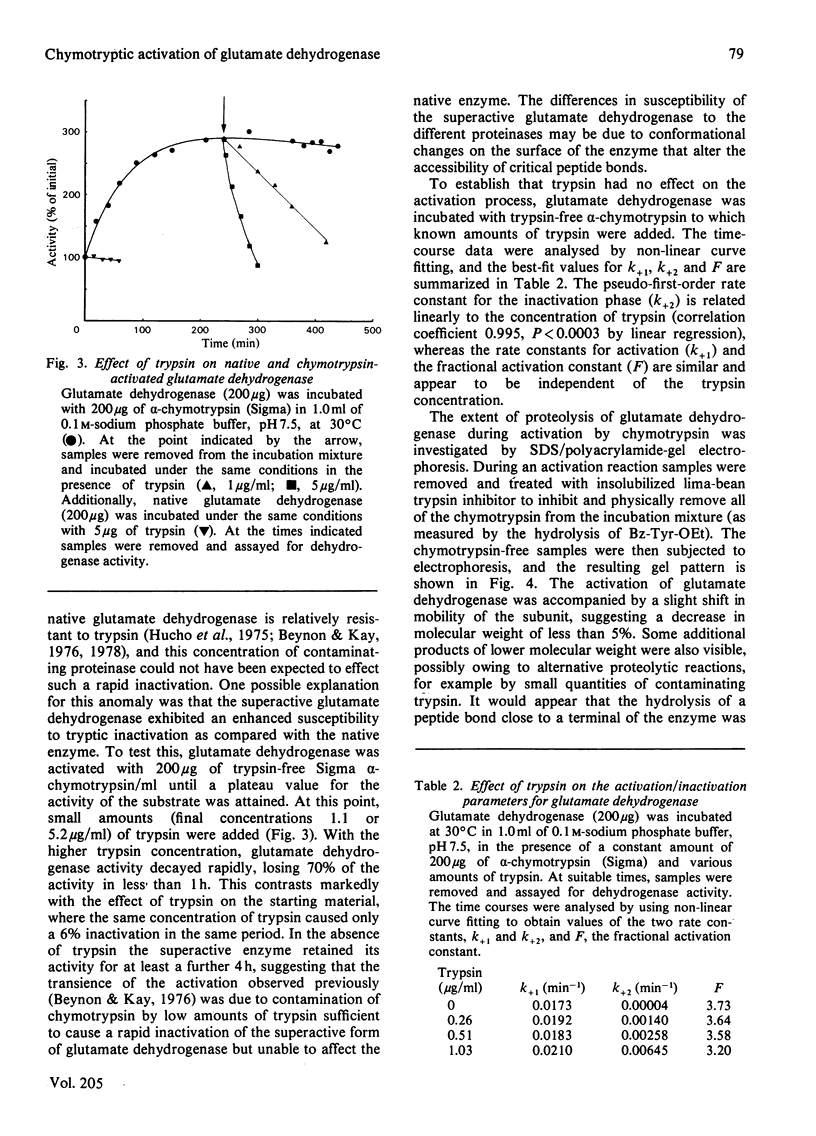

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison M. J., Engel P. C. The effects of digestion by bovine pancreas alpha-chymotrypsin on native bovine glutamate dehydrogenase. Biochem Soc Trans. 1980 Oct;8(5):649–649. doi: 10.1042/bst0080649. [DOI] [PubMed] [Google Scholar]
- Anderson W. B., Jaworski C. J., Vlahakis G. Proteolytic activation of adenylate cyclase from cultured fibroblasts. J Biol Chem. 1978 May 10;253(9):2921–2926. [PubMed] [Google Scholar]
- Anfinsen C. B., Scheraga H. A. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205–300. doi: 10.1016/s0065-3233(08)60413-1. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Kay J. The inactivation of native enzymes by a neutral proteinase from rat intestinal muscle. Biochem J. 1978 Jul 1;173(1):291–298. doi: 10.1042/bj1730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N. Conformational adaptability in enzymes. Adv Enzymol Relat Areas Mol Biol. 1973;37:397–648. doi: 10.1002/9780470122822.ch7. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Hucho F., Rasched I., Sund H. Studies of glutamate dehydrogenase: analysis of functional areas and functional groups. Eur J Biochem. 1975 Mar 17;52(2):221–230. doi: 10.1111/j.1432-1033.1975.tb03990.x. [DOI] [PubMed] [Google Scholar]
- Marshak D. R., Neer E. J. The site of alpha-chymotryptic activation of pigeon erythrocyte adenylate cyclase. J Biol Chem. 1980 May 25;255(10):4781–4785. [PubMed] [Google Scholar]
- McCarthy A. D., Johnson P., Tipton K. F. Sedimentation properties of native and proteolysed preparations of ox glutamate dehydrogenase. Biochem J. 1981 Oct 1;199(1):235–238. doi: 10.1042/bj1990235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A. D., Walker J. M., Tipton K. F. Purification of glutamate dehydrogenase from ox brain and liver. Evidence that commercially available preparations of the enzyme from ox liver have suffered proteolytic cleavage. Biochem J. 1980 Nov 1;191(2):605–611. doi: 10.1042/bj1910605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K., Tokushige M. Trypsin-catalyzed activation of aspartase. Biochem Biophys Res Commun. 1975 Nov 17;67(2):741–746. doi: 10.1016/0006-291x(75)90875-x. [DOI] [PubMed] [Google Scholar]
- Otsuka A. S., Price P. A. Removal of proteases from DNase I by chromatography over agarose with covalently attached lima bean protease inhibitor. Anal Biochem. 1974 Nov;62(1):180–187. doi: 10.1016/0003-2697(74)90379-0. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., De Flora A., Horecker B. L. Conversion of neutral to alkaline liver fructose 1,6-bisphosphatase: changes in molecular properties of the enzyme. Proc Natl Acad Sci U S A. 1973 Mar;70(3):661–664. doi: 10.1073/pnas.70.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Hager L. P., Gennis R. B. Characterization of the proteolytic activation of pyruvate oxidase. Control by specific ligands and by the flavin oxidation-reduction state. J Biol Chem. 1977 Nov 10;252(21):7877–7882. [PubMed] [Google Scholar]
- Russell P., Schrock H. L., Gennis R. B. Lipid activation and protease activation of pyruvate oxidase. Evidence suggesting a common site of interaction on the protein. J Biol Chem. 1977 Nov 10;252(21):7883–7887. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Traniello S., Melloni E., Pontremoli S., Sia C. L., Horecker R. L. Rabbit liver fructose 1,6-diphosphatase. Properties of the native enzyme and their modification by subtilisin. Arch Biochem Biophys. 1972 Mar;149(1):222–231. doi: 10.1016/0003-9861(72)90317-7. [DOI] [PubMed] [Google Scholar]



