Abstract
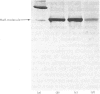
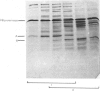
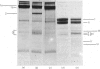
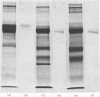
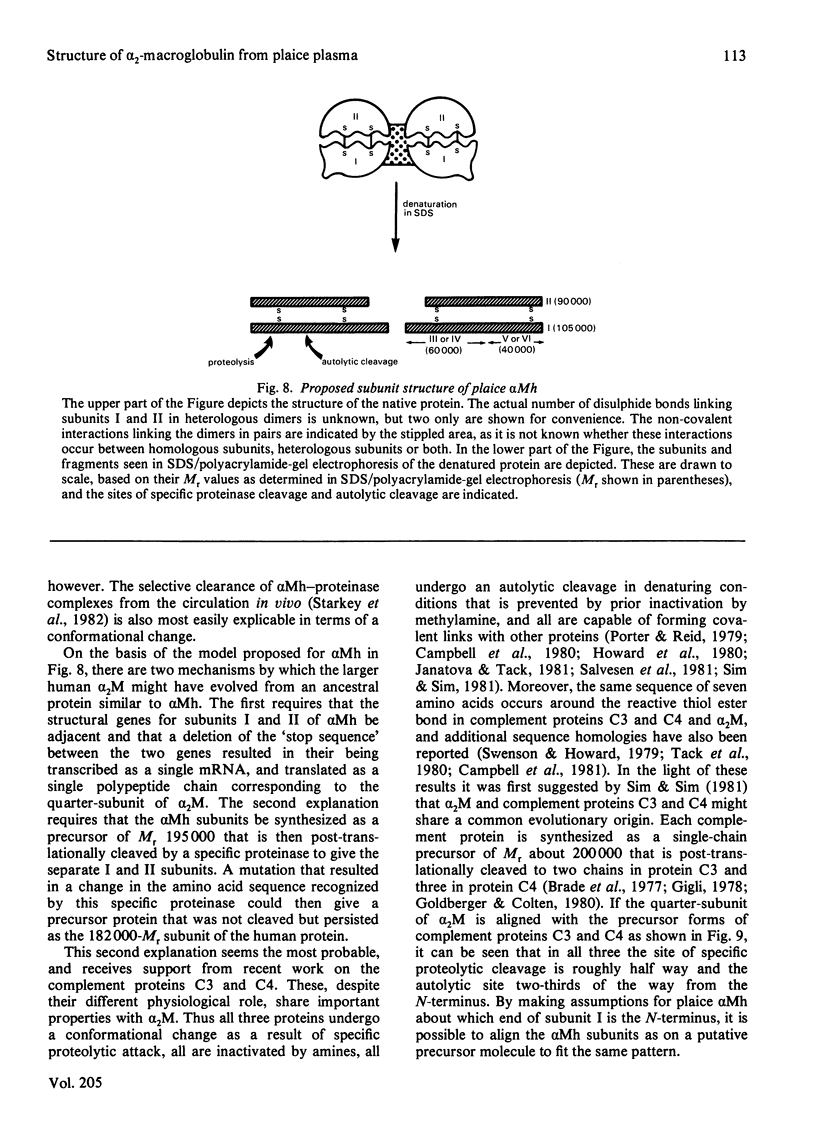
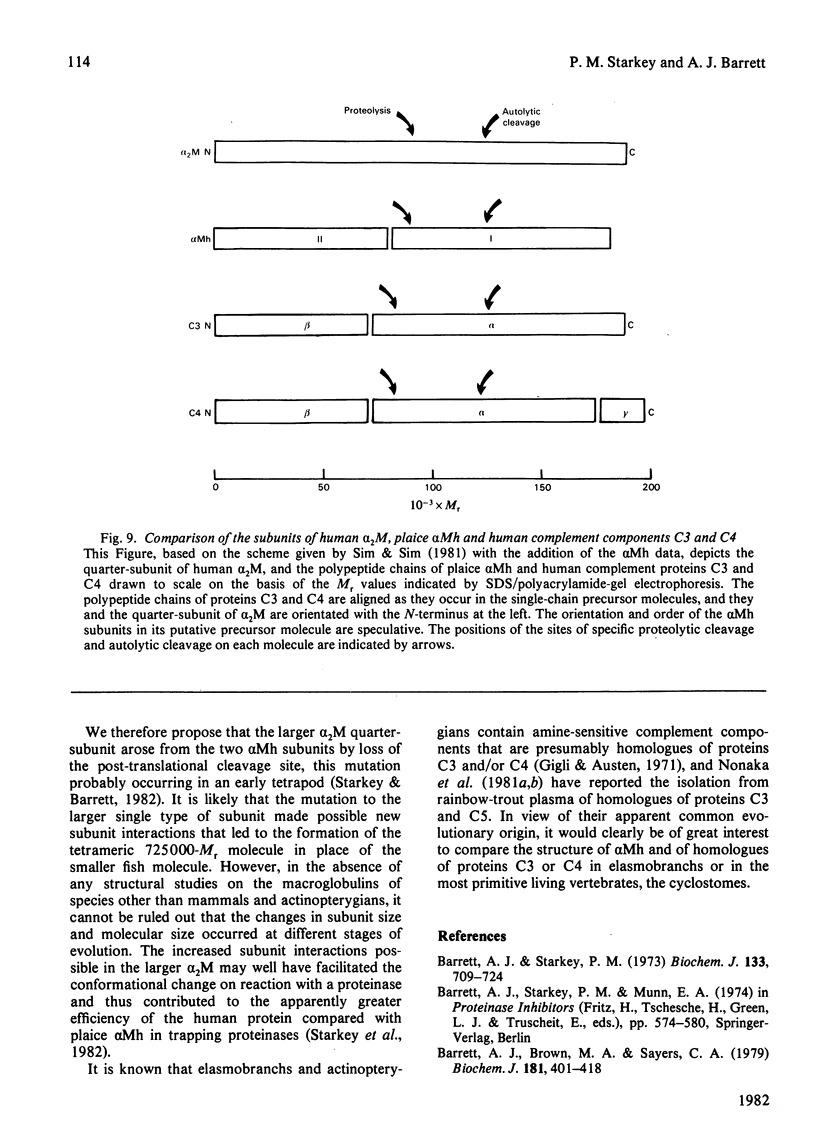
The plaice (Pleuronectes platessa L.) papain-binding protein previously demonstrated to be homologous with human α2-macroglobulin, and designated plaice α2-macroglobulin homologue or αMh, was shown to be a glycoprotein of s20,w 11.86S. In polyacrylamide-gel pore-limit electrophoresis under non-denaturing conditions plaice αMh migrated to the same position as half-molecules of human α2-macroglobulin, and treatment with methylamine or a proteinase caused no change in its electrophoretic properties. Either denaturation in urea (4m) or mild reduction by dithiothreitol (1mm) partially dissociated plaice αMh into half-molecules. Denaturation with reduction further dissociated the protein into quarter-subunits. In sodium dodecyl sulphate/polyacrylamide-gel electrophoresis under reducing conditions plaice αMh dissociated into subunits of Mr 105000 (I) and 90000 (II). Approximately equal amounts of each subunit were formed, and peptide `mapping' showed subunits I and II to be distinct polypeptide chains. Under alkaline denaturing conditions, a proportion of the I chains of αMh were cleaved into fragments of Mr about 60000 and 40000. This cleavage was favoured by reducing conditions and prevented by prior inactivation of the αMh with methylamine. [14C]Methylamine allowed to react with αMh became covalently linked to subunit I. These properties suggested the existence of an autolytic site on subunit I analogous to the autolytic site of human α2-macroglobulin. Reaction of αMh with a proteinase resulted in cleavage of a fragment of Mr 10000–15000 from subunit I. A proportion of the proteinase molecules trapped by αMh became covalently linked to the inhibitor. A scheme is proposed for the evolution of human α2-macroglobulin and plaice αMh from a common ancestral protein, which may also have been an ancestor of complement components C3 and C4.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade V., Hall R. E., Colten H. R. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977 Sep 1;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Gagnon J., Porter R. R. Amino acid sequence around the proposed thiolester bond of human complement component C4 and comparison with the corresponding sequences from C3 and alpha 2-macroglobulin. Biosci Rep. 1981 May;1(5):423–429. doi: 10.1007/BF01116192. [DOI] [PubMed] [Google Scholar]
- Clamp J. R. A gas-liquid chromatographic approach to the analysis of carbohydrates. Biochem Soc Trans. 1977;5(6):1693–1695. doi: 10.1042/bst0051693. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gigli I. A single chain precursor of C4 in human serum. Nature. 1978 Apr 27;272(5656):836–837. doi: 10.1038/272836a0. [DOI] [PubMed] [Google Scholar]
- Gigli I., Austen K. F. Phylogeny and function of the complement system. Annu Rev Microbiol. 1971;25:309–332. doi: 10.1146/annurev.mi.25.100171.001521. [DOI] [PubMed] [Google Scholar]
- Goldberger G., Colten H. R. Precursor complement protein (pro-C4) is converted in vitro to native C4 by plasmin. Nature. 1980 Jul 31;286(5772):514–516. doi: 10.1038/286514a0. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Hayes M. B., Hugli T. E. Heat-induced fragmentation of human alpha 2-macroglobulin. J Biol Chem. 1979 Sep 10;254(17):8669–8678. [PubMed] [Google Scholar]
- Howard J. B., Vermeulen M., Swenson R. P. The temperature-sensitive bond in human alpha 2-macroglobulin is the alkylamine-reactive site. J Biol Chem. 1980 May 10;255(9):3820–3823. [PubMed] [Google Scholar]
- Janatova J., Tack B. F. Fourth component of human complement: studies of an amine-sensitive site comprised of a thiol component. Biochemistry. 1981 Apr 28;20(9):2394–2402. doi: 10.1021/bi00512a005. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Barrett A. J. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980 Jun 1;187(3):695–701. doi: 10.1042/bj1870695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G. S., Sayers C. A., Barrett A. J. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J. 1981 May 1;195(2):453–461. doi: 10.1042/bj1950453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Sim E. Autolytic fragmentation of complement components C3 and C4 under denaturing conditions, a property shared with alpha 2-macroglobulin. Biochem J. 1981 Jan 1;193(1):129–141. doi: 10.1042/bj1930129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Evolution of alpha 2-macroglobulin. The demonstration in a variety of vertebrate species of a protein resembling human alpha 2-macroglobulin. Biochem J. 1982 Jul 1;205(1):91–95. doi: 10.1042/bj2050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Fletcher T. C., Barrett A. J. Evolution of alpha 2-macroglobulin. The purification and characterization of a protein homologous with human alpha 2-macroglobulin from plaice (Pleuronectes platessa L.) plasma. Biochem J. 1982 Jul 1;205(1):97–104. doi: 10.1042/bj2050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]