Abstract
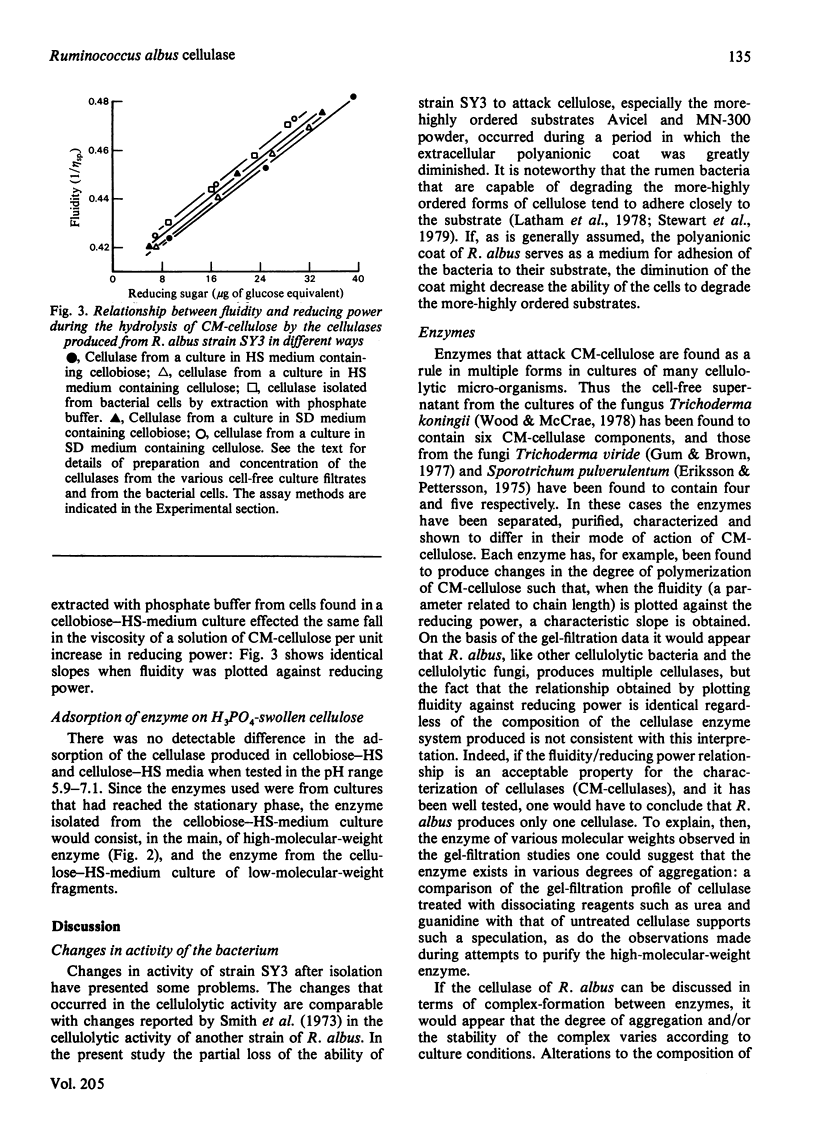
1. Most of the cellulase (CM-cellulase) elaborated by the rumen bacterium Ruminococcus albus strain SY3, which was isolated from a sheep, was cell-wall-bound. 2. The enzyme could be released readily by washing either with phosphate buffer or with water. 3. The amount of enzyme released was affected by the pH and ionic strength of the phosphate buffer. 4. The cell-wall-bound enzyme was of very high molecular weight (»1.5×106) as judged by its chromatographic behaviour on Sephacryl S-300. 5. The molecular weight of the extracellular enzyme was variable and depended on the culture conditions. 6. When cellobiose was used as the energy source and the medium contained rumen fluid (30%), the extracellular enzyme was, in the main, of high molecular weight. 7. When cellulose replaced the cellobiose, the cell-free culture filtrate contained only low-molecular-weight enzyme (Mr approx. 30000) in late-stationary-phase cultures (7 days). 8. Cultures that did not contain rumen fluid contained mainly low-molecular-weight enzyme. 9. Under some conditions the high-molecular-weight enzyme could be broken down to some extent into low-molecular-weight enzyme by treatment with dissociating agents. 10. Cell-free and cell-wall-bound enzymes showed the same relationship when the change in fluidity effected by them on a solution of CM-cellulose was plotted against the corresponding increase in reducing sugars, suggesting that the enzymes were the same. 11. It is possible that R. albus cellulase exists as an aggregate of low-molecular-weight cellulase components on the bacterial cell wall and in solution under certain conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bucht B., Eriksson K. E. Extracellular enzyme system utilized by the rot fungus Stereum sanguinolentum for the breakdown of cellulose. I. Studies on the enzyme production. Arch Biochem Biophys. 1968 Mar 20;124(1):135–141. doi: 10.1016/0003-9861(68)90312-3. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Morris E. J., Bacon J. S. Electron microscopy of the microbial populations present and their modes of attack on various cellulosic substrates undergoing digestion in the sheep rumen. Appl Environ Microbiol. 1978 Jul;36(1):160–168. doi: 10.1128/aem.36.1.160-168.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Gum E. K., Jr, Brown R. D., Jr Comparison of four purified extracellular 1,4-beta-D-glucan cellobiohydrolase enzymes from Trichoderma viride. Biochim Biophys Acta. 1977 May 27;492(1):225–231. doi: 10.1016/0005-2795(77)90229-x. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. Cellulase from Fusarium solani: purification and properties of the C1 component. Carbohydr Res. 1977 Aug;57:117–133. doi: 10.1016/s0008-6215(00)81925-4. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I., Macfarlane C. C. The isolation, purification and properties of the cellobiohydrolase component of Penicillium funiculosum cellulase. Biochem J. 1980 Jul 1;189(1):51–65. doi: 10.1042/bj1890051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The cellulase of Trichoderma koningii. Purification and properties of some endoglucanase components with special reference to their action on cellulose when acting alone and in synergism with the cellobiohydrolase. Biochem J. 1978 Apr 1;171(1):61–72. doi: 10.1042/bj1710061. [DOI] [PMC free article] [PubMed] [Google Scholar]