Abstract
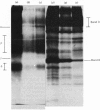
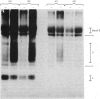
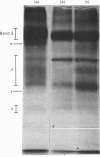
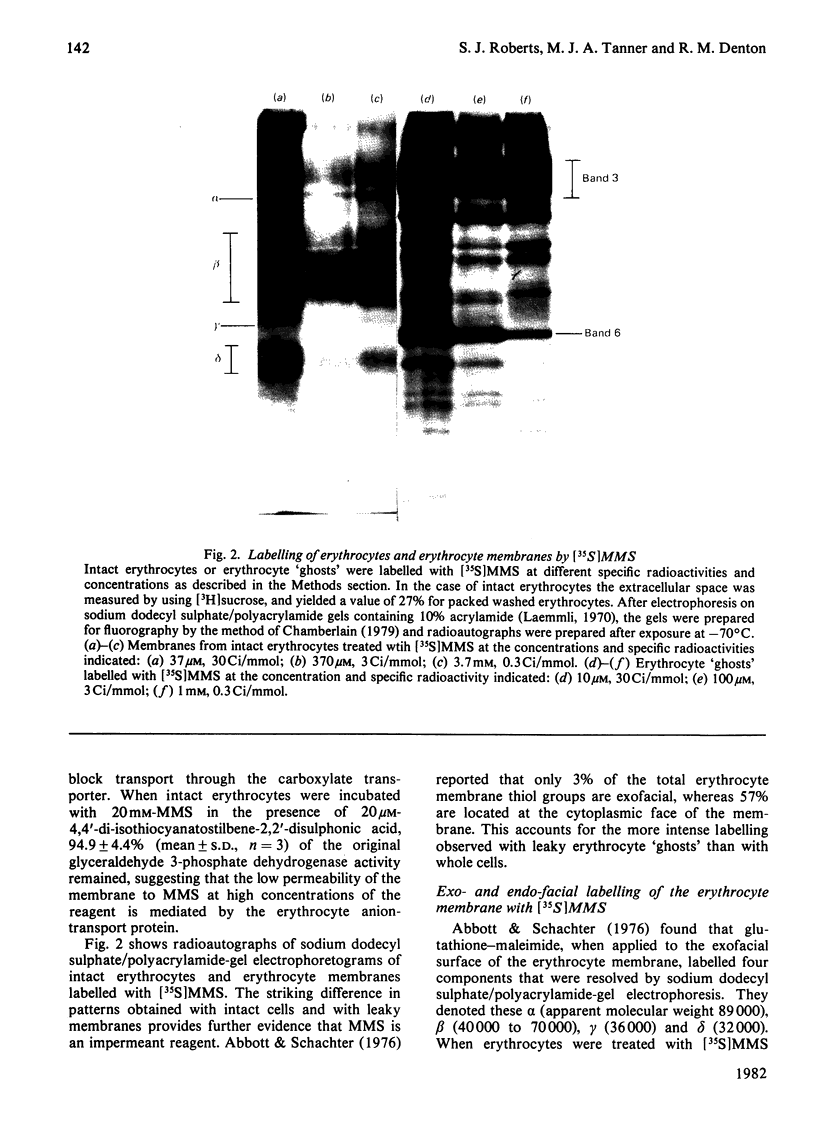
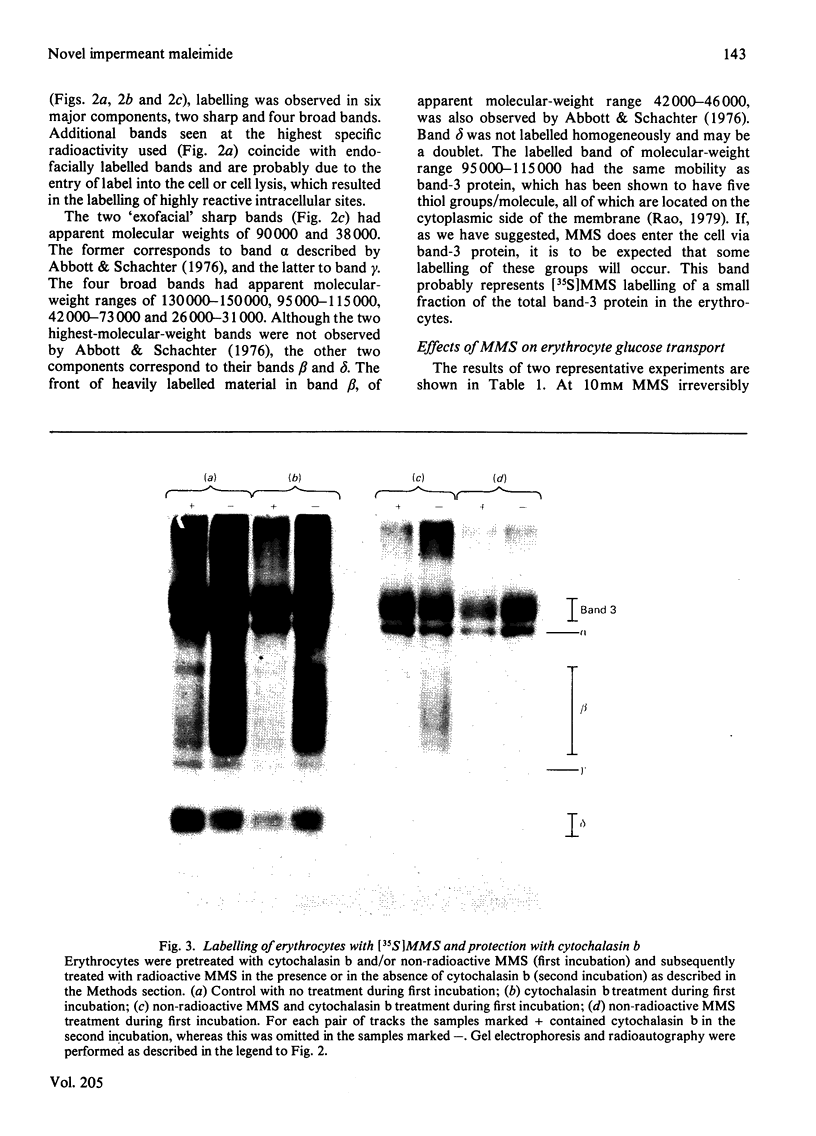
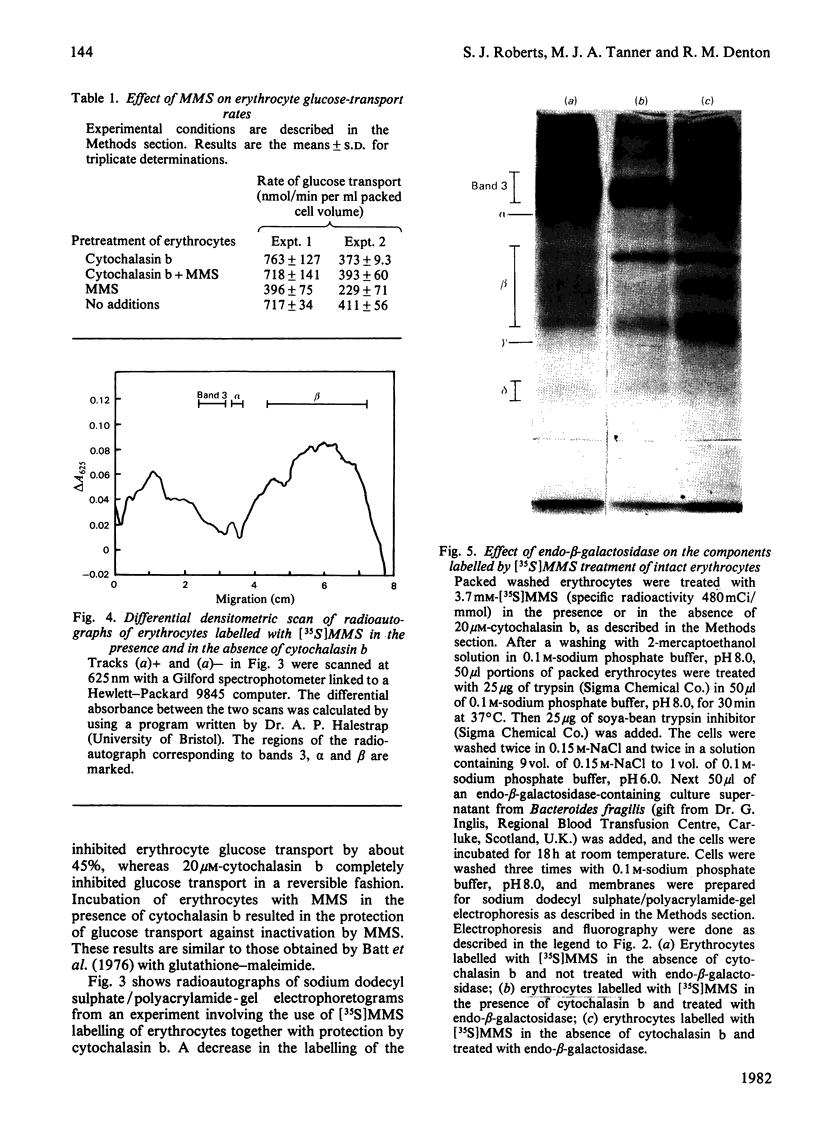
1. The synthesis of N-maleoylmethionine sulphone (MMS), a membrane-impermeant protein-labelling reagent, is described. Radioactively labelled MMS can be readily prepared at high specific radioactivity from [35S]methionine. 2. The permeability of the erythrocyte membrane to the reagent was assessed by determining the extent of inactivation of glyceraldehyde 3-phosphate dehydrogenase after treatment of erythrocytes with MMS. Some inactivation of this enzyme was found when high concentrations (20mM) of the compound were used, but this could be prevented by pretreatment of the erythrocytes with 4,4'-di-isothiocyanatostilbene-2,2'-disulphonic acid, suggesting that MMS slowly enters the cells via the anion-transport system. 3. Treatment of erythrocytes with [35S]MMS resulted in the labelling of six major components. Labelling of erythrocyte membranes resulted in the intense labelling of many additional components. 4. MMS inhibited erythrocyte glucose transport. Cytochalasin b protected glucose transport against inactivation by MMS. Labelling experiments in erythrocytes in the presence and in the absence of cytochalasin b showed that the cytochalasin b-protected material was a broad band in the band-4.5 region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. E., Schachter D. Impermeant maleimides. Oriented probes of erythrocyte membrane proteins. J Biol Chem. 1976 Nov 25;251(22):7176–7183. [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Gorga F. R., Lienhard G. E. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim Biophys Acta. 1979 Mar 23;552(1):183–188. doi: 10.1016/0005-2736(79)90257-8. [DOI] [PubMed] [Google Scholar]
- Batt E. R., Abbott R. E., Schachter D. Impermeant maleimides. Identification of an exofacial component of the human erythrocyte hexose transport mechanism. J Biol Chem. 1976 Nov 25;251(22):7184–7190. [PubMed] [Google Scholar]
- Boxer D. H., Jenkins R. E., Tanner M. J. The organization of the major protein of the human erythrocyte membrane. Biochem J. 1974 Mar;137(3):531–534. doi: 10.1042/bj1370531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Smith A. E. Biosynthesis of 35 S-L-methionine of very high specific activity. Anal Biochem. 1972 May;47(1):310–312. doi: 10.1016/0003-2697(72)90308-9. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. II. Effects of proteolytic enzymes on disulfonic stilbene sites of surface proteins. J Membr Biol. 1974;15(3):227–248. doi: 10.1007/BF01870089. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg A., Zala C. A. Reconstitution of D-glucose transport in vesicles composed of lipids and intrinsic protein (zone 4.5) of the human erythrocyte membrane. J Supramol Struct. 1977;7(3-4):287–300. doi: 10.1002/jss.400070303. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M., Stein W. D. The kinetic parameters of the monosaccharide transfer system of the human erythrocyte. Biochim Biophys Acta. 1966 Sep 26;127(1):179–193. doi: 10.1016/0304-4165(66)90488-0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Gorga F. R., Orasky J. E., Jr, Zoccoli M. A. Monosaccharide transport system of the human erythrocyte. Identification of the cytochalasin B binding component. Biochemistry. 1977 Nov 1;16(22):4921–4926. doi: 10.1021/bi00641a028. [DOI] [PubMed] [Google Scholar]
- Mueller T. J., Li Y. T., Morrison M. Effect of endo-beta-galactosidase on intact human erythrocytes. J Biol Chem. 1979 Sep 10;254(17):8103–8106. [PubMed] [Google Scholar]
- Mullins R. E., Langdon R. G. Maltosyl isothiocyanate: an affinity label for the glucose transporter of the human erythrocyte membrane. 2. Identification of the transporter. Biochemistry. 1980 Mar 18;19(6):1205–1212. doi: 10.1021/bi00547a026. [DOI] [PubMed] [Google Scholar]
- Rao A. Disposition of the band 3 polypeptide in the human erythrocyte membrane. The reactive sulfhydryl groups. J Biol Chem. 1979 May 10;254(9):3503–3511. [PubMed] [Google Scholar]
- Rich D. H., Gesellchen P. D., Tong D., Cheung A., Buckner C. K. Alkylating derivatives of amino acids and peptides. Synthesis of N-maleoylamino acids, [1-(N-maleoylglycyl)cysteinyl]oxytocin. Effects on vasopressin-stimulated water loss from isolated toad bladder. J Med Chem. 1975 Oct;18(10):1004–1010. doi: 10.1021/jm00244a011. [DOI] [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Immunological identification of the human erythrocyte glucose transporter. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5725–5729. doi: 10.1073/pnas.77.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]