Abstract
Aryl thiols have proven to be a useful class of electron donors and hydrogen atom sources in photochemical processes. However, the direct activation and functionalization of C(sp2)–S bonds in aryl thiols remains elusive in the field of photochemistry. Herein, a photochemical carboxylation of C(sp2)–S bonds in aryl thiols with CO2 is reported, providing a synthetic route to important aryl carboxylic acids. Moreover, different kinds of aryl thiol derivatives, benzeneselenol and diphenyl diselenide also show moderate-to-high reactivity in this transformation. Mechanistic studies, including DFT calculations, suggest that the in situ generated carbon dioxide radical anion (CO2•−) and disulfide might be the key intermediates, which undergo radical substitution to yield products. This reaction features mild and catalyst-free conditions, good functional group tolerance and wide substrate scope. Furthermore, the efficient degradation of polyphenylene sulfide highlights the usefulness of this methodology.
Subject terms: Synthetic chemistry methodology, Photochemistry
The photochemical transformations of aryl thiols to other functional groups have been scarcely explored. Here the authors present a carboxylation of aryl thiols using 1 atmosphere of CO2 under photoirradiative conditions, a methodology which can be extended to the degradation of polyphenylene sulfide.
Introduction
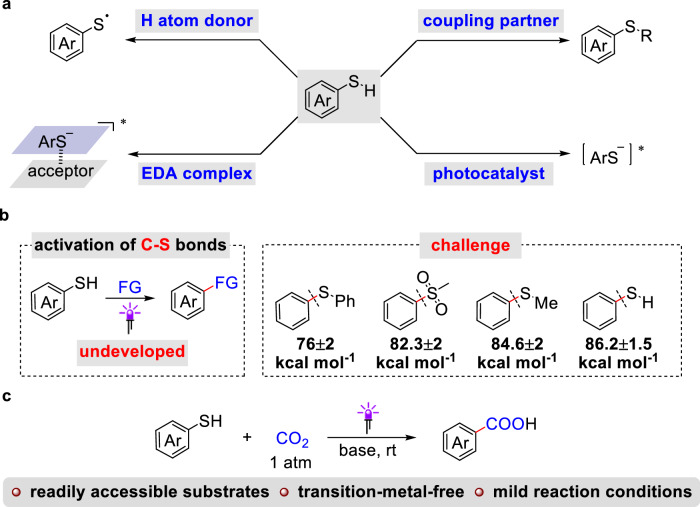
Photochemistry has experienced significant renaissance over past decades and enabled transformations that are inconceivable or even impossible by alternative means1–11. The importance of light-induced processes is also reflected in diverse fields such as biology, material science, and medicine. It is preferable for reagents used in photochemistry to be cost-effective, function well under mild reaction conditions, and possess unique properties suitable for various types of reactions. In this context, aryl thiols have recently proven to be powerful reagents with multidimensional features that can serve various types of reactions in different ways (Fig. 1a)12–16. Firstly, aryl thiols are well-known to serve as polarity reversal catalysts in the hydrogen atom transfer (HAT) process, especially in photoredox/HAT synergistic catalysis17–20. Meanwhile, aryl thiols and corresponding thiolates can readily quench the excited photocatalyst to generate thiyl radicals, which are employed for the construction of organosulfur compounds in addition to function as HAT reagents21,22. Moreover, the aryl thiolates can form the electron-donor-acceptor (EDA) complexes with substrates bearing the appropriate π acceptor (e.g., aryl, vinyl)23–26, or act directly as a photocatalyst for inert bond activation27,28. However, to the best of our knowledge, the photochemical activation and functionalization of C(sp2)−S bonds in aryl thiols have not been reported yet.
Fig. 1. Photochemical strategies for transformations of aryl thiols. FG = functional group.
a Recent advances of aryl thiols in photochemical organic synthesis. b The challenge of the photo-induced functionalization of C−S bonds in aryl thiols. c Photo-induced carboxylation of C−S bonds in aryl thiols with CO2.
In the pursuit of producing cleaner fuels, continuous efforts have been directed towards the elimination of sulfur from petroleum fractions by cleaving the carbon–sulfur (C–S) bond in organosulfur compounds29–31. Meanwhile, organosulfur compounds have been emerging as the surrogate for conventional organo halides in organic synthesis. In particular, transformations of the C(sp2)–S bonds in thiophenol derivatives, including thioethers, sulfoxides, sulfones, and sulfonium salts, are of particular interest and offer a plethora of promising synthetic methods for the construction of C–C and C–heteroatom bonds via transition metal catalysis32–37, photocatalysis38–41 or electrochemistry42. So far, the direct activation and functionalization of C(sp2)–S bonds in aryl thiols are still elusive (Fig. 1b), with only a few cases reported under harsh reaction conditions or using stoichiometric metal reagents43–45. This is due to both the inherent strong C(sp2)−S bonds in thiophenol (BDE = 86 kcal mol−1)46 and corresponding thiolates compared to thiophenol derivatives, as well as the catalyst-deactivating properties of both thiolates and sulfide leaving groups that are formed after the cleavage of C−S bonds47.
Given our ongoing interest in the activation and utilization of carbon dioxide (CO2)48–59, we wondered if it was possible that aryl thiolates could directly activate CO2 under photochemical conditions and the carbon dioxide radical anion (CO2•−)24,26,60–63, produced through single electron transfer (SET) reduction, was expected to achieve a direct carboxylation of C(sp2)–S bonds. However, this transformation is fraught with challenges. Although CO2 is an abundant, non-toxic, and renewable C1 source, its thermodynamic and kinetic stability make the coupling of these two relatively inert units difficult49–57. Furthermore, carboxylation of C(sp2)–S bonds in aryl thiols with CO2 has not been reported, and the mechanistic studies in this field are blank. In addition, competing side reactions, such as Kolbe-Schmitt type reactions64 and hydrodesulfurization44, are possible. Herein, we present a photo-induced carboxylation of C(sp2)–S bonds in aryl thiols with CO2 (Fig. 1c). A variety of aryl thiols and derivatives undergo this carboxylation reaction smoothly in the absence of transition metals.
Results
Screening of reaction conditions
In our previous research on thiolate-catalyzed carboxylation of C(sp2)–H bonds with CO226, we investigated the impact of various wavelengths of light on the reaction. During further exploration, we observed the production of aryl carboxylic acids as byproducts derived from aryl thiols under CO2 atmosphere. Based on the preliminary result, 4-tert-butylthiophenol 1a was employed as the model substrate, reacting with one atmospheric pressure of CO2 under 30 W 365 nm light-emitting diodes (LEDs) irradiation at room temperature (rt) (Table 1). After an extensive investigation of the reaction conditions, the desired carboxylation product 2a’ was obtained with 82% yield in the presence of tBuOK as the base and dimethyl sulfoxide (DMSO) as the solvent (entry 1). Control experiments revealed that the light source, base, and CO2 all played vital roles in this transformation (entries 2-4). Decreasing the reaction time and reducing the quantity of alkali both resulted in lower yields (entries 5-6). Other bases and solvents led to lower yields (entries 7-12). Light sources with other wavelengths gave poor results (entries 13-15).
Table. 1.
Optimization of reaction conditionsa
| Entry | Variations | Yield of 2a’ (%)b |
|---|---|---|
| 1 | none | 82% (84%)c |
| 2 | w/o light | n.d. |
| 3 | w/o tBuOK | n.d. |
| 4 | w/o CO2 | n.d. |
| 5 | tBuOK (2.0 eq.) | 73% |
| 6 | 6 h instead of 12 h | 71% |
| 7 | tBuONa instead of tBuOK | 65% |
| 8 | tBuOLi instead of tBuOK | 68% |
| 9 | Cs2CO3 instead of tBuOK | 58% |
| 10 | NMP instead of DMSO | 66% |
| 11 | DMF instead of DMSO | 61% |
| 12 | DMAc instead of DMSO | 69% |
| 13 | 450 nm instead of 365 nm | trace |
| 14 | 415 nm instead of 365 nm | 24% |
| 15 | 395 nm instead of 365 nm | 25% |
aConditions: 1a (0.2 mmol), tBuOK (3.5 eq.), DMSO (2.0 mL), 1 atm of CO2, 365 nm LEDs, rt, 12 h, then CH3I (5.0 eq.), 65 ˚C, 3 h. bDetermined by GC with dodecane as the internal standard. cIsolated yield of corresponding carboxylic acid 2a. n.d. = not detected. DMF = N,N-dimethylformamide. DMAc = N,N-dimethylacetamide. NMP = N-methyl-2-pyrrolidone.
Substrate scope
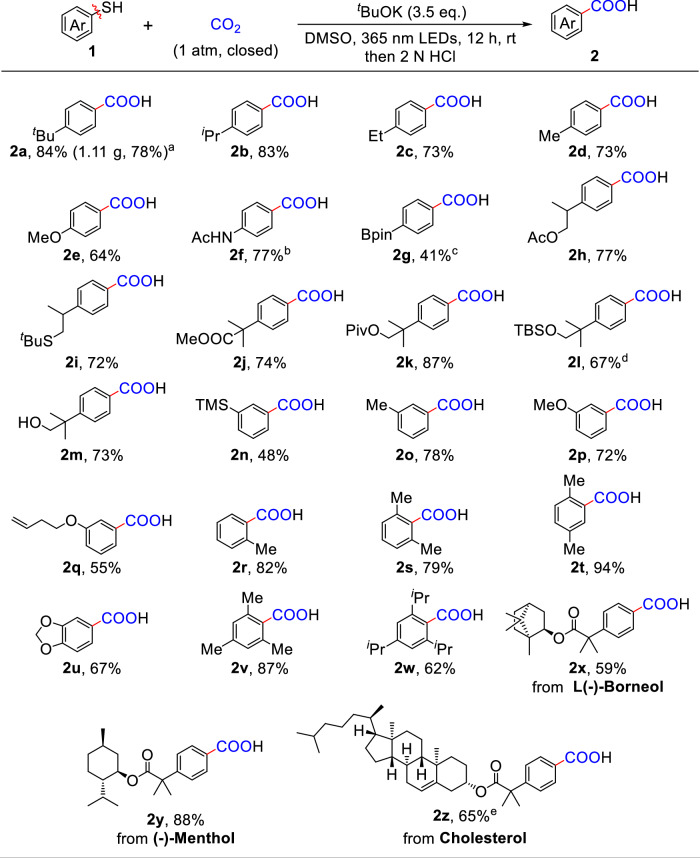
With the optimal reaction conditions in hand, we first examined the scope of aryl thiols with different substituents. As shown in Fig. 2, a broad range of para- and meta-substituted aryl thiols (1a-1q) were effectively transformed with CO2, producing the desired carboxylic acids 2a-2q in moderate to good yields (41%-87%), and the carboxylation of 1a with CO2 can be easily scaled up to afford 2a in 78 % yield. Notably, diverse functional groups, including ether (1e), amide (1f), boronic ester (1g), ester (1h, 1j, and 1k), thioether (1i), silyloxy (1l), trimethylsilyl (1n), and even unprotected hydroxy group (1m), were well tolerated in the reaction, demonstrating the application practicability of this methodology. It is noteworthy that the aryl thiol 1q bearing an unactivated vinyl group was competent for this carboxylation reaction, with the vinyl group remaining intact, affording desired product 2q in a satisfactory yield. The sterically hindered aryl thiol 1r bearing ortho methyl group could also undergo carboxylation with high efficiency. Beyond mono-substituted aryl thiols, di-substituted aryl thiols bearing methyl (1s-1t), and heterocycle (1u) were also amenable to this transformation, furnishing the desired carboxylic acids 2s-2u in 67-94% yields. Additionally, tri-substituted aryl thiols were successfully converted into the corresponding carboxylic acids 2v-2w. Furthermore, aryl thiols derived from biologically active molecules including derivatives from borneol (1x), menthol (1y), and cholesterol (1z) were compatible in this reaction, yielding the desired products in good yields.
Fig. 2. Photo-induced carboxylation of C(sp2)−S Bonds in aryl thiols with CO2.
Reaction Conditions: 1a (0.2 mmol), tBuOK (3.5 eq.), DMSO (2.0 mL), 1 atm of CO2, 365 nm LEDs, rt, 12 h, then 2 N HCl. Yields of isolated products. aGram-scale reaction, 36 h. btBuOK (5.0 eq.). cCs2CO3 (3.5 eq.). dtBuOLi (3.5 eq.). eDMSO/THF (2:1, v/v, 3 mL).
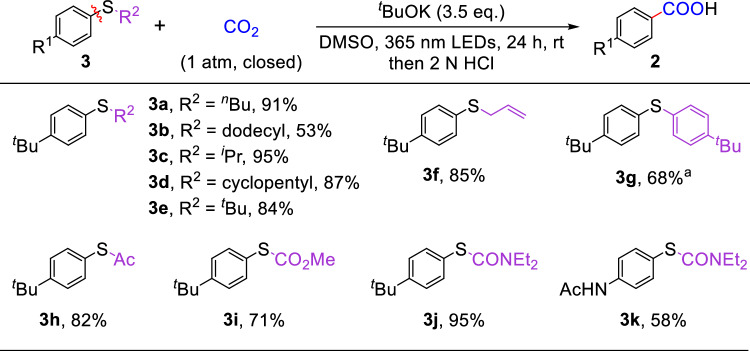
Encouraged by the above success, we further expanded the substrate scope to arylthiol derivatives. To our delight, a variety of aryl thioethers, which are challenging substrates in previous photo-induced selective cleavage of C−S bonds, underwent smooth carboxylation with CO2 under the optimized reaction conditions (Fig. 3). Different alkylthioarenes bearing primary (3a, 3b), secondary (3c, 3d), and tertiary (3e) alkyl groups were selectively transformed to 2a in 53-95% yields. It is worth noting that a thiophenol-derived alkene 3f is compatible under the present conditions. Next, diaryl sulfide 3g was tested to afford the carboxylic product 2a in 68% yield. Moreover, thioesters (3h, 3i) were successfully subjected to the reaction conditions, leading to the corresponding products in good yields. Notably, thiocarbamates 3j and 3k could also undergo the C(sp2)–S bond carboxylation smoothly, yielding the corresponding products in 95% and 58% yields, respectively.
Fig. 3. Photo-induced carboxylation of C(sp2)−S bonds in arylthiol derivatives with CO2.
Reaction Conditions: 3 (0.2 mmol), tBuOK (3.5 eq.), DMSO (2.0 mL), 1 atm of CO2, 365 nm LEDs, rt, 24 h, then 2 N HCl. Yields of isolated products. a72 h, the isolated yield is on phenyl group basis.
Synthetic applications
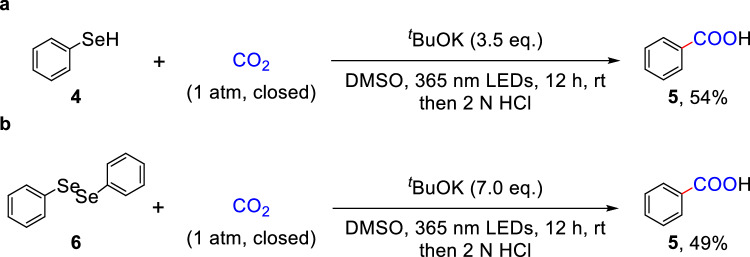
To further underscore the practicality of this strategy, carboxylation of analogues of aryl thiols were carried out (Fig. 4). For instance, the carboxylation of benzeneselenol 4 was performed to provide the corresponding carboxylic acid 5, albeit in a moderate yield (Fig. 4a). Interestingly, diphenyl diselenide, a commonly used radical scavenger, was found to be amenable to Se−Se bond cleavage and carboxylation of C(sp2)–Se bond with CO2 (Fig. 4b)58,65.
Fig. 4. Carboxylation of C(sp2)–Se bonds with CO2.
a Carboxylation of C(sp2)–Se bond in benzeneselenol. b Carboxylation of C(sp2)–Se bond in diphenyl diselenide.
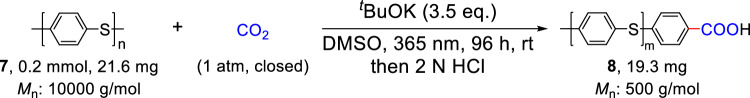
Polyphenylene sulfide (PPS) is a high-performance engineering plastic that is extensively utilized in the fields of flame retardancy, heat insulation, and dielectric insulation66,67. PPS possesses many desirable characteristics, such as excellent high-temperature stability, flame retardant properties, chemical corrosion resistance, as well as good mechanical, and electrical properties. However, the degradation of PPS typically requires intense conditions. In light of above progress, we wonder whether we could extend this photochemical strategy to the degradation of PPS. To our delight, when PPS was subjected to the similar reaction conditions, it could be degraded into product 8 effectively (Fig. 5).
Fig. 5. Degradation and carboxylation of polyphenylene sulfide with CO2.
Reaction Conditions: 7 (0.2 mmol), tBuOK (3.5 eq.), DMSO (2.0 mL), 1 atm of CO2, 365 nm LEDs, rt, 96 h, Mn: Number-average molecular weight.
Mechanistic investigations
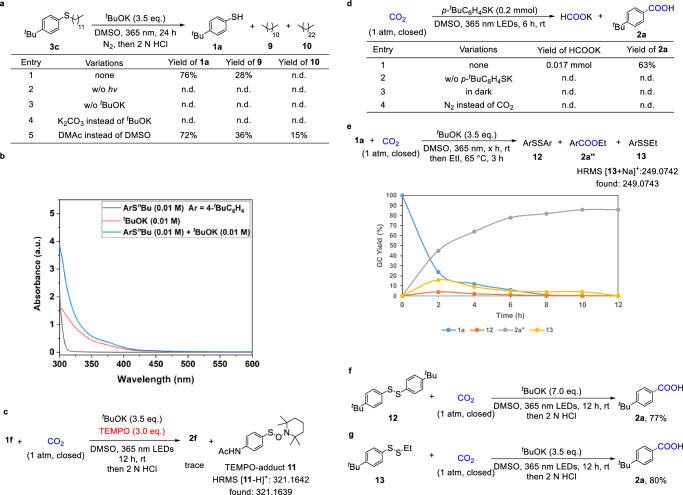
To gain more insights into the reaction mechanism, a series of mechanistic studies were conducted (Fig. 6). Firstly, the control experiments demonstrated that both light and tBuOK were crucial for the cleavage of C−S bonds in aryl thioether 3c. (Fig. 6a, entries 2–4). The formation of 9 and 10 was determined by GC, suggesting that the cleavage of the alkyl C−S bond in thioether and the generation of a corresponding alkyl radical were involved in this transformation68. We further conducted the ultraviolet-visible spectroscopic measurements (Fig. 6b). Compared to the spectrum of thioether 3a, a bathochromic shift was observed for the mixture of 3a and tBuOK. These results suggested the formation of an electron donor-acceptor (EDA) complex between thioether 3a and tBuOK. After that, the photo-induced carboxylation was obviously suppressed in the presence of 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO), indicating the involvement of a radical process (Fig. 6c). Next, the generation of formate (HCO2−) was detected only in the presence of thiolates and CO2, indicating that CO2•− might be generated from the SET between CO2 and photoactivated thiolate (Fig. 6d). The reaction profile demonstrated the formation of disulfide 12 and the anionic species p-tBuC6H4SS−, which reacted with iodoethane to produce 13 during the reaction (Fig. 6e). Compounds 12 and 13 were then subjected to standard reaction conditions, resulting in 77% and 80% yields of the desired acid 2a, respectively. These results indicated that 12 and p-tBuC6H4SS− act as the potential intermediates in this transformation (Fig. 6f, g).
Fig. 6. Mechanistic investigations.
a Study of C−S bond cleavage of 3c. b UV-vis spectroscopic measurement in DMSO. c Radical inhibition experiment. d Detection of potassium formate. e Reaction profile of aryl thiol 1a with CO2. f Identification of possible intermediate 12. g Identification of possible intermediate derivative 13.
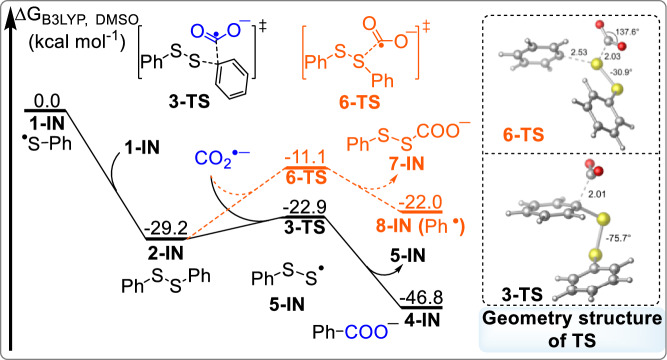
To have a better understanding of this process, computational studies were performed to investigate the cleavage of C−S bond and subsequent carboxylation with CO2.[24,26] According to the experimental observations (Fig. 6c, d), CO2•− and a thiyl radical are very likely generated as the key intermediates in this reaction. Preliminary calculations were carried out using thiyl radical 1-IN as the starting zero point (Fig. 7). 1-IN can undergo a kinetically favored radical dimerization to form disulfide intermediate 2-IN (-29.2 kcal mol-1). The carbon atom of CO2•− exhibits strong nucleophilicity69, which might react with disulfide 2-IN via radical-type nucleophilic substitution to realize the cleavage of C−S bond70. The DFT calculation clearly reveals that the substitution of CO2•− on the phenyl ring in 2-IN via transition state 3-TS requires an energy barrier of 6.3 kcal mol-1, which is 11.8 kcal mol-1 lower than that at sulfur atom via transition state 6-TS, suggesting that the reaction via 6-TS could be kinetically excluded. The geometry structure in 3-TS shows that the length of C1−C2 and C1−S1 bonds are 2.01 Å and 1.83 Å, respectively. As a result, the carboxylate intermediate 4-IN was generated via nucleophilic aromatic substitution transition state 3-TS by the release of disulfur radical 5-IN, formation of C(sp2)−COO bond and the cleavage of C(sp2)–S bond. The radical-type substitution to cleavage S−S bond via transition state 3-TSa is also considered (Please see more information in supporting information (SI)), which would result in the generation of thiyl radical 1-IN and unstable thiocarbonate 4-INa. However, the computational results reveal an invalid conversion process, which can be neglected. Further DFT calculation demonstrates that disulfur radical 5-IN could preferentially undergo reduction, S−S bond dissociation71 to fulfill the carboxylation transformation of a molecule of C−S bond (Please see more information in SI).
Fig. 7. Free energy profiles of carboxylation of C(sp2)−S bonds in disulfide with CO2.
Calculations were performed using Gaussian 16 at the M06/6-311 + G(d,p)/SMD(DMSO)//B3LYP/6-31 + G(d)/SMD(DMSO) level of theory. All energies are in kcal mol-1 and bond lengths are shown in angstroms (Å).
Based on the mechanistic studies and previous reports24,26, a plausible pathway for the cleavage and carboxylation of C(sp2)–S bonds in aryl thiols with CO2 is proposed (Fig. 8). Initially, upon the irradiation with 365 nm light, a single electron transfer event between the excited thiolate II and CO2 would occur to afford CO2•− and thiyl radical III. The thiyl radical III then undergoes dimerization to form the disulfide intermediate 12. Subsequently, the radical addition of CO2•− to disulfide 12 would generate the desired carboxylate IV and radical intermediate V. Intermediate V is then reduced by CO2•− via a SET process to produce intermediate VI, which can be trapped by EtI. Finally, intermediate VI would be converted to thiolate I under strong basic conditions. At this stage, we could not exclude other reaction pathways. Further mechanistic studies to elucidate and refine the intricacies of the mechanism are undergoing in our laboratory.
Fig. 8. Proposed mechanism.
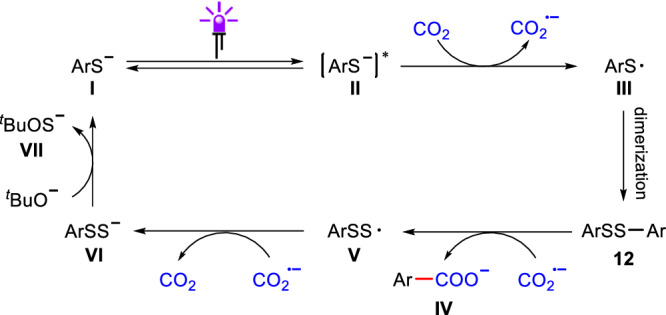
Plausible reaction mechanism of CO2 and aryl thiols in the presence of base and light.
Discussion
In summary, we have developed a photochemical carboxylation of C(sp2)–S bonds in thiols with CO2. A diverse array of readily accessible aryl thiols and derivatives were successfully converted into the corresponding carboxylic acids with high yields. Notably, the carboxylation of C(sp2)–Se bonds in benzeneselenol and diphenyl diselenide with CO2 was also achieved. This transition-metal-free and operationally simple reaction process features a broad substrate scope and good functional group tolerance. Furthermore, the degradation of PPS demonstrates the potential applications of this method in material science.
Methods
General procedure for the synthesis of 2a–2z from 1
To a 10 mL oven-dried Schlenk tube equipped with a magnetic stir bar was charged with arylthiols 1 (0.2 mmol, 1.0 eq. for solid substrates). Then, the tube was transferred to glovebox to add tBuOK (0.7 mmol, 78.6 mg, 3.5 eq.). After being taken out of the glovebox, the tube was then evacuated and back-filled with CO2 atmosphere three times. Anhydrous DMSO (2 mL) was added under CO2 atmosphere followed by liquid arylthiols 1, and the tube was sealed at atmospheric pressure of CO2 (1 atm). The reaction was stirred and irradiated with a 30 W 365 nm LEDs lamp (0.5 cm away, with a cooling fan to keep the reaction temperature at 25-30 °C and keeping the reaction region located in the center of LEDs lamp) for 12 hours. Upon completion of the reaction, the reaction mixture was diluted with 2 mL EA and quenched by 7 mL 2 N HCl. The mixture was extracted with EA four times and the combined organic phases were concentrated in vacuo. The residue was purified by a silica gel flash column chromatography (PE/EA/AcOH 30/1/0 ~ 7/1/0.2%) to give the pure desired products 2.
General procedure for the synthesis of 2 from 3
To a 10 mL oven-dried Schlenk tube equipped with a magnetic stir bar was charged with thioesters 3 (0.2 mmol, 1.0 eq. for solid substrates). Then, the tube was transferred to glovebox to add tBuOK (0.7 mmol, 78.6 mg, 3.5 eq.). After being taken out of the glovebox, the tube was then evacuated and back-filled with CO2 atmosphere three times. Anhydrous DMSO (2 mL) was added under CO2 atmosphere followed by arylthiol derivatives 3, and the tube was sealed at atmospheric pressure of CO2 (1 atm). The reaction was stirred and irradiated with a 30 W 365 nm LEDs lamp (0.5 cm away, with a cooling fan to keep the reaction temperature at 25-30 °C and keeping the reaction region located in the center of LEDs lamp for 24 hours). Upon completion of the reaction, the reaction mixture was diluted with 2 mL EA and quenched by 7 mL 2 N HCl. The mixture was extracted with EA four times and the combined organic phases were concentrated in vacuo. The residue was purified by a silica gel flash column chromatography (PE/EA/AcOH 30/1/0 ~ 7/1/0.2%) to give the pure desired products 2.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Financial support is provided by the National Natural Science Foundation of China (22225106, 22101191, 22201027, 22301193), State Key Laboratory of Polymer Materials Engineering (sklpme-2024-1-07), Fundamental Research Funds from Sichuan University (2020SCUNL102), the Fundamental Research Funds for the Central Universities. We also thank Xiaoyan Wang from the Analysis and Testing Center of Sichuan University, Jing Li and Dongyan Deng from College of Chemistry at Sichuan University for compound testing as well as Prof. Jianbo Zhu from College of Chemistry at Sichuan University for testing of polymers.
Author contributions
D.G.Y. and J.H.Y. conceived and designed the study. J.L., W.W., L.L.L.,W.Z., J.P.Y., Y.L. & X.W.C. performed the experiments, mechanistic studies and wrote the manuscript. All authors contributed to the analysis and interpretation of the data.
Peer review
Peer review information
Nature Communications thanks Ding Du, Yasunori Minami, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. B3LYP geometries for all the optimized structures and transition states are provided in the source date file. Source data are provided with this paper. Extra data are available from the author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jie Liu, Wei Wang, Li-Li Liao.
Contributor Information
Jian-Heng Ye, Email: jhye@scu.edu.cn.
Da-Gang Yu, Email: dgyu@scu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53351-w.
References
- 1.Ciamician, G. The photochemistry of the future. Science36, 385–394 (1912). [DOI] [PubMed] [Google Scholar]
- 2.Stephenson, C., Yoon, T. & MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry. (Wiley-VCH, 2018).
- 3.Goddard, J.-P., Ollivier, C. & Fensterbank, L. Photoredox catalysis for the generation of carbon centered radicals. Acc. Chem. Res.49, 1924–1936 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Hopkinson, M. N., Tlahuext-Aca, A. & Glorius, F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res.49, 2261–2272 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Ravelli, D., Protti, S. & Fagnoni, M. Carbon–carbon bond forming reactions via photogenerated intermediates. Chem. Rev.116, 9850–9913 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev.116, 10075–10166 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res.49, 1546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, Q. & Wu, L.-Z. Recent advances in visible-light-driven organic reactions. Natl Sci. Rev.4, 359–380 (2017). [Google Scholar]
- 9.Chen, Y., Lu, L.-Q., Yu, D.-G., Zhu, C.-J. & Xiao, W.-J. Visible light-driven organic photochemical synthesis in China. Sci. China Chem.62, 24–57 (2019). [Google Scholar]
- 10.Jiang, H. & Studer, A. Chemistry with N-centered radicals generated by single-electron transfer-oxidation using photoredox catalysis. CCS Chem.1, 38–49 (2019). [Google Scholar]
- 11.Goti, G., Manal, K., Sivaguru, J. & Dell’Amico, L. The impact of UV light on synthetic photochemistry and photocatalysis. Nat. Chem. 10.1038/s41557-024-01472-6 (2024). [DOI] [PubMed]
- 12.Dénès, F., Pichowicz, M., Povie, G. & Renaud, P. Thiyl radicals in organic synthesis. Chem. Rev.114, 2587–2693 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Breder, A. & Depken, C. Light-driven single-electron transfer processes as an enabling principle in sulfur and selenium multicatalysis. Angew. Chem. Int. Ed.58, 17130–17147 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Crisenza, G. E. M., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc.142, 5461–5476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, S., Wang, H. & König, B. Light-induced single-electron transfer processes involving sulfur anions as catalysts. J. Am. Chem. Soc.143, 15530–15537 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Li, H., Liu, Y. & Chiba, S. Leveraging of sulfur anions in photoinduced molecular transformations. JACS Au1, 2121–2129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan, X., Lacôte, E., Lalevée, J. & Curran, D. P. Polarity reversal catalysis in radical reductions of halides by N-heterocyclic carbene boranes. J. Am. Chem. Soc.134, 5669–5674 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Vogt, O. D. B., Seath, C. P., Wang, H. & Jui, N. T. Selective C–F functionalization of unactivated trifluoromethylarenes. J. Am. Chem. Soc.141, 13203–13211 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sap, J. B. I. et al. Organophotoredox hydrodefluorination of trifluoromethylarenes with translational applicability to drug discovery. J. Am. Chem. Soc.142, 9181–9187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen, Z. et al. General light-mediated, highly diastereoselective piperidine epimerization: from most accessible to most stable stereoisomer. J. Am. Chem. Soc.143, 126–131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santandrea, J., Minozzi, C., Cruché, C. & Collins, S. K. Photochemical dual-catalytic synthesis of alkynyl sulfides. Angew. Chem. Int. Ed.56, 12255–12259 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Chen, H. et al. A visible-light-harvesting covalent organic framework bearing single nickel sites as a highly efficient sulfur–carbon cross-coupling dual catalyst. Angew. Chem. Int. Ed.60, 10820–10827 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Liu, B., Lim, C.-H. & Miyake, G. M. Visible-light-promoted C–S cross-coupling via intermolecular charge transfer. J. Am. Chem. Soc.139, 13616–13619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, H. et al. Visible light-driven anti-markovnikov hydrocarboxylation of acrylates and styrenes with CO2. CCS Chem.3, 1746–1756 (2021). [Google Scholar]
- 25.Saux, E. L., Georgiou, E., Dmitriev, I. A., Hartley, W. C. & Melchiorre, P. Photochemical Organocatalytic Functionalization of Pyridines via Pyridinyl Radicals. J. Am. Chem. Soc.145, 47–52 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, Y.-X. et al. Visible-light-driven synthesis of N-heteroaromatic carboxylic acids by thiolate-catalysed carboxylation of C(sp²)–H bonds using CO2. Nat. Synth.3, 394–405 (2024). [Google Scholar]
- 27.Liu, C., Li, K. & Shang, R. Arenethiolate as a dual function catalyst for photocatalytic defluoroalkylation and hydrodefluorination of trifluoromethyls. ACS Catal.12, 4103–4109 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majhi, J. et al. Practical, scalable, and transition metal-free visible light-induced heteroarylation route to substituted oxindoles. Chem. Sci.14, 897–902 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churchill, D. G., Bridgewater, B. M. & Parki, G. Modeling Aaspects of hydrodesulfurization at molybdenum: carbon−sulfur bond cleavage of thiophenes by ansa molybdenocene complexes. J. Am. Chem. Soc.122, 178–179 (2000). [Google Scholar]
- 30.Wang, H. & Iglesia, E. Thiophene hydrodesulfurization catalysis on supported Ru clusters: Mechanism and site requirements for hydrogenation and desulfurization pathways. J. Catal.273, 245–256 (2010). [Google Scholar]
- 31.Al-Jamimi, H. A., BinMakhashen, G. M. & Saleh, T. A. Multiobjectives optimization in petroleum refinery catalytic desulfurization using Machine learning approach. Fuel322, 124088 (2022). [Google Scholar]
- 32.Modha, S. G., Mehta, V. P. & Van der Eycken, E. V. Transition metal-catalyzed C–C bond formation via C–S bond cleavage: an overview. Chem. Soc. Rev.42, 5042–5055 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Lou, J. et al. Transition-metal mediated carbon–sulfur bond activation and transformations: an update. Chem. Soc. Rev.49, 4307–4359 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Huang, S., Wang, M. & Jiang, X. Ni-catalyzed C–S bond construction and cleavage. Chem. Soc. Rev.51, 8351–8377 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Yorimitsu, H. Catalytic transformations of sulfonium salts via C-S bond activation. Chem. Rec.21, 3356–3369 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Aukland, M. H. et al. An interrupted pummerer/nickel-catalysed cross-coupling sequence. Angew. Chem. Int. Ed.57, 9785–9789 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Tang, S., Zhao, X., Yang, L., Li, B. & Wang, B. Copper-catalyzed carboxylation of aryl thianthrenium salts with CO2. Angew. Chem. Int. Ed.61, e202212975 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Allen, A. R., Noten, E. A. & Stephenson, C. R. J. Aryl transfer strategies mediated by photoinduced electron transfer. Chem. Rev.122, 2695–2751 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engl, P. S. et al. C–N cross-couplings for site-selective late-stage diversification via Aryl sulfonium salts. J. Am. Chem. Soc.141, 13346–13351 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Yan, J. et al. A radical smiles rearrangement promoted by neutral eosin Y as a direct hydrogen atom transfer photocatalyst. J. Am. Chem. Soc.142, 11357–11362 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Hervieu, C. et al. Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres. Nat. Chem.13, 327–334 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Kong, X., Chen, Y., Liu, Q., Wang, W. & Zhang, S. Selective fluorosulfonylation of thianthrenium salts enabled by electrochemistry. Org. Lett.25, 581–586 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Wenkert, E., Ferreira, T. W. & Michelotti, E. L. Nickel-induced conversion of carbon–sulphur into carbon–carbon bonds. One-step transformations of enol sulphides into olefins and benzenethiol derivatives into alkylarenes and biaryls. J. Chem. Soc. Chem. Commun. 637-638 (1979).
- 44.Van Buren, R. L., Baltisberger, R. J., Woolsey, N. F. & Stenberg, V. I. Formic acid and the high-temperature reductive desulfurization of aromatic sulfides. J. Org. Chem.47, 4107–4110 (1982). [Google Scholar]
- 45.Xu, H. et al. Nickel-catalyzed cross-electrophile coupling of aryl thiols with aryl bromides via C–S bond activation. Org. Chem. Front.10, 5171–5179 (2023). [Google Scholar]
- 46.The bond energy data comes from the http://ibond.nankai.edu.cn.
- 47.Murray, S. G. & Hartley, F. R. Coordination chemistry of thioethers, selenoethers, and telluroethers in transition-metal complexes. Chem. Rev.81, 365 (1981). [Google Scholar]
- 48.Aresta, M. Carbon Dioxideas Chemical Feedstock, (Wiley-VCH, Weinheim 2010).
- 49.Huang, K., Sun, C.-L. & Shi, Z.-J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev.40, 2435–2452 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Appel, A. M. et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev.113, 6621–6658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, Q., Wu, L., Jackstell, R. & Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun.6, 5933 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Tortajada, A., Juliá-Hernández, F., Börjesson, M., Moragas, T. & Martin, R. Transition-metal-catalyzed carboxylation reactions with carbon dioxide. Angew. Chem. Int. Ed.57, 15948–15982 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Grignard, B., Gennen, S., Jérôme, C., Kleij, A. W. & Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev.48, 4466–4514 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Wang, S. & Xi, C. Recent advances in nucleophile-triggered CO2-incorporated cyclization leading to heterocycles. Chem. Soc. Rev.48, 382–404 (2019). [DOI] [PubMed] [Google Scholar]
- 55.He, X., Qiu, L.-Q., Wang, W.-J., Chen, K.-H. & He, L.-N. Photocarboxylation with CO2: an appealing and sustainable strategy for CO2 fixation. Green. Chem.22, 7301–7320 (2020). [Google Scholar]
- 56.Ye, J.-H., Ju, T., Huang, H., Liao, L.-L. & Yu, D.-G. Radical carboxylative cyclizations and carboxylations with CO2. Acc. Chem. Res.54, 2518–2531 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Sun, G.-Q. et al. Recent Advances in Electrochemical Carboxylation with CO2. Acc. Chem. Res.57, 2728–2745 (2024). [DOI] [PubMed] [Google Scholar]
- 58.Song, L. et al. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal.5, 832–838 (2022). [Google Scholar]
- 59.Sun, G.-Q. et al. Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations. Nature615, 67–72 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao, W., Zhang, J. & Wu, J. Recent advances in reactions involving carbon dioxide radical anion. ACS Catal.13, 15991–16011 (2023). [Google Scholar]
- 61.Seo, H., Katcher, M. H. & Jamison, T. F. Photoredox activation of carbon dioxide for amino acid synthesis in continuous flow. Nat. Chem.9, 453–456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, H., Gao, Y., Zhou, C. & Li, G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. J. Am. Chem. Soc.142, 8122–8129 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Alektiar, S. N. & Wickens, Z. K. Photoinduced hydrocarboxylation via thiol-catalyzed delivery of formate across activated alkenes. J. Am. Chem. Soc.143, 13022–13028 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Figuly, G. D., Loop, C. K. & Martin, J. C. Directed ortho-lithiation of lithium thiophenolate. New methodology for the preparation of ortho-substituted thiophenols and related compounds. J. Am. Chem. Soc.111, 654–658 (1989). [Google Scholar]
- 65.Wang, C. et al. Synthesis and application dichalcogenides as radical reagents with photochemical technology. Molecules28, 1998 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanthapanichakoon, W., Hata, M., Nitta, K., Furuuchi, M. & Otani, Y. Mechanical degradation of filter polymer materials: polyphenylene sulfide. Polym. Degrad. Stabil.91, 2614–2621 (2006). [Google Scholar]
- 67.Yan, P. et al. Investigation on thermal degradation mechanism of poly(phenylene sulfide). Polym. Degrad. Stabil.197, 109863 (2022). [Google Scholar]
- 68.Kuzmin, J. et al. Electroreductive desulfurative transformations with thioethers as alkyl radical precursors. Angew. Chem. Int. Ed.62, e202304272 (2023). [DOI] [PubMed] [Google Scholar]
- 69.Zhang, W. et al. Electroreductive dicarboxylation of unactivated skipped dienes with CO2. Angew. Chem. Int. Ed.62, e202301892 (2023). [DOI] [PubMed] [Google Scholar]
- 70.Liu, S., Kumar, N., Robert, F. & Landais, Y. Thiochromane formation via visible-light-mediated intramolecular δ-C(sp3)–H bond arylation of sulfonamides. Org. Lett.25, 3072–3077 (2023). [DOI] [PubMed] [Google Scholar]
- 71.Zhang, G. et al. Trisulfur radical anion as the key intermediate for the synthesis of thiophene via the interaction between elemental sulfur and NaOtBu. Org. Lett.16, 6156–6159 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. B3LYP geometries for all the optimized structures and transition states are provided in the source date file. Source data are provided with this paper. Extra data are available from the author upon request.