Abstract
Purpose
This case describes the unique course and management of a patient with progressive, refractory multi-system sarcoidosis that initially presented with ocular and dermatologic findings.
Observations
A 47-year-old male presented with acute anterior uveitis and was found to have simultaneous inflammation of his skin at a tattoo site. Diagnosis of ocular sarcoidosis was confirmed through skin biopsy. Treatment with prednisone and adalimumab was initiated. Despite systemic immunosuppression and control of systemic inflammation, the patient had refractory ocular disease and developed bilateral disc edema and peripapillary choroidal neovascular membranes with subretinal fluid, so oral methotrexate and as-needed intravitreal bevacizumab injections were added. However, the patient manifested hepatic and worsening pulmonary involvement, prompting discontinuation of both methotrexate and adalimumab. Biopsy of the liver later confirmed hepatic sarcoidosis over drug-induced liver injury. Out of precaution for methotrexate-associated hepatic toxicity, mycophenolate mofetil was initiated instead, which then led to resolution of the subretinal fluid and disease quiescence.
Conclusions and Importance
This case offers insight into the varying presentation and recalcitrant course of sarcoidosis. It documents a clinicopathologic case with unique initial presentation with biopsies of multiple organs, escalation of therapy with several immunomodulatory agents, and multidisciplinary collaboration to achieve ocular and systemic quiescence.
Keywords: Ocular sarcoid, Uveitis, Tattoo, Choroidal neovascularization
1. Introduction
Sarcoidosis is a granulomatous disease with multi-system involvement. It most commonly affects the pulmonary system, followed by the integumentary and lymphatic systems.1 Ocular sarcoidosis is rarely the initial presentation; however, its prevalence in patients with diagnosed sarcoidosis can be up to 50 %.2,3
The hallmark of sarcoidosis is noncaseating granulomatous inflammation, diagnosed via biopsy. Noncaseating granulomas often manifest in the lungs, which can be asymptomatic or lead to pulmonary fibrosis with significantly decreased lung function.4 Skin manifestations include macropapules, subcutaneous nodules, scarring, erythema nodosum, and lupus pernio, and when biopsied, can allow for early diagnosis of sarcoidosis.5 Hepatic granulomas can develop in sarcoidosis patients, leading to elevated liver enzymes and subsequent portal hypertension.6 Five to ten percent of patients can have nervous system involvement, resulting in a wide range of presentations including changes in cognition, ataxia, or endocrine disturbances.7
Ocular manifestations of sarcoidosis vary widely, ranging from dry eye disease to severe granulomatous uveitis, neuro-ophthalmic manifestations, retinal vasculitis, and/or macular edema.8 Intraocular signs suggestive of ocular sarcoidosis (OS), as determined by the International Workshop on Ocular Sarcoidosis (IWOS), are: 1) mutton-fat keratic precipitates and/or iris nodules, 2) trabecular meshwork nodules and/or tent-shaped peripheral anterior synechiae, 3) snowballs or string of pearls vitreous opacities, 4) chorioretinal peripheral lesions, 5) nodular and/or segmental periphlebitis and/or macroaneurysms, 6) nodules of the optic disc or choroid, and 7) bilaterality.9,10 There are three diagnostic categories of ocular sarcoidosis according to the IWOS, each conveying a varying level of certainty. Definite OS is a diagnosis supported by biopsy with compatible uveitis. Presumed OS is a diagnosis that is not biopsy proven but involves bilateral hilar lymphadenopathy and two of the seven intraocular signs suggestive of OS.10 Finally, probable OS is a diagnosis not supported by biopsy or bilateral hilar lymphadenopathy but rather three intraocular signs and two systemic manifestations.10 Diagnosis of OS is particularly difficult given that impacted intraocular sites are not always easily accessible for biopsy. Biopsies of conjunctival, eyelid, and lacrimal regions can be performed if presenting with involvement. However, since sarcoidosis is a diagnosis of exclusion, if these sites are not involved or if biopsies are difficult to obtain, it can be helpful to biopsy other organ system involvement for confirmation.
Current management for OS according to the IWOS is to initiate steroids, either topical or systemic based on the location of the patient's involvement.11 For patients who are steroid-dependent, systemic immunosuppressive agents such as mycophenolate mofetil and methotrexate may be added to the regimen.8 Biologic agents such as TNF-alpha inhibitors may be added as second line agents in cases of posterior involvement as these have been shown to be beneficial for uveitis secondary to sarcoidosis as well.8,11
2. Case report
A 47-year-old Hispanic male with a history of asthma and type 2 diabetes presented to a uveitis specialist as a referral from an optometrist for several weeks of bilateral conjunctival injection, photophobia, and hazy vision. Best corrected visual acuity was 20/30 in the right eye (OD) and 20/25 in the left (OS). On examination, there were anterior chamber cells and iris nodules in both eyes, with unremarkable posterior findings. Notably, the patient was found to have elevation and erythema of the skin on his forearm around the site of tattoos (Fig. 1). He was diagnosed with chronic bilateral alternating anterior uveitis and started on prednisolone and atropine drops as well as 60 mg daily of oral prednisone with rapid taper. Investigative laboratory work-up was initiated.
Fig. 1.
Elevation and erythema of skin around site of tattoos on forearm.
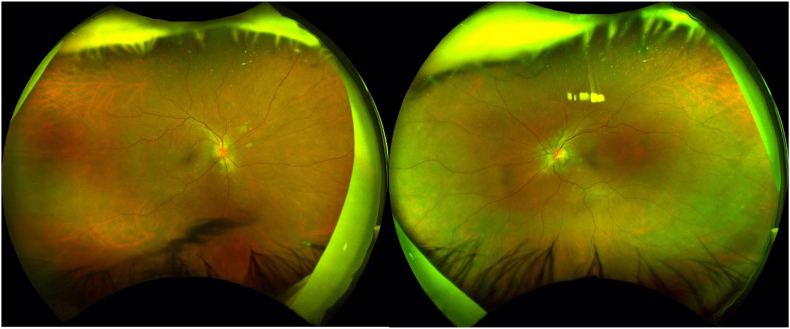
The patient returned for follow-up two weeks later with decreased vision of 20/50 OD and 20/30 OS. He was found to have bilateral disc edema and multiple hypopigmented peripapillary subretinal lesions (Fig. 2). Optical coherence tomography (OCT) of the macula further revealed intraretinal fluid in the nasal macula of both eyes, with choroidal granuloma and associated RPE irregularity and subretinal fluid adjacent to the inferotemporal arcade in the right eye.
Fig. 2.
Fundus photos of bilateral optic disc edema and peripapillary choroidal lesions.
His labs were remarkable for elevated erythrocyte sedimentation rate (88 mm/h), C-reactive protein (61.6 mg/L), angiotensin converting enzyme (110 nmol/mL/min), lysozyme (2.13 mcg/mL), alkaline phosphatase (ALP, 180 U/L), alanine aminotransferase (ALT, 86 U/L), and aspartate aminotransferase (AST, 36 U/L). Chest x-ray demonstrated hilar shadows, bronchial wall thickening, and trace right-sided pleural effusion. Given the patient's presentation and laboratory findings, sarcoidosis-associated uveitis and tattoo-associated uveitis were highly considered in the differential, with elevated liver enzymes indicating steatosis from fatty liver disease versus hepatic sarcoidosis. Other potential diagnoses included psoriasis and infectious etiologies.
A skin biopsy of his tattooed forearm revealed black tattoo pigment and numerous dermal aggregates composed of epithelioid histiocytes and Langhans-type multinucleated giant cells consistent with noncaseating granulomas (Fig. 3). There was no evidence of infection. A computed tomography scan of his chest and abdomen showed focal fibrosis of the lung, pleural thickening of the right lower lobe, and liver enlargement with steatosis but no nodules. Magnetic resonance imaging of the orbits demonstrated increased cerebrospinal fluid around the optic nerves and enhancement near the left optic nerve head indicating granulomatous infiltration. Given this constellation of findings, the patient was diagnosed with multi-system sarcoidosis affecting his ocular, dermatologic, and pulmonary systems, as well as nonalcoholic fatty liver disease in the setting of his metabolic comorbidities. He was started on subcutaneous adalimumab 40 mg every 2 weeks and continued tapering weekly from a 20 mg daily dose of oral prednisone. The patient's symptoms improved, as did his liver panel (ALP 105, ALT 62, AST 37).
Fig. 3.
Biopsy of forearm skin showing black pigment in the dermis and aggregates composed of epithelioid histiocytes and numerous Langhans-type multinucleated giant cells, consistent with noncaseating granulomas.
However, after completely tapering off the prednisone, the patient developed worsening of anterior chamber inflammation and subretinal fluid bilaterally. His prednisone was restarted at a low dose of 7.5 mg daily.
The patient continued to have active uveitis and subsequently developed choroidal neovascular membranes, which required a series of intravitreal bevacizumab injections. Despite the injections, he continued to have worsening subretinal fluid, and oral methotrexate 7.5 mg weekly was added to his management. This steroid-sparing agent was chosen over escalation of steroids to avoid steroid-induced hyperglycemia in the setting of his diabetes.
The patient showed a good response to this regimen of methotrexate 7.5mg weekly, adalimumab 40mg every 2 weeks, and low-dose prednisone (5 mg daily); however, 4 months after starting methotrexate, he was found to have worsened liver function tests (ALP 384, ALT 67, AST 61). Given concern for an adverse effect of this medication on his liver, methotrexate was discontinued. While on adalimumab monotherapy, he continued to experience active flares in the eyes, skin, and lungs, along with persistently increasing liver enzymes. The patient's adalimumab was then discontinued due to lack of efficacy. There was an additional concern for drug-induced liver injury, though the cholestatic pattern of his liver panel was consistent with hepatic sarcoidosis. A liver biopsy demonstrated noncaseating granulomas (Fig. 4), confirming hepatic sarcoidosis.
Fig. 4.
Biopsy of liver parenchyma showing noncaseating granulomatous inflammation and minimal steatosis.
Evaluation of new shortness of breath reported by the patient revealed worsening of reticular nodular interstitial markings on chest-x-ray, with corresponding moderate obstructive airway disease on pulmonary function testing. This was consistent with active pulmonary sarcoidosis. His uveitis also continued to worsen, and the patient was restarted on high dose prednisone of 40 mg daily, which when tapered to 15mg daily, resulted in flares of granulomatous inflammation.
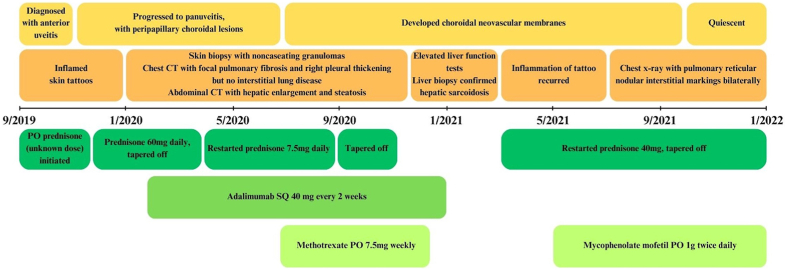
In the following months, oral mycophenolate mofetil 1000 mg twice daily was initiated in place of methotrexate, which improved his symptoms and subretinal fluid. This, in combination with low-dose prednisone 7.5mg daily controlled his multi-systemic ocular, dermatologic, hepatic, and pulmonary sarcoidosis. Fig. 5 is a graphical timeline of the patient's course. Unfortunately, the patient was lost to follow-up for one year, and during the interim, had self-discontinued his medications. He returned with active anterior uveitis and a new chorioretinal granuloma with continued regression of his choroidal neovascular membranes. He was restarted on mycophenolate mofetil and prednisone 20 mg daily with a taper down to 7.5mg daily. He was strongly counseled regarding resources to improve medication adherence.
Fig. 5.
Timeline of patient's key symptoms and findings, along with treatment regimen. Ocular findings appear in yellow. Systemic findings appear in orange. Treatment course appears in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
This patient manifested the myriad findings of sarcoidosis, initially presenting with uveitis as well as inflammation of a tattoo site. Given this combined presentation, we also considered a diagnosis of tattoo-associated uveitis, which can present with rapid onset of both skin and ocular findings, typically within a year after a tattoo is placed.12 As granulomas in tattoo-associated uveitis and sarcoidosis remain indistinguishable on histopathology, it has been questioned whether the former represents a limited form of the latter, rather than separate entities. Tattoo-associated uveitis remains a diagnosis of exclusion. This patient presented with uveitis with concomitant inflammation of his tattoo sites, but he later developed liver and lung involvement, which pointed toward a diagnosis of sarcoidosis due to multi-system involvement.
Recommended first line treatment for sarcoidosis is corticosteroids13; however, this patient required multiple immunosuppressive agents to achieve ocular and systemic quiescence. Adalimumab was initially added to his steroid regimen, but persistent disease activity and steroid-induced hyperglycemia prompted supplementation with an antimetabolite, initially methotrexate and then mycophenolate. The addition of these steroid-sparing agents enabled the patient to achieve quiescence on a lower and safer dose of steroid.
Although the patient experienced good response to this medication regimen, routine monitoring began to reveal elevated liver enzymes. This raised concern for drug induced liver injury (DILI) secondary to prolonged methotrexate use. The medication was discontinued, but biopsy of the liver later revealed that liver damage was due to progression of his sarcoidosis. Pathologically, some DILI can mimic granulomas of sarcoidosis, though DILI often presents with less defined and irregular borders as well as increased presence of lymphocytes and eosinophils.14 Additionally, methotrexate-induced liver injury on biopsy tends to demonstrate steatosis or fibrosis as opposed to granulomas.14
Though methotrexate was discontinued, his treatment continued with adalimumab, a biologic immunomodulatory medication that has been shown to be effective in patients with refractory ocular sarcoidosis.15 However, the patient's pulmonary and ocular inflammation still recurred, and adalimumab was also stopped due to lack of efficacy. The patient was transitioned to mycophenolate mofetil, and with this, the patient achieved quiescence of his uveitis. Interestingly, the First-line Antimetabolites as Steroid-sparing Treatment (FAST) uveitis trial found no statistically significant differences between mycophenolate mofetil and methotrexate for control of inflammation in 100 cases of noninfectious posterior uveitis or panuveitis, though treatment success was seen in more patients treated with methotrexate (74 % vs 55 %).16
Appropriate treatment regimen as well as patient compliance with medications and follow-up are important to prevent complications associated with prolonged untreated ocular inflammation. Chronic inflammation can impact any part of the eye in sarcoidosis and lead to synechiae formation, cataract, choroidal neovascular membrane, glaucoma, and cystoid macular edema, with resultant profound vision loss.8,17 This patient unfortunately developed choroidal neovascular membranes while tailoring his treatment regimen prior to achieving quiescence of his uveitis.
This case demonstrates a unique course of multi-system sarcoidosis that originally presented with ocular and dermatologic manifestations and, despite immunomodulatory treatment, developed worsening ocular and systemic disease. It documents a clinicopathologic case with biopsies of multiple organs, escalation of therapy with several immunomodulatory agents, and collaboration of a multidisciplinary team to achieve ocular, and multi-systemic, quiescence.
CRediT authorship contribution statement
Arthi Rao: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Jodi Hwang: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Joyce Wen: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Sarah Edminster: Methodology, Investigation. Maria Sibug-Saber: Writing – review & editing, Investigation. Narsing Rao: Writing – review & editing, Investigation, Conceptualization. Brian C. Toy: Writing – review & editing, Investigation, Conceptualization.
Patient consent
The patient consented to publication of the case with images.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Funding
Unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness; BCT reports support from the National Eye Institute of the National Institutes of Health (K23EY032985)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Baughman R.P., Teirstein A.S., Judson M.A., et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 2.Salah S., Abad S., Monnet D., Brézin A.P. Sarcoidosis. J Fr Ophtalmol. 2018;41(10):e451–e467. doi: 10.1016/j.jfo.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Sève P., Jamilloux Y., Tilikete C., Gerfaud-Valentin M., Kodjikian L., El Jammal T. Ocular sarcoidosis. Semin Respir Crit Care Med. 2020;41(5):673–688. doi: 10.1055/s-0040-1710536. [DOI] [PubMed] [Google Scholar]
- 4.Pulmonary sarcoidosis - ClinicalKey. https://www-clinicalkey-com.libproxy1.usc.edu/#!/content/playContent/1-s2.0-S221326001830064X?returnurl=null&referrer=null
- 5.Mañá J., Marcoval J. Skin manifestations of sarcoidosis. Presse Medicale Paris Fr 1983. 2012;41(6 Pt 2):e355–e374. doi: 10.1016/j.lpm.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 6.Sarcoidosis and the liver - ClinicalKey. https://www-clinicalkey-com.libproxy1.usc.edu/#!/content/playContent/1-s2.0-S1089326118301168?returnurl=null&referrer=null
- 7.Pirau L., Lui F. StatPearls. StatPearls Publishing; 2022. Neurosarcoidosis.http://www.ncbi.nlm.nih.gov/books/NBK534768/ [Google Scholar]
- 8.Pasadhika S., Rosenbaum J.T. Ocular sarcoidosis. Clin Chest Med. 2015;36(4):669–683. doi: 10.1016/j.ccm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbort C.P., Rao N.A., Mochizuki M., the members of the Scientific Committee of the First International Workshop on Ocular Sarcoidosis (IWOS) International criteria for the diagnosis of ocular sarcoidosis: results of the first international Workshop on ocular sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17(3):160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 10.Mochizuki M., Smith J.R., Takase H., Kaburaki T., Acharya N.R., Rao N.A. Revised criteria of international Workshop on ocular sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2019;103(10):1418–1422. doi: 10.1136/bjophthalmol-2018-313356. [DOI] [PubMed] [Google Scholar]
- 11.Takase H., Acharya N.R., Babu K., et al. Recommendations for the management of ocular sarcoidosis from the international Workshop on ocular sarcoidosis. Br J Ophthalmol. 2021;105(11):1515–1519. doi: 10.1136/bjophthalmol-2020-317354. [DOI] [PubMed] [Google Scholar]
- 12.Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32(11):1852–1861. doi: 10.1111/jdv.15070. [DOI] [PubMed] [Google Scholar]
- 13.Gerke A.K. Treatment of sarcoidosis: a multidisciplinary approach. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.545413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiner D.E. Drug-induced liver injury: the hepatic pathologist's approach. Gastroenterol Clin N Am. 2017;46(2):273–296. doi: 10.1016/j.gtc.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riancho-Zarrabeitia L., Calvo-Río V., Blanco R., et al. Anti-TNF-α therapy in refractory uveitis associated with sarcoidosis: multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45(3):361–368. doi: 10.1016/j.semarthrit.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Rathinam S.R., Gonzales J.A., Thundikandy R., et al. Effect of corticosteroid-sparing treatment with mycophenolate mofetil vs methotrexate on inflammation in patients with uveitis: a randomized clinical trial. JAMA. 2019;322(10):936–945. doi: 10.1001/jama.2019.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simakurthy S., Tripathy K. StatPearls. StatPearls Publishing; 2022. Ocular sarcoidosis.http://www.ncbi.nlm.nih.gov/books/NBK580538/ [Google Scholar]