Abstract
Nanotechnology plays a pivotal role in food science, particularly in the nanoencapsulation of bioactive compounds, to enhance their stability, bioavailability, and therapeutic potential. This review aims to provide a comprehensive analysis of the encapsulation of bioactive compounds, emphasizing the characteristics, food applications, and implications for human health. This work offers a detailed comparison of polymers such as sodium alginate, gum Arabic, chitosan, cellulose, pectin, shellac, and xanthan gum, while also examining both conventional and emerging encapsulation techniques, including freeze-drying, spray-drying, extrusion, coacervation, and supercritical anti-solvent drying. The contribution of this review lies in highlighting the role of encapsulation in improving system stability, controlling release rates, maintaining bioactivity under extreme conditions, and reducing lipid oxidation. Furthermore, it explores recent technological advances aimed at optimizing encapsulation processes for targeted therapies and functional foods. The findings underline the significant potential of encapsulation not only in food supplements and functional foods but also in supportive medical treatments, showcasing its relevance to improving human health in various contexts.
Keywords: Encapsulation systems, Bioactive compounds, Nutritional benefits, Nanocarriers, Functional foods
Highlights
-
•
Encapsulation is widely used to enhance the bioavailability of nutrients and functional ingredients.
-
•
Bioactive compounds are protected from environmental factors (such as light, heat, and oxygen) that can degrade their nutritional value.
-
•
Many encapsulated compounds have antioxidant properties that help combat oxidative stress, potentially reducing the risk of various diseases.
-
•
Continued research on safety and efficacy will influence regulatory frameworks for encapsulated products in the food industry.
1. Introduction
Encapsulation technology has significantly advanced the food industry by facilitating the incorporation and stabilization of bioactive compounds within various food systems. This method involves enclosing bioactive substances within a carrier material, enhancing their stability, bioavailability, and controlled release. Bioactive compounds, such as vitamins, minerals, antioxidants, and probiotics, are recognized for their health-promoting properties, making their efficient delivery essential for maximizing their benefits. Encapsulation not only maintains the functional properties of these compounds but also protects them from adverse environmental conditions and interactions with other food components (McClements & Gumus, 2016; Zhang et al., 2024).
The nutritional benefits of bioactive compounds are extensively documented, with many studies emphasizing their role in disease prevention and health promotion. For instance, antioxidants like polyphenols and carotenoids have been shown to reduce oxidative stress and inflammation, thereby decreasing the risk of chronic diseases such as cardiovascular disease and cancer (Gasa-Falcon et al., 2020; Wang et al., 2022). Similarly, probiotics support gut health by maintaining a balanced microbiota, which is crucial for proper digestion, immune function, and overall well-being (Burgain et al., 2011). Encapsulation technology is crucial in enhancing the effectiveness of these bioactive compounds by ensuring their stability and bioavailability throughout the digestive process (Davidov-Pardo et al., 2015).
In terms of food applications, encapsulation is widely used in the development of functional foods and beverages. Functional foods are designed to provide additional health benefits beyond basic nutrition, often containing ingredients that can enhance physical and mental health (McClements, 2020). Encapsulation enables the incorporation of sensitive bioactive compounds into these foods without compromising their stability or sensory properties. For example, omega-3 fatty acids, which are prone to oxidation, can be encapsulated to prevent degradation and off-flavors in food products (Sarubbo et al., 2022). Similarly, encapsulating vitamins and minerals can prevent their interaction with other food components, thereby preserving their nutritional value (Hashim et al., 2023).
The impact of encapsulated bioactive compounds on human health is substantial and multifaceted. By improving the bioavailability and targeted delivery of these compounds, encapsulation technology can enhance their therapeutic effects (Suhag et al., 2021). Research indicates that encapsulated bioactive compounds show improved absorption and retention in the body compared to their non-encapsulated forms (Munin & Edwards-Lévy, 2011). This is particularly important for compounds with low solubility or stability, such as curcumin and resveratrol, which exhibit enhanced bioactivity when encapsulated (Samborska et al., 2021). Additionally, encapsulation can aid in developing personalized nutrition strategies, where bioactive compounds are tailored to meet individual health needs (Chew et al., 2019). Recent advancements in encapsulation technologies, such as nanoencapsulation and microencapsulation, have further expanded the potential applications and benefits of this technique.
Recent progress in encapsulation technology has broadened its applications in fields such as medicine, food, and cosmetics. In the food sector, encapsulation helps safeguard sensitive bioactives like omega-3 fatty acids, vitamins, and probiotics from degradation. This technique extends the shelf life and maintains the nutritional and sensory qualities of products. Encapsulated probiotics, for instance, are protected from harsh gastrointestinal conditions, increasing their viability and effectiveness (Bauer-Estrada et al., 2023). However, encapsulation technologies are not without challenges. High production costs, complex manufacturing, and scalability issues are common obstacles. Additionally, interactions between encapsulated compounds and their environment can occasionally diminish effectiveness. Addressing these issues is crucial to further improve the technology and expand its practical use across industries (Fangmeier et al., 2019). Nanoencapsulation, in particular, offers better control over the release and targeting of bioactive compounds, enabling the design of more effective and efficient delivery systems (Shamloo et al., 2023; Singh, 2016). These advancements hold significant promise for the future of food science and nutrition, facilitating the development of innovative products that meet the growing consumer demand for health-promoting foods (Sultana et al., 2022).
As the food industry continues to evolve, the integration of encapsulation technologies will be crucial in shaping the future of health-promoting food products (Burgain et al., 2011). Therefore, we investigated the encapsulation of bioactive compounds and related issues, including food applications, nutritional benefits and health effects.
2. Bioactive compounds
Bioactive compounds are naturally occurring substances in plants and certain foods each providing unique health benefits and playing a role in disease prevention: Antioxidant, anti-inflammatory, and disease-preventing properties highlight their importance in a balanced diet.
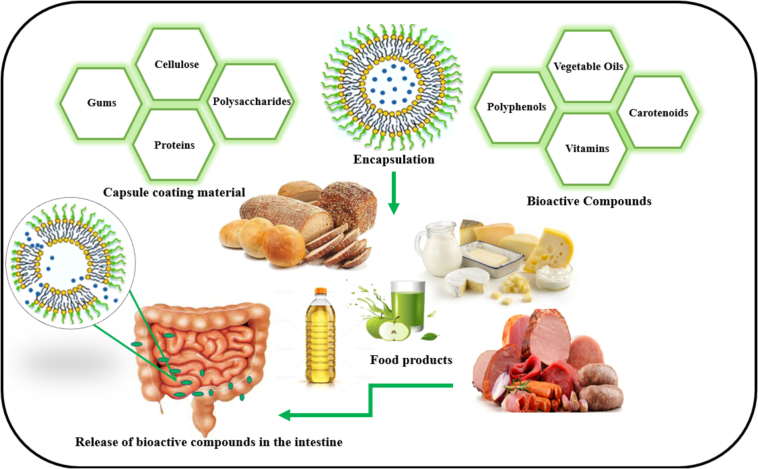
Bioactive compounds that require encapsulation include antioxidants (such as vitamins C and E), polyphenols (such as flavonoids, quercetin, and phenolic acids), omega-3 and omega-6 fatty acids (like EPA, DHA, and linoleic acid), carotenoids (such as beta-carotene, lycopene, and astaxanthin), curcumin, resveratrol, catechins, coenzyme Q10, probiotics, isoflavones (like genistein in soy), sulfur compounds (such as allicin in garlic and onion), essential oils (like peppermint and rosemary oil), and sensitive vitamins like D, K, and folic acid. Due to their instability under light, temperature, oxygen, and digestive conditions, these compounds require encapsulation to prevent degradation, improve absorption, and reduce undesirable taste and odor (Fraj et al., 2021; Jana et al., 2017; Lohith Kumar et al., 2020; Premjit et al., 2023; Qian et al., 2024; Sasi et al., 2023; Tavares et al., 2021; Venugopalan et al., 2021; Zhang et al., 2022).
2.1. Essential fatty acids from vegetable oils
Vegetable oils are rich in essential fatty acids, crucial for cellular health. Omega-3 and omega-6 fatty acids found in oils such as flaxseed, soybean, and canola oil are vital for cardiovascular health, inflammation control, and brain function (Abdi-Moghadam et al., 2023). Research indicates that these fatty acids can reduce chronic disease risks like heart disease and arthritis by lowering blood pressure, reducing triglycerides, and minimizing inflammation (Altememy et al., 2022; Bahmani et al., 2022; Darvishi et al., 2022; Falahi et al., 2019; Khan et al., 2023; Saini et al., 2021). For instance, alpha-linolenic acid (ALA) in flaxseed oil is linked to lower stroke and heart disease risks (Jayedi & Shab-Bidar, 2020). Similarly, omega-6 fatty acids in sunflower and safflower oils are essential for skin health and cell membrane integrity (Djuricic & Calder, 2022; Naderi et al., 2024).
2.2. Vitamins
Vitamins are organic compounds essential for various metabolic processes. Fat-soluble vitamins (A, D, E, and K) and water-soluble vitamins (C and B-complex) are critical for immune function, energy production, and cell repair. Vitamin D, sourced from sunlight and fortified foods, promotes calcium absorption and bone health, reducing osteoporosis risk (Jodar et al., 2023; Sutherland et al., 2021). Moreover, vitamin D modulates the immune response, potentially lowering autoimmune disease risks (Sîrbe et al., 2022). Vitamin C, abundant in citrus fruits, is a powerful antioxidant that boosts immune function by stimulating white blood cell production (Jafari et al., 2019). Additionally, vitamin E in nuts and seeds protects cells from oxidative damage, potentially lowering chronic disease risks such as heart disease and cancer (Didier et al., 2023).
2.3. Polyphenols
Polyphenols, found in fruits, vegetables, tea, coffee, and wine, are known for their antioxidant properties. These compounds, including flavonoids, phenolic acids, and stilbenes, can neutralize free radicals, protecting cells from oxidative stress and reducing chronic disease risks such as cancer and cardiovascular diseases (Fraga et al., 2019; Rana et al., 2022; Roman et al., 2019). For example, green tea polyphenols, particularly catechins, improve endothelial function and reduce atherosclerosis risk (Farhan, 2022). Resveratrol, a polyphenol in red wine and grapes, is associated with anti-aging effects and improved cardiovascular health by enhancing mitochondrial function and reducing oxidative stress (Balanov, Smotraeva, Abdullaeva, Volkova, & Ivanchenko, 2021).
2.4. Carotenoids
Carotenoids, including beta-carotene, lycopene, lutein, and zeaxanthin, are pigments responsible for the red, yellow, and orange colors in many fruits and vegetables. These potent antioxidants help reduce certain cancers and eye diseases. Beta-carotene in carrots and sweet potatoes is a precursor to vitamin A, essential for vision and immune function (Johra et al., 2020; Mrowicka et al., 2022). Lycopene in tomatoes is linked to lower prostate cancer and cardiovascular disease risks (Cheng et al., 2019; Mirahmadi et al., 2020). Lutein and zeaxanthin in green leafy vegetables like spinach and kale are crucial for eye health, potentially reducing age-related macular degeneration (AMD) risks (Mrowicka et al., 2022).
2.5. Pigments
Natural food colorants are bioactive substances extracted from plants, animals, and microorganisms that add color to food products. With growing consumer interest in healthier, more natural ingredients, the food industry increasingly favors natural pigments over synthetic dyes. These natural pigments not only improve the appearance of food but also contribute health benefits, such as antioxidant, anti-inflammatory, and antimicrobial effects.
2.5.1. Curcumin
Curcumin, the primary polyphenol in turmeric, is widely recognized for its extensive therapeutic properties. This compound, responsible for turmeric's distinct yellow hue, has been the focus of numerous studies due to its anti-inflammatory, antioxidant, and anticancer activities. Curcumin's anti-inflammatory effects are largely due to its ability to inhibit key molecular pathways, including transcription factors such as NF-κB and enzymes like COX-2 and iNOS. These mechanisms lead to a reduction in pro-inflammatory cytokines, which are crucial in the pathogenesis of chronic inflammatory diseases like rheumatoid arthritis and inflammatory bowel disease (Hewlings & Kalman, 2017).
In addition to its anti-inflammatory role, curcumin exhibits strong antioxidant properties. It neutralizes reactive oxygen species (ROS) and promotes the activity of antioxidant enzymes, thereby protecting cells from oxidative stress (Rezagholizade-shirvan et al., 2022). This function is vital in slowing the aging process and in the prevention of neurodegenerative conditions, such as Alzheimer's disease (Gupta et al., 2013). Curcumin has also demonstrated significant potential in cancer therapy, where it induces apoptosis and inhibits the proliferation of cancer cells by regulating signaling pathways like PI3K/Akt and Wnt/β-catenin. However, its clinical use is hindered by poor bioavailability, prompting ongoing research into advanced delivery systems, such as nanoparticles and liposomes, to enhance its therapeutic potential (Maleki Dizaj et al., 2022; Rezagholizade-Shirvan, Kalantarmahdavi, & Amiryousefi, 2023).
2.5.2. Anthocyanins
Among the most commonly used natural pigments are anthocyanins, which provide red, purple, and blue colors in fruits and vegetables like berries, grapes, and red cabbage. As members of the flavonoid family, anthocyanins exhibit strong antioxidant properties (Khoo, Azlan, Tang, & Lim, 2017).
These pigments play a key role in counteracting oxidative stress by neutralizing free radicals, thereby protecting cells from damage. Additionally, anthocyanins' anti-inflammatory effects contribute to lowering the risk of cancer and cardiovascular issues. Recent studies have also linked them to improved cognitive and visual functions, with evidence suggesting that they can enhance memory and support eye health (Ma et al., 2021).
In the food industry, anthocyanins are increasingly used as natural colorants, providing a safer alternative to synthetic dyes. However, their practical application is limited by instability under environmental factors like pH changes, heat, and light exposure. To overcome these challenges, new techniques such as encapsulation are being developed to improve anthocyanin stability and bioavailability (Pereira et al., 2024). Anthocyanins face challenges in therapeutic use due to low absorption rates in the human body. Researchers are investigating methods to enhance their bioavailability, including structural modifications and encapsulation technologies (Salehi et al., 2020).
2.5.3. Chlorophyll
Chlorophyll is a group of green pigments essential for photosynthesis, allowing plants, algae, and cyanobacteria to convert sunlight into chemical energy. The main types—chlorophylls a, b, c, d, and f vary slightly in their chemical structure, with chlorophyll-a being the most prominent for energy capture. Spirulina and other microalgae are particularly rich sources of chlorophyll, often used in nutritional products (Ke et al., 2021).
Chlorophyll is a well-known pigment primarily associated with photosynthesis in green plants. Beyond imparting a green color to foods like spinach, kale, and green beans, chlorophyll has been explored for its potential health benefits, such as promoting detoxification. However, its application in food is limited by its instability under light and acidic conditions, which complicates its use as a colorant (Cortez et al., 2017). This pigment also offers several health benefits, acting as an antioxidant and anti-inflammatory agent. It has been linked to the prevention of cardiovascular diseases, regulation of blood sugar, and protection against neurodegenerative disorders (Ebrahimi et al., 2023; Martins et al., 2023).
In food and pharmaceutical industries, chlorophyll is valued as a natural colorant and health supplement, providing a safer alternative to synthetic dyes. Its bioactivity is influenced by the source, with microalgae-derived chlorophyll often showing greater effectiveness than plant-based chlorophyll (Yang et al., 2024). However, commercial production faces challenges, such as extraction efficiency and stability during processing. Advances in biotechnology are helping to overcome these limitations, making chlorophyll a promising component in health and industrial applications (Mehdipoor Damiri et al., 2021).
2.5.4. Betalains
Betalains, found in beets, Swiss chard, and amaranth, include pigments like betacyanins and betaxanthins. These pigments are valued for their antioxidant and anti-inflammatory properties, and research suggests they may help reduce oxidative stress and inflammation, contributing to the prevention of chronic diseases They are categorized into two main types: betacyanins, which impart red to violet colors, and betaxanthins, responsible for yellow to orange hues (Gengatharan et al., 2015).
Beetroots are a significant commercial source of betalains, particularly betanin, but the use of beetroot extracts can come with challenges, including earthy flavors and limited color variety. On the other hand, cactus fruits such as dragon fruit and prickly pear provide a broader spectrum of colors with fewer taste issues, making them appealing options for food applications (Fu et al., 2020).
However, betalains are sensitive to various environmental factors, including pH levels, temperature, and light exposure, which can affect their stability. They tend to be more stable in slightly acidic conditions, whereas high temperatures or light can lead to degradation. Innovations like enzymatic pre-treatment and pulsed electric field technology are being investigated to enhance extraction efficiency and preserve the integrity of these pigments during processing(Calva-Estrada et al., 2022). The growing interest in natural food additives, combined with the health-promoting properties of betalains, has led to their potential use as functional ingredients. Their antioxidant capabilities may help mitigate oxidative stress, which is linked to chronic conditions such as cancer and cardiovascular diseases (Esquivel, 2024).
Natural food colorants are generally less stable than synthetic dyes. This instability can compromise the visual quality of food products. Additionally, the processes for extracting and purifying natural pigments are often more complex and expensive than those for synthetic alternatives. Nevertheless, the increasing consumer demand for natural ingredients is driving innovations to improve the stability and application of these pigments in the food industry (Esquivel, 2024; Novais et al., 2022).
2.6. Probiotics, prebiotics, and postbiotics
The human gut microbiota, a complex ecosystem of microorganisms residing in the gastrointestinal tract, significantly influences overall health. Recent research has increasingly focused on dietary strategies to modulate this microbiota, specifically through the use of probiotics, prebiotics, and postbiotics. Each of these elements plays a distinct role in promoting gut health and maintaining balance within the microbiota (Hajiagha et al., 2022; Shojaeimeher et al., 2024).
Probiotics are defined as live microorganisms that confer health benefits when administered in sufficient quantities. Commonly utilized strains include Lactobacillus and Bifidobacterium. These probiotics help sustain a healthy microbial equilibrium in the gut by suppressing pathogenic bacteria and reinforcing the intestinal barrier. Additionally, they positively influence immune function and reduce inflammation, which has implications for managing gastrointestinal conditions such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). The effectiveness of probiotics can vary by strain and is influenced by factors such as the individual's health status and diet (Hill et al., 2014). Prebiotics are non-digestible components, typically fibers like inulin, fructooligosaccharides (FOS), and galactooligosaccharides (GOS), that selectively enhance the growth and activity of beneficial gut bacteria. These prebiotics reach the colon intact and are fermented by the microbiota, resulting in the production of short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate. SCFAs play a crucial role in gut health by lowering the intestinal pH, which inhibits pathogenic bacteria, and by providing energy to colonic cells (Slavin, 2013; You et al., 2022). Furthermore, prebiotics support the proliferation of probiotics, thereby amplifying their positive effects.
Postbiotics refer to the metabolic by-products or bioactive substances produced by probiotics during fermentation processes. These include SCFAs, bioactive peptides, enzymes, and cell wall components. Postbiotics exhibit various beneficial properties, such as anti-inflammatory, antioxidant, and antimicrobial effects, and they can aid in the management of metabolic disorders and gastrointestinal conditions (Yousefi et al., 2022). Unlike probiotics, postbiotics do not involve live microorganisms, thus mitigating issues related to the viability and stability of probiotics throughout storage and gastrointestinal transit (Aguilar-Toalá et al., 2018; Chaudhari & Dwivedi, 2022).
3. Encapsulation techniques
Encapsulation techniques have found extensive applications across various industries. In the food sector, they are used to enhance the stability and bioavailability of functional ingredients, such as vitamins, antioxidants, and probiotics (Reque & Brandelli, 2021). In pharmaceuticals, encapsulation improves the delivery and controlled release of drugs, thus enhancing therapeutic efficacy (Zhang, Hai, et al., 2021). The future of encapsulation lies in developing more efficient and sustainable techniques, such as using biopolymers and green solvents, to meet the increasing demand for natural and eco-friendly products (Dahiya et al., 2023). Encapsulation technology in the food sector faces several significant drawbacks that can limit its effectiveness. One of the primary challenges is the elevated production costs associated with advanced techniques, which may discourage smaller food manufacturers from adopting these methods(Misra et al., 2021; Tarone et al., 2020).
Variations in encapsulation efficiency can complicate product formulations, necessitating comprehensive testing to achieve desired outcomes (Bhatt et al., 2018; Fernandes et al., 2024).
Moreover, incomplete release of the encapsulated active ingredients during storage or consumption can diminish their effectiveness, particularly in applications focused on flavor enhancement or nutritional benefits (Bao et al., 2019; Boostani & Jafari, 2021). This issue is especially relevant for bioactive compounds, where their efficacy relies on precise release mechanisms. Among the various encapsulation techniques, spray-drying, freeze-drying, coacervation, and emulsification stand out due to their effectiveness and versatility (Premjit et al., 2022). The encapsulation of bioactive compounds is an efficient method to improve their stability, bioavailability, and controlled release. However, the effectiveness of these methods can be influenced by factors such as the type of bioactive compound, polymer selection, and processing conditions. Bioactive compounds with diverse chemical and physical properties (such as solubility and sensitivity to temperature or pH) require different encapsulation methods to be effectively protected against environmental conditions and to provide desirable functional characteristics (Dib et al., 2023; Pateiro et al., 2021). Each technique offers unique advantages and is chosen based on the specific requirements of the bioactive compound and the desired application. Ongoing research and technological advancements will continue to enhance these methods, making them more efficient and sustainable, thereby expanding their applications and benefits (Table 1) (See Fig. 1).
Table 1.
Investigations into the nanoencapsulation of bioactive substances utilizing various nanocarriers.
| Enriched/Fortified food | Bioactive compound | Capsule wall compositions | Encapsulation Techniques | Reference | |
|---|---|---|---|---|---|
 Meat products |
Fresh bovine meat | Tunisian thyme essential oil | Chitosan, gum Arabic /Median-chain triacyglycerols | Nanoemulsion | (Snoussi et al., 2022) |
| Chilled pork | Satureja montana L. essential oil | Soy soluble polysaccharide | Hydrogel | (Cui et al., 2021) | |
| Sheep meat hamburger | Olive leaf extract (Olea europaea L.) | Gelatin/tragacanth gum | Coacervation | (Oliveira et al., 2022) | |
| Chicken nuggets | Fish oil | Carrageenan /Gum tragacanth | Freeze dryer | (Pourashouri et al., 2021) | |
| Pork meat (fresh post-rigor raw pork, mixture of biceps femoris, semimembranosus, semitendinosus, gracilis and aductor) and pork backfat | Olive leaves extract | Healthy oil blend (olive oil, linseed oil and fish oil, polyglycerol ester of polyricinoleic acid) | Double emulsions | (Robert et al., 2019) | |
| Hamburger-like meat products | Thyme (Thymus vulgaris) essential oil | Casein/maltodextrin | Spray drying | (Radünz et al., 2020) | |
| Minced beef | Laurus nobilis leaf extract | Lecithin | Nano-liposome | (Tometri et al., 2020) | |
| Reduced-fat meat batter | Murraya koenigii berry extract | Table oil | Double emulsion | (Kumar & Kumar, 2020) | |
| Beef | Pomegranate peel extract | Chitosan/Poly (ethylene oxide) | Electrospun | (Surendhiran et al., 2020) | |
| Chicken meat | Peel extract of Punica granatum L. | Alginate/ Calcium chloride | Nanoemulsion | (Rahnemoon et al., 2021) | |
| Beef meat | Rosemary extract | Basil seed gum /Soybean protein | Freeze dryer | (Rashidaie Abandansarie et al., 2019) | |
| Chicken meat | Phlorotannin | Sodium alginate/Poly(ethyleneoxide) | Electrospinning technique | (Surendhiran et al., 2019) | |
| Patty-type products based on beef (gluteus medius) | Fish Oil | Alginate/Chitosan/ P. alba exudate gum | Emulsion | (Vasile et al., 2019) | |
| Beef Burger Patties | Prickly Pear Fruit Extract | Alginate | Spray-Drying | (Parafati et al., 2019) | |
| Sausage | Animal fat | Pectin | Emulsification process | (Santiaguín-Padilla et al., 2019) | |
| Fresh meat | Eugenol | Gelatin-based emulgel | Spray-Drying | (Wan et al., 2020) | |
 Dairy products |
Milk powders | Curcumin | Casein micelles | Spray-drying |
(Neves et al., 2019) |
| Cow milk / goat milk | Bifidobacterium longum | Alginate | Extrusion method | (Prasanna & Charalampopoulos, 2018) | |
| Whole milk | Isoflavone | Maltodextrin /Gum acacia | Spray-Drying | (Mazumder & Ranganathan, 2020) | |
| Probiotic fermented milk | Lactobacillus casei | Alginate | Extrusion method | (Dimitrellou et al., 2019) | |
| Goats' milk yogurt | Bifidobacterium | Alginate/Goats' milk/inulin | Freeze dryer | (Pradeep Prasanna & Charalampopoulos, 2019) | |
| Lassi (A milk based beverage) | Vitamin D3 | Caprylic-/Capric triglyceride | Nano-liposome | (Maurya & Aggarwal, 2019) | |
| Yogurt | Beta vulgaris | Maltodextrin/ inulin | Freeze dryer | (Flores-Mancha et al., 2021) | |
| Yogurt | Red pepper waste | Whey protein | Freeze dryer | (Šeregelj et al., 2019) | |
| Yogurt | Lacticaseibacillus rhamnosus | Ionic gelation | Emulsification process | (Romero-Chapol et al., 2022) | |
| Yogurt | Carrot waste extract | Alginate | Freeze dryer | (Šeregelj, Pezo, et al., 2021) | |
| Yogurt | Lactobacillus bulgaricus | Sodium alginate/pectin | Freeze dryer | (Hu et al., 2021) | |
| Yogurt | Anthocyanin | Gum arabic | Spray-Drying | (Barretto et al., 2020) | |
| Yogurt | Fish protein hydrolysate | Complex of whey protein concentrate / Inulin | Emulsification process | (Jamshidi et al., 2019) | |
| Cheddar cheese | Bifidobacterium bifidum | Kepa carrageenan /sodium alginate | Freeze dryer | (Afzaal et al., 2020) | |
| Ricotta Cheese | Saffron Extract | Soy lecithin | Liposome | (Siyar et al., 2021) | |
| White soft cheese | Olive polyphenolic extract | Maltodextrin / Whey protein isolate /Skim milk powder | Freeze dryer | (Farrag et al., 2020) | |
| Ricotta cheese | Broccoli sprout extract | Basil seeds gum | Freeze dryer | (Azarashkan et al., 2022) | |
| Iranian white cheese | Lactobacillus plantarum and phytosterols | Whey protein isolate / Gum Arabic | Freeze dryer | (Sharifi et al., 2021) | |
 Cereal products |
Cereal cookies | Bacteriophages | Alginate / Calcium ions / /Milk proteins | Spray-Drying | (Schubert et al., 2022) |
| Bread | Lemongrass essential oil | Cassava starch fibers | Electrospinning | (da Cruz et al., 2023) | |
| Bread | Lactobacillus acidophilus | Alginate/Fish Gelatin | Liposome | (Hadidi et al., 2021) | |
| Gluten-free sorghum bread | Lactobacillus acidophilus /Lactobacillus plantarum | Tragacanth gum /Sago starch | Spray-Drying | (Ghasemi et al., 2022) | |
| Bread / Biscuits | Fenugreek oil | Okra / Psyllium mucilage / Whey protein / Gum Arabic | Freeze dryer | (Mohammad et al., 2024) | |
| Wheat bread | Rosehip extract | Sodium alginate | Freeze dryer | (Şimşek & Özgen, 2023) | |
| Durum Wheat Pasta | Carrot Waste | Sunflower oil | Spray-Drying | (Šeregelj et al., 2022) | |
| Pasta | Wild Garlic (Allium ursinum) | Maltodextrin | Spray-Drying | (Filipčev et al., 2023) | |
| Modified pasta | Apple pomace /finger millet | Maltodextrin / Hydroxypropyl Methylcellulose | Spray-Drying | (Kumari, Arya, et al., 2023) | |
| Eggless cake with chia | Flax seed oil | Gum Arabic / Soy-protein isolate / Sodium caseinate / Sodium alginate | Spray-Drying | (Murugkar et al., 2021) | |
| Cake | Lacticaseibacillus rhamnous | Flaxseed oil | Spray-Drying | (Łopusiewicz et al., 2021) | |
| Biscuits | mangosteen (Garcinia mangostana L.) peel extract | Arabic gum | Spray-Drying | (Indiarto et al., 2023) | |
| Millet biscuit | Lactobacillus acidophilus | Maltodextrin / Gum arabic | Spray-Drying | (Arepally et al., 2023) | |
 Drinks |
Apple Juice | Lacticaseibacillus rhamnosus | Ionic gelation | Emulsification process | (Romero-Chapol et al., 2022) |
| Apple Juice | Lactobacillus gasseri | Alginate | Emulsification process | (Varela-Pérez et al., 2022) | |
| Orange juice | Lactobacillus casei tocotrienol | Calcium alginate-Carboxymethyl cellulose hydrogel beads | Emulsification process | (Sultana et al., 2023) | |
| Raspberry juice | Fresh raspberry fruits | Gum Arabic / Maltodextrin / Waxy starch | Freeze dryer | (Nthimole et al., 2022) | |
| Clidemia japurensis / Clidemia hirta juices | Bioactive compounds | Maltodextrin | Freeze dryer | (Mar et al., 2020) | |
| Bitter melon juice | Indian bitter melon | Maltodextrin /Gum Arabic / Pectin/Soy protein isolate | Spray-Drying | (Wang et al., 2021) | |
| Orange-carrot juice | Arctium lappa L. root extracts | Maltodextrin /Gum Arabic | Spray-drying /Freeze-drying | (Esmaeili et al., 2022) | |
| Orange juice | Resveratrol / Epigallocatechin gallate | Low methoxyl pectin | Liposome | (Feng et al., 2020) | |
|
Orange juice |
Lacticaseibacillus rhamnosus | Alginate | Freeze dryer | (Bonaccorso et al., 2021) | |
|
Goldenberry (Physalis peruviana L.) juice |
Carotenoids | Maltodextrin/ Modified starch/ Inulin/Alginate/ Gum arabic | Spray-Drying | (Etzbach et al., 2020) | |
| The spray dried fruits juice powders | Vitamin C | Maltodextrin | Spray-Drying | (Rohini et al., 2024) | |
| Orange juice | Lactobacillus rhamnosus | Alginate / Xanthan gum | Emulsification process | (Ali et al., 2023) | |
Fig. 1.
Encapsulation of bioactive compounds for food enrichment
3.1. Spray-drying
Spray-drying is a prevalent encapsulation method involving the atomization of a liquid mixture into a hot drying chamber, causing rapid solvent evaporation and dry particle formation. This method is preferred for its cost-efficiency and scalability. In the food industry, spray-drying is extensively used to encapsulate flavors, probiotics, and vitamins. For example, encapsulating probiotics using spray-drying enhances their thermal stability and survival during storage and digestion (Furuta & Neoh, 2021). Additionally, spray-dried encapsulates exhibit excellent rehydration properties, making them ideal for instant food products (Kandasamy & Naveen, 2022).
However, a significant limitation of this method is the high temperatures required, which can degrade heat-sensitive bioactives, reducing their bioactivity and overall effectiveness. To mitigate this issue, researchers have explored several strategies. One approach involves using heat-resistant wall materials, like maltodextrin or milk proteins, which act as thermal barriers and partially protect sensitive compounds from heat. Another strategy includes optimizing spray-drying parameters, such as lowering the inlet air temperature and increasing the feed flow rate, to decrease thermal stress. Additionally, alternative drying methods, such as freeze-drying, provide a gentler option, though they are more costly. The use of two-stage encapsulation techniques is also promising, as it minimizes the direct exposure of bioactive compounds to high temperatures, thereby improving their stability and preserving their functional properties in food applications (Akbarbaglu et al., 2021; Kandasamy & Naveen, 2022).
3.2. Freeze-drying
Freeze-drying, or lyophilization, involves freezing a solution and then lowering the surrounding pressure to allow the frozen solvent to sublimate directly from solid to gas. This technique preserves the structural integrity and activity of sensitive compounds, making it suitable for encapsulating heat-sensitive substances such as enzymes and probiotics (Rezvankhah et al., 2020). Freeze-drying produces porous particles that can enhance the dissolution rate of poorly soluble drugs, thereby improving their bioavailability (Jakubowska & Lulek, 2021). Moreover, freeze-dried encapsulates are characterized by their long shelf life and stability under ambient conditions (Zhang et al., 2020).
This technique is significantly more expensive compared to alternatives like spray-drying, primarily due to its high energy consumption and extended processing times necessary for water removal through sublimation. The process requires specialized equipment and precise control of temperature and pressure, leading to increased operational costs. Despite these challenges, freeze-drying remains valuable for applications where maintaining the bioactivity and quality of the compounds is crucial, such as in the food and pharmaceutical industries, as the resulting products are highly stable and retain their functional properties effectively (El-Messery et al., 2020; Eun et al., 2020; Rezvankhah et al., 2020).
3.3. Coacervation
Coacervation is a liquid-liquid phase separation process that forms a coacervate phase, rich in encapsulating material, and a supernatant phase. This technique is often employed for encapsulating hydrophobic and sensitive bioactive compounds. Coacervation can be simple or complex, depending on whether it involves a single or multiple polymers (Gómez-Mascaraque et al., 2022). For example, complex coacervation using gelatin and gum arabic has been used to encapsulate essential oils, enhancing their stability against oxidation and volatilization (Khatibi et al., 2021). Coacervates can form microcapsules with controlled release properties, beneficial for applications in controlled drug delivery (Muhoza et al., 2022).
3.4. Emulsification
Emulsification involves dispersing one liquid in another immiscible liquid, typically resulting in oil-in-water (O/W) or water-in-oil (W/O) emulsions. This technique is crucial for encapsulating hydrophobic compounds within a hydrophilic matrix. Emulsification can be achieved through various methods, including high-shear mixing, ultrasonic emulsification, and membrane emulsification (Santosa et al., 2021). Nanoemulsions, with their small droplet size, offer improved stability and bioavailability of encapsulated compounds (Pateiro et al., 2021). Emulsification is extensively used in the food industry for delivering flavors, colors, and nutraceuticals in beverages and dairy products (Tan & McClements, 2021).
4. Nano-carrier systems for encapsulation
This technique involves encasing active ingredients within nanometer-sized carriers, which enhances their stability, controlled release, and targeted delivery. One key advantage of nanoencapsulation is the protection it provides to bioactive compounds from environmental factors like light, oxygen, and pH changes, thereby extending the shelf life of sensitive ingredients such as vitamins, antioxidants, and probiotics (Lin et al., 2023). The nanoscale size of these carriers also allows for better penetration and absorption in biological systems, enhancing the bioavailability of the encapsulated compounds (Le et al., 2021).
Controlled release is another important feature of nanoencapsulation. By adjusting the composition and structure of nanocarriers, it is possible to achieve sustained and targeted release of active ingredients, reducing required dosages and minimizing potential side effects. This is particularly beneficial in pharmaceutical applications, such as cancer therapy, where targeted delivery of chemotherapeutic agents to tumor cells can improve treatment efficacy and reduce systemic toxicity (Mohtashami et al., 2024; Parveen et al., 2023).
In the food industry, nanoencapsulation is used to fortify foods with essential nutrients without altering taste, texture, or appearance. It also allows for more efficient and controlled incorporation of flavors, colors, and preservatives (Hosseini et al., 2021). Additionally, this technology supports the development of functional foods with enhanced health benefits by delivering bioactive compounds more effectively to the site of action within the body (Hosseini et al., 2021). Some of the most important carrier systems are described below:
4.1. Liposome
Liposomes are spherical vesicles composed of one or more phospholipid bilayers. They are extensively used as delivery systems for bioactive compounds due to their biocompatibility, biodegradability, and ability to encapsulate both hydrophilic and hydrophobic substances (Subramani & Ganapathyswamy, 2020). The formation of liposomes involves several steps designed to optimize encapsulation efficiency and the stability of the encapsulated compounds.
The process typically starts with dissolving phospholipids in an organic solvent. Phosphatidylcholine, a common phospholipid found in cell membranes, is frequently used (Kirby & Gregoriadis, 2019). The solvent is then evaporated under reduced pressure to form a thin lipid film on the walls of a round-bottom flask. This lipid film is hydrated with an aqueous solution containing the active ingredient, followed by agitation, leading to the formation of multilamellar vesicles (MLVs) due to the hydrophobic interactions between the phospholipid tails and the aqueous medium (Tagrida et al., 2021).
To achieve uniform and smaller vesicles, the MLVs undergo processes such as sonication or extrusion. Sonication uses ultrasonic waves to break down MLVs into small unilamellar vesicles (SUVs), while extrusion forces the vesicle suspension through membranes with defined pore sizes to produce vesicles of uniform size (Olson et al., 1979; Sarode et al., 2022). These methods enhance the encapsulation efficiency and stability of liposomes.
Additionally, advancements in liposome preparation techniques, such as the ethanol injection method and reverse-phase evaporation, have improved control over liposome size and encapsulation parameters (ENARU et al. 2023). These methods facilitate the creation of vesicles with varying bilayer compositions and surface modifications, enabling targeted delivery and controlled release of encapsulated compounds (De Leo et al., 2021).
4.2. Hydrogel
Hydrogels are three-dimensional polymer networks that can absorb significant amounts of water, making them excellent candidates for encapsulation applications. Their ability to simulate natural tissue environments and regulate the release of encapsulated substances has led to their widespread use in drug delivery, tissue engineering, and food processing (Zheng et al., 2021).
The process of forming hydrogels usually involves polymerizing monomers in the presence of cross-linking agents, which connect polymer chains to create a network structure. Both natural polymers, such as alginate, chitosan, and gelatin, and synthetic ones like polyvinyl alcohol (PVA) and polyethylene glycol (PEG) are utilized due to their biocompatibility and customizable characteristics (Tang et al., 2022). The selection of polymer and cross-linker, along with the polymerization conditions, greatly affects the mechanical properties and swelling behavior of the hydrogel (Xue et al., 2022).
Encapsulation within hydrogels can occur through various techniques, including physical entrapment, covalent bonding, or ionic gelation. The simplest method is physical entrapment, where the bioactive substance is mixed into the polymer solution prior to gelation. In covalent bonding, bioactive molecules form stable covalent bonds with the polymer network through functional groups (Ahmed, 2015; Montà-González et al., 2022). Ionic gelation, commonly employed with alginate, relies on the interaction between polymer chains and multivalent cations, such as calcium ions, leading to rapid gel formation and encapsulation (Milivojević et al., 2023).
Hydrogels can be designed to respond to specific stimuli, including pH, temperature, or enzymatic activity, allowing for controlled release of encapsulated substances. This feature is particularly advantageous in drug delivery systems, where targeted and sustained release is essential (Li & Mooney, 2016).
4.3. Hybrid Nano-carriers
Hybrid nano-carriers, which combine both organic and inorganic materials, are emerging as key technologies in the encapsulation of food ingredients. These advanced systems enhance the stability, bioavailability, and functionality of various components by utilizing the distinct advantages of both organic and inorganic substances (Assadpour & Jafari, 2019; Khosravi-Darani et al., 2019).
Typically, hybrid nano-carriers consist of organic materials such as polysaccharides, proteins, or lipids combined with inorganic elements like silica, metal nanoparticles, or clay. This combination allows for the development of nano-carriers with improved properties, such as greater mechanical strength, thermal stability, and controlled release capabilities (Farhadi et al., 2022; Mohtashami et al., 2024; Rout et al., 2022; Shokri et al., 2024). For example, silica nanoparticles contribute structural stability and protection, while organic polymers enhance the carrier's biocompatibility and processing ease (Kankala et al., 2020). A significant use of hybrid nano-carriers in food encapsulation is the protection of sensitive bioactive compounds, including vitamins, probiotics, and antioxidants. These compounds are vulnerable to degradation from light, oxygen, or high temperatures during food processing and storage. By encapsulating these ingredients in hybrid nano-carriers, their stability and efficacy can be preserved, thereby extending their shelf life (Meng, 2017; Rezagholizade-Shirvan et al., 2024). For instance, hybrid nano-carriers have been used to improve the stability and release properties of vitamin C, ensuring its effectiveness in various food products (Comunian et al., 2022).
Moreover, hybrid nano-carriers enable controlled and sustained release of encapsulated substances. Through the precise design of these carriers, it is possible to achieve a gradual release of ingredients over time, enhancing the functionality of food products. This controlled release can be utilized to improve flavor or nutritional content by providing a steady release of components. Additionally, hybrid nano-carriers can be engineered to respond to specific environmental triggers, such as changes in pH or temperature, allowing for targeted ingredient release in different food matrices (Men et al., 2019). A study focused on the use of F-ZnO nanoparticles (NPs) as antibacterial agents, which were immobilized onto a pectin/cellulose nanofiber (CNF) aerogel skeleton through the adhesive properties of mussel-inspired dopamine. The resulting antimicrobial mats were designed for safety, long-lasting efficacy, and high performance. This aerogel was lightweight, maintained its shape on petals, featured a uniform pore structure, and exhibited strong tensile and compressive properties alongside significant antibacterial and antifungal activity. When strawberries were packaged in the F-ZnO@D-CNF aerogel, they enjoyed an extended shelf life of five days. Additionally, strawberries showed notable advantages in weight loss, color retention, and firmness compared to other groups. The final zinc ion concentration in the strawberries was measured at 3.71 ± 0.28 μg/g, which is well below the recommended dietary intake, ensuring safety for consumers (Wu et al., 2023).
5. Absorption, stability, functionality, bio accessibility and bioavailability of encapsulated bioactive compounds
The encapsulation of bioactive compounds is increasingly vital in food science and pharmaceuticals, aimed at improving the effectiveness and delivery of nutrients and active ingredients. Compounds such as polyphenols, vitamins, and essential fatty acids are prone to environmental degradation, low solubility, and poor bioavailability, which limits their therapeutic impact (Ghorbani et al., 2024; Klojdová et al., 2023).
Stability is essential for maintaining the effectiveness of bioactive compounds. Encapsulation methods like spray drying, freeze drying, and coacervation provide a protective barrier against factors such as oxidation, heat, and light (Eun et al., 2020). For instance, encapsulated curcumin shows significantly better stability compared to its non-encapsulated form. Research by Chen et al. (2020) indicates that encapsulation shields curcumin from hydrolytic degradation, prolonging its shelf life and preserving its antioxidant properties (Chen et al., 2020).
Functionality of bioactive compounds depends on their ability to remain biologically active after ingestion. Enhancing functionality is critical for creating effective nutraceuticals and functional foods that provide health benefits (Banwo et al., 2021). Encapsulation can enhance the solubility and dispersibility of hydrophobic compounds in water, improving their functionality. For example, the solubility and antioxidant activity of resveratrol, a hydrophobic polyphenol, are improved when encapsulated in cyclodextrin complexes (Yang et al., 2022).
Encapsulation significantly enhances bioaccessibility by protecting bioactives during digestion and facilitating their release in the intestines. Encapsulated omega-3 fatty acids in alginate beads, for instance, demonstrate improved bioaccessibility due to protection against gastric degradation and controlled release in the intestines. This ensures a higher proportion of the compound is available for absorption (Kovsari et al., 2024; Salvia-Trujillo et al., 2021).
There are instances when not all encapsulated compounds are released during digestion, which can limit their absorption and overall effectiveness. Several factors influence the release of these compounds, including the choice of encapsulating material, the gastrointestinal environment, and the interactions between the encapsulated compounds and food matrices. Incomplete release can impede the anticipated health benefits associated with these bioactive compounds. To improve the efficacy of encapsulated products, researchers are focusing on developing innovative delivery systems that enhance the controlled release of these compounds throughout the digestive tract, thereby boosting their absorption and maximizing their therapeutic potential in various applications, including functional foods and nutraceuticals(Carrasco-Sandoval et al., 2021; Gonçalves et al., 2021).
Bioavailability refers to the fraction of a bioactive compound that reaches the systemic circulation and exerts its physiological effects. Techniques such as liposomes, nanoparticles, and polymeric micelles are used to address challenges related to poor solubility, instability, and low permeability (Gorantla et al., 2021; Yang et al., 2020). For example, liposomal encapsulation of quercetin enhances its bioavailability by increasing its solubility and facilitating its transport across intestinal barriers (Tomou et al., 2023). However, their stability can be compromised under certain conditions, such as fluctuations in temperature, pH, or ionic strength, which can result in the leakage of encapsulated compounds. This leakage can severely impact the efficacy of bioactive substances, thereby undermining the advantages of encapsulation. To overcome these limitations, researchers are investigating various approaches to enhance the stability of liposomes, including the incorporation of stabilizing agents, optimization of formulation parameters, and the development of hybrid systems that integrate liposomes with other encapsulation techniques. By improving liposomal stability, it becomes feasible to preserve the integrity of encapsulated compounds and enhance their delivery and effectiveness in diverse applications, including pharmaceuticals and functional foods(Hamad et al., 2024; Maritim et al., 2021). Additionally, polymeric nanoparticles improve the oral bioavailability of poorly soluble drugs by enhancing their dissolution rate and protecting them from enzymatic degradation (Bhalani et al., 2022).
Besides enhancing bioactive properties, encapsulation also enables targeted delivery and controlled release, which are crucial for maximizing therapeutic efficacy and minimizing side effects. Stimuli-responsive delivery systems, which release their payload in response to specific triggers like pH, temperature, or enzymatic activity, are particularly promising. For example, pH-responsive polymeric nanoparticles have been designed to release encapsulated compounds in the acidic environment of the stomach, ensuring site-specific delivery and improved therapeutic outcomes (Ding et al., 2022; Kong et al., 2020).
Actually, encapsulating bioactive compounds offers a comprehensive approach to enhancing their stability, functionality, bioaccessibility, and bioavailability. By protecting bioactives from environmental and physiological degradation, improving solubility, and allowing for controlled release, encapsulation technologies significantly enhance the efficacy of bioactive compounds in food and pharmaceutical applications. Future research should aim to optimize encapsulation methods and materials to further improve the delivery and effectiveness of bioactive compounds, with a focus on the interactions between encapsulated bioactives and biological systems.
6. Compositions of the capsule wall
6.1. Polysaccharides
Polysaccharides are large carbohydrate molecules made up of long chains of monosaccharides linked by glycosidic bonds. These macromolecules exhibit a wide range of structural and functional diversity, playing essential roles in various biological systems. Polysaccharides can be categorized into two main types based on their monosaccharide composition: homopolysaccharides, which consist of identical monosaccharides, and heteropolysaccharides, which are made up of different monosaccharides (Shokri et al., 2023; Silva et al., 2021).
Polysaccharide-based encapsulation systems are often chosen for their biocompatibility and ability to effectively encapsulate bioactive compounds. However, a major limitation of these systems is their potential lack of mechanical strength, which can lead to capsule rupture under stress or during handling. This susceptibility may cause premature release of the encapsulated compounds, compromising their stability and effectiveness. Factors influencing the mechanical weakness of polysaccharide capsules include the type of polysaccharide used, the encapsulation method, and the drying and storage conditions. To mitigate these issues, researchers are investigating various strategies to enhance the mechanical properties of polysaccharide-based systems. Approaches such as cross-linking with other materials, integrating reinforcing agents, and optimizing encapsulation techniques are being explored to improve the robustness of these capsules, ensuring effective delivery of bioactive compounds in diverse applications, including food products and pharmaceuticals(Junaid et al., 2024; Papagiannopoulos & Sotiropoulos, 2022; Visan & Cristescu, 2023).
In Table 1 polysaccharides, including alginates, chitosan, amylopectin, and amylose, are widely used for food encapsulation. Amylopectin and amylose, create gel-like matrices that are useful for encapsulating oils and oxygen-sensitive substances. Amylose has a linear and unbranched structure, which makes it less soluble in water and results in the formation of a more stable gel. In contrast, amylopectin has a branched structure that allows it to dissolve more easily in water, leading to a gel with lower viscosity and less stability. (Zhu, 2017). Alginates, derived from brown seaweeds, can form stable gels in the presence of calcium ions, making them suitable for encapsulating probiotics, nutrients, and flavors (Bennacef et al., 2021).
Other notable polysaccharides include pectin, found in fruit cell walls and used in the food industry for gelling, and chitin, a structural polysaccharide in the exoskeletons of arthropods and fungal cell walls. Hyaluronic acid, a heteropolysaccharide, is vital in the extracellular matrix of connective tissues, helping maintain hydration and elasticity (Van Audenhove et al., 2023).
Chitosan is a biopolymer obtained from the deacetylation of chitin, which is mainly sourced from the shells of crustaceans. Owing to its non-toxic, biodegradable, and biocompatible properties, chitosan has become highly valued in various biomedical fields, particularly in drug delivery and encapsulation. In the context of encapsulation, chitosan acts as an efficient vehicle for active compounds, improving their stability, bioavailability, and providing controlled release mechanisms. Its cationic nature at acidic pH enables it to bind with anionic molecules, making it suitable for encapsulating a wide range of substances, including pharmaceuticals, proteins, and vaccines. Also, it is valued for its antimicrobial properties and biodegradability, ideal for encapsulating probiotics and extending food shelf life (Maleki et al., 2022).
Gums are natural polysaccharides that are extensively used in the food industry, particularly for the encapsulation of various food ingredients. Sourced from plants, seaweeds, and microbial processes, these biopolymers are known for their ability to gel, stabilize emulsions, and regulate the release of encapsulated materials (Taheri & Jafari, 2019b).
Gum arabic, extracted from the acacia tree, is a versatile emulsifier and film-forming agent, often used for encapsulating flavors, essential oils, and vitamins. Its low viscosity and high solubility in water make it particularly suitable for spray drying, where it forms a stable matrix that protects bioactive compounds, thereby enhancing their stability and shelf life (Taheri & Jafari, 2019a).
Xanthan gum, produced by Xanthomonas campestris, is another key gum in encapsulation, known for its high viscosity and gel-forming capabilities. It is particularly effective in encapsulating hydrophilic compounds and regulating their release within the digestive system. Xanthan gum is frequently combined with other gums or proteins to strengthen the encapsulation matrix (Zabot et al., 2022).
Guar gum, derived from guar seeds, is a galactomannan known for its high water-binding and thickening properties. It is commonly used to create gel matrices that encapsulate and protect sensitive ingredients such as probiotics, enzymes, and vitamins. Its strong gel formation at low concentrations makes it a cost-effective option for large-scale applications (Rezagholizade-Shirvan, Kalantarmahdavi, & Amiryousefi, 2023; Rostamabadi et al., 2023).
Alginate and carrageenan, both seaweed-derived, are popular in the creation of encapsulation beads and capsules. Alginate is particularly valued for its ability to gel in the presence of calcium ions, making it ideal for encapsulating probiotics and enzymes in matrices that resist acid. Carrageenan, known for forming thermally reversible gels, is often used in controlled release systems for flavors and other bioactive (Dong et al., 2021; Li et al., 2021).
The application of these gums in encapsulation not only enhances the stability and shelf life of bioactive compounds but also improves their bioavailability and controlled release. These qualities are essential in the production of functional foods, which require precise delivery of health-promoting ingredients (Jayakody et al., 2022).
6.2. Proteins
Proteins such as gelatin and whey protein are essential in food encapsulation due to their emulsifying and gelling properties. Gelatin, sourced from animal collagen, forms gels at low temperatures, making it useful for encapsulating flavors, nutrients, and in producing gummy candies and processed meats (Akbarbaglu et al., 2021). A significant limitation is gelatin tendency to denature at elevated temperatures. This denaturation can negatively impact their encapsulating capabilities, resulting in compromised structural integrity and diminished stability of the encapsulated bioactive compounds. When proteins denature, their three-dimensional structures unfold, which can impair their ability to encapsulate and safeguard sensitive bioactive ingredients from degradation. Factors such as the type of protein, temperature, and exposure duration significantly influence the degree of denaturation. To address these challenges, researchers are investigating various strategies to enhance the thermal stability of protein-based encapsulation systems, including the incorporation of stabilizing additives, cross-linking techniques, and the creation of hybrid encapsulation materials(Luo et al., 2022; Milano et al., 2023; Mohanto et al., 2023).
Bovine serum albumin (BSA) and human serum albumin (HSA) are increasingly recognized for their application in the nanoencapsulation of bioactive compounds within the food sector. These serum proteins are particularly beneficial due to their excellent biocompatibility, stability, and capacity to form robust nanoparticles that can encapsulate a diverse range of bioactive substances, such as vitamins, antioxidants, and essential fatty acids. Utilizing BSA and HSA in nanoencapsulation offers significant benefits, including the protection of sensitive bioactive compounds from environmental stressors like light, oxygen, and moisture, which can otherwise compromise their effectiveness. In food industry applications, BSA and HSA nanoparticles enhance the stability and bioavailability of these bioactive compounds, ensuring their efficacy throughout the product's shelf life. This method not only prolongs the shelf life of the bioactives but also boosts their absorption in the digestive system, thereby amplifying their nutritional and health benefits. Furthermore, the use of BSA and HSA for nanoencapsulation allows for the controlled release of bioactive compounds, which can be customized for specific delivery needs, such as targeted release during digestion. This feature is particularly advantageous for developing functional foods with improved health benefits. The natural origin and safety of BSA and HSA make them suitable for use in food applications, complying with regulatory standards and ensuring consumer safety (Chen et al., 2023; Mainardes & Khalil, 2019; Visentini et al., 2023).
Casein, a primary protein found in milk, is extensively utilized in the food industry due to its versatile functional properties, such as its capacity to form gels, emulsions, and micelles. One of the key applications of casein is in the encapsulation of bioactive compounds, which is particularly important in preserving the nutritional value and health benefits of food products. This encapsulation protects sensitive bioactives like vitamins, probiotics, and antioxidants—from environmental factors that could cause degradation. The use of casein in encapsulation offers numerous benefits. Its amphiphilic structure allows it to effectively encapsulate both hydrophilic and hydrophobic bioactive compounds. Moreover, casein's natural micelle-forming ability contributes to the stabilization and controlled release of these compounds. Additionally, casein's biocompatibility and ease of digestion ensure that these bioactives are efficiently delivered and released in the gastrointestinal tract, thereby improving their bioavailability and absorption. Casein's application in nanoencapsulation is particularly promising for the development of functional foods that aim to deliver health-enhancing compounds in a controlled manner. As research advances, the potential for casein-based encapsulation systems to improve the stability, efficacy, and delivery of bioactive compounds in the food industry continues to grow (Mohite & Waghmare, 2020; Sadiq et al., 2021).
6.3. Fats and oils
The continuous innovation in lipid-based encapsulation technologies holds promise for the development of next-generation functional foods that deliver enhanced health benefits. Triglycerides and liposomes are employed to encapsulate fat-soluble compounds like vitamins A, D, E, K, antioxidants, and omega-3 fatty acids (Nowak et al., 2021). Lipid-based encapsulation systems, such as liposomes, solid lipid nanoparticles, and nanoemulsions, have been extensively researched and applied in functional foods. These systems not only protect bioactives from oxidation, light, and temperature fluctuations but also enhance their absorption in the gastrointestinal tract. Moreover, the use of edible oils and fats aligns with consumer demand for natural and safe food ingredients, making them suitable for clean-label products. Different oils and fats, including those derived from plants (e.g., soybean oil, coconut oil) and animals (e.g., fish oil), are employed based on their unique physicochemical properties. These lipids are particularly effective in encapsulating hydrophobic bioactives due to their ability to form stable emulsions and lipid-based nanoparticles. The selection of specific oils or fats depends on factors such as the desired release profile, the compatibility with the encapsulated compound, and the intended application in food products (Assadpour & Jafari, 2019; Barroso et al., 2021; Gasa-Falcon et al., 2020). A study compared three preparation methods dispersion, ultrasonication, and microfluidization for encapsulating citral in nanostructured lipid carriers (NLC). Results showed that ultrasonication produced NLC with superior stability and lower citral release rates over 35 days compared to microfluidization. The optimized NLC had a particle size of 192.8 nm and an encapsulation efficiency of 78.37 % (Feng et al., 2023).
A significant limitation is lipid-based encapsulation systems susceptibility to oxidation, which can lead to the deterioration of both the encapsulated bioactive substances and the lipids themselves. Oxidative degradation can generate free radicals and harmful by-products, compromising the stability and efficacy of the encapsulated ingredients. Factors such as the type of lipid employed, along with exposure to light, oxygen, and heat, can accelerate oxidation rates. To address these concerns, researchers are exploring various strategies to enhance the oxidative stability of lipid-based encapsulation systems. These strategies may include the incorporation of antioxidants, the use of oxygen-resistant materials for encapsulation, and optimizing processing conditions to minimize oxidative stress. By improving the oxidative stability of these lipid-based systems, the integrity and functionality of encapsulated bioactive compounds can be maintained, thereby enhancing their effectiveness in applications across food, pharmaceuticals, and nutraceuticals(Da Costa De Quadros et al., 2023; Gbian & Omri, 2022; Patel et al., 2024).
6.4. Biodegradable synthetic polymers
Polylactic acid (PLA) is a biodegradable polymer made from renewable resources like corn starch. PLA is used for producing biodegradable packaging and encapsulating food ingredients, offering an environmentally friendly alternative to traditional plastics (Chen et al., 2022). Also, alginate dissolves well in water and can be used to produce biopolymer films, nanoparticles, and microcapsules.
6.5. Cellulose and derivatives
Cellulose, a common homopolysaccharide composed of β-d-glucose, is a key structural element in plant cell walls, providing support and strength. Another significant polysaccharide is starch, which also consists of glucose but in the form of α-d-glucose. (Chaudhary et al., 2022). Cellulose derivatives, such as methylcellulose, hydroxypropyl methylcellulose (HPMC), and carboxymethylcellulose (CMC), are increasingly recognized for their roles in the food industry, particularly in the encapsulation of bioactive compounds like vitamins, probiotics, and antioxidants. These derivatives are valuable for enhancing the stability, solubility, and bioavailability of these compounds.
The ability of cellulose derivatives to form films facilitates the production of micro- and nano-capsules, which function as carriers for bioactive substances. This encapsulation approach not only extends the shelf life of these compounds but also supports their controlled release, ensuring that they are delivered to their target locations in the digestive system. Additionally, cellulose derivatives are favored for their safety, non-toxicity, and environmental friendliness, making them ideal for food applications where consumer safety and sustainability are critical (Casalini & Giacinti Baschetti, 2023; Grover, 2020; Liu et al., 2021; Yi et al., 2020).
6.6. Starch and Derivates
Starch and its derivatives, including hydroxypropyl starch, acetylated starch, and cross-linked starch, are increasingly utilized in food encapsulation due to their adaptable properties (Liang et al., 2024; Witczak et al., 2016). These derivatives are particularly valued for their ability to form stable gels and films, which are crucial for effective encapsulation processes. Starch-based encapsulation methods offer multiple benefits, such as protecting sensitive bioactive compounds from environmental damage, enabling controlled release, and enhancing the stability of the encapsulated materials over time. Additionally, starch derivatives are noted for their safety, biodegradability, and environmental friendliness, making them suitable for food applications where health and sustainability are important. Recent studies demonstrate that starch-based encapsulation systems are effective in preserving the functionality and bioavailability of various food components, indicating their significant potential for advancing food formulation and processing (Egharevba, 2019, Liang et al., 2024).
7. Application of encapsulation in food industries
Encapsulated flavors and colors can be precisely controlled to achieve the desired sensory attributes without compromising the stability of the compounds. For example, essential oils encapsulated in biodegradable polymers improve stability and allow for controlled flavor release, enhancing the sensory experience of foods (Ghosh et al., 2021; Premjit et al., 2022). Different encapsulation methods in the food industry are selected based on the type of bioactive compound, production conditions, and desired properties of the final product. These methods help enhance the stability of sensitive compounds, and allow for controlled release (Timilsena et al., 2020).
In spray drying process, the bioactive compound mixed with a liquid carrier is sprayed into a chamber with high-velocity hot air, which rapidly evaporates the solvent and produces dry particles. These tiny particles, which contain the active compound, are more stable against environmental factors and can be easily incorporated into various food products (Piñón-Balderrama et al., 2020).
Freeze drying is ideal for preserving heat-sensitive compounds, commonly used for encapsulating probiotics, enzymes, and other thermosensitive ingredients. This results in dry particles that retain their biological properties and remain stable under environmental conditions, enhancing the shelf life of food products containing these bioactive ingredients(da Silva et al., 2019; Kuo et al., 2022; Rajam & Subramanian, 2022). Lipid-based encapsulation uses liposomes, lipid nanoparticles, and emulsions to encapsulate fat-soluble compounds. This method is suitable for encapsulating fatty acids, fat-soluble vitamins, and antioxidants. Due to their bilayer structure, liposomes can easily pass through cellular barriers, increasing the absorption and bioavailability of bioactive compounds (Alu'datt et al., 2022; Barroso et al., 2021; Wijekoon et al., 2023). Additionally, nanoemulsions improve the texture and mouthfeel of beverages and dairy products by providing a smooth, creamy consistency (Aliabbasi & Emam-Djomeh, 2024). In their study, Raeisi et al., nanoencapsulated fish oil and garlic essential oil using various percentages of chitosan and Persian gum-chitosan. They then investigated the stability of the emulsion. The results showed that the 2:1 w/w ratio of Persian gum and chitosan will offer the best performance for use in the food industry (Raeisi et al., 2019). According to Oprea et al., nanoencapsulation is a practical method for utilizing citrus essential oil in industrial applications. This essential oil is used in the food industry as flavoring, antioxidant and antibacterial and its antibacterial properties have been proven to produce edible films and preserve food. Therefore, nanoencapsulation of citrus essential oil can facilitate the use of this natural flavoring over artificial compounds in the industry (Oprea et al., 2022). The application of natural pigments and stabilization methods of anthocyanins for food applications has been explored, demonstrating the potential of nanoparticles in enhancing food safety and quality (Chatterjee et al., 2021; Fierri et al., 2023; Rosales et al., 2021; Yao et al., 2021). In the research conducted by Salah et al., anthocyanins and β-lactoglobulin from red raspberry pomace were encapsulated to investigate the effect of desolvation. The efficiency of nanoencapsulation was 77 %, and the SEM images showed a square shape. Despite the significant benefits of nanoencapsulation in the food industry, Comprehensive risk assessments and toxicity studies are essential to evaluate the potential health impacts of nanoencapsulated ingredients. Regulatory agencies such as the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) provide guidelines and frameworks for the safe use of nanotechnology in food products (Committee et al., 2018; Kumari, Arya, et al., 2023).
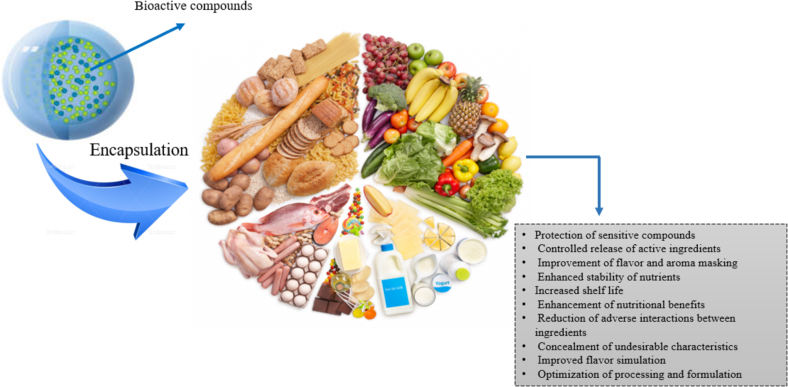
8. Advantages of encapsulation in the food area
8.1. Sensory improvement
When designing a food product, key factors to consider include the taste, solubility, and stability of the active ingredients. Modifying taste involves altering how taste characteristics are perceived and has a wide range of applications within the food sector (Fig. 2). For instance, it is used to improve the palatability of fortified foods, reduce sugar and fat content, mask the bitterness of pharmaceutical ingredients (particularly in pediatric formulations), preserve flavors, and protect against the degradation and oxidation of both flavors and active compounds. Flavors in foods are volatile compounds that play a significant role in human sensory perception and are extensively used across various products. Due to their volatile nature and sensitivity to factors such as pH, temperature, and oxidation, they require proper protection to extend their shelf life. Encapsulation is a highly effective method for taste modification by trapping undesirable functional ingredients. This technique is crucial for protecting food flavors, enhancing their thermal and oxidative stability, reducing issues related to volatility, and ensuring controlled release, which ultimately improves the bioavailability of these compounds in food products (Rezagholizade-shirvan, Masrournia, et al., 2023; Szymczak, 2023).
Fig. 2.
Advantages and Applications of Encapsulation in the Food Industries.
8.2. Extend shelf life
The shelf life of food products is impacted by numerous factors, particularly chemical and microbial processes, leading to a significant decrease in consumer acceptance. There is a growing trend toward replacing synthetic preservatives with natural, plant-based bioactive compounds that are considered safer. However, the full integration of these natural ingredients is hindered by several challenges, such as their low chemical stability, undesirable taste, poor solubility, and limited effectiveness over time. Encapsulation provides a potential solution to mitigate these limitations.
This review discusses the latest trends in utilizing naturally encapsulated ingredients for food preservation, categorized by components such as essential oils, plant extracts, phenolics, and carotenoids, with a focus on their role in extending shelf life through antimicrobial, anti-browning, and antioxidant properties. Encapsulation methods, particularly nanoencapsulation, present a promising approach to overcoming the limitations of these bioactive ingredients. Combining proteins and polysaccharides as encapsulating materials yields better results compared to using single polymers. The encapsulation technique and choice of encapsulants significantly influence the release mechanisms and physicochemical properties of the bioactive compounds. Optimizing these factors, along with refining the encapsulation process, results in a cost-effective, well-encapsulated ingredient that is more effective than its unencapsulated form in extending shelf life. Well-designed encapsulation systems have been shown to enhance the shelf-life-promoting properties of bioactive ingredients, primarily by improving their solubility, distribution within food matrices, and interaction with spoilage agents. Additionally, they prolong the presence of these compounds during food storage and processing by enhancing their thermal and processing stability, while controlling their release on food surfaces or within packaging (Hosseini et al., 2021).
8.3. Increase nutritional value
Numerous companies and research institutions are actively exploring new ingredients that offer potential health benefits. Examples include phytochemicals, wood-derived compounds like phytosterols, as well as pro- and prebiotics, novel carotenoids, trace minerals, and polyphenols. Many of these components are currently available or will become available in purified forms within the next decade. Incorporating them into food products will frequently necessitate technological advancements. Encapsulation is expected to play a significant role in this process, although it may increase the cost of using such ingredients, and careful consideration of bioavailability is essential (Rezaei, Nickfar, et al., 2023).
8.4. Food preservation
Nano-encapsulation is a nanoscale technique that involves packaging materials within nanocapsules, ensuring the functionality of the final product through controlled release mechanisms. Encapsulated ingredients offer several benefits, such as the sequential delivery of multiple active components, extended shelf life, enhanced stability, and pH-triggered controlled release. The primary goal of encapsulation is to maintain the stability of active compounds during processing and storage, while also preventing unfavorable interactions with the food matrix (Rezaei, Salari, et al., 2023).
8.5. Functional foods
Functional foods, which contain natural antioxidants, unsaturated fatty acids, probiotics, bioactive compounds, and vitamins, represent a key trend in the food industry. However, many of these components are unstable under typical conditions or have residual tastes that limit their use. These functional ingredients can enhance the nutritional value and functionality of food products. Encapsulation is a process that forms a protective barrier (a matrix or polymer coating) around the active component, preventing chemical interactions, shielding it from environmental factors (such as temperature, pH, enzymes, and oxygen), and enabling the controlled release of the component under specific conditions. The primary goals of this approach are to enhance the stability of bioactive compounds against unfavorable environmental factors, to facilitate their incorporation into food matrices, thus imparting functional properties to the products, and to ensure their controlled release at targeted sites in the gastrointestinal tract following consumption (Corrêa-Filho et al., 2019).
9. Encapsulation: Safety and cytotoxicity
In general, the toxicity and safety of various encapsulation methods depend on the type of encapsulation materials and the production processes. The use of biodegradable and natural polymers, precise control of production processes, and comprehensive toxicity testing are essential to assess the potential risks associated with each method.
Nanoparticles (NPs) are increasingly being used in various domains such as medicine, electronics, and environmental science due to their distinctive physical and chemical attributes. However, their minute size and extensive surface area raise significant concerns regarding their safety and potential cytotoxicity. The interaction of nanoparticles with biological systems can lead to harmful effects, highlighting the importance of evaluating their safety profiles (Salatin et al., 2015). The safety of nanoparticles is influenced by their physical and chemical properties, including size, shape, surface charge, and composition. Nanoparticles small size can easily penetrate cell membranes and interact with internal cellular components, which may result in cytotoxic effects. Research indicates that the surface properties of nanoparticles, such as the type of functional groups and coatings, are critical in determining their compatibility with biological systems (Ahmad et al., 2022; Mosquera et al., 2018).
Cytotoxicity is a significant issue as nanoparticles can cause oxidative stress, inflammation, and cell death. Oneway nanoparticles exert cytotoxic effects is through the generation of reactive oxygen species (ROS). They can also inflict cellular damage by disrupting mitochondrial function and cell membranes (Manke et al., 2013; Yu et al., 2020; Zhang, Yu, et al., 2021). To mitigate these risks, thorough in vitro and in vivo evaluations are conducted to assess the biocompatibility of nanoparticles. Risk assessments focus on analyzing cellular reactions and establishing safe dosage levels to reduce adverse effects. Ongoing development of regulatory guidelines and standard protocols aims to ensure the safe use of nanoparticles in various applications(Ajdary et al., 2018). A comprehensive understanding of how nanoparticle properties affect their biological interactions is crucial for the creation of safer nanomaterials and their effective application in technological innovations (Manke et al., 2013).
Early research on nanomaterials mostly investigated their short-term effects in cultured cells or live models. Recently, however, studies have begun examining their long-term biological impacts. Nutrition and energy metabolism, which are crucial to human health, aging, and longevity, are regulated by complex signaling pathways that could be influenced by prolonged nanomaterial exposure (Chen et al., 2014; Ghebretatios et al., 2021; Strojny et al., 2015).
Nanoparticles can pose significant long-term health risks to humans. Exposure through inhalation, ingestion, or skin contact can lead to oxidative stress, inflammation, DNA damage, and cell death. Specific types are linked to neurotoxicity, cellular membrane damage, and reduced cell viability. Additionally, nanoparticles can disrupt cell-to-cell interactions in tissue, while others may trigger immune responses. These effects underscore the need for more studies and regulatory monitoring of nanoparticle use (Kakakhel et al., 2021; Lamas et al., 2020; Rahman et al., 2021; Rajput et al., 2020).
In other encapsulation methods, such as microencapsulation and macroencapsulation, toxicity concerns are generally lower than with nanoencapsulation due to the larger particle size. However, there are still safety considerations associated with the encapsulation materials and production processes, which can influence the safety and toxicity of these approaches. In microencapsulation, materials like gelatin, alginate, starch, casein, and other biocompatible polymers are commonly used, which are generally non-toxic. These materials are widely used for food and pharmaceutical applications due to their high biocompatibility and biodegradability, resulting in minimal toxicity. However, certain chemicals used in microencapsulation production, such as organic solvents or surfactants, may induce cytotoxicity. Therefore, it is preferred to use non-toxic solvents or solvent-free production methods (Stern & Becher, 2018; Xu, Feng, & Fang, 2024).
Another challenge in microencapsulation is the potential formation of toxic by-products during polymerization processes, which can result from chemical changes in the encapsulation materials. To prevent this, careful control of the process conditions and selection of safe raw materials are essential (Petkova, Mihaylova, & Desseva, 2022; Valdés et al., 2018). Macroencapsulation is commonly used for food ingredients or supplements, and due to the larger capsule size, it generally poses fewer toxicity concerns than nano- and microencapsulation. This method often employs natural and edible coatings, such as gelatin, waxes, or gum coatings, which are relatively safe. However, in some cases, synthetic polymer coatings are used, which may pose toxic risks at high doses or upon degradation (Aftab et al., 2023; Espona-Noguera et al., 2019; Loudovaris, 2022; Smith et al., 2018).
Other encapsulation techniques, such as liposomal encapsulation and dual polymer encapsulation, also present distinct safety considerations. In liposomal encapsulation, phospholipid layers are used, which have high compatibility with biological tissues and are generally non-toxic. However, liposomes may undergo oxidative degradation, leading to chemical changes that could increase the likelihood of generating toxic compounds(Chang et al., 2021; Courtois et al., 2019; Ibrahim et al., 2022; Saraswat & Maher, 2020).
10. Effect of Encapsulated Bioactive Compounds on health human
10.1. Diabetes
Encapsulation protects compounds, especially those with easily lost chemical structure or biological activities. Polyphenols, known for health benefits but low shelf life, are used in medicinal applications as functional food and nutraceuticals (Datta et al., 2022).
Phenolic compounds have numerous biological properties, including antioxidant, antibacterial, anti-cancer, anti-inflammatory, and anti-diabetic effects. They can help combat oxidative stress and diabetes by increasing insulin secretion and sensitivity. However, their health benefits and anti-diabetic effects are conflicting due to low bioavailability and intrinsic limitations. Nanomaterials are being used to improve bioavailability.
Rambaran's research (Rambaran, 2020) on encapsulation in diabetes treatment found that nanotechnology enhanced the therapeutic potential of polyphenols, regardless of the type of polyphenol, nanocarrier, encapsulation technique, size, drug delivery system, dose, encapsulation efficiency, loading capacity, and study duration. The anti-diabetic benefits were evident in all but four of the 33 studies evaluated (Medina-Pérez et al., 2020). Microencapsulated polyphenols can address deficits in free chemicals. Encapsulation improves the protection of active chemicals, paving the way for industrial uses of active packaging. For example, gallic acid, a potent antidiabetic phenolic compound, can be encapsulated in nanochitosan nanoparticles, which have an encapsulation efficiency of about 50.76 % and can inhibit -glycosidase activity in 28.87 % of cases (Fachriyah et al., 2017).
10.2. Alzheimer's disease
Alzheimer's disease (AD) is a progressive cognitive loss caused by the accumulation of amyloid-beta 42 and neurofibrillary tangles of hyper-phosphorylated tau protein. Current therapy focuses on reducing symptoms, but drug delivery is a challenge. Nanotechnology-based treatments address this issue by targeting specific targets. Phenolic compounds, such as epicatechin and flavonoid inhibitors, have been shown to be beneficial in AD treatment. Consuming flavonoid and polyphenol in diets can reduce amyloid formation and prevent toxicity. Encapsulation of these compounds is needed for their effectiveness in AD prevention and treatment (Bandaru et al., 2022; Marino et al., 2022).
10.3. Hypertension diseases
Hypertension is a leading cause of death worldwide and is linked to various cardiovascular diseases. Eating habits play a significant role in the progression of hypertension, and dietary supplements and nutraceuticals have been used to prevent and treat these diseases. Some nutraceuticals are rich in phenolic compounds, which have cardioprotective activities such as vasodilation, antiplatelet effects, and the ability to lower blood pressure and LDL cholesterol. Phenolic compounds exert cardioprotective activities through endothelial nitric oxide synthase (eNOS) effects, gene expression, and antioxidant response element (Nrf2-ARE). Cocoa products, fruits, vegetables, and raisins are also rich in phenols and phenolic compounds. However, polyphenols must be available and present at pharmacological amounts to be effective in the human body. Nano-encapsulated polyphenols loaded in food-grade polymers and lipids have been found to be safe and effective in pre-clinical studies (Maaliki et al., 2019; Mattioli et al., 2020).
10.4. Cancer
Phenolic compounds like curcumin, resveratrol, kaempferol, EGCG, apigenin, and quercetin have been found to be pro-oxidants in cancer prevention due to their reactive oxygen species (ROS) scavenging activity. These compounds also inhibit insulin-like growth factor, modulate signal transduction pathways, and modulate proinflammatory cytokines. Isoflavones have multifunctional actions against cancer, including inhibiting hormone-dependent malignancies and changing estrogen receptor expression. However, their clinical use is limited due to poor pharmacokinetics, water solubility, and low stability. Nanocarrier systems have been proposed as a viable approach, providing regulated release and increased curcumin bioavailability. Studies have shown that encapsulation of phenolic compounds could represent novel approaches to cancer prevention and treatment, with potential biomedical applications (Andrabi et al., 2020; Jung et al., 2015; Oršolić & Jazvinšćak Jembrek, 2022).
11. Challenges and future perspectives
The application of inorganic materials, particularly metal nanoparticles, in encapsulation methods presents numerous benefits, such as enhanced stability and improved release characteristics for bioactive compounds. Nonetheless, the use of these materials raises significant environmental concerns related to their disposal and the potential long-term impacts on ecosystems and human health. Metal nanoparticles can accumulate in the environment and may pose toxicity risks to aquatic organisms, soil microbes, and humans upon exposure. To mitigate these issues, researchers are increasingly focused on developing biodegradable and environmentally friendly alternatives to traditional metal nanoparticles, alongside rigorous safety assessments and regulatory measures governing their use. By promoting the responsible application of inorganic materials in encapsulation, it is possible to leverage their advantages while reducing environmental impacts and fostering sustainability across various sectors, including food and pharmaceuticals (Idrees et al., 2020; Meena et al., 2020; Nemiwal & Kumar, 2021; Paris et al., 2019).There is no global legislation for the risk assessment of nano-products, which poses a risk to human health and the environment. The European Union and the U.S. Food and Drug Administration have issued guidelines on the use of nanotechnology in food. A database for nano-engineered food and beverages in the European market was established in 2012, but further studies are needed to improve methods, formulations, and encapsulation systems (Hansen, 2017; Shishir et al., 2018). The application of nano-products in food and biological systems is also being explored. The potential benefits of nano encapsulation of bioactive compounds highlight the need for worldwide regulation for safe use and marketing.
While encapsulation provides initial protection to bioactive peptides, the long-term stability of these encapsulated systems requires further investigation. Future studies must assess how storage conditions and time impact the quality and bioactivity of these peptides over extended periods.
To overcome the instability of nanoparticles in encapsulation, several strategies can be employed to improve their robustness and efficacy within food matrices. Nanoparticle instability often results from environmental factors, which can lead to aggregation, degradation, or loss of bioactivity (Le Priol et al., 2021).
One of the most effective ways to enhance the stability of nanoparticles in food systems is through multilayer encapsulation (Carpenter et al., 2019). For example, the Layer-by-Layer (LbL) assembly method allows for precise control over the number of layers and the types of materials used, which can significantly increase the stability and bioavailability of encapsulated compounds (Li et al., 2022; Liu et al., 2019).
Surface modification involves altering the surface properties of nanoparticles to improve their interaction with the food matrix and reduce aggregation. For example, coating nanoparticles with surfactants or biocompatible ligands can help prevent clumping and enhance their dispersion in aqueous environments (Koyani et al., 2018). Polyethylene glycol (PEG) is often used as a surface modifier to improve the solubility and biocompatibility of nanoparticles, which helps maintain stability during storage and processing (D'souza & Shegokar, 2016; Shi et al., 2021).
Nanoparticle size plays a crucial role in their stability. Smaller particles tend to have higher surface energy, which can lead to aggregation. However, optimizing the size of nanoparticles can minimize this risk (Wei et al., 2022). Designing pH-sensitive nanoparticles can provide additional stability and controlled release in food systems (Xiong et al., 2020). There are instances when not all encapsulated compounds are released during digestion, which can limit their absorption and overall effectiveness. Several factors influence the release of these compounds, including the choice of encapsulating material, the gastrointestinal environment, and the interactions between the encapsulated compounds and food matrices. Incomplete release can impede the anticipated health benefits associated with these bioactive compounds. To improve the efficacy of encapsulated products, researchers are focusing on developing innovative delivery systems that enhance the controlled release of these compounds throughout the digestive tract, thereby boosting their absorption and maximizing their therapeutic potential in various applications, including functional foods and nutraceuticals.
Crosslinking is another approach to improve nanoparticle stability. By crosslinking polymers used in the encapsulation process (such as using calcium ions in alginate gels), the structural integrity of the nanoparticles can be reinforced. Crosslinking strengthens the encapsulation matrix, making it more resistant to environmental stressors like heat, pH changes, and mechanical forces during processing or storage (Girón-Hernández et al., 2021).
In the following, it can be stated that the high costs associated with encapsulation remain a barrier to its widespread application in the food industry (Timilsena et al., 2020). To address this, future research should consider the following strategies (Altay et al., 2024; Getachew & Chun, 2016; Grgić et al., 2020; Safeer Abbas et al., 2023; Šeregelj, Ćetković, et al., 2021; Weng et al., 2024):
11.1. Use of affordable materials
Utilizing naturally-derived biopolymers such as alginate, chitosan, and natural gums could significantly reduce the cost of encapsulation. These materials are not only affordable but also sustainable, making them ideal candidates for industrial-scale encapsulation processes.
11.2. Optimization of industrial processes
Process optimization, such as employing more energy-efficient methods like microwave and ultrasonic-assisted encapsulation, could lower production costs. These technologies have the potential to reduce energy consumption and material usage while maintaining product quality.
11.3. Scalable and economical techniques
Development of scalable and cost-efficient encapsulation methods is critical for commercialization. Techniques such as nanoemulsions and nanogels, which are more economical compared to traditional encapsulation methods, could play a vital role in making encapsulation technologies more accessible to the food industry.
12. Conclusion
Encapsulation is a growing field of study aimed at delivering bioactive agents or cells in a targeted manner. It is being used in various clinical procedures, including cancer treatment and regenerative medicine. Encapsulation technologies for functional food preparations include capsules, particles, emulsions, liposomes, and lipid nanoparticles. These systems can administer sensitive agents like drugs, probiotics, antioxidants, and cells. The most commonly used encapsulation technologies include emulsions-spray drying, emulsions-freeze drying, complex coacervation, and supercritical microencapsulation, followed by others. Encapsulated products can contribute to targeted disease treatment and prevention, and are expected to be used in tailored therapies and diets. Encapsulation strategies aim to improve stability, solubility, bioavailability, sensorial properties, retention of bioactive properties, and shelf life. More studies are needed on bioavailability and bioactivity effectiveness in food and agricultural applications.
The future outlook for encapsulation in the food industry is vast and promising. With the growing demand for healthier and longer-lasting food products, encapsulation technology plays a crucial role in improving food quality, increasing the stability of bioactive compounds, and creating functional foods. Encapsulation can lead to the development of innovative and smart foods designed to meet specific consumer needs (such as sports or therapeutic nutrition). These products can release particular nutrients and bioactive compounds at specific times or in targeted areas of the digestive system. This technology has wide applications not only in the food industry but also in the pharmaceutical and cosmetic industries. In the future, we expect to see more interaction between these sectors to develop multifunctional products and increase efficiency. Also, the success of future research will depend on technological advancements, reducing production costs, and raising consumer awareness of the benefits of encapsulated products.
CRediT authorship contribution statement
Alieh Rezagholizade-shirvan: Writing – review & editing, Writing – original draft, Methodology, Investigation. Mahya Soltani: Writing – review & editing, Writing – original draft, Methodology, Investigation. Samira Shokri: Writing – review & editing, Writing – original draft, Methodology, Investigation. Ramin Radfar: Writing – review & editing, Writing – original draft, Methodology, Investigation. Masoumeh Arab: Writing – review & editing, Writing – original draft, Methodology, Investigation. Ehsan Shamloo: Writing – review & editing, Writing – original draft, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge the financial and technical support of the Neyshabur University of Medical Sciences, Neyshabur, Iran (Grant number: 1403-01-411).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Compliance with ethical standards.
This article lacks research involving human or animal subjects.
Data availability
The data that has been used is confidential.
References
- Abdi-Moghadam Z., Mazaheri Y., Rezagholizade-Shirvan A., Mahmoudzadeh M., Sarafraz M., Mohtashami M.…Darroudi M. The significance of essential oils and their antifungal properties in the food industry: A systematic review. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aftab A., Ahmad B., Bashir S., Rafique S., Bashir M., Ghani T.…Sajini A.A. Comparative study of microscale and macroscale technique for encapsulation of Calotropis gigantea extract in metal-conjugated nanomatrices for invasive ductal carcinoma. Scientific Reports. 2023;13:13474. doi: 10.1038/s41598-023-39330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzaal M., Saeed F., Ateeq H., Ahmed A., Ahmad A., Tufail T.…Anjum F.M. Encapsulation of Bifidobacterium bifidum by internal gelation method to access the viability in cheddar cheese and under simulated gastrointestinal conditions. Food Science & Nutrition. 2020;8:2739–2747. doi: 10.1002/fsn3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Toalá J., Garcia-Varela R., Garcia H., Mata-Haro V., González-Córdova A., Vallejo-Cordoba B., Hernández-Mendoza A. Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology. 2018;75:105–114. [Google Scholar]
- Ahmad F., Salem-Bekhit M.M., Khan F., Alshehri S., Khan A., Ghoneim M.M.…Elbagory I. Unique properties of surface-functionalized nanoparticles for bio-application: Functionalization mechanisms and importance in application. Nanomaterials. 2022;12:1333. doi: 10.3390/nano12081333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed E.M. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdary M., Moosavi M.A., Rahmati M., Falahati M., Mahboubi M., Mandegary A.…Varma R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials. 2018;8:634. doi: 10.3390/nano8090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarbaglu Z., Peighambardoust S.H., Sarabandi K., Jafari S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129965. [DOI] [PubMed] [Google Scholar]
- Ali U., Saeed M., Ahmad Z., F-u-H S., Rehman M.A., Mehmood T.…Rahman A. Stability and survivability of alginate gum-coated Lactobacillus rhamnosus GG in simulated gastrointestinal conditions and probiotic juice development. Journal of Food Quality. 2023;2023 [Google Scholar]
- Aliabbasi N., Emam-Djomeh Z. Application of nanotechnology in dairy desserts and ice cream formulation with the emphasize on textural, rheological, antimicrobial, and sensory properties. eFood. 2024;5 [Google Scholar]
- Altay Ö., Köprüalan Ö., İlter I., Koç M., Ertekin F.K., Jafari S.M. Spray drying encapsulation of essential oils; process efficiency, formulation strategies, and applications. Critical Reviews in Food Science and Nutrition. 2024;64:1139–1157. doi: 10.1080/10408398.2022.2113364. [DOI] [PubMed] [Google Scholar]
- Altememy D., Patra I., Hussam F., Jabr H.S., Alwan N.H., Dawood A.H.…Darvishi M. Determination and evaluation of total antioxidant capacity of oak (Quercus brantii), thyme (Tymbra spicata), and watermelon (Citrullus colocynthis) native of Ilam. Advancements in Life Sciences. 2022;9:373–379. [Google Scholar]
- Alu’datt M.H., Alrosan M., Gammoh S., Tranchant C.C., Alhamad M.N., Rababah T.…Tan T.-C. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and health-promoting ingredients. Food Bioscience. 2022;50 [Google Scholar]
- Andrabi S.M., Majumder S., Gupta K.C., Kumar A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids and Surfaces B: Biointerfaces. 2020;195 doi: 10.1016/j.colsurfb.2020.111263. [DOI] [PubMed] [Google Scholar]
- Arepally D., Reddy R.S., Coorey R., Goswami T.K. Modelling inactivation kinetics of free and encapsulated probiotic cells in millet biscuit under different baking conditions. Food Research International. 2023;174 doi: 10.1016/j.foodres.2023.113573. [DOI] [PubMed] [Google Scholar]
- Assadpour E, Jafari SM (2019): An overview of lipid-based nanostructures for encapsulation of food ingredients. Lipid-based nanostructures for food encapsulation purposes, 1-34.
- Azarashkan Z., Farahani S., Abedinia A., Akbarmivehie M., Motamedzadegan A., Heidarbeigi J., Hayaloğlu A.A. Co-encapsulation of broccoli sprout extract nanoliposomes into basil seed gum: Effects on in vitro antioxidant, antibacterial and anti-Listeria activities in ricotta cheese. International Journal of Food Microbiology. 2022;376 doi: 10.1016/j.ijfoodmicro.2022.109761. [DOI] [PubMed] [Google Scholar]
- Bahmani M., Shokri S., Akhtar Z.N., Abbaszadeh S., Manouchehri A. The effect of pomegranate seed oil on human health, especially epidemiology of polycystic ovary syndrome; a systematic review. JBRA assisted reproduction. 2022;26:631. doi: 10.5935/1518-0557.20210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru L.J.M., Ayyalasomayajula N., Murumulla L., Challa S. Mechanisms associated with the dysregulation of mitochondrial function due to lead exposure and possible implications on the development of Alzheimer’s disease. Biometals. 2022;35:1–25. doi: 10.1007/s10534-021-00360-7. [DOI] [PubMed] [Google Scholar]
- Banwo K., Olojede A.O., Adesulu-Dahunsi A.T., Verma D.K., Thakur M., Tripathy S.…Aguilar C.N. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Bioscience. 2021;43 [Google Scholar]
- Bao C., Jiang P., Chai J., Jiang Y., Li D., Bao W.…Li Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Research International. 2019;120:130–140. doi: 10.1016/j.foodres.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Barretto F.J.F.P., Clemente H.A., Santana A.L.B.D., Vasconcelo M.A.S. Stability of encapsulated and non-encapsulated anthocyanin in yogurt produced with natural dye obtained from Solanum melongena L. Bark. Revista Brasileira de Fruticultura. 2020;42:e–137. [Google Scholar]
- Barroso L., Viegas C., Vieira J., Ferreira-Pêgo C., Costa J., Fonte P. Lipid-based carriers for food ingredients delivery. Journal of Food Engineering. 2021;295 [Google Scholar]
- Bauer-Estrada K., Sandoval-Cuellar C., Rojas-Muñoz Y., Quintanilla-Carvajal M.X. The modulatory effect of encapsulated bioactives and probiotics on gut microbiota: Improving health status through functional food. Food & Function. 2023;14:32–55. doi: 10.1039/d2fo02723b. [DOI] [PubMed] [Google Scholar]
- Bennacef C., Desobry-Banon S., Probst L., Desobry S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocolloids. 2021;118 [Google Scholar]
- Bhalani D.V., Nutan B., Kumar A., Singh Chandel A.K. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines. 2022;10:2055. doi: 10.3390/biomedicines10092055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P., Lalani R., Vhora I., Patil S., Amrutiya J., Misra A., Mashru R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. International Journal of Pharmaceutics. 2018;536:95–107. doi: 10.1016/j.ijpharm.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Bonaccorso A., Russo N., Romeo A., Carbone C., Grimaudo M.A., Alvarez-Lorenzo C.…Caggia C. Coating Lacticaseibacillus rhamnosus GG in alginate systems: An emerging strategy towards improved viability in orange juice. AAPS PharmSciTech. 2021;22:1–14. doi: 10.1208/s12249-021-01996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boostani S., Jafari S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends in Food Science & Technology. 2021;109:303–321. [Google Scholar]
- Burgain J., Gaiani C., Linder M., Scher J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. Journal of Food Engineering. 2011;104:467–483. [Google Scholar]
- Calva-Estrada S., Jiménez-Fernández M., Lugo-Cervantes E. Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry. Food Chemistry: Molecular Sciences. 2022;4 doi: 10.1016/j.fochms.2022.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter J., George S., Saharan V.K. Curcumin encapsulation in multilayer oil-in-water emulsion: Synthesis using ultrasonication and studies on stability and antioxidant and release activities. Langmuir. 2019;35:10866–10876. doi: 10.1021/acs.langmuir.9b01523. [DOI] [PubMed] [Google Scholar]
- Carrasco-Sandoval J., Aranda-Bustos M., Henríquez-Aedo K., López-Rubio A., Fabra M.J. Bioaccessibility of different types of phenolic compounds co-encapsulated in alginate/chitosan-coated zein nanoparticles. Lwt. 2021;149 [Google Scholar]
- Casalini S., Giacinti Baschetti M. The use of essential oils in chitosan or cellulose-based materials for the production of active food packaging solutions: A review. Journal of the Science of Food and Agriculture. 2023;103:1021–1041. doi: 10.1002/jsfa.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Meikle T.G., Drummond C.J., Yang Y., Conn C.E. Comparison of cubosomes and liposomes for the encapsulation and delivery of curcumin. Soft Matter. 2021;17:3306–3313. doi: 10.1039/d0sm01655a. [DOI] [PubMed] [Google Scholar]
- Chatterjee N.S., Dara P.K., Perumcherry Raman S., Vijayan D.K., Sadasivam J., Mathew S.…Anandan R. Nanoencapsulation in low-molecular-weight chitosan improves in vivo antioxidant potential of black carrot anthocyanin. Journal of the Science of Food and Agriculture. 2021;101:5264–5271. doi: 10.1002/jsfa.11175. [DOI] [PubMed] [Google Scholar]
- Chaudhari A., Dwivedi M.K. Elsevier; 2022. The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics, probiotics in the prevention and management of human diseases; pp. 1–11. [Google Scholar]
- Chaudhary S., Jain V.P., Jaiswar G. Elsevier; 2022. The composition of polysaccharides: Monosaccharides and binding, group decorating, polysaccharides chains, innovation in nano-polysaccharides for eco-sustainability; pp. 83–118. [Google Scholar]
- Chen M., Yan X., Cheng M., Zhao P., Wang Y., Zhang R.…Chen M. Preparation, characterization and application of poly (lactic acid)/corn starch/eucalyptus leaf essential oil microencapsulated active bilayer degradable film. International Journal of Biological Macromolecules. 2022;195:264–273. doi: 10.1016/j.ijbiomac.2021.12.023. [DOI] [PubMed] [Google Scholar]
- Chen N., Wang H., Huang Q., Li J., Yan J., He D.…Song H. Long-term effects of nanoparticles on nutrition and metabolism. Small. 2014;10:3603–3611. doi: 10.1002/smll.201303635. [DOI] [PubMed] [Google Scholar]
- Chen S., Wu J., Tang Q., Xu C., Huang Y., Huang D.…Weng Z. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydrate Polymers. 2020;228 doi: 10.1016/j.carbpol.2019.115398. [DOI] [PubMed] [Google Scholar]
- Chen X., Yu C., Zhang Y., Wu Y.-C., Ma Y., Li H.-J. Co-encapsulation of curcumin and resveratrol in zein-bovine serum albumin nanoparticles using a pH-driven method. Food & Function. 2023;14:3169–3178. doi: 10.1039/d2fo03929j. [DOI] [PubMed] [Google Scholar]
- Cheng H.M., Koutsidis G., Lodge J.K., Ashor A.W., Siervo M., Lara J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Critical Reviews in Food Science and Nutrition. 2019;59:141–158. doi: 10.1080/10408398.2017.1362630. [DOI] [PubMed] [Google Scholar]
- Chew S.-C., Tan C.-H., Pui L.-P., Chong P.-N., Gunasekaran B., Nyam K. Encapsulation technologies: A tool for functional foods development. International Journal of Innovative Technology and Exploring Engineering. 2019;8:154–162. [Google Scholar]
- Committee E.S., Hardy A., Benford D., Halldorsson T., Jeger M.J., Knutsen H.K.…Ockleford C. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA Journal. 2018;16 doi: 10.2903/j.efsa.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian T., Babazadeh A., Rehman A., Shaddel R., Akbari-Alavijeh S., Boostani S., Jafari S. Protection and controlled release of vitamin C by different micro/nanocarriers. Critical Reviews in Food Science and Nutrition. 2022;62:3301–3322. doi: 10.1080/10408398.2020.1865258. [DOI] [PubMed] [Google Scholar]
- Corrêa-Filho L.C., Moldão-Martins M., Alves V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Applied Sciences. 2019;9:571. [Google Scholar]
- Cortez R., Luna-Vital D.A., Margulis D., Gonzalez de Mejia E. Natural pigments: Stabilization methods of anthocyanins for food applications. Comprehensive Reviews in Food Science and Food Safety. 2017;16:180–198. doi: 10.1111/1541-4337.12244. [DOI] [PubMed] [Google Scholar]
- Courtois A., Garcia M., Krisa S., Atgié C., Sauvant P., Richard T., Faure C. Encapsulation of ε-viniferin in onion-type multi-lamellar liposomes increases its solubility and its photo-stability and decreases its cytotoxicity on Caco-2 intestinal cells. Food & Function. 2019;10:2573–2582. doi: 10.1039/c9fo00420c. [DOI] [PubMed] [Google Scholar]
- da Cruz E.P., Pires J.B., dos Santos F.N., Fonseca L.M., Radünz M., Dal Magro J.…Dias A.R.G. Encapsulation of lemongrass essential oil into cassava starch fibers for application as antifungal agents in bread. Food Hydrocolloids. 2023;145 [Google Scholar]
- Cui H., Wang Y., Li C., Chen X., Lin L. Antibacterial efficacy of Satureja montana L. essential oil encapsulated in methyl-β-cyclodextrin/soy soluble polysaccharide hydrogel and its assessment as meat preservative. Lwt. 2021;152 [Google Scholar]
- Da Costa De Quadros C., De Sousa Araujo A.C., Latorres J.M., Michelon M., De Las Mercedes Salas-Mellado M. Lipid Nanocarriers as an alternative for the delivery of bioactive compounds beneficial to health. Current Bioactive Compounds. 2023;19:16–29. [Google Scholar]
- Dahiya D., Terpou A., Dasenaki M., Nigam P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustainable Food Technology. 2023;1:500–510. [Google Scholar]
- Darvishi M., Jasim S.A., Sarimsakov M.I., Ibrahim N.J., Hadi S.J., Al-Sammarra’e A.…Adhab Z.H. Evaluation of the total antioxidant capacity of Oliveria decumbens and Capparis spinosa. Journal of biological research-Bollettino della Società Italiana di biologia. Sperimentale. 2022;95 [Google Scholar]
- Datta S., Bhattacharjee S., Seal T. Anti-diabetic, anti-inflammatory and anti-oxidant properties of four underutilized ethnomedicinal plants of West Bengal, India: An in vitro approach. South African Journal of Botany. 2022;149:768–780. [Google Scholar]
- Davidov-Pardo G., Joye I.J., McClements D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 1: Preparation and characterization. Food Hydrocolloids. 2015;45:309–316. [Google Scholar]
- De Leo V., Milano F., Agostiano A., Catucci L. Recent advancements in polymer/liposome assembly for drug delivery: From surface modifications to hybrid vesicles. Polymers. 2021;13:1027. doi: 10.3390/polym13071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib T., Pan H., Chen S. Recent advances in pectin-based nanoencapsulation for enhancing the bioavailability of bioactive compounds: Curcumin oral bioavailability. Food Reviews International. 2023;39:3515–3533. [Google Scholar]
- Didier A.J., Stiene J., Fang L., Watkins D., Dworkin L.D., Creeden J.F. Antioxidant and anti-tumor effects of dietary vitamins a, C, and E. Antioxidants. 2023;12:632. doi: 10.3390/antiox12030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrellou D., Kandylis P., Lević S., Petrović T., Ivanović S., Nedović V., Kourkoutas Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. Lwt. 2019;116 [Google Scholar]
- Ding H., Tan P., Fu S., Tian X., Zhang H., Ma X.…Luo K. Preparation and application of pH-responsive drug delivery systems. Journal of Controlled Release. 2022;348:206–238. doi: 10.1016/j.jconrel.2022.05.056. [DOI] [PubMed] [Google Scholar]
- Djuricic I., Calder P.C. Polyunsaturated fatty acids and metabolic health: Novel insights. Current Opinion in Clinical Nutrition & Metabolic Care. 2022;25:436–442. doi: 10.1097/MCO.0000000000000865. [DOI] [PubMed] [Google Scholar]
- Dong Y., Wei Z., Xue C. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends in Food Science & Technology. 2021;112:348–361. [Google Scholar]
- D’souza A.A., Shegokar R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opinion on Drug Delivery. 2016;13:1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- Ebrahimi P., Shokramraji Z., Tavakkoli S., Mihaylova D., Lante A. Chlorophylls as natural bioactive compounds existing in food by-products: A critical review. Plants. 2023;12:1533. doi: 10.3390/plants12071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egharevba H.O. Chemical properties of starch and its application in the food industry. Chemical properties of starch. 2019;9 [Google Scholar]
- El-Messery T.M., Altuntas U., Altin G., Özçelik B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocolloids. 2020;106 [Google Scholar]
- Esmaeili F., Hashemiravan M., Eshaghi M.R., Gandomi H. Encapsulation of Arctium lappa L. root extracts by spray-drying and freeze-drying using maltodextrin and gum Arabic as coating agents and it’s application in synbiotic orange-carrot juice. Journal of Food Measurement and Characterization. 2022;16:2908–2921. [Google Scholar]
- Espona-Noguera A., Ciriza J., Canibano-Hernandez A., Villa R., Del Burgo L.S., Alvarez M., Pedraz J.L. 3D printed polyamide macroencapsulation devices combined with alginate hydrogels for insulin-producing cell-based therapies. International Journal of Pharmaceutics. 2019;566:604–614. doi: 10.1016/j.ijpharm.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Esquivel P. Elsevier; 2024. Betalains, handbook on natural pigments in food and beverages; pp. 147–167. [Google Scholar]
- Etzbach L., Meinert M., Faber T., Klein C., Schieber A., Weber F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Current Research in Food Science. 2020;3:73–81. doi: 10.1016/j.crfs.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun J.-B., Maruf A., Das P.R., Nam S.-H. A review of encapsulation of carotenoids using spray drying and freeze drying. Critical Reviews in Food Science and Nutrition. 2020;60:3547–3572. doi: 10.1080/10408398.2019.1698511. [DOI] [PubMed] [Google Scholar]
- Balanov P.E., Smotraeva I.V., Abdullaeva M.S., Volkova D.A., Ivanchenko O.B. Study on resveratrol content in grapes and wine products, E3S web of conferences. EDP Sciences. 2021;247:01063. [Google Scholar]
- Fachriyah E, Eviana I, Eldiana O, Amaliyah N, Sektianingrum A (2017): Antidiabetic activity from gallic acid encapsulated nanochitosan, IOP conference series: Materials science and engineering. IOP Publishing, pp. 012042.
- Falahi E., Delshadian Z., Ahmadvand H., Shokri Jokar S. AIMS Agriculture and Food; 2019. Head space volatile constituents and antioxidant properties of five traditional Iranian wild edible plants grown in west of Iran. [Google Scholar]
- Fangmeier M., Lehn D.N., Maciel M.J., Volken de Souza C.F. Encapsulation of bioactive ingredients by extrusion with vibrating technology: Advantages and challenges. Food and Bioprocess Technology. 2019;12:1472–1486. [Google Scholar]
- Farhadi L., Mohtashami M., Saeidi J., Azimi-nezhad M., Taheri G., Khojasteh-Taheri R.…Ghasemi A. Green synthesis of chitosan-coated silver nanoparticle, characterization, antimicrobial activities, and cytotoxicity analysis in cancerous and normal cell lines. Journal of Inorganic and Organometallic Polymers and Materials. 2022;32:1637–1649. [Google Scholar]
- Farhan M. Green tea catechins: nature’s way of preventing and treating cancer. International Journal of Molecular Sciences. 2022;23:10713. doi: 10.3390/ijms231810713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag A.F., Zahran H., Al-Okaby M.F., El-Sheikh M.M., Soliman T.N. Physicochemical properties of white soft cheese supplemented with encapsulated olive phenolic compounds. Egyptian Journal of Chemistry. 2020;63:2921–2931. [Google Scholar]
- Feng S., Sheng J., Yu J., Lin Y., Shao P. Encapsulation and release of citral using nanostructured lipid carriers: A study on the impact of different preparation methods. Food Bioscience. 2023;56 [Google Scholar]
- Feng S., Sun Y., Wang P., Sun P., Ritzoulis C., Shao P. Co-encapsulation of resveratrol and epigallocatechin gallate in low methoxyl pectin-coated liposomes with great stability in orange juice. International Journal of Food Science & Technology. 2020;55:1872–1880. [Google Scholar]
- Fernandes J.G., Rodrigues R.C., Pereira L., Stringheta P.C., Campelo P.H., Martins E. Encapsulation of hydrophobic active ingredients in plant proteins: Modulation of interfacial properties and encapsulation efficiency. Current opinion in food. Science. 2024;57 [Google Scholar]
- Fierri I., De Marchi L., Chignola R., Rossin G., Bellumori M., Perbellini A.…Saorin A. Nanoencapsulation of anthocyanins from red cabbage (Brassica oleracea L. var. Capitata f. rubra) through Coacervation of whey protein isolate and apple high Methoxyl pectin. Antioxidants. 2023;12:1757. doi: 10.3390/antiox12091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipčev B., Kojić J., Miljanić J., Šimurina O., Stupar A., Škrobot D.…Pojić M. Wild garlic (Allium ursinum) preparations in the design of novel functional pasta. Foods. 2023;12:4376. doi: 10.3390/foods12244376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mancha M.A., Ruíz-Gutiérrez M.G., Sánchez-Vega R., Santellano-Estrada E., Chávez-Martínez A. Effect of encapsulated beet extracts (Beta vulgaris) added to yogurt on the physicochemical characteristics and antioxidant activity. Molecules. 2021;26:4768. doi: 10.3390/molecules26164768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga C.G., Croft K.D., Kennedy D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food & Function. 2019;10:514–528. doi: 10.1039/c8fo01997e. [DOI] [PubMed] [Google Scholar]
- Fraj J., Petrović L., Đekić L., Budinčić J.M., Bučko S., Katona J. Encapsulation and release of vitamin C in double W/O/W emulsions followed by complex coacervation in gelatin-sodium caseinate system. Journal of Food Engineering. 2021;292 [Google Scholar]
- Fu Y., Shi J., Xie S.-Y., Zhang T.-Y., Soladoye O.P., Aluko R.E. Red beetroot betalains: Perspectives on extraction, processing, and potential health benefits. Journal of Agricultural and Food Chemistry. 2020;68:11595–11611. doi: 10.1021/acs.jafc.0c04241. [DOI] [PubMed] [Google Scholar]
- Furuta T., Neoh T.L. Microencapsulation of food bioactive components by spray drying: A review. Drying Technology. 2021;39:1800–1831. [Google Scholar]
- Gasa-Falcon A., Odriozola-Serrano I., Oms-Oliu G., Martín-Belloso O. Nanostructured lipid-based delivery systems as a strategy to increase functionality of bioactive compounds. Foods. 2020;9:325. doi: 10.3390/foods9030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbian D.L., Omri A. Lipid-based drug delivery systems for diseases managements. Biomedicines. 2022;10:2137. doi: 10.3390/biomedicines10092137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengatharan A., Dykes G.A., Choo W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT- Food Science and Technology. 2015;64:645–649. [Google Scholar]
- Getachew A.T., Chun B.-S. Optimization of coffee oil flavor encapsulation using response surface methodology. LWT. 2016;70:126–134. [Google Scholar]
- Ghasemi L., Nouri L., Mohammadi Nafchi A., Al-Hassan A.A. The effects of encapsulated probiotic bacteria on the physicochemical properties, staling, and viability of probiotic bacteria in gluten-free bread. Journal of Food Processing and Preservation. 2022;46 [Google Scholar]
- Ghebretatios M., Schaly S., Prakash S. Nanoparticles in the food industry and their impact on human gut microbiome and diseases. International Journal of Molecular Sciences. 2021;22:1942. doi: 10.3390/ijms22041942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani A., Mahmoudifar K., Shokri S., Mazaheri Y., Shamloo E., Rezagholizade-Shirvan A., Elhamirad A.H. Effect of Allium Jesdianum’s extract on the physicochemical, antioxidant, antimicrobial and sensory properties of sausage characteristics. Food Chemistry: X. 2024;22 doi: 10.1016/j.fochx.2024.101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Sarkar T., Das A., Chakraborty R. Micro and nanoencapsulation of natural colors: A holistic view. Applied Biochemistry and Biotechnology. 2021;193:3787–3811. doi: 10.1007/s12010-021-03631-8. [DOI] [PubMed] [Google Scholar]
- Girón-Hernández J., Gentile P., Benlloch-Tinoco M. Impact of heterogeneously crosslinked calcium alginate networks on the encapsulation of β-carotene-loaded beads. Carbohydrate Polymers. 2021;271 doi: 10.1016/j.carbpol.2021.118429. [DOI] [PubMed] [Google Scholar]
- Gómez-Mascaraque L.G., Balanč B., Djordjević V., Bugarski B. Encapsulation techniques for food purposes. Encapsulation in Food Processing and Fermentation. 2022:37–80. [Google Scholar]
- Gonçalves A., Estevinho B.N., Rocha F. Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds. Trends in Food Science & Technology. 2021;114:510–520. [Google Scholar]
- Gorantla S., Wadhwa G., Jain S., Sankar S., Nuwal K., Mahmood A.…Singhvi G. Recent advances in nanocarriers for nutrient delivery. Drug Delivery and Translational Research. 2021:1–26. doi: 10.1007/s13346-021-01097-z. [DOI] [PubMed] [Google Scholar]
- Grgić J., Šelo G., Planinić M., Tišma M., Bucić-Kojić A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants. 2020;9:923. doi: 10.3390/antiox9100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover J.A. CRC Press; 2020. Methylcellulose (MC) and hydroxypropylmethylcellulose (HPMC), food hydrocolloids; pp. 121–154. [Google Scholar]
- Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. The AAPS Journal. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadidi M., Majidiyan N., Jelyani A.Z., Moreno A., Hadian Z., Mousavi Khanegah A. Alginate/fish gelatin-encapsulated Lactobacillus acidophilus: A study on viability and technological quality of bread during baking and storage. Foods. 2021;10:2215. doi: 10.3390/foods10092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiagha M.N., Taghizadeh S., Asgharzadeh M., Dao S., Ganbarov K., Köse Ş., Kafil H.S. Gut microbiota and human body interactions; its impact on health: A review. Current Pharmaceutical Biotechnology. 2022;23:4–14. doi: 10.2174/1389201022666210104115836. [DOI] [PubMed] [Google Scholar]
- Hamad I., Harb A.A., Bustanji Y. Liposome-based drug delivery Systems in Cancer Research: An analysis of global landscape efforts and achievements. Pharmaceutics. 2024;16:400. doi: 10.3390/pharmaceutics16030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S.F. React now regarding nanomaterial regulation. Nature Nanotechnology. 2017;12:714–716. doi: 10.1038/nnano.2017.163. [DOI] [PubMed] [Google Scholar]
- Hashim N.A., Mudalip S.K.A., Man R.C., Sulaiman S.Z. Menhaden fish oil encapsulation by spray drying process: Influence of different biopolymer materials, inlet air temperature and emulsion ratios. Journal of Chemical Technology & Biotechnology. 2023;98:2726–2733. [Google Scholar]
- Hewlings S.J., Kalman D.S. Curcumin: A review of its effects on human health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B.…Salminen S. Nature reviews Gastroenterology & hepatology; 2014. Expert consensus document: The international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. [DOI] [PubMed] [Google Scholar]
- Hosseini S.F., Ramezanzade L., McClements D.J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends in Food Science & Technology. 2021;109:322–339. [Google Scholar]
- Hu X., Liu C., Zhang H., Hossen M.A., Sameen D.E., Dai J.…Li S. In vitro digestion of sodium alginate/pectin co-encapsulated Lactobacillus bulgaricus and its application in yogurt bilayer beads. International Journal of Biological Macromolecules. 2021;193:1050–1058. doi: 10.1016/j.ijbiomac.2021.11.076. [DOI] [PubMed] [Google Scholar]
- Ibrahim M., Abuwatfa W.H., Awad N.S., Sabouni R., Husseini G.A. Encapsulation, release, and cytotoxicity of doxorubicin loaded in liposomes, micelles, and metal-organic frameworks: A review. Pharmaceutics. 2022;14:254. doi: 10.3390/pharmaceutics14020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees H., Zaidi S.Z.J., Sabir A., Khan R.U., Zhang X., Hassan S.-u. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials. 2020;10:1970. doi: 10.3390/nano10101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiarto R., Reni R., Utama G.L., Subroto E., Pangawikan A.D., Djali M. The physicochemical, antioxidant, and sensory properties of chocolate biscuits incorporated with encapsulated mangosteen (Garcinia mangostana L.) peel extract. International Journal of Food Properties. 2023;26:122–138. [Google Scholar]
- Jafari D., Esmaeilzadeh A., Mohammadi-Kordkhayli M., Rezaei N. Vitamin C and the immune system. Nutrition and immunity. 2019:81–102. [Google Scholar]
- Jakubowska E., Lulek J. The application of freeze-drying as a production method of drug nanocrystals and solid dispersions–a review. Journal of Drug Delivery Science and Technology. 2021;62 [Google Scholar]
- Jamshidi A., Shabanpour B., Pourashouri P., Raeisi M. Optimization of encapsulation of fish protein hydrolysate and fish oil in W1/O/W2 double emulsion: Evaluation of sensory quality of fortified yogurt. Journal of Food Processing and Preservation. 2019;43 [Google Scholar]
- Jana S., Gandhi A., Jana S. Nanotechnology in bioactive food ingredients: Its pharmaceutical and biomedical approaches. Nanotechnology applications in food. Elsevier. 2017:21–41. [Google Scholar]
- Jayakody M.M., Vanniarachchy M.P.G., Wijesekara I. Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: A review. Journal of Food Measurement and Characterization. 2022;16:1195–1227. [Google Scholar]
- Jayedi A., Shab-Bidar S. Fish consumption and the risk of chronic disease: An umbrella review of meta-analyses of prospective cohort studies. Advances in Nutrition. 2020;11:1123–1133. doi: 10.1093/advances/nmaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodar E., Campusano C., De Jongh R.T., Holick M.F. Calcifediol: A review of its pharmacological characteristics and clinical use in correcting vitamin D deficiency. European Journal of Nutrition. 2023;62:1579–1597. doi: 10.1007/s00394-023-03103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johra F.T., Bepari A.K., Bristy A.T., Reza H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants. 2020;9:1046. doi: 10.3390/antiox9111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junaid P.M., Dar A.H., Dash K.K., Rohilla S., Islam R.-u., Shams R.…Zaidi S. Polysaccharide-based hydrogels for microencapsulation of bioactive compounds: A review. Journal of Agriculture and Food Research. 2024;9:101038. [Google Scholar]
- Jung K.-H., Lee J.H., Park J.W., Quach C.H.T., Moon S.-H., Cho Y.S., Lee K.-H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. International Journal of Pharmaceutics. 2015;478:251–257. doi: 10.1016/j.ijpharm.2014.11.049. [DOI] [PubMed] [Google Scholar]
- Kakakhel M.A., Wu F., Sajjad W., Zhang Q., Khan I., Ullah K., Wang W. Long-term exposure to high-concentration silver nanoparticles induced toxicity, fatality, bioaccumulation, and histological alteration in fish (Cyprinus carpio) Environmental Sciences Europe. 2021;33:1–11. [Google Scholar]
- Kandasamy S., Naveen R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. Journal of Food Process Engineering. 2022;45 [Google Scholar]
- Kankala R.K., Han Y.H., Na J., Lee C.H., Sun Z., Wang S.B.…Chen A.Z. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Advanced Materials. 2020;32 doi: 10.1002/adma.201907035. [DOI] [PubMed] [Google Scholar]
- Ke W., Yin Y., Chen X., Qiu B. Chlorophylls. Research methods of environmental physiology in aquatic sciences. 2021:95–106. [Google Scholar]
- Khan I., Hussain M., Jiang B., Zheng L., Pan Y., Hu J.…Zou X. Omega-3 long-chain polyunsaturated fatty acids: Metabolism and health implications. Progress in Lipid Research. 2023;92 doi: 10.1016/j.plipres.2023.101255. [DOI] [PubMed] [Google Scholar]
- Khatibi S.A., Ehsani A., Nemati M., Javadi A. Microencapsulation of Zataria multiflora Boiss. Essential oil by complex coacervation using gelatin and gum arabic: Characterization, release profile, antimicrobial and antioxidant activities. Journal of Food Processing and Preservation. 2021;45 [Google Scholar]
- Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Darani K., Gomes da Cruz A., Shamloo E., Abdimoghaddam Z., Mozafari M. Green synthesis of metallic nanoparticles using algae and microalgae. Letters in Applied NanoBioScience. 2019;8:666–670. [Google Scholar]
- Kirby C.J., Gregoriadis G. CRC Press; 2019. A simple procedure for preparing liposomes capable of high encapsulation efficiency under mild conditions, liposome technology; pp. 19–27. [Google Scholar]
- Klojdová I., Milota T., Smetanová J., Stathopoulos C. Encapsulation: A strategy to deliver therapeutics and bioactive compounds? Pharmaceuticals. 2023;16:362. doi: 10.3390/ph16030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Peng X., Cui H., Liu P., Pang B., Zhang K. pH-responsive polymeric nanoparticles with tunable sizes for targeted drug delivery. RSC Advances. 2020;10:4860–4868. doi: 10.1039/c9ra10280a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovsari E., Gilani P.S., Shokri S., Borazgh A.M., Rezagholizade-Shirvan A., Nia A.P. Influence of green pepper extract on the physicochemical, antioxidant, and sensory properties of stirred yogurt. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2023.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyani R.D., Andrade M., Quester K., Gaytán P., Huerta-Saquero A., Vazquez-Duhalt R. Surface modification of protein enhances encapsulation in chitosan nanoparticles. Applied Nanoscience. 2018;8:1197–1203. [Google Scholar]
- Kumar Y., Kumar V. Effects of double emulsion (W1/O/W2) containing encapsulated Murraya koenigii berries extract on quality characteristics of reduced-fat meat batter with high oxidative stability. LWT. 2020;127 [Google Scholar]
- Kumari M., Arya P., Khatkar S.K., Kumar P. Development and characterization of apple pomace and finger millet-based pasta enriched with encapsulated micronutrient. Food Chemistry Advances. 2023;3 [Google Scholar]
- Kuo C.-C., Clark S., Qin H., Shi X. Development of a shelf-stable, gel-based delivery system for probiotics by encapsulation, 3D printing, and freeze-drying. Lwt. 2022;157 [Google Scholar]
- Lamas B., Martins Breyner N., Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Particle and Fibre Toxicology. 2020;17:1–22. doi: 10.1186/s12989-020-00349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H., Karakasyan C., Jouenne T., Le Cerf D., Dé E. Application of polymeric nanocarriers for enhancing the bioavailability of antibiotics at the target site and overcoming antimicrobial resistance. Applied Sciences. 2021;11:10695. [Google Scholar]
- Le Priol L., Gmur J., Dagmey A., Morandat S., El Kirat K., Saleh K., Nesterenko A. Co-encapsulation of vegetable oils with phenolic antioxidants and evaluation of their oxidative stability under long-term storage conditions. LWT. 2021;142 [Google Scholar]
- Li D., Wei Z., Xue C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Comprehensive Reviews in Food Science and Food Safety. 2021;20:5345–5369. doi: 10.1111/1541-4337.12840. [DOI] [PubMed] [Google Scholar]
- Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nature Reviews Materials. 2016;1:1–17. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Lei X., Feng H., Li B., Kong J., Xing M. Layer-by-layer cell encapsulation for drug delivery: The history, technique basis, and applications. Pharmaceutics. 2022;14:297. doi: 10.3390/pharmaceutics14020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Chen L., McClements D.J., Peng X., Xu Z., Meng M., Jin Z. Bioactive delivery systems based on starch and its derivatives: Assembly and application at different structural levels. Food Chemistry. 2024;432 doi: 10.1016/j.foodchem.2023.137184. [DOI] [PubMed] [Google Scholar]
- Lin Z., Chen H., Li S., Li X., Wang J., Xu S. Electrospun food polysaccharides loaded with bioactive compounds: Fabrication, release, and applications. Polymers. 2023;15:2318. doi: 10.3390/polym15102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Wang Y., Zhong W., Li B., Mequanint K., Luo G., Xing M. Biomedical applications of layer-by-layer self-assembly for cell encapsulation: Current status and future perspectives. Advanced Healthcare Materials. 2019;8 doi: 10.1002/adhm.201800939. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ahmed S., Sameen D.E., Wang Y., Lu R., Dai J.…Qin W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends in Food Science & Technology. 2021;112:532–546. [Google Scholar]
- Lohith Kumar D., Mitra J., Roopa S. Nanoencapsulation of food carotenoids. Environmental Nanotechnology. 2020;3:203–242. [Google Scholar]
- Łopusiewicz Ł., Bogusławska-Wąs E., Drozłowska E., Trocer P., Dłubała A., Mazurkiewicz-Zapałowicz K., Bartkowiak A. The application of spray-dried and reconstituted flaxseed oil cake extract as encapsulating material and carrier for probiotic Lacticaseibacillus rhamnosus GG. Materials. 2021;14:5324. doi: 10.3390/ma14185324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudovaris T. Encapsulation devices to enhance graft survival: The latest in the development of micro and macro encapsulation devices to improve clinical, xeno, and stem cell transplantation outcomes. Pancreas and Beta Cell Replacement. Elsevier. 2022:125–152. [Google Scholar]
- Luo Q., Hossen M.A., Zeng Y., Dai J., Li S., Qin W., Liu Y. Gelatin-based composite films and their application in food packaging: A review. Journal of Food Engineering. 2022;313 [Google Scholar]
- Ma Z., Du B., Li J., Yang Y., Zhu F. An insight into anti-inflammatory activities and inflammation related diseases of anthocyanins: A review of both in vivo and in vitro investigations. International Journal of Molecular Sciences. 2021;22:11076. doi: 10.3390/ijms222011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaliki D., Shaito A.A., Pintus G., El-Yazbi A., Eid A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Current Opinion in Pharmacology. 2019;45:57–65. doi: 10.1016/j.coph.2019.04.014. [DOI] [PubMed] [Google Scholar]
- Mainardes R.M., Khalil N.M. Nanoparticles of bovine serum albumin for encapsulation of food ingredients, biopolymer nanostructures for food encapsulation purposes. Elsevier. 2019:169–186. [Google Scholar]
- Maleki Dizaj S., Alipour M., Dalir Abdolahinia E., Ahmadian E., Eftekhari A., Forouhandeh H.…Zununi Vahed S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytotherapy Research. 2022;36:1156–1181. doi: 10.1002/ptr.7389. [DOI] [PubMed] [Google Scholar]
- Maleki G., Woltering E.J., Mozafari M. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends in Food Science & Technology. 2022;120:88–99. [Google Scholar]
- Manke A., Wang L., Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Research International. 2013;2013 doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J.M., Silva L.S., Rabelo M.S., Muniz M.P., Nunomura S.M., Correa R.F.…Sanches E.A. Encapsulation of Amazonian blueberry juices: Evaluation of bioactive compounds and stability. Lwt. 2020;124 [Google Scholar]
- Marino A., Battaglini M., Moles N., Ciofani G. Natural antioxidant compounds as potential pharmaceutical tools against neurodegenerative diseases. ACS Omega. 2022;7:25974–25990. doi: 10.1021/acsomega.2c03291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritim S., Boulas P., Lin Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. International Journal of Pharmaceutics. 2021;592 doi: 10.1016/j.ijpharm.2020.120051. [DOI] [PubMed] [Google Scholar]
- Martins T., Barros A.N., Rosa E., Antunes L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules. 2023;28:5344. doi: 10.3390/molecules28145344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli R., Francioso A., Mosca L., Silva P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25:3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya V.K., Aggarwal M. Fabrication of nano-structured lipid carrier for encapsulation of vitamin D3 for fortification of ‘Lassi’; a milk based beverage. The Journal of Steroid Biochemistry and Molecular Biology. 2019;193 doi: 10.1016/j.jsbmb.2019.105429. [DOI] [PubMed] [Google Scholar]
- Mazumder M.A.R., Ranganathan T.V. Encapsulation of isoflavone with milk, maltodextrin and gum acacia improves its stability. Current Research in Food Science. 2020;2:77–83. doi: 10.1016/j.crfs.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements D.J. Nano-enabled personalized nutrition: Developing multicomponent-bioactive colloidal delivery systems. Advances in Colloid and Interface Science. 2020;282 doi: 10.1016/j.cis.2020.102211. [DOI] [PubMed] [Google Scholar]
- McClements D.J., Gumus C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Advances in Colloid and Interface Science. 2016;234:3–26. doi: 10.1016/j.cis.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Medina-Pérez G., Estefes-Duarte J.A., Afanador-Barajas L.N., Fernández-Luqueño F., Zepeda-Velázquez A.P., Franco-Fernández M.J.…Campos-Montiel R.G. Encapsulation preserves antioxidant and antidiabetic activities of cactus acid fruit bioactive compounds under simulated digestion conditions. Molecules. 2020;25:5736. doi: 10.3390/molecules25235736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena J., Gupta A., Ahuja R., Singh M., Bhaskar S., Panda A.K. Inorganic nanoparticles for natural product delivery: A review. Environmental Chemistry Letters. 2020;18:2107–2118. [Google Scholar]
- Mehdipoor Damiri G.R., Motamedzadegan A., Safari R., Shahidi S.A., Ghorbani A. Evaluation of stability, physicochemical and antioxidant properties of extracted chlorophyll from Persian clover (Trifolium resupinatum L.) Journal of Food Measurement and Characterization. 2021;15:327–340. [Google Scholar]
- Men W., Zhu P., Dong S., Liu W., Zhou K., Bai Y.…Zhang S. Fabrication of dual pH/redox-responsive lipid-polymer hybrid nanoparticles for anticancer drug delivery and controlled release. International Journal of Nanomedicine. 2019:8001–8011. doi: 10.2147/IJN.S226798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. An overview of molecular layer deposition for organic and organic–inorganic hybrid materials: Mechanisms, growth characteristics, and promising applications. Journal of Materials Chemistry A. 2017;5:18326–18378. [Google Scholar]
- Milano F., Masi A., Madaghiele M., Sannino A., Salvatore L., Gallo N. Current trends in gelatin-based drug delivery systems. Pharmaceutics. 2023;15:1499. doi: 10.3390/pharmaceutics15051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojević M., Popović A., Pajić-Lijaković I., Šoštarić I., Kolašinac S., Stevanović Z.D. Alginate gel-based carriers for encapsulation of carotenoids: On challenges and applications. Gels. 2023;9:620. doi: 10.3390/gels9080620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirahmadi M., Azimi-Hashemi S., Saburi E., Kamali H., Pishbin M., Hadizadeh F. Potential inhibitory effect of lycopene on prostate cancer. Biomedicine & Pharmacotherapy. 2020;129 doi: 10.1016/j.biopha.2020.110459. [DOI] [PubMed] [Google Scholar]
- Misra S., Pandey P., Mishra H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends in Food Science & Technology. 2021;109:340–351. [Google Scholar]
- Mohammad A.A., Mehaya F.M., Salem S.H., Amer H.M. Psyllium and okra mucilage as co-carrier wall materials for fenugreek oil encapsulation and its utilization as fat replacers in pan bread and biscuit production. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanto S., Narayana S., Merai K.P., Kumar J.A., Bhunia A., Hani U.…Ahmed M.G. Advancements in gelatin-based hydrogel systems for biomedical applications: A state-of-the-art review. International Journal of Biological Macromolecules. 2023;253 doi: 10.1016/j.ijbiomac.2023.127143. [DOI] [PubMed] [Google Scholar]
- Mohite D., Waghmare R. Encapsulation techniques for De-livery of bioactive compounds in Milk and dairy products-a review. Journal of Dairy Research & Technology. 2020;3:1–9. [Google Scholar]
- Mohtashami M., Rezagholizade-Shirvan A., Hojati Bonab Z., Amiryousefi M.R., Darroudi M., Ahmadi Solimani M.S.…Momtazi-Borojeni A.A. Green synthesis of silver nanoparticles using Cirsium Congestum extract modified by chitosan/alginate: Bactericidal activity against pathogenic Bacteria and cytotoxicity analysis in Normal cell line. Current Pharmaceutical Design. 2024;30:1610–1623. doi: 10.2174/0113816128304460240408085736. [DOI] [PubMed] [Google Scholar]
- Montà-González G., Sancenón F., Martínez-Máñez R., Martí-Centelles V. Purely covalent molecular cages and containers for guest encapsulation. Chemical Reviews. 2022;122:13636–13708. doi: 10.1021/acs.chemrev.2c00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera J., García I., Liz-Marzán L.M. Cellular uptake of nanoparticles versus small molecules: A matter of size. Accounts of Chemical Research. 2018;51:2305–2313. doi: 10.1021/acs.accounts.8b00292. [DOI] [PubMed] [Google Scholar]
- Mrowicka M., Mrowicki J., Kucharska E., Majsterek I. Lutein and zeaxanthin and their roles in age-related macular degeneration—Neurodegenerative disease. Nutrients. 2022;14:827. doi: 10.3390/nu14040827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhoza B., Qi B., Harindintwali J.D., Koko M.Y.F., Zhang S., Li Y. Combined plant protein modification and complex coacervation as a sustainable strategy to produce coacervates encapsulating bioactives. Food Hydrocolloids. 2022;124 [Google Scholar]
- Munin A., Edwards-Lévy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3:793–829. doi: 10.3390/pharmaceutics3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugkar D.A., Zanwar A.A., Shrivastava A. Effect of nano-encapsulation of flaxseed oil on the stability, characterization and incorporation on the quality of eggless cake. Applied Food Research. 2021;1 [Google Scholar]
- Naderi M., Mazaheri Y., Torbati M., Azadmard-Damirchi S., Rezagholizade-Shirvan A., Shokri S. Evaluation of the qualitative properties of the oil extracted from the mixture of Helianthus annuus and Nigella sativa seeds during heating. Scientific Reports. 2024;14:17573. doi: 10.1038/s41598-024-68463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemiwal M., Kumar D. TiO2 and SiO2 encapsulated metal nanoparticles: Synthetic strategies, properties, and photocatalytic applications. Inorganic Chemistry Communications. 2021;128 [Google Scholar]
- Neves M.I.L., Desobry-Banon S., Perrone I.T., Desobry S., Petit J. Encapsulation of curcumin in milk powders by spray-drying: Physicochemistry, rehydration properties, and stability during storage. Powder Technology. 2019;345:601–607. [Google Scholar]
- Novais C., Molina A.K., Abreu R.M., Santo-Buelga C., Ferreira I.C., Pereira C., Barros L. Natural food colorants and preservatives: A review, a demand, and a challenge. Journal of Agricultural and Food Chemistry. 2022;70:2789–2805. doi: 10.1021/acs.jafc.1c07533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J.K., Sobkowiak P., Drzymała-Czyż S., Krzyżanowska-Jankowska P., Sapiejka E., Skorupa W.…Kurek S. Fat-soluble vitamin supplementation using liposomes, cyclodextrins, or medium-chain triglycerides in cystic fibrosis: A randomized controlled trial. Nutrients. 2021;13:4554. doi: 10.3390/nu13124554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nthimole C.T., Kaseke T., Fawole O.A. Micro-encapsulation and characterization of anthocyanin-rich raspberry juice powder for potential applications in the food industry. Processes. 2022;10:1038. [Google Scholar]
- Oliveira F.M., Oliveira R.M., Buchweitz L.T.G., Pereira J.R., dos Santos Hackbart H.C., Nalério É.S.…Zambiazi R.C. Encapsulation of olive leaf extract (Olea europaea L.) in gelatin/tragacanth gum by complex coacervation for application in sheep meat hamburger. Food Control. 2022;131 [Google Scholar]
- Olson F., Hunt C., Szoka F., Vail W., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1979;557:9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Oprea I., Fărcaș A.C., Leopold L.F., Diaconeasa Z., Coman C., Socaci S.A. Nano-encapsulation of citrus essential oils: Methods and applications of interest for the food sector. Polymers. 2022;14:4505. doi: 10.3390/polym14214505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oršolić N., Jazvinšćak Jembrek M. Molecular and cellular mechanisms of propolis and its polyphenolic compounds against cancer. International Journal of Molecular Sciences. 2022;23:10479. doi: 10.3390/ijms231810479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannopoulos A., Sotiropoulos K. Current advances of polysaccharide-based nanogels and microgels in food and biomedical sciences. Polymers. 2022;14:813. doi: 10.3390/polym14040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parafati L., Palmeri R., Trippa D., Restuccia C., Fallico B. Quality maintenance of beef burger patties by direct addiction or encapsulation of a prickly pear fruit extract. Frontiers in Microbiology. 2019;10:1760. doi: 10.3389/fmicb.2019.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J.L., Baeza A., Vallet-Regí M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opinion on Drug Delivery. 2019;16:1095–1112. doi: 10.1080/17425247.2019.1662786. [DOI] [PubMed] [Google Scholar]
- Parveen R., Mohapatra S., Ahmad S., Husain S.A. Amalgamation of nanotechnology for delivery of bioactive constituents in solid tumors. Current Drug Delivery. 2023;20:457–482. doi: 10.2174/1567201819666220425093102. [DOI] [PubMed] [Google Scholar]
- Pateiro M., Gómez B., Munekata P.E., Barba F.J., Putnik P., Kovačević D.B., Lorenzo J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules. 2021;26:1547. doi: 10.3390/molecules26061547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Garala K., Singh S., Prajapati B.G., Chittasupho C. Lipid-based nanoparticles in delivering bioactive compounds for improving therapeutic efficacy. Pharmaceuticals. 2024;17:329. doi: 10.3390/ph17030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A.R., Fernandes V.C., Delerue-Matos C., de Freitas V., Mateus N., Oliveira J. Exploring acylated anthocyanin-based extracts as a natural alternative to synthetic food dyes: Stability and application insights. Food Chemistry. 2024;461 doi: 10.1016/j.foodchem.2024.140945. [DOI] [PubMed] [Google Scholar]
- Petkova D., Mihaylova D., Desseva I. Microencapsulation in food industry–an overview, BIO web of conferences. EDP Sciences. 2022;25:02005. [Google Scholar]
- Piñón-Balderrama C.I., Leyva-Porras C., Terán-Figueroa Y., Espinosa-Solís V., Álvarez-Salas C., Saavedra-Leos M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes. 2020;8:889. [Google Scholar]
- Pourashouri P., Shabanpour B., Heydari S., Raeisi S. Encapsulation of fish oil by carrageenan and gum tragacanth as wall materials and its application to the enrichment of chicken nuggets. Lwt. 2021;137 [Google Scholar]
- Pradeep Prasanna P., Charalampopoulos D. Encapsulation in an alginate–goats’ milk–inulin matrix improves survival of probiotic Bifidobacterium in simulated gastrointestinal conditions and goats’ milk yoghurt. International Journal of Dairy Technology. 2019;72:132–141. [Google Scholar]
- Prasanna P., Charalampopoulos D. Encapsulation of Bifidobacterium longum in alginate-dairy matrices and survival in simulated gastrointestinal conditions, refrigeration, cow milk and goat milk. Food Bioscience. 2018;21:72–79. [Google Scholar]
- Premjit Y., Pandey S., Mitra J. Recent trends in folic acid (vitamin B9) encapsulation, controlled release, and mathematical modelling. Food Reviews International. 2023;39:5528–5562. [Google Scholar]
- Premjit Y., Pandhi S., Kumar A., Rai D.C., Duary R.K., Mahato D.K. Current trends in flavor encapsulation: A comprehensive review of emerging encapsulation techniques, flavour release, and mathematical modelling. Food Research International. 2022;151 doi: 10.1016/j.foodres.2021.110879. [DOI] [PubMed] [Google Scholar]
- Qian Y.L., Hua G.K., Dung J.K., Qian M. Encapsulation of garlic oil and diallyl disulfide with β-cyclodextrin for white rot control in Allium crops. Crop Protection. 2024;178 [Google Scholar]
- Radünz M., dos Santos Hackbart H.C., Camargo T.M., Nunes C.F.P., de Barros F.A.P., Dal Magro J.…da Rosa Z.E. Antimicrobial potential of spray drying encapsulated thyme (Thymus vulgaris) essential oil on the conservation of hamburger-like meat products. International Journal of Food Microbiology. 2020;330 doi: 10.1016/j.ijfoodmicro.2020.108696. [DOI] [PubMed] [Google Scholar]
- Raeisi S., Ojagh S.M., Quek S.Y., Pourashouri P., Salaün F. Nano-encapsulation of fish oil and garlic essential oil by a novel composition of wall material: Persian gum-chitosan. LWT. 2019;116 [Google Scholar]
- Rahman A., Sarkar A., Yadav O.P., Achari G., Slobodnik J. Potential human health risks due to environmental exposure to nano-and microplastics and knowledge gaps: A scoping review. Science of the Total Environment. 2021;757 doi: 10.1016/j.scitotenv.2020.143872. [DOI] [PubMed] [Google Scholar]
- Rahnemoon P., Sarabi-Jamab M., Bostan A., Mansouri E. Nano-encapsulation of pomegranate (Punica granatum L.) peel extract and evaluation of its antimicrobial properties on coated chicken meat. Food Bioscience. 2021;43 [Google Scholar]
- Rajam R., Subramanian P. Encapsulation of probiotics: Past, present and future. Beni-Suef University Journal of Basic and Applied Sciences. 2022;11:46. [Google Scholar]
- Rajput V., Minkina T., Mazarji M., Shende S., Sushkova S., Mandzhieva S.…Jatav H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Annals of Agricultural Sciences. 2020;65:137–143. [Google Scholar]
- Rambaran T.F. Nanopolyphenols: A review of their encapsulation and anti-diabetic effects. SN Applied Sciences. 2020;2:1335. [Google Scholar]
- Rana A., Samtiya M., Dhewa T., Mishra V., Aluko R.E. Health benefits of polyphenols: A concise review. Journal of Food Biochemistry. 2022;46 doi: 10.1111/jfbc.14264. [DOI] [PubMed] [Google Scholar]
- Rashidaie Abandansarie S.S., Ariaii P., Charmchian Langerodi M. Effects of encapsulated rosemary extract on oxidative and microbiological stability of beef meat during refrigerated storage. Food Science & Nutrition. 2019;7:3969–3978. doi: 10.1002/fsn3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reque P.M., Brandelli A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends in Food Science & Technology. 2021;114:1–10. [Google Scholar]
- Rezaei Z., Nickfar F., Salari A., Yousefi M., Khodaparast M.H.H., Shamloo E. Feasibility of biofilm production capacity by Levilactobacillus brevis isolated from motal cheese and evaluation of biofilm resistance produced in vitro and in yogurt. Arabian Journal of Chemistry. 2023;16 [Google Scholar]
- Rezaei Z., Salari A., Khanzadi S., Rhim J.W., Shamloo E. Preparation of milk-based probiotic lactic acid bacteria biofilms: A new generation of probiotics. Food Science & Nutrition. 2023;11:2915–2924. doi: 10.1002/fsn3.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezagholizade-Shirvan A., Kalantarmahdavi M., Amiryousefi M.R. Evaluation of the effect of basil seed gum, tragacanth gum, pectin, and coating formulation with corn flour on oil absorption and sensory properties of watermelon rind chips. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezagholizade-shirvan A., Masrournia M., Fathi Najafi M., Behmadi H. Synthesis and characterization of nanoparticles based on chitosan-biopolymers systems as nanocarrier agents for curcumin: Study on pharmaceutical and environmental applications. Polymer Bulletin. 2023;80:1495–1517. [Google Scholar]
- Rezagholizade-Shirvan A., Mohammadi M., Mazaheri Y., Fallahizadeh S., Ghorbani H., Shokri S.…Shamloo E. Employing a magnetic chitosan/molybdenum disulfide nanocomposite for efficiently removing polycyclic aromatic hydrocarbons from milk samples. Scientific Reports. 2024;14:15054. doi: 10.1038/s41598-024-66087-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezagholizade-shirvan A., Najafi M.F., Behmadi H., Masrournia M. Preparation of nano-composites based on curcumin/chitosan-PVA-alginate to improve stability, antioxidant, antibacterial and anticancer activity of curcumin. Inorganic Chemistry Communications. 2022;145 [Google Scholar]
- Rezvankhah A., Emam-Djomeh Z., Askari G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Drying Technology. 2020;38:235–258. [Google Scholar]
- Robert P., Zamorano M., González E., Silva-Weiss A., Cofrades S., Giménez B. Double emulsions with olive leaves extract as fat replacers in meat systems with high oxidative stability. Food Research International. 2019;120:904–912. doi: 10.1016/j.foodres.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Rohini C., Kanchana S., Geetha P., Mini M. Optimize the process for encapsulated spray dried lemon juice powder to enhance the vitamin C. World Journal of Advanced Research and Reviews. 2024;21:2064–2072. [Google Scholar]
- Roman G.C., Jackson R., Gadhia R., Román A., Reis J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Revue Neurologique. 2019;175:724–741. doi: 10.1016/j.neurol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Romero-Chapol O.O., Varela-Pérez A., Castillo-Olmos A.G., García H.S., Singh J., García-Ramírez P.J.…Cano-Sarmiento C. Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic survival, in vitro digestion and viability in apple juice and yogurt. Applied Sciences. 2022;12:2141. [Google Scholar]
- Rosales T.K.O., da Silva M.P., Lourenço F.R., Hassimotto N.M.A., Fabi J.P. Nanoencapsulation of anthocyanins from blackberry (Rubus spp.) through pectin and lysozyme self-assembling. Food Hydrocolloids. 2021;114 [Google Scholar]
- Rostamabadi M.M., Falsafi S.R., Nishinari K., Rostamabadi H. Seed gum-based delivery systems and their application in encapsulation of bioactive molecules. Critical Reviews in Food Science and Nutrition. 2023;63:9937–9960. doi: 10.1080/10408398.2022.2076065. [DOI] [PubMed] [Google Scholar]
- Rout S.R., Gowtham K., Sheikh A., Parvez S., Dandela R., Kesharwani P. Recent advances and future prospective of hybrid drug delivery systems. Hybrid nanomaterials for Drug Delivery. 2022:357–374. [Google Scholar]
- Sadiq U., Gill H., Chandrapala J. Casein micelles as an emerging delivery system for bioactive food components. Foods. 2021;10:1965. doi: 10.3390/foods10081965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safeer Abbas M., Afzaal M., Saeed F., Asghar A., Jianfeng L., Ahmad A.…Shah Y.A. Probiotic viability as affected by encapsulation materials: Recent updates and perspectives. International Journal of Food Properties. 2023;26:1324–1350. [Google Scholar]
- Saini R.K., Prasad P., Sreedhar R.V., Akhilender Naidu K., Shang X., Keum Y.-S. Omega− 3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants. 2021;10:1627. doi: 10.3390/antiox10101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salatin S., Maleki Dizaj S., Yari Khosroushahi A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biology International. 2015;39:881–890. doi: 10.1002/cbin.10459. [DOI] [PubMed] [Google Scholar]
- Salehi B., Sharifi-Rad J., Cappellini F., Reiner Ž., Zorzan D., Imran M.…Fahmy N.M. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Frontiers in Pharmacology. 2020;11:1300. doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvia-Trujillo L., McClements D.J., Martín-Belloso O. Nanoemulsion design for the delivery of omega-3 fatty acids: Formation, oxidative stability, and digestibility. Omega-3 delivery systems. 2021:295–319. [Google Scholar]
- Samborska K., Boostani S., Geranpour M., Hosseini H., Dima C., Khoshnoudi-Nia S.…Akbari-Alavijeh S. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends in Food Science & Technology. 2021;108:297–325. [Google Scholar]
- Santiaguín-Padilla A.J., Peña-Ramos E.A., Pérez-Gallardo A., Rascón-Chu A., González-Ávila M., González-Ríos H.…Islava-Lagarda T. In vitro digestibility and quality of an emulsified meat product formulated with animal fat encapsulated with pectin. Journal of Food Science. 2019;84:1331–1339. doi: 10.1111/1750-3841.14626. [DOI] [PubMed] [Google Scholar]
- Santosa T.P., Cejasb C.M., Cunhaa R.L. Microfluidics as a platform to assess and induce emulsion destabilization. Faculdade de Engenharia de Alimentos. 2021;21 [Google Scholar]
- Saraswat A.L., Maher T.J. Development and optimization of stealth liposomal system for enhanced in vitro cytotoxic effect of quercetin. Journal of Drug Delivery Science and Technology. 2020;55 [Google Scholar]
- Sarode C., Jagtap Y., Gogate P. Springer; 2022. Ultrasound for improved encapsulation and crystallization with focus on pharmaceutical applications, optimization of pharmaceutical processes; pp. 193–229. [Google Scholar]
- Sarubbo L.A., Maria da Gloria C.S., Durval I.J.B., Bezerra K.G.O., Ribeiro B.G., Silva I.A.…Banat I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochemical Engineering Journal. 2022;181 [Google Scholar]
- Sasi M., Kumar S., Hasan M., Garcia-Gutierrez E., Kumari S., Prakash O.…Dahuja A. Current trends in the development of soy-based foods containing probiotics and paving the path for soy-synbiotics. Critical Reviews in Food Science and Nutrition. 2023;63:9995–10013. doi: 10.1080/10408398.2022.2078272. [DOI] [PubMed] [Google Scholar]
- Schubert C., Fischer S., Dorsch K., Teßmer L., Hinrichs J., Atamer Z. Microencapsulation of bacteriophages for the delivery to and modulation of the human gut microbiota through milk and cereal products. Applied Sciences. 2022;12:6299. [Google Scholar]
- Šeregelj V., Ćetković G., Čanadanović-Brunet J., Šaponjac V.T., Vulić J., Lević S.…Hidalgo A. Encapsulation of carrot waste extract by freeze and spray drying techniques: An optimization study. LWT. 2021;138 [Google Scholar]
- Šeregelj V., Pezo L., Šovljanski O., Lević S., Nedović V., Markov S.…Šaponjac V.T. New concept of fortified yogurt formulation with encapsulated carrot waste extract. Lwt. 2021;138 [Google Scholar]
- Šeregelj V., Škrobot D., Kojić J., Pezo L., Šovljanski O., Tumbas Šaponjac V.…Čanadanović-Brunet J. Quality and sensory profile of durum wheat pasta enriched with carrot waste encapsulates. Foods. 2022;11:1130. doi: 10.3390/foods11081130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šeregelj V., Tumbas Šaponjac V., Lević S., Kalušević A., Ćetković G., Čanadanović-Brunet J.…Vidaković A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. Journal of Microencapsulation. 2019;36:704–714. doi: 10.1080/02652048.2019.1668488. [DOI] [PubMed] [Google Scholar]
- Shamloo E., Nickfar F., Mahmoudzadeh M., Sarafraz M., Salari A., Darroudi M.…Rezaei Z. Investigation of heavy metal release from variety cookware into food during cooking process. International Journal of Environmental Analytical Chemistry. 2023:1–17. [Google Scholar]
- Sharifi S., Rezazad-Bari M., Alizadeh M., Almasi H., Amiri S. Use of whey protein isolate and gum Arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: Enhanced viability of probiotic in Iranian white cheese. Food Hydrocolloids. 2021;113 [Google Scholar]
- Shi L., Zhang J., Zhao M., Tang S., Cheng X., Zhang W.…Wang Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 2021;13:10748–10764. doi: 10.1039/d1nr02065j. [DOI] [PubMed] [Google Scholar]
- Shishir M.R.I., Xie L., Sun C., Zheng X., Chen W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology. 2018;78:34–60. [Google Scholar]
- Shojaeimeher S., Babashahi M., Shokri S., Mirlohi M., Zeinali T. Optimizing the production of probiotic yogurt as a new functional food for diabetics with favorable sensory properties using the response surface methodology. Probiotics and Antimicrobial Proteins. 2024;16:413–425. doi: 10.1007/s12602-023-10051-z. [DOI] [PubMed] [Google Scholar]
- Shokri S., Shariatifar N., Molaee-Aghaee E., Khaniki G.J., Sadighara P., Faramarzi M.A. Author correction: Modeling sunset yellow removal from fruit juice samples by a novel chitosan-nickel ferrite nano sorbent. Scientific Reports. 2024;14 doi: 10.1038/s41598-024-55343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri S., Shariatifar N., Molaee-Aghaee E., Khaniki G.J., Sadighara P., Faramarzi M.A.…Rezagholizade-Shirvan A. Synthesis and characterization of a novel magnetic chitosan–nickel ferrite nanocomposite for antibacterial and antioxidant properties. Scientific Reports. 2023;13:15777. doi: 10.1038/s41598-023-42974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I.M., Machado F., Moreno M.J., Nunes C., Coimbra M.A., Coreta-Gomes F. Polysaccharide structures and their hypocholesterolemic potential. Molecules. 2021;26 doi: 10.3390/molecules26154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva T.M., de Deus C., de Souza F.B., Lopes E.J., Cichoski A.J., Esmerino E.A.…de Menezes C.R. The effect of enzymatic crosslinking on the viability of probiotic bacteria (Lactobacillus acidophilus) encapsulated by complex coacervation. Food Research International. 2019;125 doi: 10.1016/j.foodres.2019.108577. [DOI] [PubMed] [Google Scholar]
- Şimşek S.T., Özgen S. Vacuum modification of wheat bread with encapsulated rosehip extract: Evaluation of physicochemical and microstructural properties. Journal of Cereal Science. 2023;112 [Google Scholar]
- Singh H. Nanotechnology applications in functional foods; opportunities and challenges. Preventive nutrition and food science. 2016;21:1. doi: 10.3746/pnf.2016.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sîrbe C., Rednic S., Grama A., Pop T.L. An update on the effects of vitamin D on the immune system and autoimmune diseases. International Journal of Molecular Sciences. 2022;23:9784. doi: 10.3390/ijms23179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siyar Z., Motamedzadegan A., Mohammadzadeh Milani J., Rashidinejad A. The effect of the liposomal encapsulated saffron extract on the physicochemical properties of a functional ricotta cheese. Molecules. 2021;27:120. doi: 10.3390/molecules27010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.E., Johnson R.C., Papas K.K. Update on cellular encapsulation. Xenotransplantation. 2018;25 doi: 10.1111/xen.12399. [DOI] [PubMed] [Google Scholar]
- Snoussi A., Chouaibi M., Koubaier H.B.H., Bouzouita N. Encapsulation of Tunisian thyme essential oil in O/W nanoemulsions: Application for meat preservation. Meat Science. 2022;188 doi: 10.1016/j.meatsci.2022.108785. [DOI] [PubMed] [Google Scholar]
- Stern A.J., Becher D.Z. Pesticide Formulation and Adjuvant Technology; 2018. Microencapsulation technology and future trends; pp. 93–114. [Google Scholar]
- Strojny B., Kurantowicz N., Sawosz E., Grodzik M., Jaworski S., Kutwin M.…Chwalibog A. Long term influence of carbon nanoparticles on health and liver status in rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani T., Ganapathyswamy H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. Journal of Food Science and Technology. 2020;57:3545–3555. doi: 10.1007/s13197-020-04360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhag Y., Nayik G.A., Karabagias I.K., Nanda V. Development and characterization of a nutritionally rich spray-dried honey powder. Foods. 2021;10:162. doi: 10.3390/foods10010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana M., Chan E.S., Janarthanan P., Choo W.S. Functional orange juice with Lactobacillus casei and tocotrienol-enriched flaxseed oil co-encapsulation: Physicochemical properties, probiotic viability, oxidative stability, and sensorial acceptability. Lwt. 2023;188 [Google Scholar]
- Sultana M., Chan E.-S., Pushpamalar J., Choo W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends in Food Science & Technology. 2022;123:69–86. [Google Scholar]
- Surendhiran D., Cui H., Lin L. Encapsulation of Phlorotannin in alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packaging and Shelf Life. 2019;21 [Google Scholar]
- Surendhiran D., Li C., Cui H., Lin L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packaging and Shelf Life. 2020;23 [Google Scholar]
- Sutherland J.P., Zhou A., Leach M.J., Hyppönen E. Differences and determinants of vitamin D deficiency among UK biobank participants: A cross-ethnic and socioeconomic study. Clinical Nutrition. 2021;40:3436–3447. doi: 10.1016/j.clnu.2020.11.019. [DOI] [PubMed] [Google Scholar]
- Szymczak L.C. Encapsulation for taste modification. Microencapsulation in the Food Industry. 2023:555–562. [Google Scholar]
- Tagrida M., Prodpran T., Zhang B., Aluko R.E., Benjakul S. Liposomes loaded with betel leaf (Piper betle L.) ethanolic extract prepared by thin film hydration and ethanol injection methods: Characteristics and antioxidant activities. Journal of Food Biochemistry. 2021;45 doi: 10.1111/jfbc.14012. [DOI] [PubMed] [Google Scholar]
- Taheri A., Jafari S.M. Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Advances in Colloid and Interface Science. 2019;269:277–295. doi: 10.1016/j.cis.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Taheri A., Jafari S.M. Nanostructures of gums for encapsulation of food ingredients, biopolymer nanostructures for food encapsulation purposes. Elsevier. 2019:521–578. [Google Scholar]
- Tan C., McClements D.J. Application of advanced emulsion technology in the food industry: A review and critical evaluation. Foods. 2021;10:812. doi: 10.3390/foods10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Zhang X., Li X., Ma C., Chu X., Wang L., Xu W. A review on recent advances of protein-polymer hydrogels. European Polymer Journal. 2022;162 [Google Scholar]
- Tarone A.G., Cazarin C.B.B., Junior M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Research International. 2020;133 doi: 10.1016/j.foodres.2020.109092. [DOI] [PubMed] [Google Scholar]
- Tavares L., Santos L., Noreña C.P.Z. Microencapsulation of organosulfur compounds from garlic oil using β-cyclodextrin and complex of soy protein isolate and chitosan as wall materials: A comparative study. Powder Technology. 2021;390:103–111. [Google Scholar]
- Timilsena Y.P., Haque M.A., Adhikari B. Encapsulation in the food industry: A brief historical overview to recent developments. Food and Nutrition Sciences. 2020;11:481–508. [Google Scholar]
- Tometri S.S., Ahmady M., Ariaii P., Soltani M.S. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. Journal of Food Measurement and Characterization. 2020;14:3333–3344. [Google Scholar]
- Tomou E.-M., Papakyriakopoulou P., Saitani E.-M., Valsami G., Pippa N., Skaltsa H. Recent advances in nanoformulations for quercetin delivery. Pharmaceutics. 2023;15:1656. doi: 10.3390/pharmaceutics15061656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés A., Ramos M., Beltran A., Garrigos M.C. Recent trends in microencapsulation for smart and active innovative textile products. Current Organic Chemistry. 2018;22:1237–1248. [Google Scholar]
- Van Audenhove J., Bernaerts T., Putri N.I., Van Loey A.M., Hendrickx M.E. The functionalisation of fruit and vegetable cell wall material as texturizing agent: The role of pectin depletion and particle size reduction techniques. Food Hydrocolloids. 2023;142 [Google Scholar]
- Varela-Pérez A., Romero-Chapol O.O., Castillo-Olmos A.G., García H.S., Suárez-Quiroz M.L., Singh J.…Cano-Sarmiento C. Encapsulation of Lactobacillus gasseri: Characterization, probiotic survival, in vitro evaluation and viability in apple juice. Foods. 2022;11:740. doi: 10.3390/foods11050740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile F.E., Romero A.M., Judis M.A., Mazzobre M.F. Physicochemical, nutritional, and stability aspects of a meat product (gluteus medius) enriched with encapsulated fish oil in polyelectrolyte beads containing Prosopis alba exudate gum. Food and Bioprocess Technology. 2019;12:654–664. [Google Scholar]
- Venugopalan V.K., Gopakumar L.R., Kumaran A.K., Chatterjee N.S., Soman V., Peeralil S.…Nagarajarao R.C. Encapsulation and protection of omega-3-rich fish oils using food-grade delivery systems. Foods. 2021;10:1566. doi: 10.3390/foods10071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visan A.I., Cristescu R. Polysaccharide-based coatings as drug delivery systems. Pharmaceutics. 2023;15:2227. doi: 10.3390/pharmaceutics15092227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentini F.F., Perez A.A., Santiago L.G. Bioactive compounds: Application of albumin nanocarriers as delivery systems. Critical Reviews in Food Science and Nutrition. 2023;63:7238–7268. doi: 10.1080/10408398.2022.2045471. [DOI] [PubMed] [Google Scholar]
- Wan J., Pei Y., Hu Y., Ai T., Sheng F., Li J., Li B. Microencapsulation of eugenol through gelatin-based emulgel for preservation of refrigerated meat. Food and Bioprocess Technology. 2020;13:1621–1632. [Google Scholar]
- Wang L., Clardy A., Hui D., Wu Y. Physiochemical properties of encapsulated bitter melon juice using spray drying. Bioactive Carbohydrates and Dietary Fibre. 2021;26 [Google Scholar]
- Wang X., Gao S., Yun S., Zhang M., Peng L., Li Y., Zhou Y. Microencapsulating alginate-based polymers for probiotics delivery systems and their application. Pharmaceuticals. 2022;15:644. doi: 10.3390/ph15050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Wang C., Liu X., Mackie A., Zhang M., Dai L.…Gao Y. Co-encapsulation of curcumin and β-carotene in Pickering emulsions stabilized by complex nanoparticles: Effects of microfluidization and thermal treatment. Food Hydrocolloids. 2022;122 [Google Scholar]
- Weng Y., Li Y., Chen X., Song H., Zhao C.-X. Encapsulation of enzymes in food industry using spray drying: Recent advances and process scale-ups. Critical Reviews in Food Science and Nutrition. 2024;64:7941–7958. doi: 10.1080/10408398.2023.2193982. [DOI] [PubMed] [Google Scholar]
- Wijekoon M.J.O., Mahmood K., Ariffin F., Nafchi A.M., Zulkurnain M. Recent advances in encapsulation of fat-soluble vitamins using polysaccharides, proteins, and lipids: A review on delivery systems, formulation, and industrial applications. International Journal of Biological Macromolecules. 2023;241 doi: 10.1016/j.ijbiomac.2023.124539. [DOI] [PubMed] [Google Scholar]
- Witczak M., Ziobro R., Juszczak L., Korus J. Starch and starch derivatives in gluten-free systems–a review. Journal of Cereal Science. 2016;67:46–57. [Google Scholar]
- Wu W., Zhou Y., Pan J., Wu Y., Goksen G., Shao P. Multibranched flower-like ZnO anchored on pectin/cellulose nanofiber aerogel skeleton for enhanced comprehensive antibacterial capabilities. Carbohydrate Polymers. 2023;322 doi: 10.1016/j.carbpol.2023.121320. [DOI] [PubMed] [Google Scholar]
- Xiong K., Zhou L., Wang J., Ma A., Fang D., Xiong L., Sun Q. Construction of food-grade pH-sensitive nanoparticles for delivering functional food ingredients. Trends in Food Science & Technology. 2020;96:102–113. [Google Scholar]
- Xu M., Feng G., Fang J. Microcapsules based on biological macromolecules for intestinal health: A review. International Journal of Biological Macromolecules. 2024;276:133956. doi: 10.1016/j.ijbiomac.2024.133956. [DOI] [PubMed] [Google Scholar]
- Xue X., Hu Y., Wang S., Chen X., Jiang Y., Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioactive Materials. 2022;12:327–339. doi: 10.1016/j.bioactmat.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Dong Y., Wang F., Zhang Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules. 2020;25:4613. doi: 10.3390/molecules25204613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Shen J., Liu J., Yang Y., Hu A., Ren N.…Liu W. Spray-drying of hydroxypropyl β-cyclodextrin microcapsules for co-encapsulation of resveratrol and piperine with enhanced solubility. Crystals. 2022;12:596. [Google Scholar]
- Yang Z., Li F., Shen S., Wang X., Nihmot Ibrahim A., Zheng H.…Zhang Y. Natural chlorophyll: A review of analysis methods, health benefits, and stabilization strategies. Critical Reviews in Food Science and Nutrition. 2024:1–15. doi: 10.1080/10408398.2024.2356259. [DOI] [PubMed] [Google Scholar]
- Yao L., Xu J., Zhang L., Liu L., Zhang L. Nanoencapsulation of anthocyanin by an amphiphilic peptide for stability enhancement. Food Hydrocolloids. 2021;118 [Google Scholar]
- Yi T., Zhao H., Mo Q., Pan D., Liu Y., Huang L.…Song H. From cellulose to cellulose nanofibrils—A comprehensive review of the preparation and modification of cellulose nanofibrils. Materials. 2020;13:5062. doi: 10.3390/ma13225062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S., Ma Y., Yan B., Pei W., Wu Q., Ding C., Huang C. The promotion mechanism of prebiotics for probiotics: A review. Frontiers in Nutrition. 2022;9:1000517. doi: 10.3389/fnut.2022.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi M., Hosseini H., Khorshidian N., Rastegar H., Shamloo E., Abdolshahi A. Effect of time and incubation temperature on ability of probiotics for removal of polycyclic aromatic hydrocarbon in phosphate buffer saline. Journal of Microbiology, Biotechnology and Food Sciences. 2022;12:1918. [Google Scholar]
- Yu Z., Li Q., Wang J., Yu Y., Wang Y., Zhou Q., Li P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Research Letters. 2020;15:115. doi: 10.1186/s11671-020-03344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabot G.L., Schaefer Rodrigues F., Polano Ody L., Vinícius Tres M., Herrera E., Palacin H.…Olivera-Montenegro L. Encapsulation of bioactive compounds for food and agricultural applications. Polymers. 2022;14:4194. doi: 10.3390/polym14194194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yu D., Regenstein J.M., Xia W., Dong J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends in Food Science & Technology. 2021;112:690–707. [Google Scholar]
- Zhang R., Zhou L., Li J., Oliveira H., Yang N., Jin W.…He J. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: Characterization, stability and bioaccessibility. Lwt. 2020;123 [Google Scholar]
- Zhang X., Wang Y., Li Z., Li Y., Qi B. Effects of polysaccharide type on the structure, interface behavior, and foam properties of soybean protein isolate hydrolysate-polysaccharide Maillard conjugates. Food Hydrocolloids. 2024;151 [Google Scholar]
- Zhang Y., Hai Y., Miao Y., Qi X., Xue W., Luo Y.…Yue T. The toxicity mechanism of different sized iron nanoparticles on human breast cancer (MCF7) cells. Food Chemistry. 2021;341 doi: 10.1016/j.foodchem.2020.128263. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Li X., Sang S., McClements D.J., Chen L., Long J.…Qiu C. Polyphenols as plant-based nutraceuticals: Health effects, encapsulation, nano-delivery, and application. Foods. 2022;11:2189. doi: 10.3390/foods11152189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Yu C., Wei H. Injectable hydrogels as three-dimensional network reservoirs for osteoporosis treatment. Tissue Engineering Part B: Reviews. 2021;27:430–454. doi: 10.1089/ten.TEB.2020.0168. [DOI] [PubMed] [Google Scholar]
- Zhu F. Encapsulation and delivery of food ingredients using starch based systems. Food Chemistry. 2017;229:542–552. doi: 10.1016/j.foodchem.2017.02.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.