Abstract
This study performed advanced toxicological assessments of the new substance AP-238 using nine ‘green’ in silico methods, focusing on acute toxicity, organ-specific effects, skin and eye irritation, genetic toxicity, and cardiotoxicity. A quantitative assessment of AP-238’s acute toxicity (AT) was performed by predicting theoretical LD50 values for both rats and mice across different administration routes using various in silico methods. Results indicated the highest toxicity via intravenous administration in mice, with a t-LD50 of 53 mg/kg, while oral administration in rats exhibited a lower toxicity range, with t-LD50 values between 666.43 and 1838.77 mg/kg, depending on the predictive model used. The identification of toxicophores (the fragment connecting the benzene ring to the piperazine ring, including the α, β, and γ carbon atoms near the nitrogen atom) in AP-238 suggests a high likelihood of lung toxicity (61%), with additional risks to the cardiovascular (58%) and renal systems (56%), emphasizing specific molecular fragments associated with these adverse effects. Genotoxic evaluations presented a mixed view, with low to moderate probabilities of a positive Ames test, suggesting some uncertainty but generally indicating a reduced risk of genetic toxicity. Eye and skin irritation risks were deemed minimal, supported by several models with high confidence. Cardiotoxicity assessments revealed varied information on the potential effects of AP-238 on the hERG channel, with some studies suggesting a nonsignificant impact, while others indicated moderate risk, although with low reliability in the predictions. This highlights the nuanced challenges in assessing the safety of novel substances through ‘green’ in silico methods.
Subject terms: Cheminformatics, Preclinical research, Computational science, Chemical safety, Chemical safety, Computational chemistry
Introduction
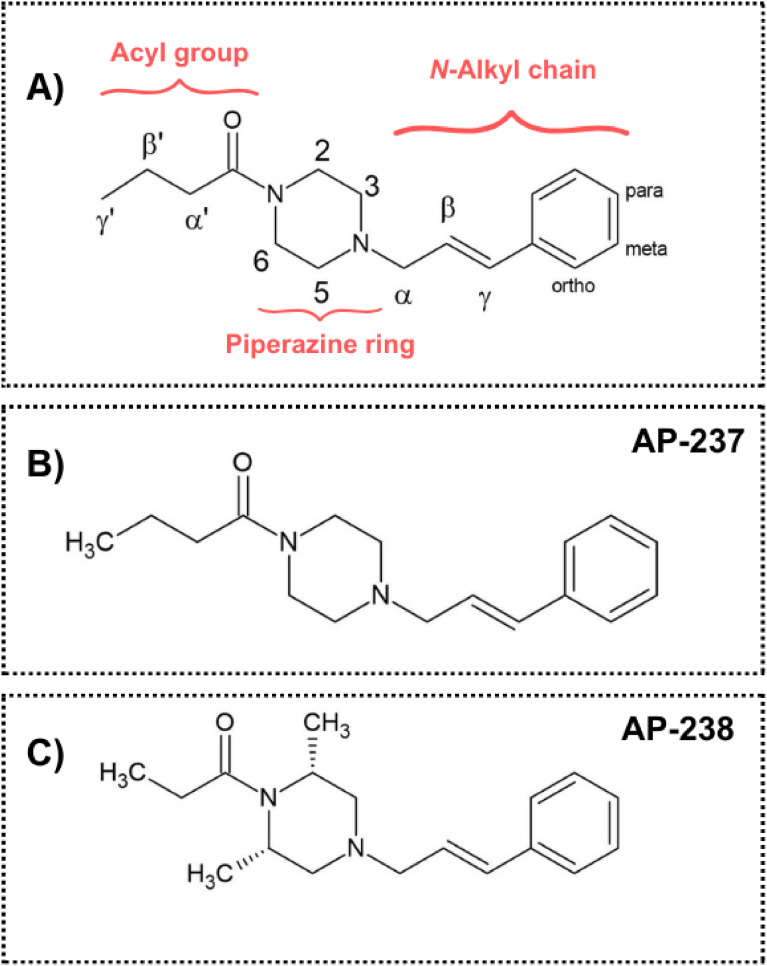
AP-238 (N26WML8AR7; UNII-N26WML8AR7; 1-(4-Cinnamyl-2,6-dimethylpiperazin-1-yl)propan-1-one) is an example of a new synthetic opioid (NSO) that has recently emerged in the realm of new psychoactive substances (NPS)1. The evolution of NPS is a rapidly changing phenomenon, constantly challenging both public health and law enforcement agencies. NSOs, represent a significant threat due to their potential for extremely high potency and the risk of causing respiratory depression, a common and severe adverse effect associated with opioid overdose. The genesis of AP-238 can be traced back to the legal restrictions imposed on fentanyl analogues (FAs). These restrictions led to the rise of a new generation of ‘legal’ opioids, notably within the class of cinnamylpiperazines – Fig. 1A.
Fig. 1.
Chemical structure of (A) Cinnamylpiperazines; (B) Bucinnazine, AP-237; (C) AP-238.
(C) AP-238.
Expanding on the chemical and pharmacological characteristics of AP-238, it is essential to understand its structural distinctions from other opioids, particularly (FAs). AP-238 belongs to a distinct class characterised by a piperazine core fused with a cinnamyl moiety – Fig. 1C), diverging from the typical piperidine core and phenethyl chain found in FAs. This unique chemical structure significantly influences the pharmacological and toxicological properties of AP-238. The prototype of this structural class is AP-237, also known as bucinnazine - Fig, with the IUPAC name 1-{4-[(2E)-3-phenylprop-2-en-1-yl]piperazine-1-yl}butan-1-one. First synthesized in 19682, AP-237 was initially recognized for its potent analgesic properties in animal models. Its efficacy in pain management was found to be superior by oral administration and exhibited a lower dependence liability compared to morphine. In China, AP-237 has been approved for medical use as a moderate intensity analgesic for various pain conditions, including migraine, traumatic pain, trigeminal neuralgia, and cancer-related pain. However, its approval has not been extended to other countries. AP-238, identified as the 2,6-dimethyl propionyl analogue of AP-237 (Fig. 1B), emerged more recently in 2020. It gained attention after being circulating via Internet shops and being involved in a fatal case of alcohol. The pharmacological and toxicological profiles of AP compounds, including AP-238, are not fully understood, reflecting a gap in the available literature: the known physicochemical properties of AP-238 are summarised in Table 1.
Table 1.
Known physicochemical properties of AP-238.
| Name(s) | AP-238 |
|---|---|
| CAS | 140924-11-4 |
| SMILES | CCC(= O)N1C(CN(CC1C)CC = CC2 = CC = CC = C2)C |
| IUPAC name | 1-[(2 S,6R)-2,6-dimethyl-4-[(E)-3-phenylprop-2-enyl]piperazin-1-yl]propan-1-one |
| Molecular formula | C18H26N2O |
| Structural formula |
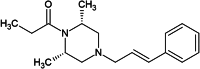
|
| Acronym(s) | N26WML8AR7; AP-238; UNII-N26WML8AR7; 1-(4-Cinnamyl-2,6-dimethylpiperazin-1-yl)propan-1-one |
| Molecular Weight, g/mol | 286.4 |
The adverse effects associated with the abuse of NSOs such as AP-238 are multiple. In addition to the high risk of respiratory depression, these substances can cause a variety of other serious health problems, varying according to their specific pharmacological profiles. The continued evolution, increasing popularity, and high availability of these NSOs constitute a serious concern, as they pose an imminent hazard to public health. AP-238, as with other NSOs, underscores the challenges faced in monitoring, regulating, and addressing the risks posed by new psychoactive substances. The dynamic nature of this drug market requires ongoing research, surveillance, and legislative adaptation to effectively mitigate public health risks associated with these potent and evolving compounds.
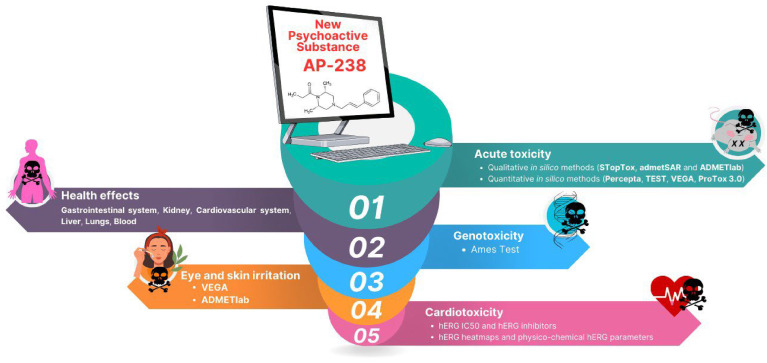
Due to a significant knowledge gap regarding the toxicity of AP-238, it is justified and even necessary to immediately focus on utilising various in silico methods (qualitative and quantitative) to determine its main toxicity endpoints: acute toxicity (AT), skin and eye irritation, genetic toxicity, health effects, and cardiotoxicity. These parameters are of utmost importance from a medical point of view, serving two critical areas: clinical toxicology and forensic toxicology. In clinical toxicology, understanding these endpoints of toxicity is crucial to predict what to expect after the use of AP-238, both in the short term and long term. From the perspective of forensic toxicology, these toxicity endpoints provide crucial evidence in legal cases. In incidents involving fatalities (referred to as ‘denat cases’), an in-depth understanding of AP-238’s toxicological profile is essential. The symptoms observed in deceased individuals need to be scientifically linked to the substance in question for accurate legal interpretation and judgment. Hence, the justification for the application of in silico methods in AP-238 toxicity prediction is fully warranted and essential, as it offers several advantages, particularly in alignment with the 3R concept (Replace, Reduce and Refinement) in modern toxicological studies. This approach emphasises the importance of reducing the reliance on animal testing, advocating for alternative methods that are more ethical, efficient and often more precise. The idea of this work is to carry out rapid in silico toxicological tests using various methods to determine the unknown toxicological profile of AP-238, taking into account key parameters: acute toxicity (AT as LD50 for different species and route of exposure), skin and eye irritation, genetic toxicity (Ames test), health effects (gastrointestinal system, kidneys, liver, blood, lungs, and cardiovascular system) and cardiotoxicity (hERG blocker/inhibitor and IC50). The workflow of conducted in silico studies is schematically shown in Fig. 2.
Fig. 2.
Workflow with idea of toxicity prediction of AP-238 with applied in silico methods.
Results and discussion
Acute toxicity (AT)
Acute toxicity (AT) refers to the adverse effects3 that arise from the administration of a single dose of a substance orally or dermally, or from multiple doses within a 24-hour period, or from inhalation exposure lasting 4 hours. AT values are typically expressed as the median lethal dose rate 50; LD50 (for oral and dermal route of administration) or as the median lethal concentration rate 50; LC50 (for inhalation route of administration). Rats are generally preferred test species for assessing AT via oral exposure in most in silico methods (probably due to the fact that most databases are based on LD50 for oral exposure for rats). The classification of chemicals into six toxicity categories (Globally Harmonized System of Classification and Labeling of Chemicals; GHS) is determined by their AT through the oral, dermal, or inhalation route, using specific numeric cut-off criteria4. Category 1 represents the highest level of toxicity (fatal if swallowed; LD50 = 5 mg/ kg bw), while category 6 is assigned to chemicals with relatively low AT that can still pose a hazard to vulnerable populations under certain circumstances (Class VI: non-toxic; LD50 > 5000 mg/kg bw). As AT stands as one of the well-recognized and most important parameters in toxicity assessment of novel substances, the present study undertook predictions of AT using in silico toxicology methods. Subsequently, AT was quantitatively evaluated through the computation of theoretical LD50 (t-LD50) values for rats and mice via various routes of administration.
Quality assessment of AT
For quality assessment of AT, specialised scientific software (STopTox, admetSAR, and ADMETlab) were applied to predict the quality results of AT for AP-238; i.e. classification with probability and prediction of fragment contribution (toxicophores, labeled as red structural fragments) – Table 2.
Table 2.
Prediction of acute toxicity (AT) for AP-238 using qualitative in silico methods (STopTox, admetSAR and ADMETlab).
| Type of acute toxicity (AT) | STopTox | AdmetSAR 2.0 | ADMETlab | |||||
|---|---|---|---|---|---|---|---|---|
| Prediction | Confidence, % | Applicability domain (AD) | Predicted fragment contribution | Classification | Probablility, % | Probability | Probability, % | |
| =AOT | Toxic (+) | 67 |

|

|
III | 69.34 | Non-toxic | NA |
| ADT | Non-Toxic (-) | 63 |
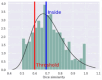
|

|
NA | NA | Sensitizer | NA |
| AIT | Toxic (+) | 64 |

|
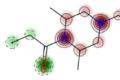
|
NA | NA | Toxic | NA |
These software allow the prognosis of qualitative AT related to acute oral toxicity (AOT), acute dermal toxicity (ADT), and acute inhalation toxicity (AIT) including all route of administration for rats. The data presented in Table 2 reveal that STopTox provides the most comprehensive information, capable of predicting toxicity across all three exposure routes. Although two in silico methods (STopTox and AdmetSAR 2.0) suggest that AP-238 can be classified by toxic category 3 for oral administration, ADMETlab contradicts this by indicating non-toxic nature. Category 3, which AP-238 may fall under, represents a moderate level of AT. Chemicals in this category are defined by oral LD50 values between 50 and 300 mg/kg, dermal LD50 values between 200 and 1,000 mg/kg, and inhalation LC50 values between 0.5 and 1.0 mg/L for dusts or mists. Substances classified in Category 3 can cause significant harm at relatively low doses but are less acutely toxic than those in Categories 1 and 2. For novel psychoactive substances like AP-238, this classification indicates a need for strict handling precautions due to potential health risks. The results for ADT are suggesting potential dermal sensitization. In terms of AIT, both STopTox (confidence 64%) and ADMETlab (probability not-known/not applicable) confirming the hypothetical toxic nature via inhalation for AP-238. Hence, overall evaluations indicates that AP-238 is very likely toxic when administered orally (and hypothetically by inhalation), which suggested that further research should be done to know possible life-threatening doses administered via various routes of exposure. It may also be a dermal irritant which may be a threat to the first, basic barrier of the body.
Quantitative assessment of acute toxicity (AT) as t-LD50
After the qualitative assessment of AT of the investigated compound was confirmed, a quantitative assessment (prediction of theoretical LD50 for mice and rats in different route of administration) was conducted using different scientific in silico methods. The results of the obtained values of LD50 (i.e. t-LD50, theoretical median lethal dose, rate 50) expressed as milligrams per kilogram of body weight (mg/kg bw) are summarized in Table 3.
Table 3.
Results for prediction of acute toxicity (AT) for AP-238. Using quantitative in silico methods (Percepta, TEST, VEGA, ProTox 3.0, AdmetSar 3.0).
| Species | Route of administration | t-LD50, mg/kg bw | Method | Additional information |
|---|---|---|---|---|
| Rat | Intraperitoneal | 150.00 | Percepta | Moderate, RI = 0.55 |
| Oral | 850.00 | Percepta | Borderline, RI = 0.45 | |
| 982.68 | TEST - Consensus | N/A | ||
| 1056.16 | TEST - Nearest neighbor | N/A | ||
| 1838.77 | TEST - Hierarchical clustering | N/A | ||
| 666.43 | VEGA | N/A | ||
| 693.00 | ProTox 3.0 | Moderate, RI = 72.9 | ||
| 1023.00 | AdmetSar 3.0 | RI = 69.34 | ||
| Mouse | Intraperitoneal | 300.00 | Percepta | High, RI = 1 (experimental value) |
| Oral | 620.00 | Percepta | Moderate, RI = 0.73 | |
| Intravenous | 53.00 | Percepta | High, RI = 0.95 | |
| Subcutaneous | 440.00 | Percepta | Moderate, RI = 0.75 |
t-LD50–theoretical LD50.
In the case of rats, oral distribution is the most commonly applied route of administration in toxicological studies. Similarly, most results were observed with oral intake in rat experiments. The VEGA software yielded the lowest t-LD50 value of 666.43 mg/kg body weight, while the highest t-LD50 value of 1838.77 mg/kg bw was obtained using the TEST - Hierarchical clustering program. For the i.p. route of exposure in rats, only Percepta provided a result, indicating a t-LD50 value of 150 mg/kg bw. However, it is currently unfeasible to make a direct comparison between the achieved outcome and experimental data due to the absence of conducted tests. Consequently, the evaluation of this result can only be regarded as moderate, given that the reliability index (RI) stands at 55. Nevertheless, the availability of results for other routes of exposure in rats is currently unknown. In silico studies investigating potential mouse intoxication routes were conducted exclusively using Percepta. The intravenous administration of AP-238 in mice resulted in the highest toxicity, with a t-LD50 of 53 mg/kg. This indicates that this route of administration poses the highest risk in terms of toxicity. On the other hand, the intraperitoneal administration exhibited a t-LD50 of 300 mg/kg, while the subcutaneous administration showed a t-LD50 of 440 mg/kg.
These findings suggest that both intraperitoneal and subcutaneous routes of administration are less toxic compared to intravenous administration. Furthermore, the oral administration of AP-238 demonstrated the least toxicity, with a t-LD50 of 620 mg/kg. This implies that oral administration is the least toxic route for AP-238 administration. Hence, it should be emphasized that only Percepta provided wide range of parameters for other possible routes of exposure, including intraperitoneal (ip) administration for rats and intraperitoneal, oral, intravenous, and subcutaneous administration for mice. Since no results were obtained for routes of administration other than oral use in rats, it is impossible to verify the accuracy of the obtained results. The majority of results obtained through Percepta demonstrated an RI index greater than 0.7, signifying a high level of reliability.
Health effects
The use of NPS carries grave health dangers, especially for the younger population, as these substances exhibit unpredictable clinical properties and have the potential to lead to addiction5. Moreover, among young NPS users, cases of serious health incidents have been observed, including seizures, cardiotoxic issues, and even fatal poisonings. The fact that NPS are often marketed as safe alternatives to traditional drugs further contributes to their popularity, despite the growing number of reports on severe side effects and the life-threatening dangers they pose. Regarding NPS substances, a major challenge is the lack of knowledge about their important health effects from clinical point of view, which remain largely unknown in most instances. Therefore, having data on the likelihood of specific effects occurring would be invaluable in clinical and forensic settings. In these cases, the only possible solution is prediction of such effects using appropriate in silico methods. For this reason, we applied Percepta software using ‘health effects’ mode. The results obtained from the Percepta as ‘Health Effects’ mode include evaluating the potential adverse health impacts of AP-238 on organ-specific health effects (gastrointestinal system, kidneys, liver, blood, lungs, and cardiovascular system). These predictions are the result of statistical analyses performed on an extensively reviewed dataset that amalgamates information from diverse toxicity studies, including acute, mid-term, and long-term investigations. The dataset encompasses a wide array of species and administration routes, covering nearly 15,000 individual unique compounds. The obtained results are summarized in Table 4. as organ or organ system effects with probability (%), specific prognosed toxic effect(s) (if available) and toxicophoric groups (indicated as red) – structural fragments responsible for contributing to each health effect.
Table 4.
Results for predicted health effects for AP-238 with probability (%), specific prognosed toxic effect(s) and toxicophoric groups (indicated as red) using percepta software.
| Organ or Organ System | Probability, % | Toxic effect(s) | Toxicophores (red/orange) |
|---|---|---|---|
| Lungs | 61 |
Toxic effect: Dyspnea, Route: Intravenous Species: Mouse Studies: Acute |

|
| Cardiovascular system | 58 | NA |
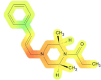
|
| Kidney | 56 | NA |
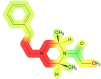
|
| Blood | 38 |
Toxic effect: Normocytic anemia Route: Subcutaneous Species: Rat Studies: Chronic |

|
| Gastrointestinal system | 21 |
Toxic effect: Hypermotility, diarrhea Route: Oral Species: Dog Studies: Chronic |

|
| Liver | 14 | NA |

|
Obtained results are crucial in visualizing the likelihood of specific structural fragments causing toxic effects (toxicophores) on targeted organs. Toxicophores6, which are qualitative structural elements, are believed to underlie a drug’s toxic characteristics, either as a direct consequence or as an indirect result of its pharmacological activity. The biological approach heavily relies on the chemical properties of the molecule, which undergoes biotransformation to form a toxicophore. By pinpointing the molecular fragments most closely associated with potential adverse outcomes, these results are crucial from a molecular point of view, due to the identification of potential toxicological liabilities linked to specific structural components of an AP-238. In most cases, the structural fragment that can be considered as toxicophoric group/fragment is consistent across all effects — this is the fragment connecting the benzene ring to the piperazine ring (up to the nitrogen atom of the piperazine ring, i.e., the nitrogen atom of piperazine and the α, β, and γ carbon atoms, as shown in Fig. 1A).
The highest probability of toxic effects occurrence (61%) was observed for the lungs, specifically, the evaluation indicates probability that AP-238 can induce dyspnea, a condition characterized by difficult or labored breathing, affecting the lung system in the studied species during acute exposure scenarios. Recent progress in the identification of toxicophores has primarily been driven by computational techniques, although the biological approach remains more prevalent. The lung-toxic fragments of the AP-238 molecule are highlighted in red (mentioned earlier fragment, with a special contribution by the α, β, and γ carbon atoms, as shown in Fig. 1A). This indicates a hypothetical concern for pulmonary toxicity associated with the compound in question, highlighting the lungs as the primary organ system at risk of adverse effects (critical organ) for AP-238 which may be important when inhaling this substance intranasally. On the other hand the cardiovascular system had the second highest probability value (58%) however, their toxic effects on human health were not predicted (hence there is no prediction to specific health effects associated with this system). Toxicophores shows that the fragment responsible for toxicity in the molecule is marked in red and orange color (this structural fragment is similar to the previous examples; however, the color is not as intense; it appears near the α carbon atom and partially β carbon atom but is clearly shifted towards the nitrogen atom of the piperazine ring). The third highest possible toxicity score was for the kidneys (56%), which are crucial for the proper functioning of the body’s excretory system. Predicted toxophores presented in Table 4. for kidney highlight in intensive red colour (including, as before, the α and β carbon atoms, as well as the N atom of the piperazine ring, along with the neighboring carbon atoms of this ring). There is 38% probability chance that toxic effects will also occur blood with special emphasis on normocytic anemia will occur in the blood (type of anemia characterized by the presence of red blood cells that are of normal size and volume, but there is a decrease in the overall number of red blood cells which may lead to reduced breathing efficiency). It should be emphasized that toxicophoric group for blood effects is analogous to predicted for cardiovascular system (α carbon atom and partially β carbon atom but is clearly shifted towards the nitrogen atom of the piperazine ring). Part responsible for toxicity in toxophores is similar to lung toxicophores. Gastrointestinal system might show toxic effects such as hypermotility and diarrhea with probability of 21%. Toxicophores show toxicity mostly on N-alkyl chain part with nitrogen atom of the piperazine ring. Among these AP-238 demonstrates the lowest predicted probability of occurrence for liver with 14% likelihood of it occurring with no specific health effects pointed by Percepta.
Genotoxicity
Genetic toxicology (genotoxicity) tests have emerged as a regulatory prerequisite for new chemical substances, driven by concerns over the carcinogenic and hereditary impacts of these substances on public health7. Numerous innovative methods can be employed to enhance the comprehension and interpretation of genotoxicity test results, thereby facilitating a better understanding of chemical mechanisms and aiding in the prediction of potential impacts on human and environmental health. A typical strategy involves the use of test batteries comprising bacterial and mammalian cell culture assays to detect genotoxic properties, complemented by in vivo studies involving rodents. The Ames test, recognized as a reliable bacterial reverse mutation assay, plays a crucial role in evaluating mutagenicity and serves as an important early indicator for screening substances that might be carcinogenic. A positive result in this test frequently suggests the possibility of carcinogenic effects in live organisms. This test is essential for the initial assessment and potential elimination of dangerous substances in the early phases of research development. In the context of evaluating the genotoxic potential of AP-238, it is particularly important in the case of long-term and chronic use of this psychoactive substance, as it occurs in addictions. Obtained results for genotoxic potential for AP-238 are summarized in Table 5. using five methods: Percepta, PreADMET, VEGA, ADMETlab and OCHEM.
Table 5.
Results of genotoxicity – prediction of Ames test results for AP-238 using: Percepta, PreADMET, VEGA-Consensus, ADMETlab and OCHEM.
| Parameter | In silico method | ||||
|---|---|---|---|---|---|
| Percepta | PreADMET | VEGA - Consensus | ADMETlab | OChem | |
| Result | Probability of positive Ames test, 14.00% |
Mutagen TA100_10RLI: negative TA100_NA: negative TA1535_10RLI: negative TA1535_NA: negative |
Non-mutagenic | Non-mutagenic | Inactive |
| Reliability |
Borderline (RI = 0.46) |
NA | RI = 0.89 | NA | RI = 0.86 |
| Toxicophore |

|
NA | NA | NA | NA |
RI – Reliability Index; NA – Not Applicable.
The results obtained from Percepta are quantitatively articulated in terms of the probability (14%) of a positive Ames test and the associated reliability (46%) of this prediction for Percepta, where PreADMET shows mutagenic potential for AP-238 based on negative results on bacterial strains: TA100_10RLI, TA100_NA, TA1535_10RLI and TA1535_NA. VEGA, ADMETlab and OCHEM predictaed that AP-238 is non-mutagenic. The disparities in these predictions underscore the complexity of in silico modeling for genotoxicity, where different algorithms and databases can lead to divergent outcomes. The low probability of a positive Ames test from Percepta, juxtaposed with the non-mutagenic classifications from VEGA-Consensus and ADMETlab, and the detailed negative results from PreADMET, highlight the nuances in predicting genotoxic risk. The reliability indices provided by VEGA-Consensus and OCHEM bolster the confidence in their non-mutagenic predictions, suggesting that, according to these models, AP-238 is less likely to pose a genotoxic risk. However, the borderline reliability of the Percepta prediction and the direct classification of mutagenicity by PreADMET without corresponding reliability measures or probability scores introduce a level of uncertainty.
Eye and skin irritation
The skin serves as the largest organ of the body and acts as the primary barrier against the external environment8. It is the initial line of defense that shields the body from various harmful factors such as microbes, viruses, bacteria, and other pathogens. Similarly, the eye is equipped with crucial defense mechanisms that safeguard it against infections triggered by viruses, fungi, parasites, and bacteria. The eye’s first line of defense includes the eyelids and eyelashes, and any irritation to these structures can result in discomfort, redness, dryness of the conjunctiva, vision issues, eyelid swelling, or infections. Therefore, it is essential to assess whether a particular foreign substance causes irritation to the skin or eyes to prevent potential health complications, especially during application AP-238 in different scenario (probability of spillage). For this purpose, we conducted eye and skin irritation prediction for AP-238, summarized in Table 6.
Table 6.
Results of eye and skin irritation prediction for AP-238 using VEGA, ADMETlab and Percepta.
| Type of irritation | Prediction | Reliability, % | Method |
|---|---|---|---|
| Eye | Not irritant | 71 | VEGA |
| Not irritant | NA | ADMETlab | |
| 0.26 | NA | Percepta | |
| Skin | Non - Sensitizer | 78 | VEGA |
| Sensitizer | 73 | VEGA | |
| Sensitizer | NA | ADMETlab | |
| 0.91 | NA | Percepta |
The VEGA, ADMETlab and Percepta methods were employed to perform in silico assessments on the skin and eye irritation caused by AP-238. The findings for eye irritation clearly demonstrate the lack of any irritant effects upon contact with the substance. However, the results for skin irritation are less conclusive. The VEGA program, which utilizes different algorithm calculation methods, yielded conflicting outcomes for AP-238-induced skin irritation, with one result being negative and the other positive. Conversely, ADMETlab and Percepta indicates that this substance acts as a skin sensitizer. Regarding the prediction of eye irritation, all the methods employed indicate that AP-238 is non-irritating. However, it is worth noting that it is a skin sensitizer. The outcomes obtained from the VEGA program exhibit conflicting results, with one suggesting irritant properties and the other indicating non-irritation. Nevertheless, considering the results obtained from other programs, it is plausible to suggest that the tested substance is indeed irritating to the skin. This concludes that AP-238 user should be careful while in contact with this substance.
Cardiotoxicity
Cardiotoxicity refers to the toxicity that affects the heart, leading to various heart diseases such as angina, myocardial infarction, coronary artery disease, heart failure, hypertensive heart disease, cardiomyopathy, and arrhythmia9. Among these conditions, cardiac arrhythmia is a common indicator of heart disease, presenting life-threatening cardiovascular symptoms and disturbances in normal cardiac function, characterised by changes in heartbeat frequency and rhythm. Torsades de Pointes (TdP), a type of polymorphic ventricular tachycardia, is often associated with congenital or acquired long QT syndrome (LQTS). LQTS is typically diagnosed on the basis of an abnormal prolongation of the QT interval on an electrocardiogram (ECG). This prolongation is caused by delayed repolarisation due to reductions in the activation of the cardiac potassium current, either rapid or slow. Inhibition of the rapid activation of rectifier potassium channels (IKr) encoded by the human ether-a-go-go-go (hERG) is a common mechanism leading to drug-induced prolongation of the QT interval. IC50, a crucial parameter in cardiotoxicology10, signifies the effectiveness of a drug in blocking potassium human ether-à-go-go (hERG). It is worth mentioning that the IC50 value is subject to variation based on the experimental conditions utilised. The summary of obtained results are shown in Table 7.
Table 7.
Results for cardiotoxicity predictions – hERG IC50 and hERG inhibitors for AP-238.
| Parameter(s) | Value | Reliability | Method |
|---|---|---|---|
| hERG Blockers | No | N/A | ADMETlab |
| hERG inhibitor | 32% | Not Reliable, RI = 0.12 | Percepta |
| hERG inhibitor | Medium risk | N/A | PreADMET |
| hERG IC50 | 19.2 µM | N/A | Percepta |
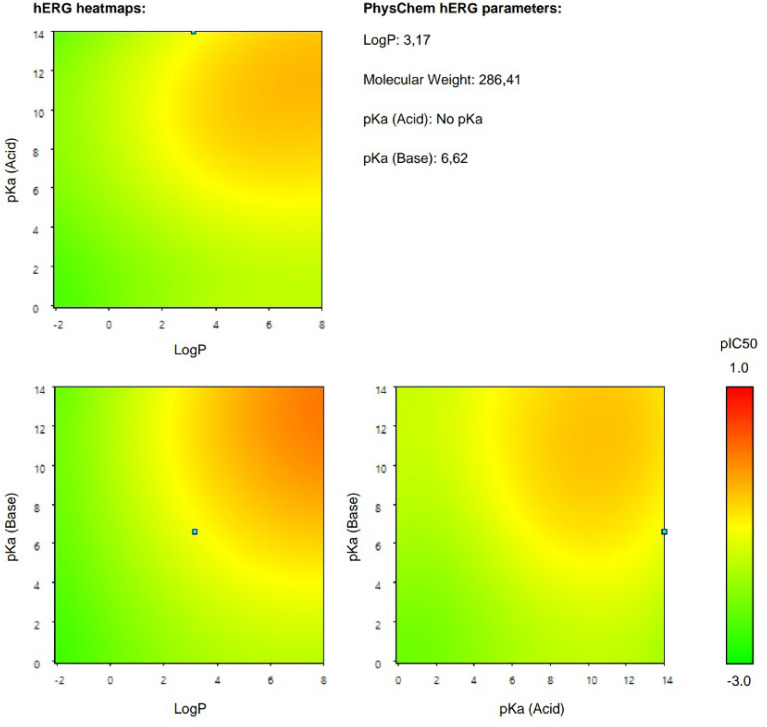
PreADMET, ADMETlab analysis suggests that AP-238 does not affect hERG blockers; however, PreADMET show that there is a medium risk of hERG inhibitor for this substance. Percepta indicated that there is a 32% chance that AP-238 inhibits the hERG channel at concentrations relevant to clinical settings. However, this prediction is considered unreliable with a reliability index (RI) of 0.12. Furthermore, the hERG IC50 value of 19.2 µM indicates a lack of significant inhibitory potency. Findings obtained from Percepta presented in Fig. 3. contains information such as PhysChem hERG parameters, ionisation parameters, and hERG heatmaps, which shows the correlation between hERG inhibition potential and lipophilicity.
Fig. 3.
Results of hERG heatmaps and physico-chemical hERG parameters for AP-238.
Conclusions
The conducted advanced toxicological studies utilizing nine in silico methods (StopTox, AdmetSAR 3.0, ACD/Labs Percepta, ProTox 3.0, PreADMET, ADMETlab 2.0, OCHEM, TEST and VEGA QSAR) encompassed a comprehensive assessment of the toxicity of the novel substance AP-238 including key toxicity parameters:
AT (qualitative and quantitative as LD50 for different species and route of exposure: AOT, ADT and AIT);
organ-specific health effects (gastrointestinal system, kidneys, liver, blood, lungs, and cardiovascular system);
skin and eye irritation;
genetic toxicity (Ames test);
cardiotoxicity (hERG blocker/inhibitor and IC50).
The in silico toxicological studies for AP-238 suggest that there are marked differences in toxicity levels depending on the species and method of administration (oral and subcutaneous in mice), as evidenced by variations in t-LD50 values. These findings indicate the complex challenges and restrictions of predictive toxicology, largely due to the lack of direct experimental data. The research also suggests a notable danger of lung toxicity from the chemical AP-238, with a significant probability (61%) of causing shortness of breath, especially in cases of short-term exposure, which makes the respiratory system particularly vulnerable. Further examination suggests that the heart and kidney systems are also hypothetically possible for damage from AP-238 (however with probabilities of toxicity at 58% and 56%, respectively). Additionally, there is a lesser but important risk of normocytic anemia and gastrointestinal problems associated with AP-238, with probabilities of 38% and 21%, respectively. When evaluating the genotoxic threat of AP-238, the predictions of various in silico models present a complex overview. The Percepta model gives a low chance (14%) of a positive Ames test result for AP-238, but with moderate confidence (46%), indicating some predictive uncertainty. However, other models like VEGA, ADMETlab, and OChem consistently deem AP-238 as non-mutagenic, suggesting a reduced risk of genetic toxicity. Regarding the potential for eye and skin irritation from HU-210, the predictions mostly indicate a low risk of irritation. Percepta suggests a 15% chance of eye irritation and a 26% chance of skin irritation, with other models such as VEGA, STopTox, and admetSAR supporting these findings with high confidence, indicating a minimal risk of irritation.
Cardiotoxicity assessments of AP-238, focussing on its potential effects on the hERG channel essential for heart safety, show varied information from different in silico studies. PreADMET and ADMETlab suggest a nonsignificant impact on hERG channel blockers, with PreADMET recognising a medium risk of hERG inhibition. On the contrary, Percepta estimates a 32% chance that AP-238 inhibits the hERG channel at relevant doses, but this comes with a low reliability score (0.12), raising questions about the accuracy of the prediction. The hERG IC50 value of 19.2 µM indicates a low inhibitory effect on the hERG channel. Despite some predictions having low reliability, the collective data suggests that, while AP-238’s interaction with the hERG channel is of some concern, the inhibition might not be significant, the need for cautious interpretation and potential further research.
The initial toxicological evaluation conducted on AP-238 using nine in silico methods offers an innovative, ‘green’ and thorough understanding of its potential health hazards. This cutting-edge computational research sheds light on the intricate toxic profile of AP-238, addressing key toxic endpoints important for clinical and forensic analysis. It is crucial to note that while this investigation paves new paths for understanding toxicology through toxicity prognosis, empirical research is needed to verify these computational predictions. Moreover, there is a gap for future in silico studies on ADME (Absorption, Distribution, Metabolism, and Excretion).
Methods
StopTox
STopTox (Systemic and Topical chemical Toxicity, https://stoptox.mml.unc.edu/), is an advanced in silico platform designed for predicting potential human AT outcomes introduced by Borba11 et al. in 2022. This comprehensive software focusses on assessing a group of key toxicological effects collectively called the “6 pack”. These evaluations cover a broad spectrum of AT endpoints, including oral, dermal, and inhalation toxicity. Additionally, STopTox assesses the risk of irritation and corrosion effects on both the skin and eyes, as well as the potential to cause skin sensitisation. The significance of STopTox lies in its ability to accurately forecast these AT outcomes using computational methods. By utilising in silico approaches, it offers an alternative to traditional, more time-consuming and ethically challenging in vivo (animal-based) testing methods. This method provides researchers and regulators with a reliable, rapid, and humane tool for toxicity assessment, crucial for ensuring public safety in relation to chemical exposures. STopTox contains a comprehensive and publicly available database of meticulously organised animal-based data. This database is instrumental in conducting wide-ranging assessments of acute human toxicity, a requirement for many regulatory bodies. Additionally, STopTox employs sophisticated machine learning models, notably enhancing its capability for rapid and effective chemical toxicity screening, thus providing essential insights in this area. Within STopTox, the AOT test, according to OECD TG protocols 401, 420, 423, and 425, uses rat models for its evaluations. This specific test incorporates the Random Forest algorithm, a machine learning technique, and employs MACCS (Molecular ACCess System) as molecular descriptors. The Random Forest algorithm functions by creating numerous decision trees during its training phase and then predicts the most common class from these trees. This method is acknowledged for its remarkable precision, resistance to overfitting, and competence in managing large data sets with various input factors. MACCS descriptors are essentially predefined keys that represent distinct structural elements in a molecule, playing a vital role in encoding molecular structures for machine learning applications, such as the Random Forest algorithm used in STopTox. These descriptors are pivotal for identifying and quantifying different structural components in chemical compounds, aiding in pinpointing specific toxicophores, the structural elements responsible for toxicity, which are highlighted in red.
AdmetSar 3.0.
AdmetSAR (https://admetlab3.scbdd.com/) is comprehensive scientific software designed to predict the ADMET properties of chemicals (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties of chemicals, enhancing the safety evaluation in drug discovery and environmental assessments but also is very useful for NPS. Since its initial launch in 2012, AdmetSAR has been enriched with more than 210,000 high-quality experimental data points for approximately 96,000 compounds. The platform stands out with its user-friendly interface, allowing users to easily retrieve chemical information through various identifiers, including SMILES, CASRN, common names, or structural inputs12,13.
The AdmetSAR 3.0, further elevates the platform’s capabilities by supporting single-molecule prediction and batch evaluations, facilitating the assessment of 51 ADMET-related endpoints through a combination of 38 binary classification models, six multilabel models, three multiclassification models, and four regression models. Each model benefits from the optimal combination of machine learning algorithms and molecular representations, ensuring high predictive accuracy and reliability.
In our research, we used the admetSAR software to determine quantitative AT specifically calculating the LD50values for rats (oral). The foundational research by Zhu14 involved compiling a dataset of 10,207 molecules, assessing their LD50 values compared to rats. Focussing on transparency and the capability for result reproduction, admetSAR 3.0 incorporates open-source cheminformatics and machine learning libraries to support the algorithms and descriptors utilised in the development of the model.
ACD/Labs Percepta.
The ACD/Labs Percepta platform is scientific software for predicting a wide range of key toxicological endpoints. The toxicity parameters analysed included AT (LD50), health effects (blood, cardiovascular, gastrointestinal, kidneys, liver, lungs), genotoxicity (determined through the Ames Test), eye and skin irritation and cardiotoxicity (assessed by hERG inhibition)15. The effectiveness of the platform is continually enhanced by the integration of new consensus models, which increases the precision of its predictions. With its structure optimisation module, featuring a comprehensive selection of ADMET filters, the platform enables users to steer their projects towards the desired outcomes more efficiently16. Percepta generates its predictions based on input data such as molecular structure, established chemical properties, and prior toxicological findings, playing an essential role in assessing chemical compounds’ potential effects on biological systems. The accuracy and dependability of the Percepta models are constantly verified and improved in light of new scientific evidence. In this study, we used Percepta version 2023.1.2, with a licence acquired by the Institute of Medical Expertise in Łódź.
ProTox 3.0.
ProTox 3.0 is scientific software (upgraded in March 2024), leverages a variety of machine learning algorithms and extensive databases to predict toxicological characteristics. These predictions span specific organ toxicities, toxic endpoints, and especially the median lethal dose (LD50for rats, oral route of exposure). ProTox 3.0 is particularly invaluable for assessing the potential toxic impact of new substances, including those within the NPS category. ProTox 3.0 uses a comprehensive approach, combining molecular similarity, fragment tendencies, common characteristics, and a machine learning framework (CLUSTER cross-validation based on fragment similarity), in a suite of 61 models, to forecast various toxicity endpoints. These include AT, specific organ toxicity, broader toxicological endpoints, initial molecular events, metabolic impacts, adverse outcome pathways (as defined by Tox21), and specific toxicity targets. The application of ProTox 3.0 in our compound analysis is justified by its comprehensive external validation, as demonstrated in publications by Drwal17et al. and Banerjee18 et al., which confirm its exceptional accuracy and reliability. Distinguished by its high sensitivity, specificity, and precision, prediction and toxicity categorisation methods include similarity and fragment-based approaches, alerting to possible toxicity targets. The results are detailed with confidence levels, an extensive toxicity radar chart, and insights into structurally similar known toxic entities. Within ProTox 3.0, the acute oral toxicity (AOT) uses two-dimensional similarity assessments against compounds with established LD50 values (mg/kg), pinpointing fragments indicative of toxicity. Its validation methodology employs a leave-one-out cross-validation technique, calculating the three closest neighbours from the training dataset for each compound based on fingerprint similarity.
PreADMET
The PreADMET19software is an advanced scientific software that offers comprehensive capabilities for toxicity assessment. This software is structured around four main components, each tailored to address specific aspects of the drug discovery and substance evaluation process, including the assessment of New Psychoactive Substances (NPS). Within the Molecular Descriptor Calculation module, powered by the TOPOMOL module, PreADMET excels in calculating physicochemical descriptors critical for ADME/Tox property predictions, such as lipophilicity (logP), molecular weight, polar surface area, and water solubility. Leveraging more than 2,500 molecular descriptors from 2D and 3D chemical structures, including constitutional, topological, electrostatic, physicochemical, and geometrical descriptors, it offers a rapid assessment tool for a wide array of compounds by processing MDL mol or sd files. The Drug-likeness prediction module of PreADMET, utilising established guidelines such as Lipinski’s Rule of Five and the Lead-like rule, adds a layer of versatility by allowing users to apply various drug-like criteria developed from the analysis of major drug databases such as the CMC, WDI, and MDDR DB. This module’s adaptability makes it especially suitable for evaluating the drug-likeness of new substances, including NPS, providing critical insights into toxicity assessment. In the Toxicity Prediction module, PreADMET demonstrates its commitment to early-stage safety assessment by developing precise models to evaluate mutagenicity in specific strains of Ames Salmonella (TA100, TA98, TA1535) and rodent carcinogenicity based on 2-year studies. This focus on early prediction of in silico toxicity is invaluable not only for traditional drugs20,21 but also for the safety evaluation of NPS, aiding in the identification of potential hazards associated with these substances.
ADMETlab 2.0.
ADMETlab 2.022 is a comprehensive scientific resource developed to forecast the ADMET characteristics of chemical compounds. This platform leverages multi-experimental data and utilises a sophisticated multitask graph attention framework to generate accurate and reliable prediction models. Designed with the user’s needs in mind, ADMETlab 2.0 supports an extensive array of ADMET-related endpoints, encompassing physicochemical properties, aspects of medicinal chemistry, ADME traits, toxicity endpoints, and rules for identifying toxicophores. With its emphasis on user-friendliness and efficiency, the platform is equipped with features for batch processing and boasts an intuitive user interface, making it an indispensable asset for medicinal chemists engaged in drug discovery and development. ADMETlab 2.0 provides comprehensive insights and actionable recommendations, facilitating a deeper understanding of a compound’s ADMET profile. The platform integrates databases curated with high-quality experimental data, guaranteeing the trustworthiness and precision of its outputs. The predictive models within ADMETlab 2.0 employ a cutting-edge multitask graph attention framework, showcasing a state-of-the-art computational technique capable of managing various prediction tasks simultaneously. This approach utilises graph-based neural networks that excel at analysing molecular information depicted as graphs. The embedded attention mechanism selectively concentrates on significant aspects of the molecular structure, significantly improving the model’s ability to identify intricate patterns and interactions within the data. As a result, ADMETlab 2.0 delivers refined and detailed predictions across a broad spectrum of ADMET-related endpoints, enhancing the drug development process with its depth of analysis and accuracy.
Online Chemical Modeling Environment (OCHEM).
The Online Chemical Modeling Environment (OCHEM) is a comprehensive scientific platform designed to automate the traditional steps necessary for creating predictive Quantitative Structure-Activity Relationship (QSAR) and Quantitative Structure-Property Relationship (QSPR) models23. This platform is comprised of two main components: a database filled with user-contributed experimental measurements and a sophisticated modeling framework24. Originally, OCHEM was tailored solely towards the processing of individual compounds. The database hosts nearly 10,000 data points concerning the density, and azeotropic behavior of binary mixtures25. Leveraging different learning methods and specially tailored descriptors for mixtures, models have been developed to predict both qualitative (azeotrope/zeotrope) and quantitative (density and bubble points) aspects of these mixtures. The predictive accuracy of these models is on par with, or exceeds, those reported in prior studies, marking a significant advancement in the modeling of chemical compound mixtures. OCHEM supports a wide range of descriptor packages, enhancing its versatility for modeling. These packages include CDK (3D, 274 descriptors), Dragon v.6 (3D, 4885 descriptors organized into 29 blocks), OEstate, ALogPS, ISIDA Fragments, GSFrag, Mera, Mersy, Chemaxon (3D, 499 descriptors), Inductive (3D), Adriana (3D, 211 descriptors), Spectrophores (3D), QNPR, Structural Alerts, and SIRMS. This extensive selection allows for a comprehensive approach to modeling, capturing the various aspects of chemical structure and behavior within QSAR frameworks. This array of descriptor packages not only underscores OCHEM’s versatility but also enables a comprehensive modeling approach that captures the diverse facets of chemical structure and behavior within QSAR and QSPR frameworks. By offering these advanced tools and resources, OCHEM is important scientific toll in the ongoing effort to predict toxicity of NPS.
Test
The Toxicity Estimation Software Tool (TEST)26–31, crafted by the US EPA and available as an open-source program, streamlines the process of chemical toxicity evaluation using SMILES or CAS numbers. With its versions 5.1.2 and 4.2.1, TEST introduces a variety of models for assessing AT, leveraging either structural analogues or multivariate regression methods to simplify and enhance the accessibility of toxicity assessments for diverse uses. TEST utilizes a comprehensive array of molecular descriptors drawn from the EPA ECOTOX databases, covering aspects like structural, constitutional, connectivity, shape, topological, molecular distance, fragments, and electrotopological properties. It evaluates acute oral LD50 toxicity in rats using three distinct QSAR strategies. Within TEST, the hierarchical approach enhances the precision of toxicity predictions by grouping compounds according to structural similarities through Ward’s method. This method aims to reduce variance within clusters by examining a training set to pinpoint compounds with similar structural attributes. The toxicity of a new compound is then estimated using a weighted average from models associated with these clusters, thus improving accuracy and customizing predictions based on a compound’s unique structural features. The nearest-neighbour technique in TEST calculates toxicity by taking the average of the three chemically closest substances in the training set to the compound under review. By focusing on structural resemblance, this method ensures that the predictions are closely matched to the specific structural traits of the chemical being assessed, refining the evaluation through comparison and averaging of toxicity figures from the nearest analogues. This reliance on structural similarity augments the specificity and relevance of the predictions.
The consensus method in TEST compiles and averages toxicity estimates from all the QSAR models used, offering a comprehensive prediction of a compound’s toxicity. This method evaluates the domain of applicability for each model, ensuring that the final estimate represents a harmonized perspective from various analytical approaches. Acknowledged by TEST as the most trustworthy prediction, this approach effectively merges insights from hierarchical, FDA, and nearest-neighbour methods, yielding a thorough and balanced toxicity assessment.
VEGA QSAR
The VEGA (Virtual Models for the Evaluation of Chemical Properties) software is scientific computational software for the quantitative analysis of structure-activity relationships (QSAR)32,33. This is a computational platform shaped by the collective insights from EU projects like CAESAR, ORCHESTRA, and ANTARES. It integrates QSAR models alongside rule-based expert systems for forecasting various human toxicity endpoints, such as mutagenicity, carcinogenicity, developmental toxicity, and skin sensitisation. As a scientific software tool, it facilitates the prediction of chemical properties, biological activities, and environmental toxicity, leveraging a diverse array of algorithmic strategies rooted in statistical analysis and machine learning. The core of VEGA QSAR’s methodology is the application of quantitative models that correlate chemical structure with activity or property of interest. This approach utilizes a comprehensive dataset of chemically characterized entities, employing both linear and non-linear modeling techniques to predict the behavior of uncharacterized compounds. The predictive models are constructed and validated using robust statistical methods, ensuring the accuracy and reliability of the software’s outputs. To determine the reliability of its predictions, VEGA utilizes the Applicability Domain Index (ADI), which assigns values from 0 (indicating lower reliability) to 1 (signifying higher reliability). The platform automatically conducts a series of checks to flag potential issues that could compromise the accuracy or reliability of its predictions. These measures include: (a) confirming that the target compound shares a sufficient resemblance with closely related compounds in the dataset, (b) ensuring that the experimental values of similar compounds are in agreement with the prediction for the target compound, (c) evaluating the closeness of the experimental values to their respective predictions to assess precision, (d) identifying rare molecular fragments in the target compound not commonly found in the training set, (e) checking that the descriptor values of the target compound are within the acceptable range, and (f) analyzing the effect of a 10% variation in descriptor values on the predicted outcome
Author contributions
Kamil Jurowski - conceptualization, data curation, formal analysis, investigation, methodology, supervision, visualisation, writing-original draft, writing-review & editing.Alicja Krośniak - data curation, investigation, writing-original draft.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.1. Resnik, K. N. et al. Case report: Identification of AP-238 and 2-fluorodeschloroketamine in internet available powder samples sold as bucinnazine. Forensic Science International348, 111732 (2023). [DOI] [PubMed]
- 2.2. Giorgetti, A., Brunetti, P., Pelotti, S. & Auwärter, V. Detection of AP-237 and synthetic cannabinoids on an infused letter sent to a German prisoner. Drug Testing and Analysis14, 1779–1784 (2022). [DOI] [PMC free article] [PubMed]
- 3.3. Nations, U. Acute toxicity. https://www.un-ilibrary.org/content/books/9789210475655s004-c001 (2007) doi:10.18356/f1860653-en.
- 4.4. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). (United Nations, New York Geneva, 2017).
- 5.5. Orsolini, L. et al. The use of new psychoactive substances (NPS) in young people and their role in mental health care: a systematic review. Expert Review of Neurotherapeutics19, 1253–1264 (2019). [DOI] [PubMed]
- 6.6. Singh, P. K., Negi, A., Gupta, P. K., Chauhan, M. & Kumar, R. Toxicophore exploration as a screening technology for drug design and discovery: techniques, scope and limitations. Arch Toxicol90, 1785–1802 (2016). [DOI] [PubMed]
- 7.7. Turkez, H., Arslan, M. E. & Ozdemir, O. Genotoxicity testing: progress and prospects for the next decade. Expert Opinion on Drug Metabolism & Toxicology13, 1089–1098 (2017). [DOI] [PubMed]
- 8.8. Twilley, D. & Lall, N. 16 - African Plants with Dermatological and Ocular Relevance. in Toxicological Survey of African Medicinal Plants (ed. Kuete, V.) 493–512 (Elsevier, 2014). doi:10.1016/B978-0-12-800018-2.00016-9.
- 9.9. Yoon, K. S. et al. 2-(2,5-Dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe) and N-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine (25 C-NBOMe) induce adverse cardiac effects in vitro and in vivo. Toxicology Letters304, 50–57 (2019). [DOI] [PubMed]
- 10.10. Gomis-Tena, J. et al. When Does the IC50 Accurately Assess the Blocking Potency of a Drug? J. Chem. Inf. Model.60, 1779–1790 (2020). [DOI] [PMC free article] [PubMed]
- 11.11. STopTox: An in Silico Alternative to Animal Testing for Acute Systemic and Topical Toxicity | Environmental Health Perspectives | Vol. 130, No. 2. https://ehp.niehs.nih.gov/doi/10.1289/EHP9341. [DOI] [PMC free article] [PubMed]
- 12.12. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties | Journal of Chemical Information and Modeling. https://pubs.acs.org/doi/10.1021/ci300367a. [DOI] [PubMed]
- 13.13. Gu, Y., Lou, C. & Tang, Y. Chapter 14 - admetSAR—A valuable tool for assisting safety evaluation. in QSAR in Safety Evaluation and Risk Assessment (ed. Hong, H.) 187–201 (Academic Press, 2023). doi:10.1016/B978-0-443-15339-6.00004-7.
- 14.14. Quantitative Structure − Activity Relationship Modeling of Rat Acute Toxicity by Oral Exposure | Chemical Research in Toxicology. https://pubs.acs.org/doi/10.1021/tx900189p. [DOI] [PMC free article] [PubMed]
- 15.15. Predict Molecular Properties | Percepta Software. ACD/Labs https://www.acdlabs.com/products/percepta-platform/.
- 16.16. Gromek, K., Hawkins, W., Dunn, Z., Gawlik, M. & Ballabio, D. Evaluation of the predictivity of Acute Oral Toxicity (AOT) structure-activity relationship models. Regulatory Toxicology and Pharmacology129, 105109 (2022). [DOI] [PubMed]
- 17.17. Drwal, M. N., Banerjee, P., Dunkel, M., Wettig, M. R. & Preissner, R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Research42, W53–W58 (2014). [DOI] [PMC free article] [PubMed]
- 18.18. Banerjee, P., Eckert, A. O., Schrey, A. K. & Preissner, R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Research46, W257–W263 (2018). [DOI] [PMC free article] [PubMed]
- 19.19. Lee, S. et al. The PreADME Approach: Web-based program for rapid prediction of physico-chemical, drug absorption and drug-like properties. euro QSAR 2002 - Designing Drugs and Crop Protectants: Processes Problems and Solutions 418–420 (2002).
- 20.20. Yeni, Y., Supandi, S. & Merdekawati, F. In silico toxicity prediction of 1-phenyl-1-(quinazolin-4-yl) ethanol compounds by using Toxtree, pkCSM and preADMET. Pharmaciana8, 205–216 (2018).
- 21.21. Viana Nunes, A. M. et al. preADMET analysis and clinical aspects of dogs treated with the Organotellurium compound RF07: A possible control for canine visceral leishmaniasis. Environ Toxicol Pharmacol80, 103470 (2020). [DOI] [PubMed]
- 22.22. Dong, J. et al. ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. Journal of Cheminformatics10, 29 (2018). [DOI] [PMC free article] [PubMed]
- 23.23. Sushko, I. et al. Online chemical modeling environment (OCHEM): web platform for data storage, model development and publishing of chemical information. J Comput Aided Mol Des25, 533–554 (2011). [DOI] [PMC free article] [PubMed]
- 24.24. Sushko, I., Salmina, E., Potemkin, V. A., Poda, G. & Tetko, I. V. ToxAlerts: A Web Server of Structural Alerts for Toxic Chemicals and Compounds with Potential Adverse Reactions. J. Chem. Inf. Model.52, 2310–2316 (2012). [DOI] [PMC free article] [PubMed]
- 25.25. Oprisiu, I., Novotarskyi, S. & Tetko, I. V. Modeling of non-additive mixture properties using the Online CHEmical database and Modeling environment (OCHEM). Journal of Cheminformatics5, 4 (2013). [DOI] [PMC free article] [PubMed]
- 26.26. Martin, T. WebTEST (Web-services Toxicity Estimation Software Tool). (2018) doi:10.13140/RG.2.2.15742.08009.
- 27.27. Noga, M., Michalska, A. & Jurowski, K. The estimation of acute oral toxicity (LD50) of G-series organophosphorus-based chemical warfare agents using quantitative and qualitative toxicology in silico methods. Arch Toxicol (2024) doi:10.1007/s00204-024-03714-5. [DOI] [PubMed]
- 28.28. Noga, M., Michalska, A. & Jurowski, K. The acute toxicity of Novichok’s degradation products using quantitative and qualitative toxicology in silico methods. Arch Toxicol98, 1469–1483 (2024). [DOI] [PubMed]
- 29.29. Institute for Health and Consumer Protection (Joint Research Centre), Worth, A. & Fuart Gatnik, M. Review of Software Tools for Toxicity Prediction. (Publications Office of the European Union, 2010).
- 30.30. Review of QSAR models and software tools for predicting acute and chronic systemic toxicity - Publications Office of the EU. https://op.europa.eu/en/publication-detail/-/publication/940acf32-3e4d-47cf-b4a1-eebe67c79ae1/language-en.
- 31.31. Noga, M., Michalska, A. & Jurowski, K. Application of toxicology in silico methods for prediction of acute toxicity (LD50) for Novichoks. Arch Toxicol97, 1691–1700 (2023). [DOI] [PMC free article] [PubMed]
- 32.32. The VEGAHUB Platform: The Philosophy and the Tools - Alessandra Roncaglioni, Anna Lombardo, Emilio Benfenati, 2022. https://journals.sagepub.com/doi/full/10.1177/02611929221090530. [DOI] [PubMed]
- 33.33. Benfenati, E., Roncaglioni, A., Lombardo, A. & Manganaro, A. Integrating QSAR, Read-Across, and Screening Tools: The VEGAHUB Platform as an Example. in Advances in Computational Toxicology: Methodologies and Applications in Regulatory Science (ed. Hong, H.) 365–381 (Springer International Publishing, Cham, 2019). doi:10.1007/978-3-030-16443-0_18.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.