Abstract
This research aims to isolate, characterize, and analyze pure compounds from Leea macrophylla leaf extract to investigate its antibacterial, antidiabetic, cytotoxic, and phytotoxic effects. Fresh leaves were collected, dried, and subjected to methanol extraction to obtain a crude extract. From the petroleum ether fraction (PEF) of this extract, three fractions—designated LM1, LM2, and LM3—were prepared using column chromatography. The fractions were tried to be characterized in search for single compound by instrumental technique like ATR-FTIR, 1H NMR and 13C NMR but the 1H NMR and 13C NMR spectra were found complex which were difficult to interpret. To dispel the doubt and get clear idea about the structure, GC-MS analysis of the compounds was carried out whose result showed that all the three extracts were decomposed to several small organic compounds that made the structure elucidation difficult. For this complication, the characterization of the extracts was not possible. Numerous compounds were identified in the methanol extract of L.macrophylla through GC-MS analysis. Among these compounds, Benzene, 1,2,3-trimethyl- and Undecane were found in higher percentages in LM1. LM2 contained Azulene and Bicyclo [4.4.1]undeca-1,3,5,7,9-pentaene, while LM3 was characterized by the presence of 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione and 11-(2-Cyclopenten-1-yl)undecanoic acid, among others. The antibacterial activity of these fractions was evaluated against various bacterial strains, demonstrating broad-spectrum effectiveness. LM1 fraction showed the highest antibacterial activity against Proteus sp. With zone of inhibition 25 mm and weak activity against S. sonnei with zone of inhibition 5 mm. LM2 showed the highest activity to both E. cocci and P. aeruginosa with the zone of inhibition of 18 mm and comparatively lower but significant against Proteus sp. LM3 was highly active to S. sonnei with zone of inhibition 20 mm and lower but quite significant against Proteus sp. Moreover, the anti-diabetic potential was assessed, with LM1 showing the strongest α-amylase inhibitory activity, outperforming quercetin (standard). The IC50 values of LM1, LM2, LM3, and quercetin were 57.36 μg/mL, 100.66 μg/mL, 164.92 μg/mL, and 97.45 μg/mL, respectively. In addition, cytotoxicity was assessed using a brine shrimp lethality bioassay, and phytotoxicity was evaluated through seed germination and growth assays. The results suggest that L.macrophylla leaf extracts have potential applications in antimicrobial, antidiabetic, and anti-cancer contexts. This comprehensive study bridges gaps in knowledge surrounding L.macrophylla's multifaceted properties, offering insights into its therapeutic and ecological potential for healthcare and environmental management.
Keywords: Leea macrophylla, Leaf extract, Antibacterial, Antidiabetic, Cytotoxicity, Phytoxicity
Highlights
-
•
Identify antibacterial, antidiabetic, and cytotoxic potential of Leea macrophylla.
-
•
The leaves of Leea macrophylla exhibit a rich diversity of bioactive compounds.
-
•
Leea macrophylla leaf fractions effectively combat a spectrum of bacteria.
-
•
Leaf extracts exhibit strong α-amylase inhibition, highlighting antidiabetic potential.
-
•
Extracts exhibit both cytotoxicity and phytotoxicity effects.
1. Introduction
The utilization of plants for both sustenance and therapeutic purposes dates back to ancient times [1]. Among these botanical resources, Leea macrophylla Roxb. ex Hornem, locally known as Hastikarna Palasha, stands out as a noteworthy herbaceous shrub belonging to the genus Leea within the Vitaceae family. Its striking large leaves resemble those of an elephant ear [2]. L. macrophylla is prevalent in regions across South and Southeast Asia, particularly in the central and eastern areas of Nepal, Bhutan, Indo-China, Myanmar, Thailand, Cambodia, and Laos [[3], [4], [5], [6]]. Notably, certain areas in Bangladesh, such as Rajshahi, Natore, Pabna, and Jashore, are recognized for hosting L. macrophylla.
L. macrophylla Roxb. ex Hornem. Has garnered attention for its numerous ethnomedicinal and economic applications [7]. Due to its medicinal attributes, herbalists have harnessed its potential for addressing a wide range of ailments since ancient times. Various ethnobotanical claims have emerged from survey investigations conducted in tribal regions across the world. However, there remains a significant gap in our collective knowledge concerning the ethnobotanical aspects, culinary use, ethnopharmacological applications, pharmacological investigations, and economic uses of L. macrophylla.
Numerous medicinal plants have been identified as valuable sources of natural antimicrobial compounds, offering potential treatments for challenging bacterial infections [8]. L. macrophylla is one such medicinal plant. In contemporary times, medicinal plants have been explored as a means to manage diseases like diabetes, owing to their rich phytoconstituent profiles, which encompass flavonoids, terpenoids, saponins, carotenoids, alkaloids, and glycosides that may possess anti-diabetic properties [9]. The anti-diabetic effects of plant materials are often attributed to a combination of phytochemicals or individual plant extract components. Additionally, certain plants exhibit allelopathic potential, allowing them to inhibit the growth of other plants [10,11]. In this context, their allelopathic potential, often referred to as phytotoxicity, is of research interest, particularly for the development of bioherbicides. Seed germination and plant growth bioassays are the primary techniques employed to assess the phytotoxic properties of compounds [12].
Cancer, as the second leading cause of morbidity and mortality globally, necessitates various treatment modalities, including chemotherapy, which has shown promise in improving survival rates but is associated with numerous acute toxic effects [13]. Over the past decade, the significance of identifying medicinal plants with substantial cytotoxic potential for the development of cancer therapeutics has grown, resulting in expanded research in this field [14]. The brine shrimp lethality bioassay is a widely accepted method for assessing the toxicity of heavy metals, pesticides, drugs, including natural plant extracts, and other substances. It is also employed to evaluate potential antitumor, antiviral, and anticancer activities [15,16].
In our pursuit to harness the therapeutic and ecological potential of L. macrophylla leaf extract, we confront critical research gaps. Firstly, despite its historical use in traditional medicine, there is a dearth of rigorous scientific validation for its purported anti-diabetic effects. Secondly, its antibacterial potential against specific pathogens remains inadequately explored, particularly in light of the escalating issue of antibiotic resistance. Furthermore, there is a lack of comprehensive research into its phytotoxicity, a factor of paramount importance for sustainable agriculture, and its cytotoxicity, a facet with potential implications for drug development. Lastly, integrating these multifaceted properties into a single comprehensive study is essential, as previous research has predominantly focused on isolated aspects. Addressing these gaps holds the promise of unlocking the full potential of L. macrophylla for healthcare and environmental management.
The leaves of L. macrophylla have been traditionally employed to treat various conditions, including gastric tumors, goiter, lipomas, tetanus, and urinary disturbances [5,17,18]. The leaf juice is also utilized for treating ailments such as boils, arthritis, gout, and rheumatism, functioning as a local anti-inflammatory agent. Additionally, a paste made from the plant's roots, when mixed with a glass of milk and consumed once a month, has been used for birth control [19]. Reports on L. macrophylla have highlighted its diverse pharmacological effects, including antilithiatic, anti-inflammatory, anti-nociceptive, cardiotonic, anticancer, cytotoxic, antioxidant, antithrombotic, neuroprotective, and gastroprotective properties [1,20]. Hypothetically, the antioxidative potential of L. macrophylla may play a substantial role in mitigating diabetic complications. Nonetheless, no scientific study has yet been conducted to definitively establish the anti-diabetic properties of L. macrophylla. Consequently, this research aims to evaluate the potential antidiabetic effects of L. macrophylla.
Furthermore, no prior research has explored the antibacterial, antidiabetic, and phytotoxic activities of this plant comprehensively. Moreover, existing studies have primarily focused on the cytotoxic effects of crude methanol, ethanol, and aqueous extracts. Therefore, this study aims to evaluate the potent phytotoxic effects of the crude methanol extract, as well as the petroleum ether and Diaion resin-adsorbed fractions of L. macrophylla leaf extracts, on Allium cepa (onion), Cicer arietinum (gram), and Triticum aestivum (wheat). Additionally, we aim to evaluate their cytotoxic activities using the brine shrimp lethality bioassay.
2. Materials and methods
2.1. Chemicals and reagents
In this study, we obtained various chemicals and substances from Sigma-Aldrich, Germany. These included α-Amylase, acetone, absolute alcohol, chloroform, hydrochloric acid, iodine reagent, kannamycin (30 μg/disc), methanol, petroleum ether, sodium phosphate buffer, and a 1 % w/v starch solution. The bacterial strains used in our research were sourced from the Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh. For our experiments, we acquired brine shrimp (Artemia salina) eggs from an aquarium shop situated in Rajshahi, Bangladesh.
2.2. Collection and identification of plant materials
Fresh leaves of L. macrophylla were obtained from Oushodhi Gram in Natore, Bangladesh, in July 2022. They were subsequently identified by a taxonomist at the National Herbarium of Bangladesh, Mirpur, Dhaka, with a specimen voucher number assigned as 43415. Approximately 3 kg of fresh green plant leaves were gathered for use in this research study.
2.3. Preparation of plant leaf for extraction
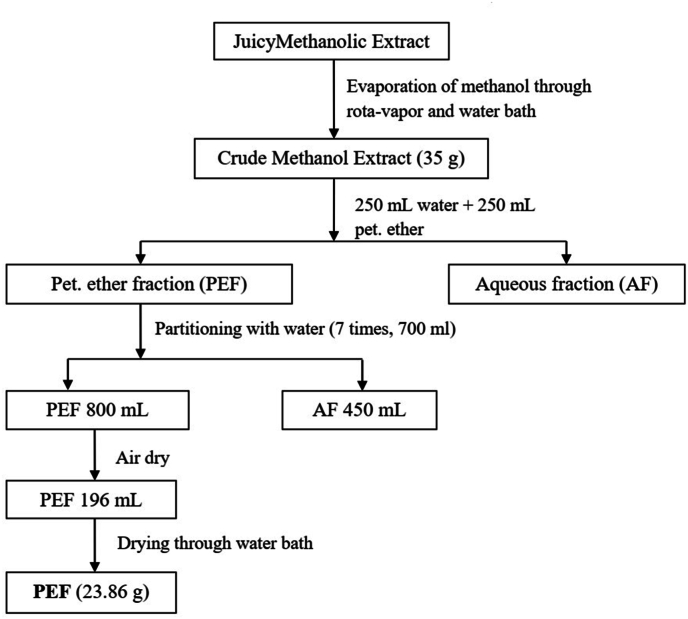
The collected leaf was dried at room temperature over a period of a month. The dried leaves were then pulverized in an electric blender after being cut into small pieces. After grinding, 647 g of dried leaf powder was obtained. Crude methanolic extract from dried leaf powder (325 g) of L. macrophylla was made using soxhlet apparatus. The methanolic extract thus collected was filtered and concentrated under reduced pressure at 45 °C using rota-vapor (BioBase) to yield 41.12 g of extract. From the crude extract pet-ether fraction was prepared according to the flowchart shown in Fig. 1. The crude extract was stored in a desiccator with calcium chloride acting as a desiccant.
Fig. 1.
Preparation of extract from Leea macrofylla leaves powder and fractionation.
2.4. Isolation and identification of compounds
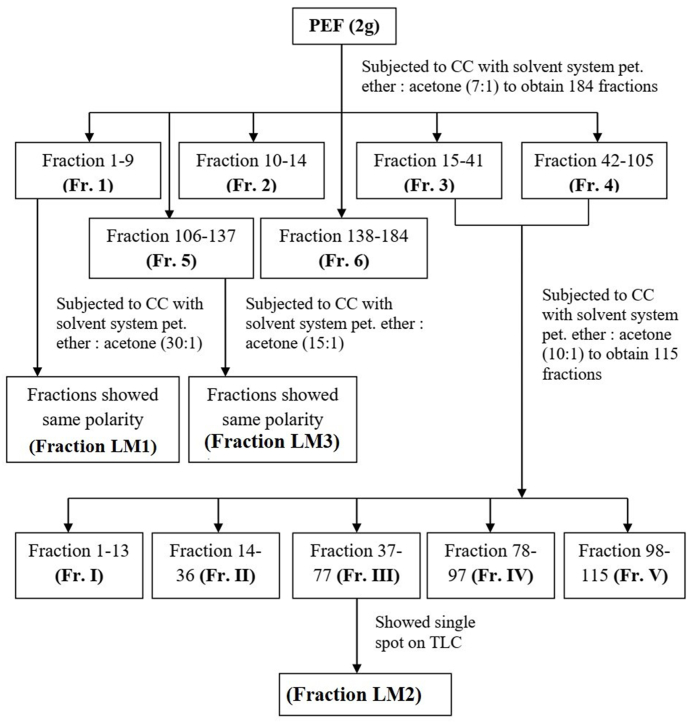
Three fractions mark as LM1, LM2 and LM3 was prepared from the pet-ether fraction following the described procedure shown in Fig. 2 by column chromatographic separation over silica gel (70–230 mesh) and eluted by petroleum ether: acetone (7:1 ratio) as solvent system. The three fractions thus obtained from column chromatography were checked by TLC with a view to get single bioactive compound and showed single spot. The FTIR spectrum of the samples was recorded using attenuated total reflection (ATR) and a diamond internal reflection element (Spectrum Two PerkinElmer Inc., USA). The spectrum region was 4000-400 cm-1 at a resolution of 4 cm-1. Spectrum 10.03.03.0139 software (PerkinElmer Inc., USA) was used to measure the spectra. All the compounds were scanned using the ATR technique. 1D 1H NMR spectrum was carried out with expansions at Bangladesh Council Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh using deuterated chloroform as solvent at 400 MHz and 13C NMR at 100 MHz NMR spectrometer. Based on the single spot both 1H NMR, 13C NMR was taken but no clear and smooth spectrum observed to identify/elucidate the structure of the bioactive compounds. The physical properties of the fraction (LM1, LM2 and LM3) L. macrofylla leaves extract are shown in Table 1.
Fig. 2.
Column chromatography of pet-ether fraction (PEF) of Leea macrofylla leaves.
Table 1.
Physical properties of the fraction (LM1, LM2 and LM3) Leea macrofylla leaves extract.
| Fraction | Physical state | Color | Yield | Solubility |
|---|---|---|---|---|
| LM1 | Oily liquid | Dark Yellow | 520 mg | Soluble in chloroform, DMSO, petroleum ether, cyclohexane, diethyl ether |
| LM2 | Amorphous solid | Black | 190 mg | Soluble in chloroform, petroleum ether, dichloromethane; sparingly soluble in DMSO, methanol, ethyl acetate |
| LM3 | Amorphous | Green | 160 mg | Sparingly soluble in chloroform, benzene, petroleum ether, diethyl ether; soluble in methanol, DMSO, acetone, ethyl acetate |
2.5. GC–MS analysis of Leea macrofylla leaves extract
The GC-MS analysis of the L. macrophylla leaves extract was conducted using a GCMS-QP2020 instrument manufactured by Shimadzu Corporation, Japan. The chromatography procedure utilized a capillary column measuring 30 m in length and 0.25 mm in inner diameter, comprising 5 % diphenyl and 95 % dimethyl poly-siloxane. The injection temperature was set at 220 °C. Initially, the oven temperature was established at 80 °C and held isothermal for 2 min, after which it was ramped up to 150 °C at a rate of 50 °C per minute, and eventually maintained at 280 °C. The ion source temperature was maintained at 280 °C. Helium gas served as the carrier gas with a flow rate of 1.72 mL per minute. A 4-micro-liter sample was injected in split-less mode with a 1:100 ratio. For GC-MS detection, the detector acquired ionizing energy at 45 m/z, and the scan interval was set to 0.30 s. The analysis of the prepared sample took approximately 50 min to complete. All detected peaks were compared against the GC-MS library database, specifically the NIST library from the 2008 and 2014 editions.
2.6. Antibacterial activities
The antimicrobial activity of L. macrophylla leaf extract was assessed using the disk diffusion method. The bacteria strains used in this study were gram positive bacteria (Bacillus cereus, Staphylococcus aureus, Enterococcus (E. cocci) and gram-negative bacteria (Escherichia coli, Shigella sonnei, Proteus sp., Pseudomonas aeruginosa, Salmonella typhi, Klebsiella pneumoniae). These strains were obtained from the Bangladesh Council scientific and Industrial Research (BCSIR), Dhaka-1205, Bangladesh. Nutrient agar medium (2.3 % w/v) was prepared, sterilized, and poured into petri dishes. Slants were also prepared in sterile test tubes. Bacterial cultures were inoculated onto the slants and incubated at 37.5 °C. Fresh cultures were used for the sensitivity test. Isolated extracts (LM1, LM2, and LM3) were dissolved in DMSO solvent. Discs loaded with 200 μg of each compound were placed on agar plates alongside a standard Kannamycin disc (30 μg/disc). The plates were placed in an incubator at a temperature of 37.5 °C for a duration of 24 h, and the assessment of antibacterial activity was conducted by measuring the diameter of the inhibition zones in millimeters.
2.7. Anti-diabetic activities
The evaluation of the anti-diabetic activity of the isolated compounds proceeded according to the established procedure [21]. In triplicate, 250 μL of each solution (at concentrations of 10, 20, 40, 80, and 160 μg/mL) was placed in a test tube. Subsequently, 250 μL of phosphate buffer (200 mM, pH 6.9, with 6 mM sodium chloride), 250 μL of phosphate buffered α-amylase (0.05 mg/mL), and 250 μL of a 1 % w/v starch solution were sequentially added. The reaction mixture was incubated for 15 min at 37 °C. To halt the enzymatic reaction, 20 μL of 1 M HCl was introduced, followed by the addition of 100 μL of an iodine reagent (comprising 5 mM I2 and 5 mM KI). Precisely 1 min after adding the iodine reagent, a noticeable color change was observed, and the absorbance was measured at 625 nm using a 1 mL cuvette. The control reaction, which represented 100 % enzyme activity, did not contain any test compound. Inhibition of enzyme activity was calculated as follows:
Where, C is the absorbance of the control and S is the absorbance of the tested compounds.
The concentration of the compounds that resulted in 50 % inhibition of enzyme activity (IC50) was determined graphically by plotting % of inhibition as a function of inhibition concentration of the tested compounds.
2.8. Cytotoxicity (brine shrimp lethality bioassay)
The brine shrimp (A. salina) lethality experiment was carried out according to the method followed by Mannan et al., 2017 [22] with some modifications. The eggs of brine shrimp were collected from an aquarium shop of Rajshahi, Bangladesh. Artificial ‘sea water’ was prepared by dissolving 38 g NaCl (sea salt) in 1L of distilled water and then filtering it off to obtain a clear solution. The pH of the ‘sea water’ was maintained between 8–9 by adding NaHCO3. Artificial sea water was added to a small tank and shrimp eggs (∼2 g/L) were added to the tank. The tank was kept open air and at a constant temperature (37 °C) by a light source for 48 h to allow the eggs to hatch and larvae to mature. The test samples dissolved in dimethyl sulphoxide (DMSO) were placed in each vial containing 10 nauplii to make the final concentration of 100, 50, 25, 12.5 and 6.25 μg/mL. Each treatment was tested in triplicate. The negative control group was prepared in DMSO without the extracts. After 24 h, the number of survived nauplii was counted using magnifying glass. From the mortality data, the LD50 values at 95 % confidence interval and regression equations were determined using MedCalc, version 20.006 statistical software.
2.9. Phytotoxicity (seed germination and growth assays)
The crude methanol extract, petroleum ether and dia-ion resin adsorbed fraction were used to prepare test solutions at different levels of concentrations (200, 150, 100, 50, 25 μg/mL) from a 1000 μg/mL stock solution by dilution method. For seed germination assay, C. arietinum and T. aestivum seeds were collected from one of the local markets of Rajshahi, Bangladesh. The seeds were washed with distilled water and 15 fresh seeds of each category were placed in different petri dishes having filter paper on the bottom. Five dishes for each treatment were prepared in this manner. 0.5 mL of the prepared solutions (200, 150, 100, 50, 25 μg/mL) of each extractive was sprayed to different petri dishes separately, covered with their lids and labeled. The petri dishes were kept under observation for 7 days at room temperature and the test solutions were sprayed after 12 h interval in case the filter papers became dry. Distilled water was used as a negative control group and three replicates of each treatment were tested. After 7 days, the number of germinated seeds was counted, and the germination rate was calculated as under:
For growth assay, 50 mL falcon tubes were filled up to the mark with the prepared solutions (200, 150, 100, 50, 25 μg/mL) of each extractive and fresh A. cepa were placed at the top of the falcon tubes in such a way that only the roots of them were dipped in the solution. Each treatment was tested in triplicate and distilled water was used as a negative control group. The falcon tubes were kept in observation at room temperature for 7 days. After 7 days, length of roots and shoots were measured with ruler and the mean value was taken [23].
2.10. Statistical analysis
The experimental data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA). Results were calculated by averaging three independent evaluations and are presented as mean ± standard deviation.
3. Results and discussion
3.1. Characterization of the fractions
The three fractions obtained from column chromatography were analyzed by TLC to verify the presence of a single bioactive compound, each showing a single spot. Based on the single spot both 1H NMR, 13C NMR was taken but no clear and smooth spectrum observed to elucidate the structure of the bioactive compounds. Therefore, the fractions were then analyzed by GC-MS and found several bioactive compounds which confirmed that the prepared column chromatographic fractions were a mixture of bioactive compounds rather than single. The FTIR spectrum was also recorded in order to identify the possible functional group presents in the compound (Table 2).
Table 2.
ATR-FTIR spectral data of the fraction (LM1, LM2 and LM3) Leea macrofylla leaves extract with assigned group.
| LM1 cm−1 | LM2 cm−1 | LM3 cm−1 | Assigned group |
|---|---|---|---|
| – | 3388 | 3449 | OH stretch alcohol/phenol |
| 2924 | 2925 | 2918 | C–H Asymmetric stretch |
| 2855 | 2855 | 2850 | C–H Symmetric stretch |
| 1734 | 1712 | 1711 | C=O stretch |
| 1608 | 1619 | 1621 | C=C stretch |
| – | – | 1557 | -NH bend |
| 1456 | 1456 | 1456 | CH2 bending |
| 1377 | 1377 | 1377 | CH3 bending |
| 1218 | 1260 | – | C–O stretch |
| 1172 | 1162 | 1115, 1188 | |
| 1095 | – | – | |
| 1011 | 1042 | – | |
| – | 900 | – | -OH bending |
| 805 | 799 | – | – |
| 741 | 723 | 717 | – |
| 698 | 605 | 618 | – |
The ATR-FTIR spectrum of fraction LM2 and LM3 showed peaks at 3388 cm−1 and 3449 cm−1 which was the characteristic absorption peaks for –OH group. All the three fractions showed two sharp and narrow bands at 2918 cm−1 to 2925 cm−1 and 2850 cm−1 to 2855 cm−1 that were characteristic to the absorption peaks for aliphatic alkanes. Usually, alkanes other than strained ring compounds give sp3 C–H stretches at frequencies less than 3000 cm-1; approximately at 2935 cm-1 (for asymmetric stretch) and 2860 cm−1 (for symmetric stretch). Besides, the bending vibrations are generally found in the fingerprint region and are often hard to identify. Methylene (CH2) groups give a characteristic bending absorption near at 1456 cm−1 whereas methyl (CH3) groups give bending absorption at 1375 cm−1. Two narrow bands at 1456 and 1377 cm−1 were observed that depicted the existence of CH2 and CH3 groups [24]. Moreover, a sharp band at 1711 cm−1to 1734 cm−1 gave an indication of C=O stretch for simple aliphatic ester group whose approximate range of absorption is 1750-1735 cm−1. They also showed weak bands within the region 1095 to 1260 cm−1 which were probably for C–O stretch [25].
3.2. GC–MS analysis of Leea macrofylla leaves extract
The fractions were subsequently analyzed by GC-MS, revealing several bioactive compounds (Table 3, Table 4, Table 5). This confirmed that the column chromatographic fractions contained a mixture of bioactive compounds rather than individual ones. The main compounds identified in the methanol extract of L. macrophylla extract (LM1 fraction) by GC-MS based on the relative contents were Benzene, 1,2,3-trimethyl- (18.52 %), Undecane (12.61 %), Azulene (9.18 %), and o-Cymene (8.23 %) (Table 3). Undecane demonstrates both antiallergic and anti-inflammatory properties, Azulene boasts antidiabetic, antimicrobial, and anticancer properties [26], and 3,7,11,15-Tetramethyl-2-hexadecen-1-ol showcases attributes with anti-inflammatory and antimicrobial effects [27].
Table 3.
Compounds identified in the methanol extract of Leea macrophylla extract (LM1 fraction) by GC-MS.
| S.N | Name of the Compound | R.T | m/z | Peak Area | Conc. (%) | Structure |
|---|---|---|---|---|---|---|
| 1 | Benzene, 1,2,3-trimethyl- | 12.18 | 105 | 454767 | 18.52 | 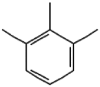 |
| 2 | Pseudocumene | 13.13 | 105 | 33363 | 1.35 | 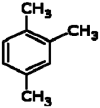 |
| 3 | Benzene, [3-(methoxymethoxy)-1-propenyl]- | – | 117 | – | nd | 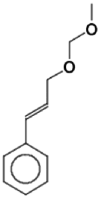 |
| 4 | Benzene, 1-methyl-3-propyl- | 13.19 | 105 | 193421 | 7.87 | 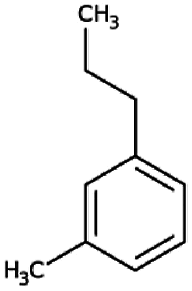 |
| 5 | Benzene, 1,3-Diethyl | 13.29 | 119 | 194375 | 7.91 | 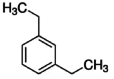 |
| 6 | Undecane | 13.56 | 57 | 298573 | 12.16 | |
| 7 | Benzene, 2-ethyl-1,4-dimethyl- | 13.71 | 119 | 115199 | 4.69 | 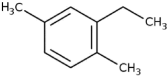 |
| 8 | o-Cymene | 13.89 | 119 | 202087 | 8.23 | 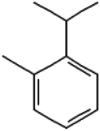 |
| 9 | Benzene, (1-methyl-1-propenyl)-, (Z)- | – | 117 | – | – | 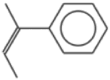 |
| 10 | 1,7,7-Trimethyl-2-vinylbicyclo [2.2.1]hept-2-ene | 14.53 | 119 | 114576 | 4.66 | 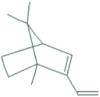 |
| 11 | 3a,6-Methano-3aH-indene, 2,3,6,7-tetrahydro- | 15.04 | 117 | 119748 | 4.87 | 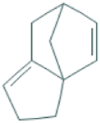 |
| 12 | Isobutyl isopentyl carbonate | – | 57 | 0 | – | 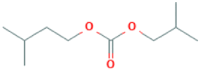 |
| 13 | Azulene | 16.31 | 128 | 225438 | 9.18 | 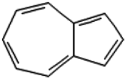 |
| 14 | Dodecane | 16.98 | 57 | 80846 | 3.29 |
Table 4.
Compounds identified in the methanol extract of Leea macrophylla extract (LM2 fraction) by GC-MS.
| S.N | Name of the Compound | R.T | m/z | Peak Area | Con. (%) | Structure |
|---|---|---|---|---|---|---|
| 1 | (S,E)-2,5-Dimethyl-4-vinylhexa-2,5-dien-1-yl REMOVE ACETATE | 8.84 | 119 | 20365 | 8.35 | 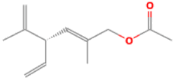 |
| 2 | Azulene | 11.12 | 128 | 76263 | 31.29 | 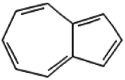 |
| 2 | Bicyclo [4.4.1]undeca-1,3,5,7,9-pentaene | 12.86 | 142 | 35189 | 14.44 | 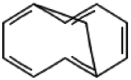 |
| 3 | 1-Octadecanesulfonyl chloride | 14.14 | 57 | 27275 | 11.19 | |
| 4 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 19.60 | 55 | 13763 | 5.64 | |
| 5 | 7-Hexadecenal, (Z)- | 20.537 | 55 | 10037 | 4.11 |  |
| 6 | 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | 28.37 | 55 | 60784 | 24.94 | 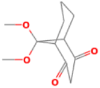 |
Table 5.
Compounds identified in the methanol extract of Leea macrophylla extract (LM3 fraction) by GC-MS.
| S.N | Name of the Compound | R.T | m/z | Peak Area | Conc. (%) | Structure |
|---|---|---|---|---|---|---|
| 1 | 11-(2-Cyclopenten-1-yl)undecanoic acid, (+)- | 7.75 | 105 | 14253 | 16.12 |  |
| 2 | 5-Methyl-6-phenyltetrahydro-1,3-oxazine-2-thione | 10.45 | 117 | 5726 | 6.47 | 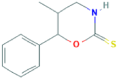 |
| 3 | Azulene | 11.12 | 128 | 11299 | 12.78 | 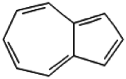 |
| 4 | Bicyclo [4.4.1]undeca-1,3,5,7,9-pentaene | 12.87 | 142 | 879 | 0.99 | 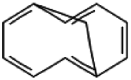 |
| 5 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 19.61 | 55 | 4589 | 5.19 | |
| 6 | 7-Hexadecenal, (Z)- | 20.53 | 55 | 4159 | 4.70 |  |
| 7 | 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | 28.38 | 55 | 47505 | 53.73 | 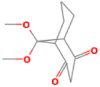 |
The main compounds identified in the methanol extract of Leea macrophylla extract (LM2 fraction) by GC-MS based on the relative contents were Azulene (31.29 %), 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione (24.94 %), Bicyclo [4.4.1]undeca-1,3,5,7,9-pentaene (14.4 %), and 1-Octadecanesulfonyl chloride (11.19 %) (Table 4).
The main compounds identified in the methanol extract of L. macrophylla extract (LM3 fraction) by GC-MS based on the relative contents were 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione (53.73 %), 11-(2-Cyclopenten-1-yl)undecanoic acid, (+)- (16.12 %), and Azulene (12.78 %) (Table 5).
3.3. Antibacterial activities
Plants possess remarkable potential to produce a diverse range of secondary metabolites, such as coumarins, alkaloids, saponins, terpenoids, glycosides, flavonoids, steroids, quinones, and tannins. The plant-derived antimicrobial substances are derived from these biomolecules [28]. One such medicinal plant is L. macrophylla. The phytochemicals present in this plant can provide antibacterial agents potentially in order to meet the increased demand for new antibacterial agents. In Table 6, the assessment of antibacterial activity of L. macrophylla extract and its successive fractions from leaves against bacterial strains is presented. These extracts exhibited a broad spectrum of activity against the bacteria, with almost all of them demonstrating significant efficacy. LM3 exhibited the strongest antibacterial activity, followed by LM1 and LM2 at higher concentrations, based on the zone of inhibition diameter. In our research study, LM1 exhibited remarkable effectiveness against Proteus sp and K. pneumoniae, but it displayed reduced efficacy against S. sonnei among the tested strains. On the other hand, LM2 demonstrated strong activity against both E. cocci and P. aeruginosa, and it also exhibited significant effectiveness against Proteus sp. LM3 displayed high activity against S. sonnei and remained notably effective against Proteus sp among the tested strains. M. B. Islam et al. (2013) reported that L. macrophylla seed extracts have antibacterial activity [29]. C. macrophylla leaf extracts had antibacterial activity against E. carotovora, P. syringae, R. solanacearum, and X. axonopodis [30].
Table 6.
Analyzing the influence of various Leeamacrophylla fractions on the inhibition zone against diverse gram-positive and gram-negative bacterial strains.
| Test bacteria |
Zones of Inhibition of the extracts (mm) |
|||
|---|---|---|---|---|
| Gram positive bacteria | LM1 (200 μg/disc) | LM2 (200 μg/disc) | LM3 (200 μg/disc) | Kannamycin (30 μg/disc) |
| Bacillus cereus | 13.8 ± 0.32 | 15.6 ± 045 | 17.2 ± 0.50 | 6.5 ± 0.65 |
| Staphylococcus aureus | 16.5 ± 0.35 | 16.4 ± 0.41 | 18.5 ± 0.35 | 25.5 ± 0.65 |
| Enterococcus (E. cocci) | 14.8 ± 0.43 | 18.6 ± 0.32 | 13.2 ± 0.50 | 20 ± 0.25 |
| Gram negative bacteria | ||||
| Escherichia coli | 15.4 ± 0.35 | 12.5 ± 0.25 | 15.3 ± 0.52 | 14.2 ± 0.81 |
| Shigella sonnei | 5.5 ± 0.28 | 14.3 ± 0.35 | 20.5 ± 0.32 | 9.4 ± 0.67 |
| Proteus sp. | 25.5 ± 0.43 | 11.2 ± 0.15 | 12.3 ± 0.25 | 14.5 ± 0.51 |
| Pseudomonas aeruginosa | 14.5 ± 0.42 | 18.2 ± 0.24 | 18.7 ± 0.35 | 12.2 ± 0.41 |
| Salmonella typhi | 16.3 ± 0.37 | 15.5 ± 0.28 | 17.1 ± 0.45 | 16.2 ± 0.50 |
| Klebsiella pneumoniae | 20.5 ± 0.32 | 15.2 ± 0.42 | 14.3 ± 0.21 | 12.5 ± 0.51 |
Data are means of three replicates (n = 3) ± standard deviation.
3.4. Antidiabetic activities
The in-vitro antidiabetic activity of the three isolated extracts from L. macrophylla leaf, measured by % α-amylase inhibition and median inhibitory concentration (IC50), along with a comparison of IC50 values of the tested compounds to quercetin (standard), is presented in Table 7. The LM1 extract from isolated L. macrophylla leaf displayed the highest α-amylase inhibition activity, followed by LM2 and LM3. These findings suggest that LM1 exhibits the strongest anti-diabetic activity. The presence of α-amylase inhibitory activity in T. populnea leaves was investigated [31]. Glucose-lowering effects of L. macrophylla leaves were investigated in a model of streptozotocin-induced albino rats [32]. The IC50 values of extract LM1, LM2, LM3 and quercetin were 57.36 μg/mL, 100.66 μg/mL, 164.92 μg/mL and 97.45 μg/mL respectively. As compared to quercetin, extract LM1 exhibited greater inhibitory effect. The anti-diabetic activities of the isolated extracts in comparison with quercetin (standard) can be represented by the following descending order: LM1> Quercetin > LM2> LM3.
Table 7.
In-vitro comparison of IC50 and % α-amylase inhibition for anti-diabetic activity in Leeamacrophylla leaf extracts with quercetin standard.
| Fractions | Conc. (μg/mL) | % of α-amylase inhibitory |
Inhibition of α-amylase (%) (Mean ± STD) | IC50 (μg/mL) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
|
LM1 |
10 | 13.53 | 13.55 | 13.50 | 13.53 ± 0.02 | 57.36 |
| 20 | 25.40 | 25.42 | 25.44 | 25.42 ± 0.02 | ||
| 40 | 49.44 | 49.48 | 49.44 | 49.45 ± 0.02 | ||
| 80 | 81.11 | 81.17 | 81.15 | 81.14 ± 0.03 | ||
| 160 |
92.55 |
92.51 |
92.50 |
92.52 ± 0.03 |
||
|
LM2 |
10 | 6.01 | 6.06 | 6.11 | 6.06 ± 0.05 | 100.66 |
| 20 | 13.88 | 13.60 | 13.66 | 13.71 ± 0.15 | ||
| 40 | 19.50 | 19.58 | 19.57 | 19.55 ± 0.02 | ||
| 80 | 40.33 | 40.36 | 40.35 | 40.35 ± 0.02 | ||
| 160 |
78.36 |
78.35 |
78.40 |
78.37 ± 0.03 |
||
|
LM3 |
10 | 7.14 | 7.14 | 7.17 | 7.15 ± 0.02 | 164.92 |
| 20 | 12.65 | 12.66 | 12.66 | 12.66 ± 0.01 | ||
| 40 | 20.07 | 20.14 | 20.12 | 20.11 ± 0.04 | ||
| 80 | 40.55 | 40.58 | 40.52 | 40.55 ± 0.03 | ||
| 160 |
43.48 |
43.47 |
43.55 |
43.50 ± 0.04 |
||
| Quercetin (standard) | 10 | 7.55 | 7.55 | 7.54 | 7.55 ± 0.01 | 97.45 |
| 20 | 11.11 | 11.10 | 11.10 | 11.10 ± 0.01 | ||
| 40 | 21.98 | 22.00 | 21.98 | 21.99 ± 0.01 | ||
| 80 | 41.88 | 41.87 | 41.87 | 41.87 ± 0.01 | ||
| 160 | 80.64 | 80.69 | 80.66 | 80.66 ± 0.03 | ||
3.5. Cytotoxicity (brine shrimp lethality bioassay)
The lethality of A. salina was determined after 24 h exposure. The LD50 values of the extractives of L. macrophylla were presented in Table 8. The levels of toxicity of the test extracts were graded as strong or significant when the value of LD50 < 20 μg/mL, moderate with LD50 20 to <50 μg/mL, low with LD50 50 to <200 μg/mL and not toxic with LD50 > 200 μg/mL [33].The results of brine shrimp bioassay (Table 8) depicted that the crude methanol extract showed low toxicity with LD50 value 90.315 μg/mL whereas the petroleum ether and dia-ion resin adsorbed fraction possessed no toxicity with LD50 values 913.551 and 636.796 μg/mL respectively. The phytochemical profiling of L. macrophylla crude extract reported that the presence of alkaloids and flavones that might possess the cytotoxic effect of crude methanol extract [13]. Thus, the cytotoxic effect of the plant extract gives the evidence that it can be chosen for further cell line assay and can be used for the treatment of tumors and cancer.
Table 8.
LD50, 95 % confidence intervals and regression equations of different extractives of Leeamacrophylla against Artemiasalina nauplii at 24h exposure.
| Extractives | Regression equation | LD50 (μg/mL) | 95 % Confidence interval |
χ2 value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Crude methanol | Y = −0.703 + 2.916X | 90.315 | 69.909 | 110.721 | 0.555 |
| Petroleum ether | Y = 1.607 + 1.146X | 913.551 | 30.491 | 1796.611 | 0.797 |
| Dia-ion resin | Y = 2.196 + 1.000X | 636.796 | −177.082 | 1450.674 | 0.552 |
Here, X is the log dose and Y is the working probit of percentage mortalities. The χ2 (chi-square) statistic depicts the difference between the observed counts and the counts expected if there were no association at all in the population. When the chi-square value is low, the correlation between two sets of data is high.
3.6. Phytotoxicity seed germination and growth assays
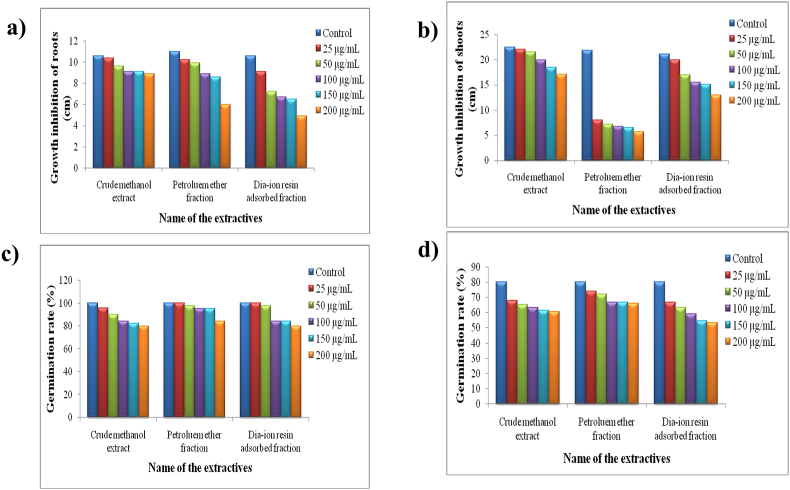
The phytotoxic potential of crude methanol extract, petroleum ether and dia-ion resin adsorbed fraction of L. macrophylla leaf varied substantially with concentration and test plants. The growth of all test plants inhibited with increasing concentration of the extractives (Fig. 3). The petroleum ether fraction showed severe growth inhibition on A. cepa shoots. The IC50 values of the extractives against the test plants ranged from 6.1 to 741.8 μg/mL (Table 9). In comparison to the crude methanol extract and dia-ion resin adsorbed fraction, the petroleum ether fraction showed strong growth inhibition to the A. cepa shoots (IC50 6.1 μg/mL). The dia-ion resin adsorbed fraction exhibited moderate growth of inhibition against all the test plants (IC50 190.8−441 μg/mL). On the other side, the crude methanol extract and petroleum ether fraction weakly inhibited the germination of C. arietinum and T. aestivum seeds (IC50 539.9−741.8 μg/mL). Previous investigation reported that the leaves of L. macrophylla contain a potent total phenolic content which might be responsible for exerting phytotoxic effect [34].
Fig. 3.
Growth of (a) Allium cepa (onion) roots and (b) shoots, germination rate of (c) Cicer arietinum (gram) and (d) Triticum aestivum (wheat) in presence and absence of Leea macrophylla extractives after 7 days of treatment.
Table 9.
The concentration of different extractives of Leea macrophylla leaf required for 50 % growth inhibition (IC50) of the test plants.
| Test plants | IC50 values of the extractives (μg/mL) |
||
|---|---|---|---|
| Crude methanol | Petroleum ether | Dia-ion resin | |
| Allium cepa (roots) | 698.6 | 262.4 | 190.8 |
| Allium cepa (shoots) | 401 | 6.1 | 268.8 |
| Cicer arietinum | 539.9 | 700 | 441 |
| Triticum aestivum | 698.8 | 741.8 | 354.6 |
4. Conclusions
In conclusion, this study aimed to isolate and characterize compounds from the leaf extract of L. macrophylla, while evaluating their antibacterial, antidiabetic, cytotoxic, and phytotoxic activities. Although the complexity of the compound spectra made complete characterization challenging, GC-MS analysis successfully identified several compounds across different fractions. The antibacterial tests demonstrated broad-spectrum efficacy, with the LM1 fraction showing the strongest activity against Proteus sp. Additionally, LM1 exhibited significant α-amylase inhibitory activity, highlighting its potential as an antidiabetic agent. The cytotoxicity and phytotoxicity assays further suggested that these extracts could have valuable applications in various fields. Overall, this study highlights the multifaceted properties of L. macrophylla leaf extracts, presenting promising prospects for both healthcare and environmental management.
CRediT authorship contribution statement
Md. Selim Reza: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Md. Badrul Islam: Funding acquisition, Methodology, Project administration, Software, Writing – review & editing, Supervision. Samia Sharmin: Conceptualization, Methodology, Investigation, Writing – review & editing. Farzana Mim: Formal analysis, Data curation, Methodology, Resources, Writing – review & editing. A.B.M. Hamidul Haque: Methodology, Resources, Visualization. Md. Sabir Hossain: Methodology, Resources, Writing – review & editing. Farha Matin Juliana: Methodology, Resources, Validation, Writing – review & editing. Subrata Banik: Methodology, Resources, Visualization. Kazi Rasel Uddin: Methodology, Resources. Md. Mahmudul Hasan: Conceptualization, Methodology. Salina Akter: Methodology, Resources, Validation. Afroza Parvin: Methodology, Resources, Visualization. Md. Omar Ali Mondal: Methodology, Resources, Visualization.
Ethical clearance
Not applicable.
Funding
This research received no external funding.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express their gratitude to Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh and the Ministry of Science and Technology, Bangladesh.
Data availability
Data will be made available on request.
References
- 1.Sarvade D.D. Leea macrophylla Roxb. ex Hornem.: an ethnomedicinal, ethnic food, economical, and pharmacological update. Int. J. Green Pharm. 2019;13 [Google Scholar]
- 2.Singh R.S., Singh A.N. On the identity and economico-medicinal uses of Hastikarnapalasa (Leea macrophylla Roxb, Family Ampelidaceae) as evidence in the ancient (Sanskrit) texts and traditions. Indian J. Hist. Sci. 1981;16:219–222. [PubMed] [Google Scholar]
- 3.Mawa J., Rahman M.A., Hashem M.A., Hosen M.J. Leea macrophylla root extract upregulates the mRNA expression for antioxidative enzymes and repairs the necrosis of pancreatic β-cell and kidney tissues in fructose-fed Type 2 diabetic rats. Biomed. Pharmacother. 2019;110:74–84. doi: 10.1016/j.biopha.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Akhter S., Rahman M.A., Aklima J., Hasan M.R., Hasan Chowdhury J.M. Antioxidative role of Hatikana (Leea macrophylla Roxb.) partially improves the hepatic damage induced by CCl 4 in Wistar Albino rats. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/356729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nizami A.N., Rahman M.A., Ahmed N.U., Islam M.S. Whole Leea macrophylla ethanolic extract normalizes kidney deposits and recovers renal impairments in an ethylene glycol–induced urolithiasis model of rats. Asian Pac. J. Tropical Med. 2012;5:533–538. doi: 10.1016/s1995-7645(12)60094-7. [DOI] [PubMed] [Google Scholar]
- 6.Joshi A., Prasad S.K., Joshi V.K., Hemalatha S. Phytochemical standardization, antioxidant, and antibacterial evaluations of Leea macrophylla: a wild edible plant. J. Food Drug Anal. 2016;24:324–331. doi: 10.1016/j.jfda.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahato D., Sharma H.P. Phytochemical profiling and antioxidant activity of Leea macrophylla Roxb. ex Hornem.-in vitro study. Indian J. Tradit. Knowl. 2019;18:493–499. [Google Scholar]
- 8.Manandhar S., Luitel S., Dahal R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019;2019 doi: 10.1155/2019/1895340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afrisham R., Aberomand M., Ghaffari M.A., Siahpoosh A., Jamalan M. Inhibitory effect of heracleum persicum and ziziphus jujuba on activity of alpha-amylase. J. Bot., Le. 2015;2015 doi: 10.1155/2015/824683. [DOI] [Google Scholar]
- 10.Cangiano T., Dellagreca M., Fiorentino A., Isidori M., Monaco P., Zarrelli A. Effect of ent-labdane diterpenes from Potamogetonaceae on Selenastrum capricornutum and other aquatic organisms. J. Chem. Ecol. 2002;28:1091–1102. doi: 10.1023/a:1016213630957. [DOI] [PubMed] [Google Scholar]
- 11.Dellagreca M., Isidori M., Lavorgna M., Monaco P., Previtera L., Zarrelli A. Bioactivity of phenanthrenes from Juncus acutus on Selenastrum capricornutum. J. Chem. Ecol. 2004;30:867–879. doi: 10.1023/b:joec.0000028437.96654.2c. [DOI] [PubMed] [Google Scholar]
- 12.Araújo A.S.F., Monteiro R.T.R. Plant bioassays to assess toxicity of textile sludge compost. Sci. Agric. 2005;62:286–290. [Google Scholar]
- 13.Islam A., Chowdhury M.M., Molla M.S., Zaman F., Das A.K., Islam M.T., Rouf R. Evaluation of cytotoxic effects of five Bangladeshi medicinal plants. 2021. [DOI]
- 14.Al-Kalaldeh J.Z., Abu-Dahab R., Afifi F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010;30:271–278. doi: 10.1016/j.nutres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Price K.S., Waggy G.T., Conway R.A. Brine shrimp bioassay and seawater BOD of petrochemicals. J. Water Pollut. Control Fed. 1974:63–77. [PubMed] [Google Scholar]
- 16.Sorgeloos P., Remiche-Van Der Wielen C., Persoone G. The use of Artemia nauplii for toxicity tests—a critical analysis. Ecotoxicol. Environ. Saf. 1978;2:249–255. doi: 10.1016/S0147-6513(78)80003-7. [DOI] [PubMed] [Google Scholar]
- 17.Garodia P., Ichikawa H., Malani N., Sethi G., Aggarwal B.B. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J. Soc. Integr. Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 18.Swarnkar S., Katewa S.S. Ethnobotanical observation on tuberous plants from tribal area of Rajasthan (India) Ethnobot. Leafl. 2008;2008:87. [Google Scholar]
- 19.Mahmud Z.A., Bachar S.C., Qais N. Evaluation of anti-nociceptive activity and brine shrimp lethality bioassay of roots of Leea macrophylla Roxb. Int. J. Pharma Sci. Res. 2011;2:3230. doi: 10.13040/IJPSR.0975-8232.2(12).3230-34. [DOI] [Google Scholar]
- 20.Malik M., Upadhyay G. Leea macrophylla: a Review on ethanobotanical uses, phytochemistry and pharmacological action. Pharm. Rev. 2020;14:33. doi: 10.5530/phrev.2020.14.6. [DOI] [Google Scholar]
- 21.Nguyen N.H., Pham Q.T., Luong T.N.H., Le H.K., Vo V.G. Potential antidiabetic activity of extracts and isolated compound from Adenosma bracteosum (Bonati) Biomolecules. 2020;10:201. doi: 10.3390/biom10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannan M., Alam M., Mustari F., Ali R., Haque A., Zaman S., Talukder D. In vitro antioxidant, antimicrobial, insecticidal and cytotoxic activities of the medicinal plants: allamanda cathartica and Mimusops elengi. Eur. J. Med. Plants. 2017;20:1–12. [Google Scholar]
- 23.Ullah A., Hassan S., Khan M.I., Rizwan M., Ullah Z., Shah M. Antioxidant, phytotoxic and cytotoxic activity of methanolic extract of Trigonella foenum-graecum. J. Coast. Life Med. 2016;4:386–389. [Google Scholar]
- 24.Nandiyanto A.B.D., Oktiani R., Ragadhita R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019;4:97–118. doi: 10.17509/ijost.v4i1.15806. [DOI] [Google Scholar]
- 25.Pavia D.L., Lampman G.M., Kriz G.S., Vyvyan J.A. Third. Cengage learning; 2014. Introduction to Spectroscopy. [Google Scholar]
- 26.Bakun P., Czarczynska-Goslinska B., Goslinski T., Lijewski S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021;30:834–846. doi: 10.1007/s00044-021-02701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko G.-A., Cho S.K. Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem. Biol. Interact. 2018;286:132–140. doi: 10.1016/j.cbi.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Elisha I.L., Botha F.S., McGaw L.J., Eloff J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Compl. Alternative Med. 2017;17:1–10. doi: 10.1186/s12906-017-1645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam M.B., Sarkar M.M.H., Shafique M.Z., Jalil M.A., Haque M.Z., Amin R. Phytochemical screening and anti-microbial activity studies on Leea macrophylla seed extracts. J. Sci. Res. 2013;5:399–405. doi: 10.3329/jsr.v5i2.13213. [DOI] [Google Scholar]
- 30.Akbar M., Ali U., Khalil T., Iqbal M.S., Amin A., Naeem R., Nazir A., Waqas H.M., Aslam Z., Jafri F.I. Cornus macrophylla, the antibacterial activity of organic leaf extracts and the characterization of the more lipophilic components by GC/MS. Molecules. 2020;25:2395. doi: 10.3390/molecules25102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangeetha R., Vedasree N. In vitro α-amylase inhibitory activity of the leaves of Thespesia populnea. Int. Sch. Res. Notices. 2012;2012 doi: 10.5402/2012/515634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman M.A., Chowdhury J.M.K.H., Aklima J., Azadi M.A. Leea macrophylla Roxb. leaf extract potentially helps normalize islet of β‐cells damaged in STZ‐induced albino rats. Food Sci. Nutr. 2018;6:943–952. doi: 10.1002/fsn3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Islam M.T., Uddin M.A. Phytochemicals are the promising tools towards drug-resistant cancers. Bol. Inf. GEUM. 2017;8:26. [Google Scholar]
- 34.Ahmed F., Akter D., Muhit M.A., Raihan S.Z., Faroque A.B.M. DPPH free-radical scavenging and cytotoxic activities of Leea macrophylla, Bangladesh med. Res. Counc. Bull. 2018;44:77–81. doi: 10.3329/bmrcb.v44i2.38690. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.