Abstract
An increasing number of studies have shown that lead is an important cardiovascular risk factor, but the impact of cardiovascular related gene polymorphisms on lead induced cardiovascular diseases is still unclear. To assess the interaction of lead exposure and related key cardiovascular regulating gene polymorphisms on blood pressure traits, three single-nucleotide polymorphisms including NOTCH1 rs3124591, Cerebral cavernous malformations 3 (CCM3) rs3804610 and Vascular endothelial growth factor receptor type 2 (VEGFR2) rs2305948 were selected and genotyped using improved multiplex ligase detection reaction method in 568 lead exposure workers in South China. General characteristics, blood lead and biochemical parameters including glucose, lipid profile and creatinine were also collected according to standard protocols. Regression analysis was used to evaluate the association of blood pressure with lead exposure, polymorphisms and their interaction. This study displayed that CCM3 rs3804610 had a positive interaction with lead and VEGFR2 rs2305948 had a negative interaction with lead. Specifcally, compared with the wild-type population, the blood lead of the genotype population carrying the risk allele increased by 1 µg/dL, systolic blood pressure increased by 0.53 mmHg (p < 0.01) and diastolic blood pressure increased by 0.34 mmHg (p < 0.05) for CCM3 rs3804610, and systolic blood pressure decreased by 0.28 mmHg (p < 0.05) and diastolic blood pressure decreased by 0.22 mmHg (p < 0.05) for VEGFR2 rs2305948. Thus our findings showed that the interaction between CCM3 rs3804610 and VEGFR2 rs2305948 and lead exposure were associated with blood pressure and may provide guidance for future research on hypertension prevention and personalized clinical treatment in lead exposed populations.
Keywords: Lead exposure, blood pressure , Cardiovascular regulating gene , Single-nucleotide polymorphism , Gene-environment interaction
Subject terms: Epidemiology, Cardiovascular diseases, Genetic markers
Introduction
Lead is a common toxic metal element that is widely present in our living or working environment. Due to its widespread application, lead pollution has become a global problem1. In China, occupational exposures in industries that still use lead, such as mining, smelting, and recycling, pose a significant risk to workers.The hazard of lead to the human body is multifaceted, among which cardiovascular diseases (CVDs) are particularly prominent2. A systematic review and meta-analysis of 37 epidemiological studies, including prospective cohorts, case-control, and nested case-control studies, found a positive correlation between environmental lead exposure and CVD risk3. A study on the health impact model of lead on cardiovascular diseases in the United States reported that approximately 16–46% of the decline in CVD-related mortality from 1999 to 2014 could be attributed to the reduction in blood lead levels4.
Hypertension plays an important role in CVDs. Long term high blood pressure can lead to changes in the structure and function of the heart and blood vessels, thereby increasing the risk of CVDs5. In recent years, the relationship between lead and hypertension has garnered significant attention. However, epidemiological studies investigating the associations between lead exposure and hypertension among occupationally exposed workers are relatively limited. In a study involving 395 molybdenum miners and iron and steel foundry workers, Lu Shi et al. observed that increased prevalence ratios for hypertension among the quartile of urinary concentrations of lead were positive6. Lamas et al. showed that systolic blood pressure (SBP) and diastolic blood pressure (DBP) increased in line with a 1 µg/dL increase in blood lead level (BLL), and a BLL ≥ 6.87 µg/dL was associated with hypertension in lead-exposed workers in the Republic of Korea7. In a repeated-measure study, Han et al. found that the average annual increase of SBP or DBP showed an upward trend in different Pb dose groups among lead-exposed workers in China8. Therefore, further studies are necessary to gain a deeper understanding of this relationship in lead-exposed workers.
Whole genome association studies (GWAS) have made significant progress in the genetic basis of complex diseases such as hypertension, but this approach also has limitations9. An important point is that the design of GWAS usually does not directly consider the impact of environmental factors10. Therefore, although GWAS may reveal some gene regions associated with disease risk, it may overlook genes that are sensitive to environmental factors such as lead exposure. A recent study confirmed the importance of the aminolevulinate dehydratase (ALAD) gene in lead kinetics even at low exposure levels11. Nunes et al. observed that polymorphisms related to DNA repair pathways may modulate lead-induced DNA damage12. Besides, the association between lead exposure and lipid profile, and associated polymorphisms were reported13. Although the above studies may have indirect implications for cardiovascular research, including hypertension, the direct association between key cardiovascular genes and lead exposure is still limited.
Notch homolog 1 (NOTCH1), cerebral cavernous malformations 3 (CCM3) and vascular endothelial growth factor receptor type 2 (VEGFR2) genes are key cardiovascular genes, playing important roles in the normal development and function of the cardiovascular system14–16. The abnormal expression or dysfunction of them may lead to the occurrence and development of cardiovascular and cerebrovascular diseases17–19. On the other hand, we and other researchers found that CCM3 and VEGF signaling played a crucial role in lead induced vascular toxicity, especially in angiogenesis, which is observed in many diseases, such as atherosclerosis, hypertension20–22. Notch signalingis regarded as a master regulator of angiogenesis and vascular remodeling. The Notch1 signaling acts to suppress the expression of VEGFR2 in adjacent endothelial cells, resulting in a situation where a single cell exhibits elevated levels of VEGFR2 compared to its neighboring cells, thereby making it highly sensitive to VEGF23. However, there is currently a lack of in-depth understanding of the role of NOTCH1 in lead-induced cardiovascular toxic effects.
In a previous study, we assessed CCM3 genetic polymorphismin arsenic exposed workers and observed that interactions between rs3804610 and arsenic exposure boosted the hazard of increased systolic pressure24. Mohamed et al. performed a systematic mutation-analysis based on DNA-sequencing of all coding exons and adjacent splice consensus sequences of NOTCH1 gene, they demonstrated that Notch1 rs3124591 play a role in bicuspid aortic valve25. Several studies explored the potential relationships between polymorphisms in VEGFR2 and coronary heart disease or major adverse cardiac events, consistent results were found for rs2305948 polymorphism26,27. However, investigations on whether these genetic polymorphisms affect the association between lead exposure and hypertension remains unexplored. In this study, We investigated the genotype distribution characteristics of NOTCH1, CCM3 and VEGFR2 polymorphisms ( rs3124591, rs3804610, rs2305948) in lead exposed populations and evaluated the impact of lead exposure and polymorphisms interaction on blood pressure. The findings may provide possible personalized treatments guideline for hypertension in lead-exposed populations.
Results
Basic characteristics of the population
Among a total of 568 workers, the blood lead level ranged from 10.37 µg/L to 864.07 µg/L, with an average of 72.89 µg/L (SD = 88.48). There were 475 male workers (83.63%). Current smokers accounted for 37.15%. Drinkers accounted for 21.48%. According to China’s body mass index (BMI) standard definition, over one-fifth of people were overweight (BMI above 24). Table 1 shows that the age, BMI, total cholesterol (TC), triglyceride (TG), lowdensity lipoprotein cholesterol (LDL-c), fasting blood glucose (FBG), and BLL of the high lead group were significantly higher than those of the low lead group. In addition, the high lead group had more smokers and drinkers. In terms of blood pressure, the high lead group showed a significant increase in SBP and DBP.
Table 1.
General characteristics of study participants (N = 568).
| N (%), Mean ± SD | P-value | |||
|---|---|---|---|---|
| Overall | Low lead exposure | High lead exposure | ||
| Age | 31.88 ± 10.55 | 25.32 ± 5.606 | 38.41 ± 10.264 | <0.001 |
| Sex (male) | 475(83.63) | 217(76.40) | 258(90.85) | <0.001 |
| Years of work | 9.12 ± 10.91 | 2.37 ± 3.687 | 15.92 ± 11.538 | <0.001 |
| Smoking (Yes) | 211(37.15) | 69(24.30) | 142(50.00) | <0.001 |
| Drinking (Yes) | 116(21.48) | 42(14.79) | 74(26.06) | 0.001 |
| BMI(kg/m2) | 22.06 ± 3.78 | 20.80 ± 2.98 | 23.30 ± 4.07 | <0.001 |
| BMI ≥ 24 | 135(23.77) | 37(13.03) | 98(34.50) | <0.001 |
| TC(mmol/L) | 4.85 ± 1.14 | 4.62 ± 1.08 | 5.09 ± 1.15 | <0.001 |
| TG(mmol/L) | 1.26 ± 1.06 | 1.00 ± 0.70 | 1.53 ± 1.28 | <0.001 |
| LDL-c(mmol/L) | 2.31 ± 1.09 | 1.88 ± 0.82 | 2.73 ± 1.15 | <0.001 |
| HDL-c(mmol/L) | 1.53 ± 1.01 | 1.53 ± 0.27 | 1.53 ± 1.24 | 0.997 |
| SCr(µmol/L) | 79.84 ± 54.27 | 77.41 ± 21.93 | 82.28 ± 73.61 | 0.311 |
| FBG(mmol/L) | 4.07 ± 1.34 | 3.93 ± 1.11 | 4.21 ± 1.52 | 0.017 |
| SBP(mmHg) | 122 ± 14 | 120 ± 12 | 123 ± 15 | 0.005 |
| DBP(mmHg) | 78 ± 10 | 77 ± 9 | 80 ± 10 | <0.001 |
| BLL(µg/L) | 72.89 ± 88.48 | 25.58 ± 5.80 | 120.21 ± 105.63 | <0.001 |
BMI, body mass index ; TC, total cholesterol; TG, triglyceride;LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol; SCr, serum creatinine; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; BLL, blood lead level. P values were calculated by independent t test for continuous variables and chi-square test for categorical variables.
Distribution characteristics of polymorphic genotypes in different lead exposed workers
Polymorphism analysis showed that the NOTCH1 rs3124591 locus did not conform to the Hardy-Weinberg equilibrium (HWE) (p < 0.05) in the overall population, but conforms to the HWE (p > 0.05) in the low lead exposure population. The CCM3 rs3804610 locus and VEGFR2 rs2305948 locus in the total population conformed to HWE (p > 0.05). There was no statistically significant difference in the distribution of genotypes between low lead exposure and high lead exposure workers (Table 2).
Table 2.
The distribution characteristics of related gene polymorphic genotypes in different lead exposed participants.
| Gene polymorphism | N (%) | P-value | ||
|---|---|---|---|---|
| Overall | Low lead exposure | High lead exposure | ||
| NOTCH1 rs3124591 | 567 | 284 | 283 | 0.320 |
| TT | 524(92.41) | 260(91.55) | 264(93.29) | |
| CT | 39(6.88) | 23(8.10) | 16(5.65) | |
| CC | 4(0.71) | 1(0.35) | 3(1.06) | |
| CCM3 rs3804610 | 567 | 284 | 283 | 0.932 |
| TT | 440(77.60) | 220(77.46) | 220(77.74) | |
| CT | 120(21.16) | 60(21.13) | 60(21.20) | |
| CC | 7(1.23) | 4(1.41) | 3(1.06) | |
| VEGFR2 rs2305948 | 566 | 284 | 282 | 0.274 |
| CC | 385(68.02) | 200(70.42) | 185(65.60) | |
| CT | 164(28.98) | 74(26.06) | 90(31.91) | |
| TT | 17(3.00) | 10(3.52) | 7(2.48) | |
| HWE test P-value | ||||
| NOTCH1 rs3124591 | 0.006 | 0.818 | 0.001 | |
| CCM3 rs3804610 | 0.934 | 0.999 | 0.887 | |
| VEGFR2 rs2305948 | 0.996 | 0.634 | 0.591 | |
The difference of basic characteristics among workers with different polymorphic genotypes
Table 3 shows the basic characteristics of the population among different gene single nucleotide polymorphisms (SNPs) and genotypes. The genotype of NOTCH1 rs3124591 carrying the risk allele had a lower proportion in males compared to the wild type, with higher BMI, TC, and blood lead, but the difference was not statistically significant. The genotype of CCM3 rs3804610 carrying risk alleles had a lower proportion of males and a higher proportion of smokers compared to the wild type, and the difference was statistically significant. Compared with the wild-type, the genotype carrying the risk allele VEGFR2 rs2305948 had a higher proportion of drinkers and lower fasting blood sugar, and the difference was statistically significant; TC and blood lead level were higher, but the difference was not statistically significant.
Table 3.
Descriptive characteristics among the different types of NOTCH1 rs3124591, CCM3 rs3804610 and VEGFR2 rs2305948 SNPs.
| Variables | NOTCH1 | CCM3 | VEGFR2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD or n(%) | TT | CT/CC | P-value | TT | CT/CC | P-value | CC | CT/TT | P-value |
| Age | 31.89 ± 10.54 | 31.49 ± 10.66 | 0.813 | 31.91 ± 10.61 | 31.65 ± 10.37 | 0.805 | 31.45 ± 10.55 | 32.66 ± 10.50 | 0.204 |
| Sex (male) | 443(84.54) | 31(72.09) | 0.051 | 378(85.91) | 96(75.59) | 0.007 | 319(82.86) | 154(85.08) | 0.545 |
| Years of service | 9.19 ± 10.93 | 7.91 ± 10.55 | 0.461 | 9.23 ± 10.91 | 8.61 ± 10.89 | 0.569 | 8.80 ± 10.88 | 9.62 ± 10.90 | 0.403 |
| Smoking (Non) | 316(61.48) | 30(69.77) | 0.328 | 279(64.43) | 67(54.03) | 0.036 | 233(61.48) | 113(63.84) | 0.639 |
| Drinking (Non) | 402(79.13) | 32(76.19) | 0.694 | 337(79.11) | 97(78.23) | 0.900 | 306(82.04) | 127(72.16) | 0.010 |
| BMI(kg/m2) | 21.98 ± 3.58 | 23.05 ± 5.65 | 0.074 | 22.11 ± 3.9 | 21.91 ± 3.35 | 0.610 | 22.22 ± 4.01 | 21.73 ± 3.23 | 0.159 |
| TC(mmol/L) | 4.84 ± 1.14 | 5.05 ± 1.17 | 0.263 | 4.84 ± 1.14 | 4.89 ± 1.15 | 0.723 | 4.80 ± 1.12 | 4.98 ± 1.18 | 0.085 |
| TG(mmol/L) | 1.25 ± 1.07 | 1.37 ± 0.98 | 0.505 | 1.28 ± 1.15 | 1.19 ± 0.67 | 0.398 | 1.26 ± 1.02 | 1.26 ± 1.16 | 0.994 |
| LDL-c(mmol/L) | 2.30 ± 1.10 | 2.41 ± 0.92 | 0.548 | 2.28 ± 1.10 | 2.40 ± 1.05 | 0.328 | 2.26 ± 1.09 | 2.41 ± 1.07 | 0.124 |
| HDL-c(mmol/L) | 1.53 ± 1.05 | 1.45 ± 0.50 | 0.709 | 1.54 ± 1.14 | 1.48 ± 0.29 | 0.663 | 1.55 ± 1.22 | 1.48 ± 0.37 | 0.558 |
| SCr(µmol/L) | 80.27 ± 55.92 | 74.42 ± 24.95 | 0.533 | 80.31 ± 60.23 | 78.28 ± 24.10 | 0.725 | 81.61 ± 64.57 | 76.18 ± 19.27 | 0.293 |
| FBG(mmol/L) | 4.07 ± 1.33 | 4.02 ± 1.51 | 0.824 | 4.03 ± 1.28 | 4.21 ± 1.54 | 0.182 | 4.16 ± 1.39 | 3.88 ± 1.22 | 0.026 |
| SBP(mmHg) | 122 ± 14 | 122 ± 11 | 0.994 | 121 ± 13 | 121 ± 15 | 0.714 | 122 ± 13 | 122 ± 14 | 0.864 |
| DBP(mmHg) | 78 ± 10 | 78 ± 8 | 0.797 | 78 ± 9 | 78 ± 11 | 0.537 | 78 ± 10 | 78 ± 10 | 0.617 |
| BLL(µg/L) | 71.64 ± 85.12 | 87.05 ± 123.26 | 0.273 | 75.2 ± 93.00 | 64.54 ± 70.62 | 0.232 | 68.46 ± 80 | 81.73 ± 104.19 | 0.096 |
The impact of the interaction between polymorphic loci of related genes and lead exposure on blood pressure
We next explored the interaction between blood lead and different SNPs.results of multiple linear regression, using SBP and DBP as dependent variables, adjusted for age, sex, BMI, smoking, alcohol consumption, TC, TG, LDL-c and FBG are shown in Table 4. The results showed that when SBP or DBP were used as the dependent variable, there were significant interactions between blood lead and CCM3 rs3804610 and VEGFR2 rs2305948 in Model 2 and Model 3, respectively (P < 0.05). In addition, the individual effect of blood lead on SBP in Model 3 was statistically significant (P < 0.01).
Table 4.
Multiple linear regression coefficients of SBP and DBP in different interaction models.
| SBP | DBP | |||||
|---|---|---|---|---|---|---|
| Models | β | SE | P-value | β | SE | P-value |
| model1 | ||||||
| BLL | 0.015 | 0.008 | 0.064 | 0.003 | 0.006 | 0.586 |
| NOTCH1 | 0.604 | 2.837 | 0.832 | 2.090 | 1.982 | 0.292 |
| NOTCH1×BLL | -0.007 | 0.021 | 0.729 | -0.018 | 0.015 | 0.215 |
| model2 | ||||||
| BLL | 0.008 | 0.008 | 0.299 | -0.003 | 0.006 | 0.600 |
| CCM3 | -2.690 | 1.918 | 0.161 | -2.164 | 1.344 | 0.108 |
| CCM3×BLL | 0.053 | 0.020 | 0.008 | 0.034 | 0.014 | 0.014 |
| model3 | ||||||
| BLL | 0.028 | 0.010 | 0.006 | 0.011 | 0.007 | 0.107 |
| VEGFR2 | 1.410 | 1.640 | 0.390 | 0.974 | 1.148 | 0.396 |
| VEGFR2×BLL | -0.028 | 0.014 | 0.038 | -0.022 | 0.010 | 0.025 |
β, regression coefficient; SE, standard error. All models were adjusted for age, years of work, sex, BMI, smoking, drinking, TC, TG, LDL-c and FBG. BLL: blood lead level.
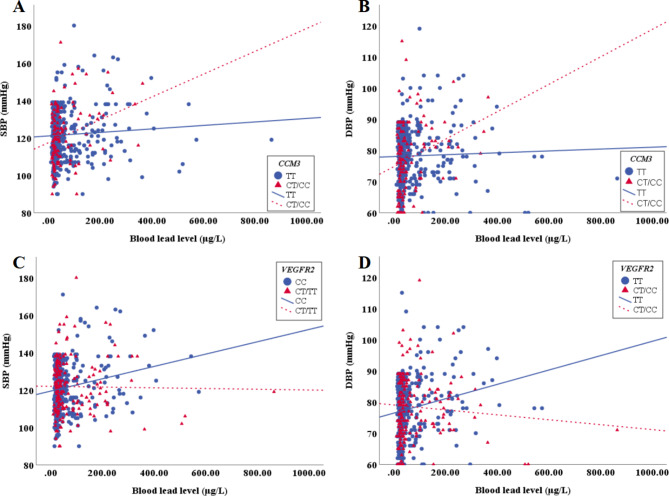
In Fig. 1A, compared with the wild-type population of CCM3 rs3804610, the blood lead of the genotype population carrying the risk allele increased by 1 µg/dL, SBP increased by 0.53 mmHg. In Fig. 1B, compared with the wild-type population of CCM3 rs3804610, the blood lead of the genotype population carrying the risk allele increased by 1 µg/dL, DBP increased by 0.34 mmHg. In Fig. 1C, compared with the wild-type population of VEGFR2 rs2305948, the blood lead of the genotype population carrying the risk allele increased by 1 µg/dL, SBP decreased by 0.28 mmHg. In Fig. 1D, compared with the wild-type population of VEGFR2 rs2305948, the blood lead of the genotype population carrying the risk allele increased by 1 µg/dL, DBP decreased by 0.22 mmHg.
Fig. 1.
The interaction effect of CCM3 rs3804610 and VEGFR2 rs2305948 on the levels of SBP and DBP in lead exposed workers. (A) CCM3 rs3804610 and SBP; (B) CCM3 rs3804610 and DBP; (C) VEGFR2 rs2305948 and SBP; (D) VEGFR2 rs2305948 and DBP.
Discussion
Industrial production is an important source of lead exposure, especially in industries such as mining, smelting, and paint manufacturing, causing serious impacts on the environment and worker health. The present study mainly found that the interaction between lead and CCM3 rs3804610 and VEGFR2 rs2305948 affected both SBP and DBP. Specifcally, CCM3 rs3804610 had a positive interaction with lead and VEGFR2 rs2305948 had a negative interaction with lead. Besides, this study also found that there were differences in basic characteristics such as sex, smoking, alcohol consumption, and FBG levels between population carrying mutated alleles and the wild-type population.
The blood lead level in the low lead group is close to that of the general population in China in this study, while the high lead group significantly increases, but rarely exceeds 400 µg/L, which is the highest BLL acceptable for leadexposed workers in national and international criteria28,29. Thus, with the improvement of production technology and the emphasis on occupational health protection, the incidence of lead poisoning in the occupational population has significantly decreased. But this does not mean that there are no health risks associated with low-level lead exposure. When comparing blood pressure, it was observed that there was a significant increase in the high lead group. Other epidemiological studies have also shown an association between lead exposure and hypertension and the evidence is sufficient to infer the causal relationship8,30–32. Some studies using chronic exposure animal models have also shown that lead exposure is a risk factor for the development of hypertension33–35. These findings suggests that for lead-exposed workers, more frequent blood pressure monitoring and/or more aggressive antihypertensive treatment strategies may be needed to prevent the occurrence and development of CVDs. Future research should explore the potential impact of this discovery on clinical practice, including the development of more precise personalized treatment plans.
Many studies have confirmed that CVDs are complex and caused by both environmental and genetic factors. NOTCH1, CCM3 and VEGFR2 are key environmental response cardiovascular genes21,28,36. Detecting their genetic polymorphism may identify subsets of individuals who are more susceptible to the risk of exposure to harmful factors. Currently, there are few studies on the polymorphism of NOTCH1 rs3124591 and CCM3 rs3804610. Abramenko et al.37 reported NOTCH1 rs3124591 did not affect the risk of chronic lymphocytic leukemia and survival parameters of the patients. Another study on ductal breast carcinoma of the Han population of China revealed that the frequency of rs3124591 CT genotype was significantly higher in invasive ductal carcinoma and ductal carcinoma in situ patients than in usual ductal hyperplasia controls38. Our previous study suggest that arsenic exposure of population can assist CCM3 polymorphism including rs3804610 in elevating SBP24. Several studies have found that VEGFR2 polymorphism rs2305948 was associated with coronary heart disease and glioma risk26,39,40. In the present study, we firstly studied the frequency of these SNP genotypes in lead exposed populations. There was no statistically significant difference in the distribution of genotypes between low exposure and high exposure populations. When we observed the allele frequencies of these SNPs, we identified NOTCH1 rs3124591 was not consistent with Hardy-Weinberg equilibrium in high exposure populations, suggesting that the distribution of allele frequencies may be related with high lead exposure and requires validation from a larger sample size population.
In addition to studying the distribution differences of genetic polymorphisms between the low lead and high lead exposure populations, we also analyzed the impact of carrying risk alleles on the basic characteristics and health indicators in overall population. Mani et al.41 reported that genes associated with lead metabolism ALAD polymorphism might contribute to increased susceptibility to high blood lead retention, and genotyping of ALAD in lead exposed subjects might be used as a prediction marker to impede tissue/organ damage due to lead toxicity. They recently found hemochromatosis(HFE) polymorphism might have a role in increasing the concentration of lead42. We found that workers carrying risk alleles had higher blood lead levels in NOTCH1 rs3124591 and VEGFR2 rs2305948 and had lower blood lead levels in CCM3 rs3804610, suggesting a role in predisposition to lead and its associated effects. Further validation is needed due to the relatively small proportion of workers carrying risk alleles. When comparing blood pressure of different genotypes, no upward or downward trend was observed. This further indicates that these polymorphisms have little effect on blood pressure alone, and exploring their interaction with environmental factors can provide a deeper understanding of their role in CVDs.
Lim et al.43 studied the impact of interaction between 42 GWAS SNPs and BMI, waist circumference, and drinking status on blood pressure in the Korean population with no interactions were found. Another study from Hollister et al. suggest a gene-environment interaction for blood pressure in African Americans44. Regression analysis of the present study observed that the effect of polymorphism or lead alone on blood pressure was not significant, but interestingly we identified CCM3 rs3804610, VEGFR2 rs2305948 interacted with lead to affect blood pressure, respectively. This may mean that they are interrelated in some way and have a common impact on blood pressure. Pathophysiological studies have extensively investigated the structural factor in hypertension, including large and small artery remodeling and functional changes45. Besides, our previous study found that CCM3 may regulate VEGFR2 receptor stability and association with apoptosis and endothelial cell growth46. DLL4-NOTCH and VEGF signaling are well-defined pathways for the regulation of vessel maturation and vessel permeability47,48. Lu et al.49 found the inactivation of VEGFR2 signaling by Notch1 contributes to the delayed angiogenesis phenotype. Moreover, the loss of CCM3 impaired DLL4-Notch signaling disrupted the homeostasis of VEGF/VEGFR-2 pathway and activated Erk1/2, which was critical to the regulation of endothelial proliferation, migration, and sprouting50. Thus, further research is needed to elucidate whether the interaction between lead exposure and polymorphism on blood pressure is related to angiogenesis from the perspective of endothelial dysfunction. Additionally, exploring other genetic polymorphisms or evaluating the performance of longitudinal studies evaluating the evolution of hypertension is also beneficial in lead-exposed population.
This study provides evidence of the impact of key cardiovascular gene polymorphisms and lead interaction on blood pressure. However, some limitations of this study should be noted. First, participants were recruited in occupational health examinations, and the low lead group was also a group exposed to lead, not a strict control group. There were significant differences in some basic characteristics compared to the high lead group, which may lead to selection bias. Secondly, considering variables not included in our study, such as diet or physical activity, the consequences of these considerations on estimates of the interaction between lead exposure and polymorphism on blood pressure may be both over- and underestimations. Thirdly, due to the relatively small sample size, we did not analyze the interaction between lead and other genetic models, including co-dominant and recessive models.
In brief, we found that CCM3 rs3804610 and VEGFR2 rs2305948 might interact with lead to affect blood pressure, suggesting that rs3804610 and rs2305948 may serve as new targets to uncover possible personalized treatments guideline for hypertension in lead-exposed populations.
Materials and methods
Study population
This study is based on cross-sectional design, and the study population was sourced from the annual health examinations of occupational populations from 2013 to 2021 in the Pearl River Delta region of South China. After signing the consent forms, participants were asked a variety of sociodemographic questions regarding occupational health, such as age, race, sex, years of work, history of smoking, and alcohol intake. This also included a family history of CVDs and a history of taking antihypertensive drugs. Each subject was examined electrocardiogram and blood pressure by professional medical staff. Blood pressure was calculated as the average of all available systolic and diastolic readings, which were measured 3 times by a standard sphygmomanometer. Subjects having histories of cardiovascular, liver, lung, kidney or other organ diseases, psychiatric disorders, or other serious health problems and taking antihypertensive drugs were excluded. A total of 568 workers was enrolled. This study was approved by the Medical Ethics Committee of the School of Public Health at Sun Yat-sen University.
According to the current “Occupational Exposure Limits for Hazardous Factors in the Workplace” standards in China29, the concentration of the lead smoke in most workshops did not exceed the permissible concentration-time weighted average (PC-TWA). Only one workshop(including 23 workers) showed that the highest lead smoke concentration of the short-time and long-term sampling was high than the permissible concentration. Thus, population grouping is mainly based on blood lead levels.
Collection and measurement of biological samples
Venous fasting blood (5mL EDTA anticoagulation, 5mL heparin sodium anticoagulation, 5mL non-anticoagulation) was taken from the workers in the morning for biochemical parameters analysis. We assessed the concentration of lead in whole blood in relation to participants’ recent exposure to lead by using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Agilent 7700X series, America). Blood samples were digested via microwave-induced digestion with nitric acid. A standard solution of lead was prepared by diluting certified standard solutions (High Purity Standards, Charleston, SC, USA). Quality control materials were also used (Seronorm Trace Elements Whole Blood L-1, Trace Elements Whole Blood L-2, Norway). The lead calibration curve ranged from 0 to 1000 µg/L. Blood biochemical parameters including glucose, lipid profile and creatinine were detected by Trilogy automatic biochemical analyzer.
SNP selection and genotyping
We selected three tag SNPs: Notch1 rs3124591, CCM3 rs3804610 and VEGFR2 rs2305948. Notch1 rs3124591 is located in the 3’-UTR of chromosome 9; CCM3 rs3804610 is located in the intron 6 of chromosome 6; VEGFR2 rs2305948 lies within the exon region of chromosome 4. All of them are meet our inclusion criteria: (1) Minor allele frequency (MAF) > 0.1; (2) The linkage disequilibrium value of r2 > 0.8; (3) Genetic balance tests (HWE) P value > 0.0001. The genotyping was done by the improved multiplex ligase detection reaction (iMLDR) method (Genesky Biotech, Shanghai) as previously described51.
Statistical analysis
In this study, all subjects were divided into two equal groups based on the 50th percentile of blood lead levels. Categorical variables are expressed in frequency (%) and analyzed using Pearson chi-square independent test. Quantitative data obey normal distributionis expressed as the mean ± SD, and analyzed by Student’s t test. The HWE tests of genotype frequencies was estimated with chi-square goodness-of-fit tests. We also conducted two independent analyses based on the presence or absence of mutated alleles to compare the difference of general characteristics. Multiple linear regression analysis was used to evaluate the association of blood pressure with lead exposure and polymorphisms. We also analyzed the interaction terms between lead and different polymorphisms on blood pressure. To address potential confounding factors that might have influenced the observed effects, we thoroughly considered several key variables in our analysis. Specifically, we identified age, years of work, sex, BMI, smoking, drinking, TC, TG, LDL-c and FBG as potential confounders based on previous research and the theoretical framework of our study, and handled by including these variables as covariates in our multiple regression models. A two-tailed p-value < 0.05 was considered significant. All statistical analysis and graphical construction were performed by Statistical Package for Social Sciences (SPSS) version 25.0 for Windows.
Acknowledgements
We are grateful to everyone who took part in the investigation.
Author contributions
Conceptualization: ZZ, YH; Methodology: XO, XL, LL; Formal analysis and investigation: XO, CX, JJ, YL, WZ; Writing—original draft preparation: XO, CX, JJ; Writing—review and editing: ZZ, YH; Supervision: ZZ, YH.
Funding
This work was supported by the Natural Science Foundation of China (No.81273097, 81472998); Guangdong Provincial Medical Research Fund Project (A2021421, A2022276); Anhui Provincial Department of Education Natural Science Research Key Project (KJ2018A0805).
Data availability
Correspondence and requests for research data should be addressed to Z.Z. or Y.H.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was acquired through all subjects included in this investigation.
Ethical approval
This study was approved by the Medical Ethics Committee of the School of Public Health at Sun Yat-sen University and this study was conducted in accordance with the guidelines of Medical Ethics Committees.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoyan Ou, Chen Xiao and Jun Jiang have contributed equally to this study.
Contributor Information
Yun He, Email: heyun7@mail.sysu.edu.cn.
Zhiqiang Zhao, Email: zhaozq68@163.com.
References
- 1.Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health. 34, 25–34. 10.1515/reveh-2018-0037 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Larsen, B. & Sanchez-Triana, E. Global health burden and cost of lead exposure in children and adults: a health impact and economic modelling analysis. Lancet Planet. Health. 7, e831–e840. 10.1016/s2542-5196(23)00166-3 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury, R. et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 362, k3310. 10.1136/bmj.k3310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, L., Lynch, M., Belova, A., Klein, R. & Chiger, A. Developing a health impact model for adult lead exposure and Cardiovascular Disease Mortality. Environ. Health Perspect. 128, 97005. 10.1289/ehp6552 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs, F. D. & Whelton, P. K. High blood pressure and Cardiovascular Disease. Hypertension. 75, 285–292. 10.1161/hypertensionaha.119.14240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi, P., Jing, H. & Xi, S. Urinary metal/metalloid levels in relation to hypertension among occupationally exposed workers. Chemosphere. 234, 640–647. 10.1016/j.chemosphere.2019.06.099 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kim, M. G., Kim, Y. W. & Ahn, Y. S. Does low lead exposure affect blood pressure and hypertension? J. Occup. Health. 62, e12107. 10.1002/1348-9585.12107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han, Z. et al. The relationships between blood pb levels and blood pressure among lead-exposed workers in China: a repeated-measure study. J. Occup. Environ. Med. 65, e759–e763. 10.1097/jom.0000000000002974 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Tam, V. et al. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 20, 467–484. 10.1038/s41576-019-0127-1 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Aschard, H. et al. Inclusion of gene-gene and gene-environment interactions unlikely to dramatically improve risk prediction for complex diseases. Am. J. Hum. Genet. 90, 962–972. 10.1016/j.ajhg.2012.04.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stajnko, A. et al. Genetic susceptibility to low-level lead exposure in men: insights from ALAD polymorphisms. Int. J. Hyg. Environ. Health. 256, 114315. 10.1016/j.ijheh.2023.114315 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Nunes, E. A. et al. Impact of DNA repair polymorphisms on DNA instability biomarkers induced by lead (pb) in workers exposed to the metal. Chemosphere. 334, 138897. 10.1016/j.chemosphere.2023.138897 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Yang, C. C. et al. Single nucleotide polymorphism of TWIST2 may be a modifier for the association between High-Density Lipoprotein Cholesterol and blood lead (pb) level. Int. J. Environ. Res. Public. Health. 1910.3390/ijerph19031352 (2022). [DOI] [PMC free article] [PubMed]
- 14.Nemir, M. & Pedrazzini, T. Functional role of notch signaling in the developing and postnatal heart. J. Mol. Cell. Cardiol. 45, 495–504. 10.1016/j.yjmcc.2008.02.273 (2008). [DOI] [PubMed] [Google Scholar]
- 15.van den Akker, N. M., Caolo, V. & Molin, D. G. Cellular decisions in cardiac outflow tract and coronary development: an act by VEGF and NOTCH. Differentiation. 84, 62–78. 10.1016/j.diff.2012.04.002 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Valentino, M., Dejana, E. & Malinverno, M. The multifaceted PDCD10/CCM3 gene. Genes Dis.8, 798–813. 10.1016/j.gendis.2020.12.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, K., Zhou, H. J. & Wang, M. CCM3 and cerebral cavernous malformation disease. Stroke Vasc Neurol.4, 67–70. 10.1136/svn-2018-000195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng, X., Wang, S., Chen, H. & Chen, M. Role of the Notch1 signaling pathway in ischemic heart disease (review). Int. J. Mol. Med. 5110.3892/ijmm.2023.5230 (2023). [DOI] [PubMed]
- 19.Miller, B. & Sewell-Loftin, M. K. Mechanoregulation of vascular endothelial growth factor receptor 2 in Angiogenesis. Front. Cardiovasc. Med. 8, 804934. 10.3389/fcvm.2021.804934 (2021). [DOI] [PMC free article] [PubMed]
- 20.Sun, Y. et al. The interaction of lead exposure and CCM3 defect plays an important role in regulating angiogenesis through eNOS/NO pathway. Environ. Toxicol. Pharmacol. 79, 103407. 10.1016/j.etap.2020.103407 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Sun, Y. et al. Lead promotes abnormal angiogenesis induced by CCM3 gene defects via mitochondrial pathway. J. Dev. Orig Health Dis. 9, 182–190. 10.1017/s2040174417000782 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Machoń-Grecka, A., Dobrakowski, M., Kasperczyk, A., Birkner, E. & Kasperczyk, S. Angiogenesis and lead (pb): is there a connection? Drug Chem. Toxicol. 45, 589–593. 10.1080/01480545.2020.1734607 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Hasan, S. S. & Fischer, A. Notch Signaling in the vasculature: angiogenesis and angiocrine functions. Cold Spring Harb Perspect. Med. 1310.1101/cshperspect.a041166 (2023). [DOI] [PMC free article] [PubMed]
- 24.Gao, Y. et al. Arsenic exposure assists ccm3 genetic polymorphism in elevating blood pressure. Oncotarget. 9, 4915–4923. 10.18632/oncotarget.23518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed, S. A. et al. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem. Biophys. Res. Commun. 345, 1460–1465. 10.1016/j.bbrc.2006.05.046 (2006). [DOI] [PubMed]
- 26.Wang, L., Ge, H., Peng, L. & Wang, B. A meta-analysis of the relationship between VEGFR2 polymorphisms and atherosclerotic cardiovascular diseases. Clin. Cardiol. 42, 860–865. 10.1002/clc.23233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirdeev, A. et al. Machine learning models for predicting risks of MACEs for myocardial infarction patients with different VEGFR2 genotypes. Front. Med. (Lausanne). 11, 1452239. 10.3389/fmed.2024.1452239 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, Y. et al. Cr(VI) promotes tight joint and oxidative damage by activating the Nrf2/ROS/Notch1 axis. Environ. Toxicol. Pharmacol. 85, 103640. 10.1016/j.etap.2021.103640 (2021). [DOI] [PubMed] [Google Scholar]
- 29.National Health Commission of the People’s Republic of China.GBZ2.1-. Occupational exposure limits for hazardous agents in the workplace—Part 1: Chemical hazardous agents. Retrieved January 20, 2024, from (2019). http://www.nhc.gov.cn/fzs/s7852d/201909/7abe11973e2149678e4419f36298a89a.shtml
- 30.Yadav, S. K. et al. Occupational lead exposure is an independent modulator of hypertension and poor pulmonary function: a cross-sectional comparative study in lead-acid battery recycling workers. Toxicol. Ind. Health. 38, 139–150. 10.1177/07482337221076248 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Camaj, P. R. et al. Long-term effects of environmental lead exposure on blood pressure and plasma soluble cell adhesion molecules in young adults: a follow-up study of a prospective cohort in Kosovo. J. Environ. Public Health 3180487. 10.1155/2018/3180487 (2018). [DOI] [PMC free article] [PubMed]
- 32.Everson, T. M. et al. Metal biomarker mixtures and blood pressure in the United States: cross-sectional findings from the 1999–2006 National Health and Nutrition Examination Survey (NHANES). Environ. Health. 20, 15. 10.1186/s12940-021-00695-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fioresi, M. et al. Chronic lead exposure increases blood pressure and myocardial contractility in rats. PLoS One. 9, e96900. 10.1371/journal.pone.0096900 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silveira, E. A. et al. Low-dose chronic lead exposure increases systolic arterial pressure and vascular reactivity of rat aortas. Free Radic Biol. Med. 67, 366–376. 10.1016/j.freeradbiomed.2013.11.021 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Sharifi, A. M., Darabi, R., Akbarloo, N., Larijani, B. & Khoshbaten, A. Investigation of circulatory and tissue ACE activity during development of lead-induced hypertension. Toxicol. Lett. 153, 233–238. 10.1016/j.toxlet.2004.04.013 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Yap, R. W. K., Lin, M. H., Shidoji, Y. & Yap, W. S. Association of Stress, Mental Health, and VEGFR-2 gene polymorphisms with cardiometabolic risk in Chinese Malaysian adults. Nutrients. 1110.3390/nu11051140 (2019). [DOI] [PMC free article] [PubMed]
- 37.Abramenko, I. V., Bilous, N. I., Chumak, A. A., Dyagil, I. S. & Martina, Z. V. Analysis of the 3’UTR region of the NOTCH1 gene in chronic lymphocytic leukemia patients. Exp. Oncol. 40, 211–217 (2018). [PubMed]
- 38.Cao, Y. W. et al. Notch1 single nucleotide polymorphism rs3124591 is associated with the risk of development of invasive ductal breast carcinoma in a Chinese population. Int. J. Clin. Exp. Pathol. 7, 4286–4294 (2014). [PMC free article] [PubMed]
- 39.Hajer, F. et al. Genetic polymorphisms in VEGFA and VEGFR2 genes associated with coronary heart disease susceptibility and severity. Mol. Biol. Rep. 50, 10169–10177. 10.1007/s11033-023-08899-z (2023). [DOI] [PubMed] [Google Scholar]
- 40.Sun, S. et al. Association of the VEGFR2 single nucleotide polymorphism rs2305948 with glioma risk. Med. (Baltim). 101, e28454. 10.1097/md.0000000000028454 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mani, M. S. et al. Modifying effects of delta-aminolevulinate dehydratase polymorphism on blood lead levels and ALAD activity. Toxicol. Lett. 295, 351–356. 10.1016/j.toxlet.2018.07.014 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Mani, M. S., Puranik, A., Kabekkodu, S. P., Joshi, M. B. & Dsouza, H. S. Influence of VDR and HFE polymorphisms on blood lead levels of occupationally exposed workers. Hum. Exp. Toxicol. 40, 897–914. 10.1177/0960327120975451 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Lim, J. E. et al. Gene-environment interactions related to blood pressure traits in two community-based Korean cohorts. Genet. Epidemiol. 43, 402–413. 10.1002/gepi.22195 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Hollister, B. M., Farber-Eger, E., Aldrich, M. C. & Crawford, D. C. A Social Determinant of Health May Modify Genetic associations for blood pressure: evidence from a SNP by Education Interaction in an African American Population. Front. Genet.10, 428. 10.3389/fgene.2019.00428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurent, S. & Boutouyrie, P. The structural factor of hypertension: large and small artery alterations. Circ. Res. 116, 1007–1021. 10.1161/circresaha.116.303596 (2015). [DOI] [PubMed] [Google Scholar]
- 46.He, Y. et al. Stabilization of VEGFR2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Sci. Signal. 3, ra26. 10.1126/scisignal.2000722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitulescu, M. E. et al. Dll4 and notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell. Biol. 19, 915–927. 10.1038/ncb3555 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Bautch, V. L. VEGF-directed blood vessel patterning: from cells to organism. Cold Spring Harb Perspect. Med. 2, a006452. 10.1101/cshperspect.a006452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu, H. et al. Angiotensin-converting enzyme inhibitor promotes angiogenesis through Sp1/Sp3-mediated inhibition of notch signaling in male mice. Nat. Commun. 14, 731. 10.1038/s41467-023-36409-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.You, C. et al. Loss of CCM3 impairs DLL4-Notch signalling: implication in endothelial angiogenesis and in inherited cerebral cavernous malformations. J. Cell. Mol. Med. 17, 407–418. 10.1111/jcmm.12022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao, Z. et al. Association between Single Nucleotide Polymorphisms in Cardiovascular Developmental critical genes and hypertension: a propensity score matching analysis. Int. J. Hypertens. 2020 (9185697). 10.1155/2020/9185697 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Correspondence and requests for research data should be addressed to Z.Z. or Y.H.