This cross-sectional study evaluates the association between continuous glucose monitoring prescriptions and individual characteristics among patients with type 1 and type 2 diabetes receiving care at federally qualified health centers (FQHCs).
Key Points
Question
What is the current state of continuous glucose monitoring (CGM) prescription for type 1 and type 2 diabetes among patients receiving care in federally qualified health centers (FQHCs)?
Findings
In this cross-sectional analysis of electronic health record data from 1168 patients with type 1 diabetes and 35 216 patients with type 2 diabetes receiving primary care in FQHCs, diabetes severity was notable, yet CGM prescription was low. Race, ethnicity, and insurance status were associated with CGM prescription in this vulnerable population.
Meaning
This study suggests low rates of CGM prescription in FQHCs, highlighting a critical need to understand inequities in diabetes technology use in this setting.
Abstract
Importance
While continuous glucose monitoring (CGM) has been found to improve diabetes care processes and outcomes, adoption remains low.
Objective
To examine the association between CGM prescriptions and individual characteristics among patients with type 1 or 2 diabetes (T1D and T2D, respectively).
Design, Setting, and Participants
Retrospective cross-sectional study using electronic health record data for patients with T1D or T1D from 275 clinic sites nationwide between January 2014 and February 2021. All participating clinics were federally qualified health centers (FQHCs), the largest US system of primary care for vulnerable populations. Data were analyzed from September 2022 to August 2024.
Main Outcomes and Measures
Sociodemographic factors, clinical characteristics, and CGM prescription orders.
Results
A total of 1168 patients with T1D (mean [SD] age, 41.8 [16.0] years; 600 [51.4%] male; 372 [31.9%] Black; 262 [22.4%] Hispanic, and 750 [64.2%] White patients) and 35 216 patients with T2D (mean [SD] age, 58.4 [13.1] years; 19 772 [56.1%] female; 12 030 [34.2%] Black; 12 979 [36.9%] Hispanic, and 20 413 [58.0] White patients) were included. Overall, CGM prescriptions were infrequent (129 [11.0%] for patients with T1D and 362 [1.0%] for those with T2D) but increased throughout the study period. Among patients with T1D, those who reported Hispanic ethnicity (odds ratio [OR], 0.30; 95% CI, 0.16-0.57), Black race (OR, 0.61; 95% CI, 0.38-0.99), or were uninsured (OR, 0.42; 95% CI, 0.23-0.74) had lower multivariable odds of receiving a CGM prescription than White or insured adults, respectively. Similar findings were observed among patients with T2D reporting Hispanic ethnicity (OR, 0.43; 95% CI, 0.32-0.57), Black race (OR, 0.76; 95% CI, 0.59-0.98), or being uninsured (OR, 0.42; 95% CI, 0.31-0.58), relative to their counterparts. Among patients with T2D, hemoglobin A1c values higher than 9.0% (OR, 3.17; 95% CI, 2.37-4.21) and a greater burden of diabetes complications were associated with higher odds of CGM prescription.
Conclusions and Relevance
In this cross-sectional study of electronic health record data, rates of CGM prescription orders were low among FQHC patients with T1D and T2D. Disparities in CGM orders were observed among patients reporting Hispanic ethnicity, Black race, and those who lacked health insurance. Future research is needed to understand the causes of infrequent CGM orders in FQHCs and drivers of observed disparities in this vulnerable patient population.
Introduction
Continuous glucose monitoring (CGM) has transformed diabetes care and improved outcomes among patients with type 1 and type 2 diabetes (T1D and T2D).1 CGM offers increased precision in diabetes management, improving glycemic control, promoting treatment satisfaction, and enhancing quality of life.1,2 There is also evidence that CGM is associated with reduced acute care utilization due to hypo- and hyperglycemic events.3 This robust evidence base now informs clinical guidelines recommending CGM use.4 Initial studies reported these positive outcomes among patients with T1D, and more recent research demonstrates similar benefits in the larger population of patients with T2D.3,5 This prior work also suggests that a greater proportion of patients with T1D have used CGM than their counterparts with T2D.6,7
Despite CGM’s potential to improve diabetes care and outcomes, this technology remains underutilized. The uptake of CGM has been greatest in endocrinology clinics, which provide diabetes care for many patients with T1D and more complex cases of T2D.7,8 The most recent estimates of CGM use in this setting are as high as 46%.9,10 Limited prior research has found lower rates of CGM utilization and wider variation in primary care clinics, where the majority of T2D treatment is provided and the acuity of disease is generally lower.7,11,12 Disparities in CGM utilization among racial and ethnic minority groups have been consistently documented across diverse health care settings, highlighting the need to examine health equity implications of this diabetes technology.9,13,14,15
Federally qualified health centers (FQHCs) represent the largest national system of primary care clinics for over 30 million medically underserved patients in the US, approximately two-thirds of whom are members of racial or ethnic minority groups with incomes below the federal poverty level.16 Almost half of FQHC patients have Medicaid insurance, which constitutes the largest payer in this setting, while approximately one-quarter of patients are uninsured.17 While FQHCs represent an important setting to study diabetes health equity, prior research on CGM use in FQHCs has been limited. We sought to describe CGM prescription patterns among patients with T1D and patients with T2D in a large national network of FQHCs, as well as model associations between CGM prescription orders and patient characteristics in this understudied primary care setting.
Methods
The study protocol was deemed exempt from review and the need for informed consent by the Northwestern University institutional review board because data were deidentified. We conducted a retrospective cross-sectional study of electronic health record (EHR) data between January 2014 and February 2021 from 19 FQHCs comprising 275 clinic sites affiliated with AllianceChicago. This health center–controlled network hosts a centralized EHR infrastructure and a common data warehouse for FQHCs in 21 US states. We included adult patients aged 18 years and older with diabetes, defined with a validated EHR phenotype that included: (1) International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnostic codes for T1D and T2D; (2) hemoglobin A1c (HbA1c; to convert to proportion of total hemoglobin, multiply by 0.01) values 6.5% or higher; random glucose 200 mg/dL or higher (to convert to mmol/L, multiply by 0.0555); and (3) antidiabetic medication prescription.18 CGM prescription status was ascertained by the presence of clinicians’ orders for CGM or Current Procedural Terminology codes for CGM placement or interpretation. The index date was defined as the first available office visit starting in January 2014, when the first CGM prescription was observed in our data, and ending in February 2021 with the last available data. The reporting of study results adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.19
The primary outcome was receipt of the first CGM prescription order or CGM-related procedure code during the study period, which was ascertained using routinely collected EHR data. We analyzed sociodemographic factors and clinical characteristics that were available in the EHR and may be associated with CGM prescription orders. Specifically, these covariates were age, sex, self-reported race and ethnicity (Black, White, and Other [Asian, American Indian or Alaska Native, Native Hawaiian, and Pacific Islander] for race; and Hispanic and non-Hispanic for ethnicity), insurance status at index visit, last available HbA1c value (≤7.0%, 7.1%-8.9%, and ≥9.0%), and the diabetes complication severity index (DCSI) score. The DCSI score and age were analyzed continuously.
Statistical Analysis
Descriptive statistics were used to examine the sociodemographic and clinical characteristics of patients with T1D and T2D, with stratification by CGM prescription status. We used logistic regression to model the odds of CGM prescription, controlling for all sociodemographic factors and clinical characteristics as potential confounders. The year of the last observed office visit was included in models as a fixed effect for year, accounting for secular trends and any year-specific effects in CGM prescribing during the study period. The odds ratios (ORs) and confidence intervals for all variables in the primary model are shown in eTable 1 in Supplement 1. In a sensitivity analysis, an interaction term in the fully adjusted models for race or ethnicity × HbA1c value was used to explore the potential for racial or ethnic disparities according to participants’ glycemic control. Further sensitivity analyses added adjustment for insulin use, the number of office visits during the study period, and the first observed HbA1c result to the primary model variables (eTable 3 in Supplement 1; descriptive analyses of these additional variables presented in eTable 2 in Supplement 1). eFigures 1 to 3 in Supplement 1 display the rates of CGM prescription orders by race and ethnicity and insurance status. A 2-sided P value less than .05 was considered significant for all statistical testing. Analyses were conducted using Stata SE version 18.5 (StataCorp). Data were analyzed from September 2022 to August 2024.
Results
Among 1168 patients with T1D, the mean (SD) age was 41.9 (16.0) years, 600 (51.4%) were male, 372 (31.9%) were Black, 262 (22%) were Hispanic, and 750 (64.2%) where White (Table 1). Among 35 216 patients with T2D, 19 772 (56.1%) were female, and the mean (SD) age was older (58.4 [13.1] years), with a similar proportion reporting Black race and a higher proportion with Hispanic ethnicity (12 030 [34.2%] Black; 12 979 [36.9%] Hispanic, and 20 413 [58.0%] White patients) (Table 2). Among patients with T1D, 359 (30.7%) were uninsured and 464 (39.8%) had Medicaid insurance, with corresponding proportions in patients with T2D of 14 428 (41.0%) without health insurance and 9602 (27.3%) with Medicaid coverage.
Table 1. Characteristics of Patients With Type 1 Diabetes by Continuous Glucose Monitor (CGM) Prescription Status.
| Characteristic | Patients, No. (%) | P valuea | ||
|---|---|---|---|---|
| Total (N = 1168) | CGM prescription (n = 129) | No CGM prescription (n = 1039) | ||
| Age, mean (SD), y | 41.8 (16.0) | 38.8 (13.4) | 42.2 (16.3) | .02 |
| Race | ||||
| Black | 372 (31.9) | 36 (27.9) | 336 (32.3) | .31 |
| White | 750 (64.2) | 89 (69.0) | 661 (63.6) | .23 |
| Otherb | 46 (3.9) | 4 (3.1) | 42 (4.0) | .60 |
| Ethnicity | ||||
| Non-Hispanic | 906 (77.6) | 116 (89.9) | 790 (76.0) | <.001 |
| Hispanic | 262 (22.4) | 13 (10.1) | 249 (24.0) | <.001 |
| Sex | ||||
| Male | 600 (51.4) | 66 (51.2) | 534 (51.4) | .96 |
| Female | 568 (48.6) | 63 (48.8) | 505 (48.6) | .96 |
| Insurance statusc | ||||
| Private | 256 (21.9) | 47 (36.4) | 209 (20.1) | <.001 |
| Medicaid | 465 (39.8) | 52 (40.3) | 413 (39.8) | .90 |
| Medicare | 88 (7.5) | 8 (6.2) | 80 (7.7) | .54 |
| Uninsured | 359 (30.7) | 22 (17.1) | 337 (32.4) | <.001 |
| Hemoglobin A1c, %d | ||||
| ≤7.0 | 195 (16.7) | 17 (13.2) | 178 (17.1) | .51 |
| 7.1-8.9 | 352 (30.1) | 39 (30.2) | 313 (30.1) | .65 |
| ≥9.0 | 621 (53.2) | 73 (56.6) | 548 (52.7) | .97 |
| Diabetes Complications Severity Index, mean (SD) | 2.2 (4.7) | 2.1 (4.3) | 2.2 (4.8) | .66 |
SI Conversion: to convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
P values are derived from t tests of the difference in patient characteristics between those with and without CGM prescriptions during the study period.
Other racial groups included Asian, American Indian or Alaska Native, Native Hawaiian, and Pacific Islander.
Insurance status was ascertained at the first visit during the study period.
Hemoglobin A1c was analyzed as the last available value during the study period.
Table 2. Characteristics of Patients With Type 2 Diabetes by Continuous Glucose Monitor (CGM) Prescription Status.
| Characteristic | Patients, No. (%) | P valuea | ||
|---|---|---|---|---|
| Total (N = 35 216) | CGM prescription (n = 362) | No CGM prescription (n = 34 854) | ||
| Age, mean (SD), y | 58.4 (13.1) | 57.4 (12.8) | 58.4 (13.1) | .15 |
| Race | ||||
| Black | 12 030 (34.2) | 139 (38.4) | 11 891 (34.1) | .09 |
| White | 20 413 (58.0) | 203 (56.1) | 20 210 (58.0) | .46 |
| Otherb | 2773 (7.9) | 20 (5.5) | 2753 (7.9) | .10 |
| Ethnicity | ||||
| Non-Hispanic | 22 237 (63.1) | 285 (78.7) | 21 952 (63.0) | <.001 |
| Hispanic | 12 979 (36.9) | 77 (21.3) | 12 902 (37.0) | <.001 |
| Sex | ||||
| Male | 15 444 (43.9) | 166 (45.9) | 15 278 (43.8) | .44 |
| Female | 19 772 (56.1) | 196 (54.1) | 19 576 (56.2) | .44 |
| Insurance statusc | ||||
| Private | 6347 (18.0) | 97 (26.8) | 6250 (17.9) | <.001 |
| Medicaid | 9602 (27.3) | 139 (38.4) | 9463 (27.2) | <.001 |
| Medicare | 4839 (13.7) | 48 (13.3) | 4791 (13.8) | .79 |
| Uninsured | 14 428 (41.0) | 78 (21.6) | 14 350 (41.2) | <.001 |
| Hemoglobin A1c, %d | ||||
| ≤7.0 | 13 663 (38.8) | 72 (19.9) | 13 591 (39.0) | <.001 |
| 7.1-8.9 | 11 402 (32.4) | 134 (37.0) | 11 268 (32.3) | .08 |
| ≥9.0 | 10 141 (28.8) | 156 (43.1) | 9995 (28.7) | .001 |
| Diabetes Complications Severity Index, mean (SD) | 1.7 (3.6) | 2.7 (4.2) | 1.7 (3.6) | <.001 |
SI Conversion: to convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
P values are derived from t tests of the difference in patient characteristics between those with and without CGM prescriptions during the study period.
Other racial groups included Asian, American Indian or Alaska Native, Native Hawaiian, and Pacific Islander.
Insurance status was ascertained at the first visit during the study period.
Hemoglobin A1c was analyzed as the last available value during the study period.
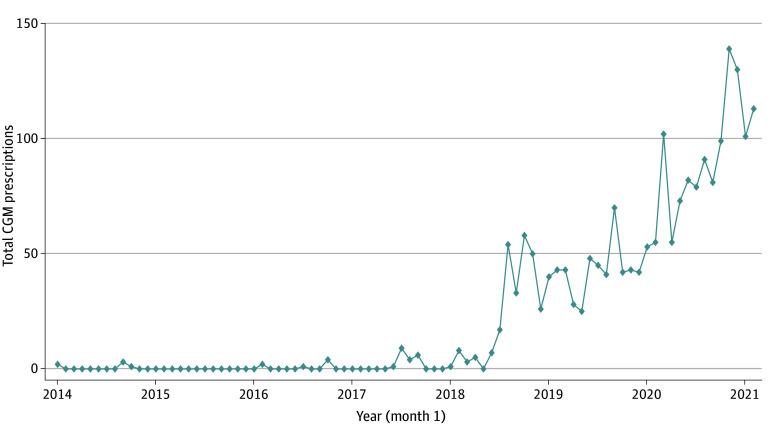
The study sample exhibited poor glycemic control, with approximately half of patients with T1D and almost one-third of patients with T2D having HbA1c values above 9.0%. Both patients with T1D and T2D experienced diabetes complications (mean [SD] DSCI scores, 2.2 [4.7] and 1.7 [3.6], respectively). Overall, CGM prescriptions were infrequent (ie, 491 patients [1.3%]), constituting 129 (11.0%) patients with T1D and only 362 (1.0%) patients with T2D. Annual CGM prescriptions increased throughout the study period from only 6 prescriptions in 2014 to 1039 in 2020, and 214 in the first 2 months of 2021. Figure 1 illustrates this trend in CGM prescriptions for the entire cohort, demonstrating very few prescription orders from 2014 to 2018 and then a steady increase beginning in 2019 and continuing through the end of the study period.
Figure 1. Total Continuous Glucose Monitor (CGM) Prescriptions by Month.
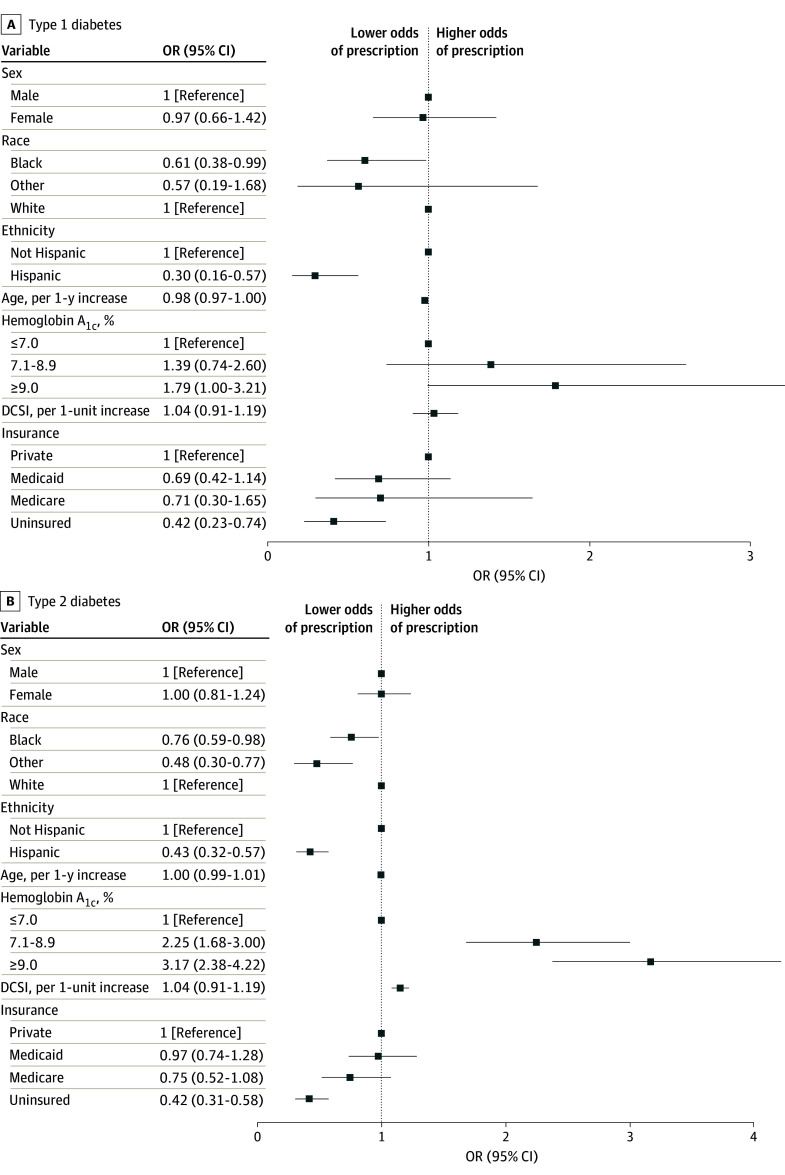
ORs displayed in Figure 2 display the independent associations of selected covariates with CGM prescriptions among patients with T1D and T2D, respectively. ORs and 95% CIs for all covariates included in the primary models are presented in eTable 1 in Supplement 1. Patients with T1D reporting Hispanic ethnicity (OR, 0.30; 95% CI, 0.16-0.57) or Black race (OR, 0.61; 95% CI, 0.38-0.99) exhibited lower odds of CGM prescription than their White counterparts (Figure 2A; eTable 1 in Supplement 1). Similar disparities in CGM prescription were also observed among Hispanic and Black patients with T2D (OR, 0.43; 95% CI, 0.32-0.57 and OR, 0.76; 95% CI, 0.59-0.98, respectively). Lacking health insurance was significantly associated with lower odds of CGM prescription than private insurance coverage among both patients with T1D and T2D (OR, 0.42; 95% CI, 0.23-0.74 and OR, 0.42; 95% CI, 0.31-0.58, respectively). There were no differences in CGM prescription orders by sex in either patients with T1D or T2D.
Figure 2. Odds of Continuous Glucose Monitor Prescription Among Patients With Diabetes.
SI Conversion: to convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01. DCSI indicates diabetes complication severity index.
The level of glycemic control and presence of diabetes complications were significantly associated with CGM prescription orders in patients with T2D. Relative to patients with T2D who had HbA1c values 7.0% or below, the odds of CGM prescription for those with HbA1c of 7.1% to 8.9% were over twice as high and over 3 times as high for those with HbA1c 9.0% or higher. The odds of CGM orders in patients with T2D increased by 15% for every point on the DCSI score. Among patients with T1D, the likelihood of CGM prescription for those with HbA1c values 9.0% or higher compared with those with HbA1c values 7.0% or below did not achieve statistical significance (OR, 1.79; 95% CI, 1.00-3.21). Interaction terms for race and ethnicity × HbA1c values were not statistically significant (data not shown). Sensitivity analyses including insulin prescriptions as a covariate, as well as the number of office visits and the first observed HbA1c value—rather than the last HbA1c value in the primary models—yielded similar findings (eTable 3 in Supplement 1).
Discussion
This study found low CGM prescription rates among patients receiving diabetes care in FQHCs, the largest national system of safety-net primary care clinics. CGM orders were greater than 10 times more common in the management of T1D than T2D. While infrequent among patients with T2D, CGM orders were significantly associated with poor glycemic control and the presence of diabetes complications. Among both patients with T1D and T2D, we observed significant disparities in CGM prescriptions among those with Black race, Hispanic ethnicity, and no health insurance. It is notable that these disparities exist among patients receiving primary care in FQHCs, which represent the main system of care for historically underserved groups. Our findings highlight the importance of expanding efforts to promote diabetes health equity in this setting.
Disparities in CGM use have also been reported in other health care settings.20,21 Most prior research documenting racial and ethnic disparities in CGM use is from academic endocrinology clinics,9,13,15,22 with a recent study reporting similar findings in Veterans Affairs (VA) outpatient clinics.14 Our study is the first that we know of to demonstrate racial and ethnic CGM disparities in a national network of FQHCs, where there are additional resources available to support the diabetes care of the vulnerable patient populations they serve.23 This may explain why the magnitude of disparities we observed among Black patients in FQHCs was smaller than those reported in other settings.13,15 The persistence of racial and ethnic disparities in CGM use across diverse health care settings suggests that intractable forces such as limited access to care and medical devices, implicit bias, and medical mistrust may play a role.24 Some prior efforts have demonstrated promise in reducing disparities in CGM uptake, including a Colorado Medicaid program that provided full subsidies for CGM devices.25 Results from this program suggest that financial barriers remain significantly associated with disparities in CGM use.
CGM uptake has expanded significantly over the last 2 decades, first in T1D and increasingly in T2D. The earliest adoption of CGMs occurred in endocrinology clinics, where less than 7% of antidiabetic medication prescriptions are written.12 New clinical trial data supports CGM use in primary care,1,26 providing an evidence base to justify the growth of this technology among a much larger diabetic population. Employing CGM effectively across outpatient settings will require overcoming systemic barriers including a lack of time, support, and expertise among clinicians.27,28 Recently, the Federal Drug Administration recently approved the first over-the-counter CGM,29 signaling a trend that may expand the market significantly. While this may promote increased CGM access, with the potential to reduce disparities in CGM use, new challenges will emerge as patients with diabetes may use these devices with less support from their health care clinicians.
Strengths and Limitations
Strengths of our analysis include the novelty of studying CGM orders among patients receiving their primary care in FQHCs. This represents an important and understudied setting for exploring diabetes health equity given the high proportion of patients from racial and ethnic minority groups and those who are uninsured or underinsured. The prevalence of uncontrolled diabetes in FQHCs, reported among 35.6% of patients nationwide,30 is higher than that observed overall among US adults with diabetes.31 This observation also underscores the importance of examining CGM prescriptions in FQHCs, given the potential of this diabetes technology to improve glycemic control among the large and particularly vulnerable patient populations they serve. While some prior studies of CGM use among FQHC patients have focused on samples from a single FQHC or a single state, our data are derived from a national FQHC network spanning 275 clinic sites in 19 US states.25,32
Our study also has notable limitations. CGM orders observed in the EHR are not equivalent to long-term use, which cannot be verified with our dataset because it does not include pharmacy claims. FQHCs offer primary care for medically underserved patients, which does not include subspecialty care by endocrinologists, who are more likely to prescribe CGM.8,33 The SARS-CoV-2 pandemic occurred during the latter part of our study period, when telemedicine visits became common.34 The study data did not include information about telehealth visits, when CGM prescriptions may have been less likely than in-person visits. However, we observed a steady increase in CGM prescriptions from 2018 to the end of the study period in 2021, and our models included a fixed effect for year that adjusted for changes in care delivery during the SARS-CoV-2 pandemic. While we adjusted for the number of office visits, we could not determine whether they were primarily associated with diabetes management. In addition, we could not adjust for state-specific trends in CGM prescribing that may have impacted our findings. Since the end of the study period in February 2021, insurance coverage for CGM has expanded, out-of-pocket costs for CGM have decreased, and primary care clinicians have become increasingly familiar with this diabetes technology. Investigation of more recent FQHC data is needed to confirm whether low use of CGMs and the CGM-related disparities reported in this study persist.
Conclusions
In this cross-sectional study, we highlighted a significant gap in implementing CGM clinical practice guidelines in FQHCs.4 We observed infrequent use of CGMs in FQHCs relative to prior data from other ambulatory settings. In addition, we reported persistent disparities in CGM prescriptions among racial and ethnic minorities, as well as those who were uninsured. Future research is needed to understand barriers to CGM use among FQHC clinicians and their patients. Prior research suggests that eliminating financial barriers significantly increases CGM uptake, while reducing racial and ethnic disparities.25,32 Changes in Medicaid policy have the greatest potential to improve CGM use in FQHCs, as it is the primary payer in this setting.17 However, additional coverage strategies and alternate mechanisms for accessing CGM will be needed given the large proportion of uninsured patients who receive primary care in FQHCs.
eTable 1. Multivariable Association of Patient Characteristics and Continuous Glucose Monitor Prescription (Primary Model)
eTable 2. Descriptive Statistics of Additional Variables in Supplementary Logistic Regression Analysis
eTable 3. Multivariable Association of Patient Characteristics and Continuous Glucose Monitor Prescription with Additional Variables
eFigure 1. CGM Prescriptions by Month and Ethnicity
eFigure 2. CGM Prescriptions by Month and Insurance Status
eFigure 3. CGM Prescriptions by Month and Race
Data Sharing Statement
References
- 1.Kieu A, King J, Govender RD, Östlundh L. The benefits of utilizing continuous glucose monitoring of diabetes mellitus in primary care: a systematic review. J Diabetes Sci Technol. 2023;17(3):762-774. doi: 10.1177/19322968211070855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin RR, Peyrot M. Treatment satisfaction and quality of life for an integrated continuous glucose monitoring/insulin pump system compared to self-monitoring plus an insulin pump. J Diabetes Sci Technol. 2009;3(6):1402-1410. doi: 10.1177/193229680900300621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273-2284. doi: 10.1001/jama.2021.6530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Professional Practice Committee . 7. Diabetes technology: standards of care in diabetes-2024. Diabetes Care. 2024;47(suppl 1):S126-S144. doi: 10.2337/dc24-S007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(S2):S4-S11. doi: 10.1089/dia.2017.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacy ME, Lee KE, Atac O, et al. Patterns and trends in continuous glucose monitoring utilization among commercially insured individuals with type 1 diabetes: 2010-2013 to 2016-2019. Clin Diabetes. 2024;42(3):388-397. doi: 10.2337/cd23-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayberry LS, Guy C, Hendrickson CD, McCoy AB, Elasy T. Rates and correlates of uptake of continuous glucose monitors among adults with type 2 diabetes in primary care and endocrinology settings. J Gen Intern Med. 2023;38(11):2546-2552. doi: 10.1007/s11606-023-08222-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunberger G, Sze D, Ermakova A, Sieradzan R, Oliveria T, Miller EM. Treatment intensification with insulin pumps and other technologies in patients with type 2 diabetes: results of a physician survey in the United States. Clin Diabetes. 2020;38(1):47-55. doi: 10.2337/cd19-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai CW, Lipman TH, Willi SM, Hawkes CP. Early racial/ethnic disparities in continuous glucose monitor use in pediatric type 1 diabetes. Diabetes Technol Ther. 2021;23(11):763-767. doi: 10.1089/dia.2021.0120 [DOI] [PubMed] [Google Scholar]
- 10.Gaulke AP, Giordano J, Grossman DS. Association of continuous glucose monitor receipt and diabetes care provider type: a cohort study of West Virginia Medicaid beneficiaries with type 1 diabetes. Med Care. 2023;61(11):760-764. doi: 10.1097/MLR.0000000000001917 [DOI] [PubMed] [Google Scholar]
- 11.Shrivastav M, Gibson W Jr, Shrivastav R, et al. Type 2 diabetes management in primary care: the role of retrospective, professional continuous glucose monitoring. Diabetes Spectr. 2018;31(3):279-287. doi: 10.2337/ds17-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann RT, Wallia A, O’Brien MJ, et al. Correlates of second-line type 2 diabetes medication selection in the USA. BMJ Open Diabetes Res Care. 2017;5(1):e000421. doi: 10.1136/bmjdrc-2017-000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanbour S, Jones M, Abusamaan MS, et al. Racial disparities in access and use of diabetes technology among adult patients with type 1 diabetes in a U.S. academic medical center. Diabetes Care. 2023;46(1):56-64. doi: 10.2337/dc22-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipska KJ, Oladele C, Zawack K, et al. Association of race and ethnicity with prescriptions for continuous glucose monitoring systems among a national sample of veterans with diabetes on insulin therapy. Diabetes Technol Ther. 2024. doi: 10.1089/dia.2024.0152 [DOI] [PubMed] [Google Scholar]
- 15.Fantasia KL, Wirunsawanya K, Lee C, Rizo I. Racial disparities in diabetes technology use and outcomes in type 1 diabetes in a safety-net hospital. J Diabetes Sci Technol. 2021;15(5):1010-1017. doi: 10.1177/1932296821995810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Association of Community Health Centers . Community health centers: providers, partners and employers of choice 2024 chartbook. 2024. Accessed October 25, 2024. https://www.nachc.org/wp-content/uploads/2024/07/2024-2022-UDS-DATA-Community-Health-Center-Chartbook.pdf
- 17.Medicaid and CHIP Payment and Access Commission . Medicaid payment policy for federally qualified health centers. 2017. Accessed October 14, 2024. https://www.macpac.gov/wp-content/uploads/2017/12/Medicaid-Payment-Policy-for-Federally-Qualified-Health-Centers.pdf
- 18.Nichols GA, Desai J, Elston Lafata J, et al. ; SUPREME-DM Study Group . Construction of a multisite datalink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110. doi: 10.5888/pcd9.110311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 20.DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D exchange real-world observational study. J Diabetes Sci Technol. 2023;17(2):322-328. doi: 10.1177/19322968211049783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306-313. doi: 10.1089/dia.2020.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremblay ES, Bernique A, Garvey K, Astley CM. A retrospective cohort study of racial/ethnic and socioeconomic disparities in initiation and meaningful use of continuous glucose monitoring among youth with type 1 diabetes. J Diabetes Sci Technol. 2023:19322968231183985. doi: 10.1177/19322968231183985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley AT, Nocon RS, O’Brien MJ. Diabetes management in community health centers: a review of policies and programs. Curr Diab Rep. 2020;20(2):8. doi: 10.1007/s11892-020-1289-0 [DOI] [PubMed] [Google Scholar]
- 24.Isaacs D, Bellini NJ, Biba U, Cai A, Close KL. Health care disparities in use of continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S3):S81-S87. doi: 10.1089/dia.2021.0268 [DOI] [PubMed] [Google Scholar]
- 25.Ni K, Tampe CA, Sol K, Richardson DB, Pereira RI. Effect of CGM access expansion on uptake among patients on Medicaid with diabetes. Diabetes Care. 2023;46(2):391-398. doi: 10.2337/dc22-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fantasia KL, Stockman MC, Ju Z, et al. Professional continuous glucose monitoring and endocrinology eConsult for adults with type 2 diabetes in primary care: results of a clinical pilot program. J Clin Transl Endocrinol. 2021;24:100254. doi: 10.1016/j.jcte.2021.100254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker AF, Hood KK, Gurka MJ, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480-1490. doi: 10.2337/dc20-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oser TK, Hall TL, Dickinson LM, et al. Continuous glucose monitoring in primary care: understanding and supporting clinicians’ use to enhance diabetes care. Ann Fam Med. 2022;20(6):541-547. doi: 10.1370/afm.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration . FDA clears first over-the-counter continuous glucose monitor. March 5, 2024. Accessed September 3, 2024. https://www.fda.gov/news-events/press-announcements/fda-clears-first-over-counter-continuous-glucose-monitor
- 30.Health Resources and Services Administration . 2020. Health Center Data. U.S. Department of Health and Human Services. Accessed September 3, 2024. https://data.hrsa.gov/tools/data-reporting/program-data/national/table?tableName=Full&year=2020
- 31.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in US adults, 1999–2018. N Engl J Med. 2021;384(23):2219-2228. doi: 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni K, Tampe CA, Sol K, Cervantes L, Pereira RI. Continuous glucose monitor: reclaiming type 2 diabetes self-efficacy and mitigating disparities. J Endocr Soc. 2024;8(8):bvae125. doi: 10.1210/jendso/bvae125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messer LH, Vigers T, Akturk HK, et al. Increasing use of diabetes devices: what do health care professionals need? Clin Diabetes. 2023;41(3):386-398. doi: 10.2337/cd22-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon J, Mohanty N, Masinter L, Hamilton A, Jain A. COVID-19: exploring the repercussions on federally qualified health center service delivery and quality. J Health Care Poor Underserved. 2021;32(1):137-144. doi: 10.1353/hpu.2021.0013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Multivariable Association of Patient Characteristics and Continuous Glucose Monitor Prescription (Primary Model)
eTable 2. Descriptive Statistics of Additional Variables in Supplementary Logistic Regression Analysis
eTable 3. Multivariable Association of Patient Characteristics and Continuous Glucose Monitor Prescription with Additional Variables
eFigure 1. CGM Prescriptions by Month and Ethnicity
eFigure 2. CGM Prescriptions by Month and Insurance Status
eFigure 3. CGM Prescriptions by Month and Race
Data Sharing Statement