Abstract
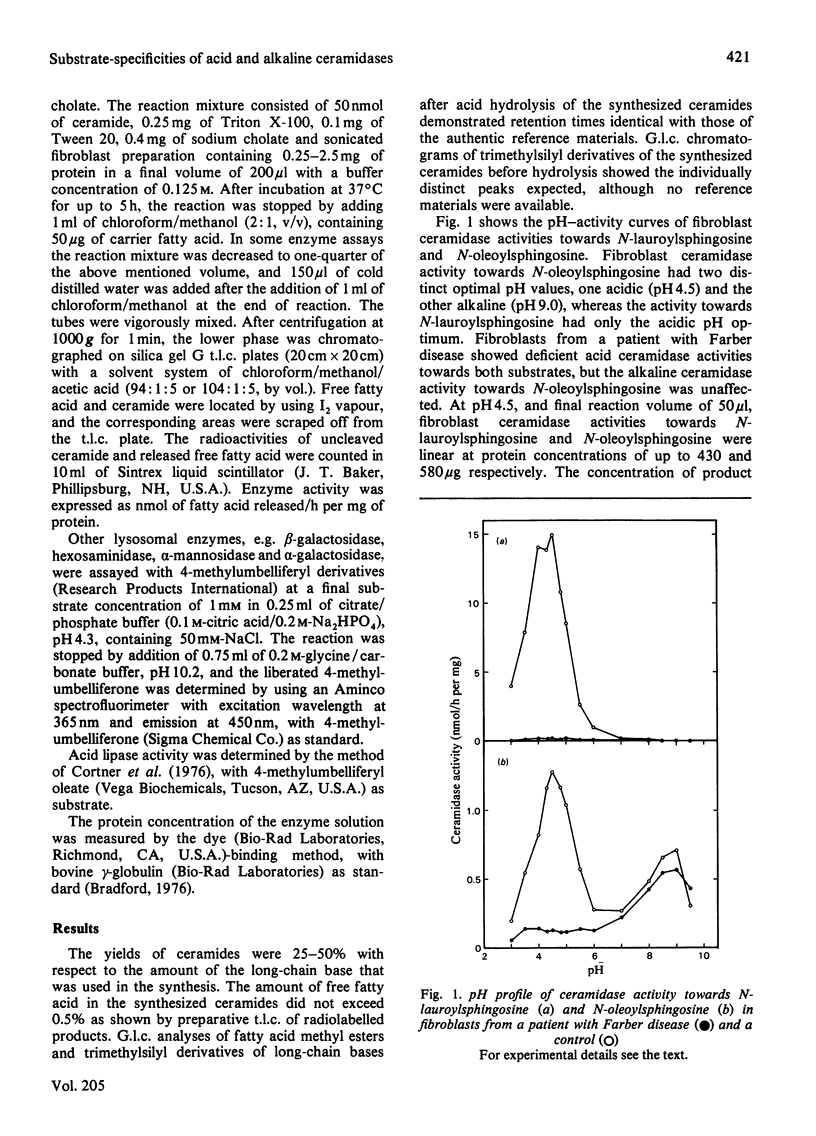
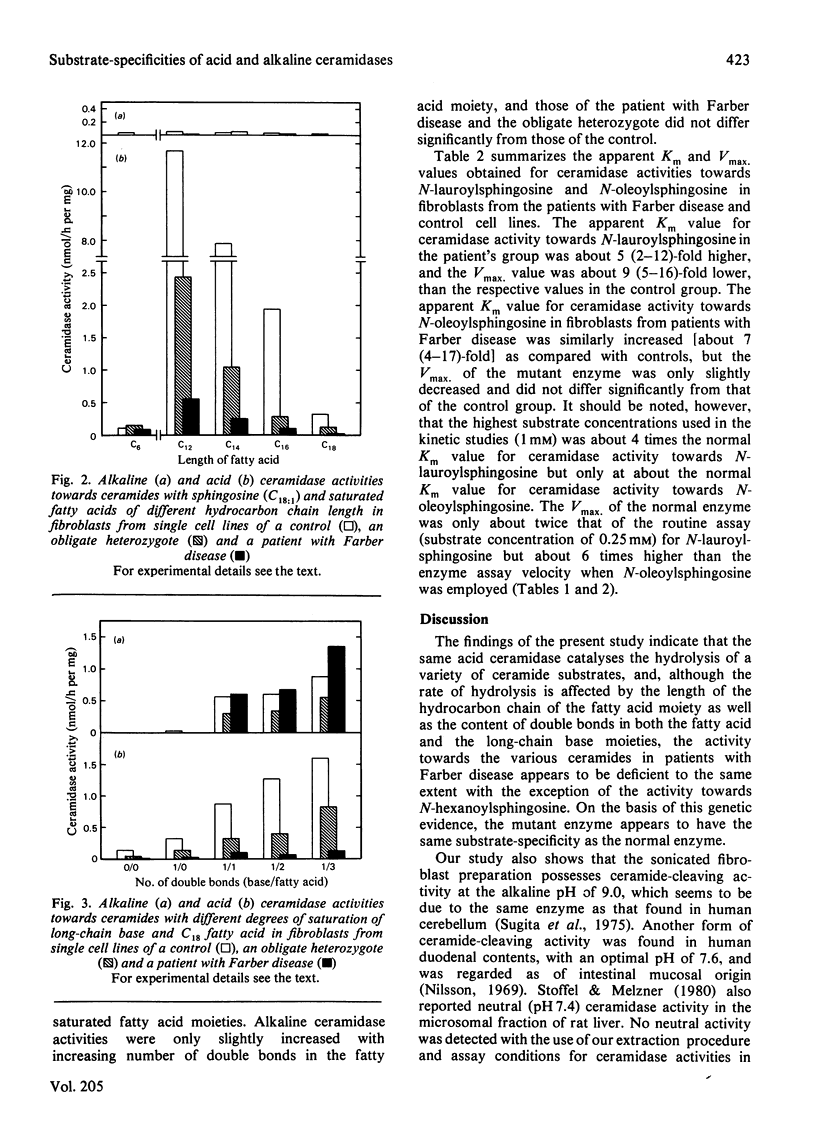
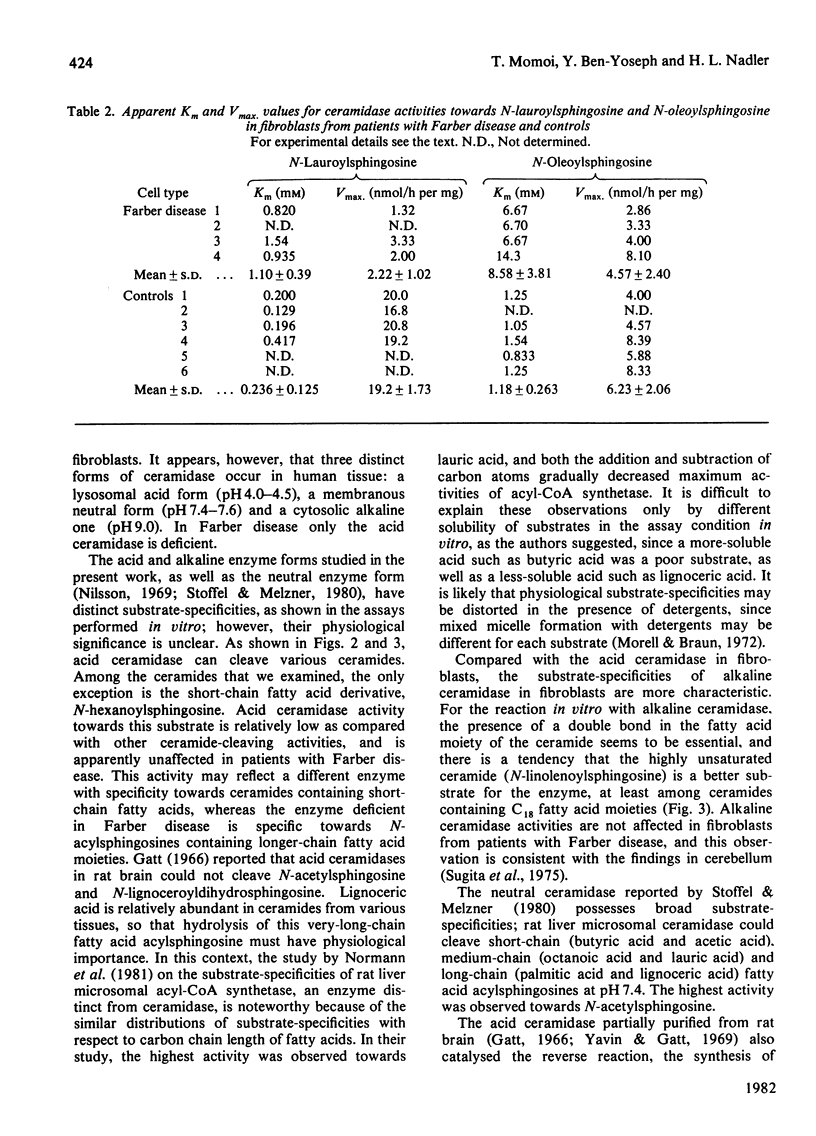
The specific activity of acid ceramidase (N-acylsphingosine deacylase, EC 3.5.1.23) was measured at pH4.5 in normal fibroblasts and in fibroblasts from patients with Farber disease and obligate heterozygotes. Greater activity was found when the synthetically made ceramide substrates contained shorter-chain fatty acids or higher content of double bonds. Acid ceramidase activities towards N-lauroyl- (C12:0), N-myristoyl- (C14:0) and N-palmitoyl- (C16:0) sphingosine (C18:1) were respectively about 38, 26 and 6 times higher than the activity towards the N-stearoyl (C18:0) substrate. The activity towards N-linolenoylsphingosine (C18:3/C18:1), N-linoleoylsphingosine (C18:2/C18:1) and N-oleoylsphingosine (C18:1/C18:1) were respectively about 5, 4 and 3 times higher than the activity towards N-stearoylsphingosine (C18:0/C18:1). The activity towards N-stearoyldihydrosphingosine (C18:0/C18:0) was about 40% of that towards N-stearoylsphingosine. Fibroblast alkaline ceramidase possessed significant activity only towards ceramides of unsaturated fatty acids, with a pH optimum of about 9.0. Deficiency of acid ceramidase activity in fibroblasts from patients with Farber disease and intermediate activities in obligate heterozygotes were demonstrated with all ceramides examined except for N-hexanoylsphingosine (C6:0/C18:1), whereas alkaline ceramidase activity was unaffected. Comparative kinetic studies of acid ceramidase activity with N-lauroylsphingosine and N-oleoylsphingosine demonstrated about 5 (2–12)-fold and 7 (4–17)-fold higher Km values in fibroblasts from patients with Farber disease as compared with normal controls. N-Lauroylsphingosine, towards which acid ceramidase activity in control fibroblasts was about 10 times higher than that towards N-oleoylsphingosine, may serve as a better substrate for enzymic diagnosis of Farber disease as well as for further characterization of the catalytically defective acid ceramidase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cortner J. A., Coates P. M., Swoboda E., Schnatz J. D. Genetic variation of lysosomal acid lipase. Pediatr Res. 1976 Nov;10(11):927–932. doi: 10.1203/00006450-197611000-00005. [DOI] [PubMed] [Google Scholar]
- Dulaney J. T., Milunsky A., Sidbury J. B., Hobolth N., Moser H. W. Diagnosis of lipogranulomatosis (Farber disease) by use of cultured fibroblasts. J Pediatr. 1976 Jul;89(1):59–61. doi: 10.1016/s0022-3476(76)80927-4. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Benson P. F., Neville B. R., Moser H. W., Moser A. E., Dulaney J. T. Prenatal diagnosis of Farber's disease. Lancet. 1979 Nov 10;2(8150):990–992. doi: 10.1016/s0140-6736(79)92562-5. [DOI] [PubMed] [Google Scholar]
- Gatt S. Enzymatic hydrolysis of sphingolipids. I. Hydrolysis and synthesis of ceramides by an enzyme from rat brain. J Biol Chem. 1966 Aug 25;241(16):3724–3730. [PubMed] [Google Scholar]
- Iwamori M., Moser H. W. Above-normal urinary excretion of urinary ceramides in Farber's disease, and characterization of their components by high-performance liquid chromatography. Clin Chem. 1975 May;21(6):725–729. [PubMed] [Google Scholar]
- Morell P., Braun P. Biosynthesis and metabolic degradation of sphingolipids not containing sialic acid. J Lipid Res. 1972 May;13(3):293–310. [PubMed] [Google Scholar]
- Moser H. W., Prensky A. L., Wolfe H. J., Rosman N. P. Farber's lipogranulomatosis. Report of a case and demonstration of an excess of free ceramide and ganglioside. Am J Med. 1969 Dec;47(6):869–890. doi: 10.1016/0002-9343(69)90202-2. [DOI] [PubMed] [Google Scholar]
- Nilsson A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969 Mar 4;176(2):339–347. doi: 10.1016/0005-2760(69)90192-1. [DOI] [PubMed] [Google Scholar]
- Normann P. T., Thomassen M. S., Christiansen E. N., Flatmark T. Acyl-CoA synthetase activity of rat liver microsomes. Substrate specificity with special reference to very-long-chain and isomeric fatty acids. Biochim Biophys Acta. 1981 May 22;664(2):416–427. doi: 10.1016/0005-2760(81)90064-3. [DOI] [PubMed] [Google Scholar]
- Rutsaert J., Tondeur M., Vamos-Hurwitz E., Dustin P. The cellular lesions of Farber's disease and their experimental reproduction in tissue culture. Lab Invest. 1977 May;36(5):474–480. [PubMed] [Google Scholar]
- Samuelsson K., Zetterström R. Ceramides in a patient with lipogranulomatosis (Farber's disease) with chronic course. Scand J Clin Lab Invest. 1971 Jun;27(4):393–405. doi: 10.3109/00365517109080235. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Krüger E., Melzner I. Studies on the biosynthesis of ceramide. Does the reversed ceramidase reaction yield ceramides? Hoppe Seylers Z Physiol Chem. 1980 May;361(5):773–779. doi: 10.1515/bchm2.1980.361.1.773. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Melzner I. Studies in vitro on the biosynthesis of ceramide and sphingomyelin. A reevaluation of proposed pathways. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):755–771. doi: 10.1515/bchm2.1980.361.1.755. [DOI] [PubMed] [Google Scholar]
- Sugita M., Connolly P., Dulaney J. T., Moser H. W. Fatty acid composition of free ceramides of kidney and cerebellum from a patient with Farber's disease. Lipids. 1973 Jul;8(7):401–406. doi: 10.1007/BF02531715. [DOI] [PubMed] [Google Scholar]
- Sugita M., Dulaney J. T., Moser H. W. Ceramidase deficiency in Farber's disease (lipogranulomatosis). Science. 1972 Dec 8;178(4065):1100–1102. doi: 10.1126/science.178.4065.1100. [DOI] [PubMed] [Google Scholar]
- Sugita M., Willians M., Dulaney J. T., Moser H. W. Ceramidase and ceramide synthesis in human kidney and cerebellum. Description of a new alkaline ceramidase. Biochim Biophys Acta. 1975 Jul 22;398(1):125–131. doi: 10.1016/0005-2760(75)90176-9. [DOI] [PubMed] [Google Scholar]
- Yavin E., Gatt S. Enzymatic hydrolysis of sphingolipids. 8. Further purification and properties of rat brain ceramidase. Biochemistry. 1969 Apr;8(4):1692–1698. doi: 10.1021/bi00832a052. [DOI] [PubMed] [Google Scholar]


