Abstract
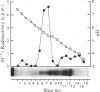
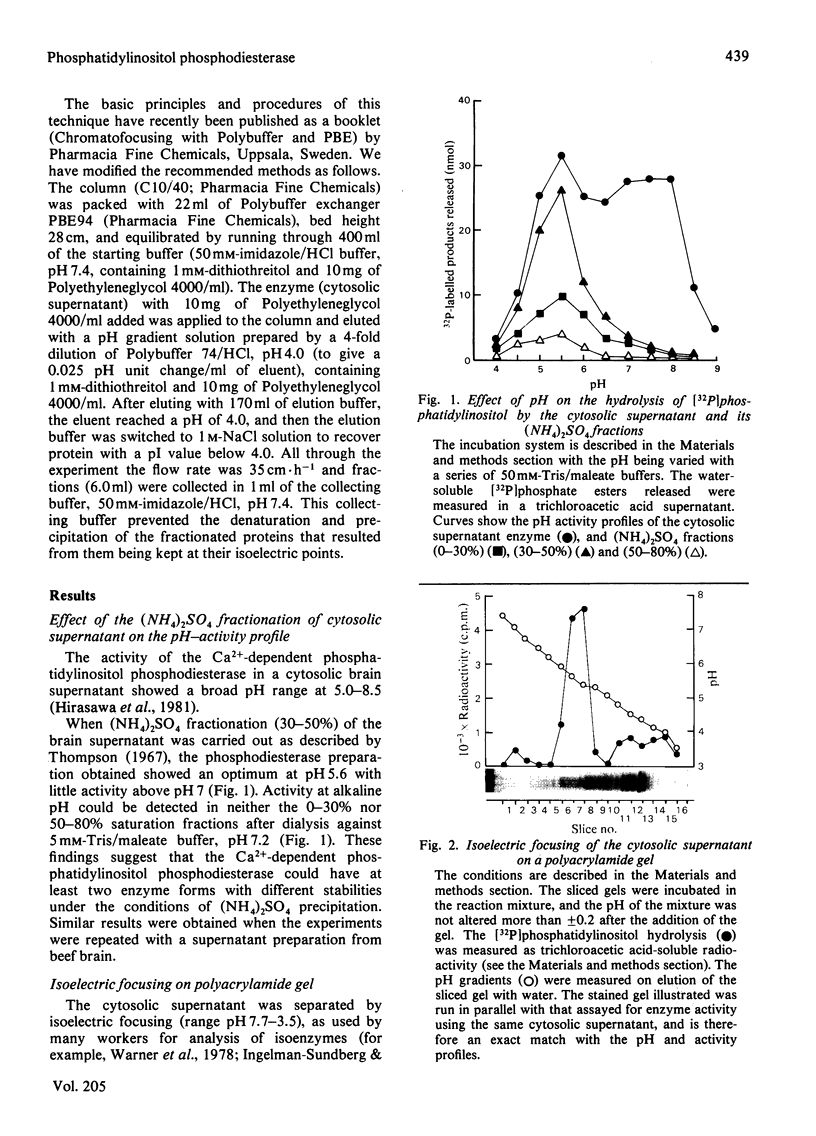
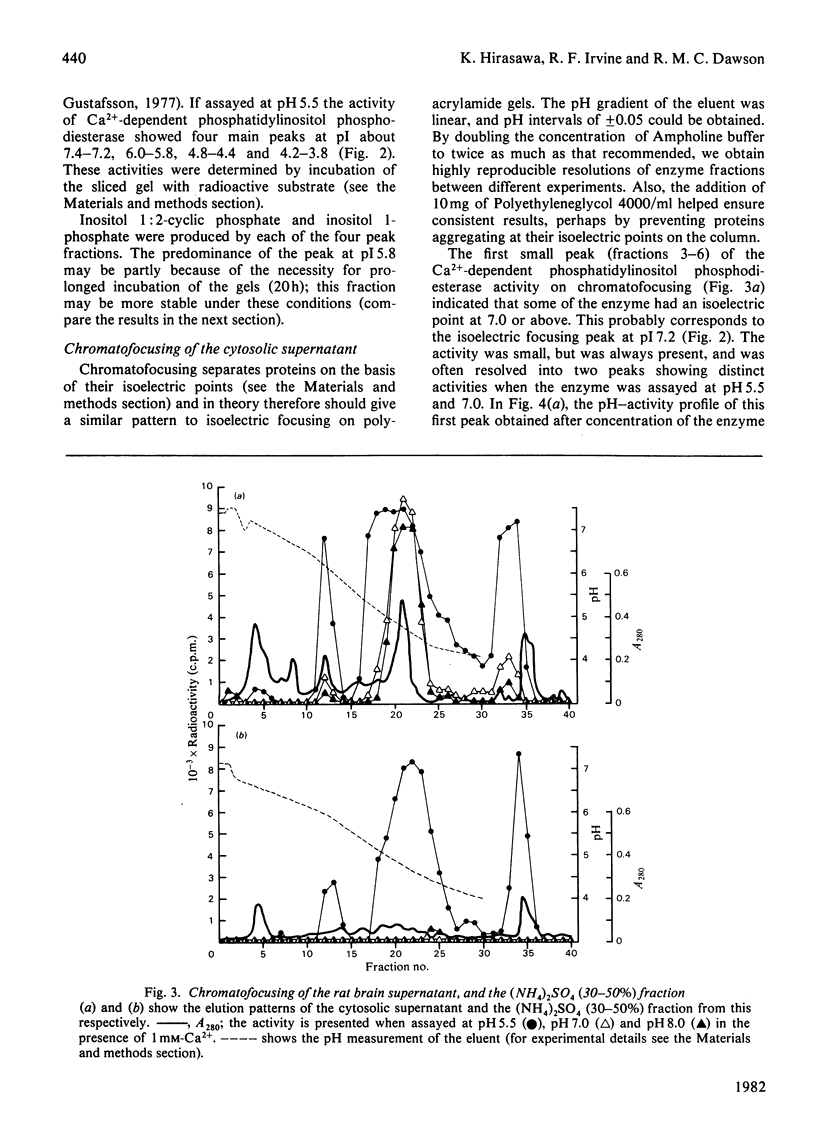
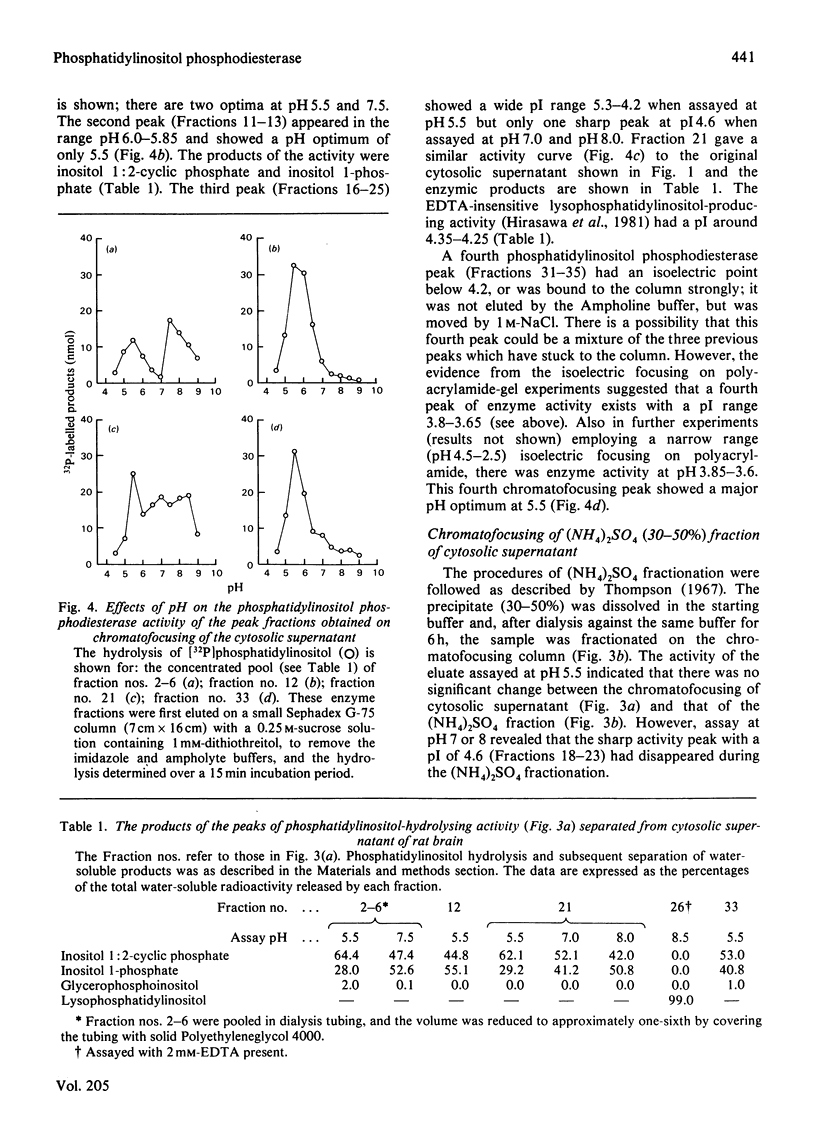
1. The Ca2+-dependent phosphatidylinositol phosphodiesterase (phospholipase C-type) from the cytosolic supernatant of rat brain was active against exogenous [32P]-phosphatidylinositol from pH5.0 to pH8.5. However, the activity in the range pH7.0–8.5 could not be recovered after precipitation with (NH4)2SO4; most of the enzyme activity was recovered in the 30–50% fraction and showed a single sharp pH optimum at 5.5. 2. The cytosolic supernatant was analysed by isoelectric focusing on acrylamide gels, and assay at pH5.5. Four peaks of phosphodiesterase activity were found at pI ranges 7.4–7.2, 6.0–5.8, 4.8–4.4 and 4.2–3.8. 3. The cytosolic supernatant was also applied to a chromatofocusing column, and again assayed at pH5.5. Four peaks were eluted: minor, but consistent, activity at the beginning of the elution with a pI of near 7.2 or above; a second peak at pH6.0–5.85; a third broad peak with a wide range pH5.3–4.2; and a fourth peak, which was eluted by washing the column with 1m-NaCl, suggesting an isoenzyme with a pI below 4.0 (supported by the result of the isoelectric focusing). 4. If all the chromatofocusing fractions were assayed at pH7.0 or 8.0 (at 1mm-Ca2+), only a single sharp peak was detected, with a pI of 4.6–4.8. This peak disappeared on (NH4)2SO4 fractionation (30–50%) of the cytosolic supernatant, whereas the four peaks with activity at pH5.5 were virtually unaffected. 5. The four activities (assayed at pH5.5) separated by chromatofocusing produced inositol 1:2-cyclic monophosphate, inositol 1-monophosphate and diacylglycerol as enzymic products. 6. We conclude that the Ca2+-dependent phosphatidylinositol phosphodiesterase exhibits considerable heterogeneity, both with respect to pH optima of activity, and its isoelectric properties.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Luke B., Smith J. P. Studies on the properties of a soluble phosphatidylinositol-phosphodiesterase of rabbit iris smooth muscle. Biochim Biophys Acta. 1980 Aug 7;614(2):425–434. doi: 10.1016/0005-2744(80)90232-6. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Phosphatidylinositol cleavage catalysed by the soluble fraction from lymphocytes. Activity at pH5.5 and pH7.0. Biochem J. 1974 Sep;142(3):591–597. doi: 10.1042/bj1420591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton R. S., Hawthorne J. N. The phosphoinositide inositolphosphohydrolase of guinea-pig intestinal mucosa. Eur J Biochem. 1968 Mar;4(1):68–75. doi: 10.1111/j.1432-1033.1968.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974 Mar;58(1):238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. Studies on the enzymic hydrolysis of monophosphoinositide by phospholipase preparations from P. notatum and ox pancreas. Biochim Biophys Acta. 1959 May;33(1):68–77. doi: 10.1016/0006-3002(59)90499-8. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. The measurement of 32P labelling of individual kephalins and lecithin in a small sample of tissue. Biochim Biophys Acta. 1954 Jul;14(3):374–379. doi: 10.1016/0006-3002(54)90195-x. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Hirasawa K., Hemington N. L. Hydrolysis of phosphatidylinositol by pancreas and pancreatic secretion. Biochim Biophys Acta. 1982 Feb 15;710(2):212–220. doi: 10.1016/0005-2760(82)90151-5. [DOI] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. The catabolism of phosphatidylinisitol by an EDTA-insensitive phospholipase A1 and calcium-dependent phosphatidylinositol phosphodiesterase in rat brain. Eur J Biochem. 1981 Nov;120(1):53–58. doi: 10.1111/j.1432-1033.1981.tb05669.x. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Gustafsson J. A. Resolution of multiple forms of phenobarbital-induced liver microsomal cytochrome P-450 by electrofocusing on granulated gels. FEBS Lett. 1977 Feb 15;74(1):103–106. doi: 10.1016/0014-5793(77)80763-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The calcium-dependent phosphatidylinositol-phosphodiesterase of rat brain. Mechanisms of suppression and stimulation. Eur J Biochem. 1979 Sep;99(3):525–530. doi: 10.1111/j.1432-1033.1979.tb13284.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J. 1978 Nov 15;176(2):475–484. doi: 10.1042/bj1760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP P., HUBSCHER G., HAWTHORNE J. N. Phosphoinositides. 3. Enzymic hydrolysis of inositol-containing phospholipids. Biochem J. 1961 Apr;79:193–200. doi: 10.1042/bj0790193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough K. M., Thompson W. Soluble and particulate forms of phosphoinositide phosphodiesterase in ox brain. Biochim Biophys Acta. 1972 Jul 7;270(3):324–336. doi: 10.1016/0005-2760(72)90197-x. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Thompson W. The hydrolysis of monophosphoinositide by extracts of brain. Can J Biochem. 1967 Jun;45(6):853–861. doi: 10.1139/o67-095. [DOI] [PubMed] [Google Scholar]
- Warner M., LaMarca M. V., Neims A. H. Chromatographic and electrophoretic heterogeneity of the cytochromes P-450 solubilized from untreated rat liver. Drug Metab Dispos. 1978 Jul-Aug;6(4):353–362. [PubMed] [Google Scholar]