Abstract
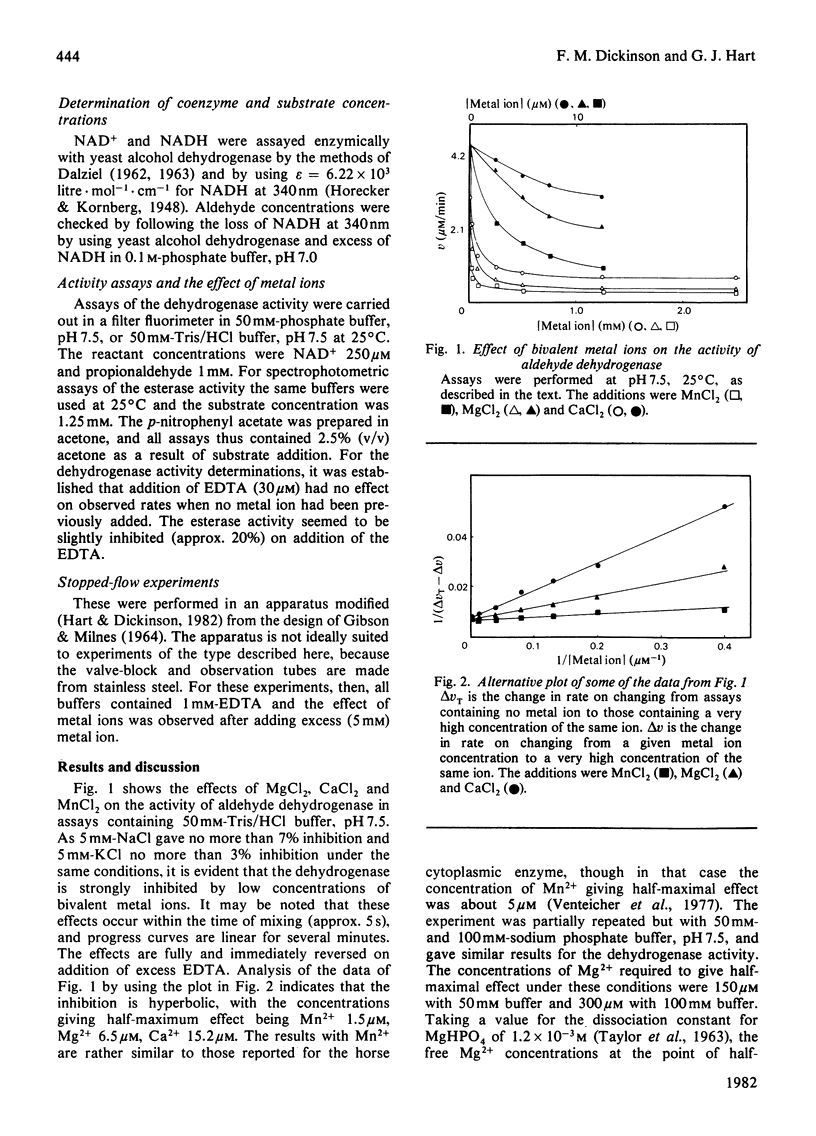
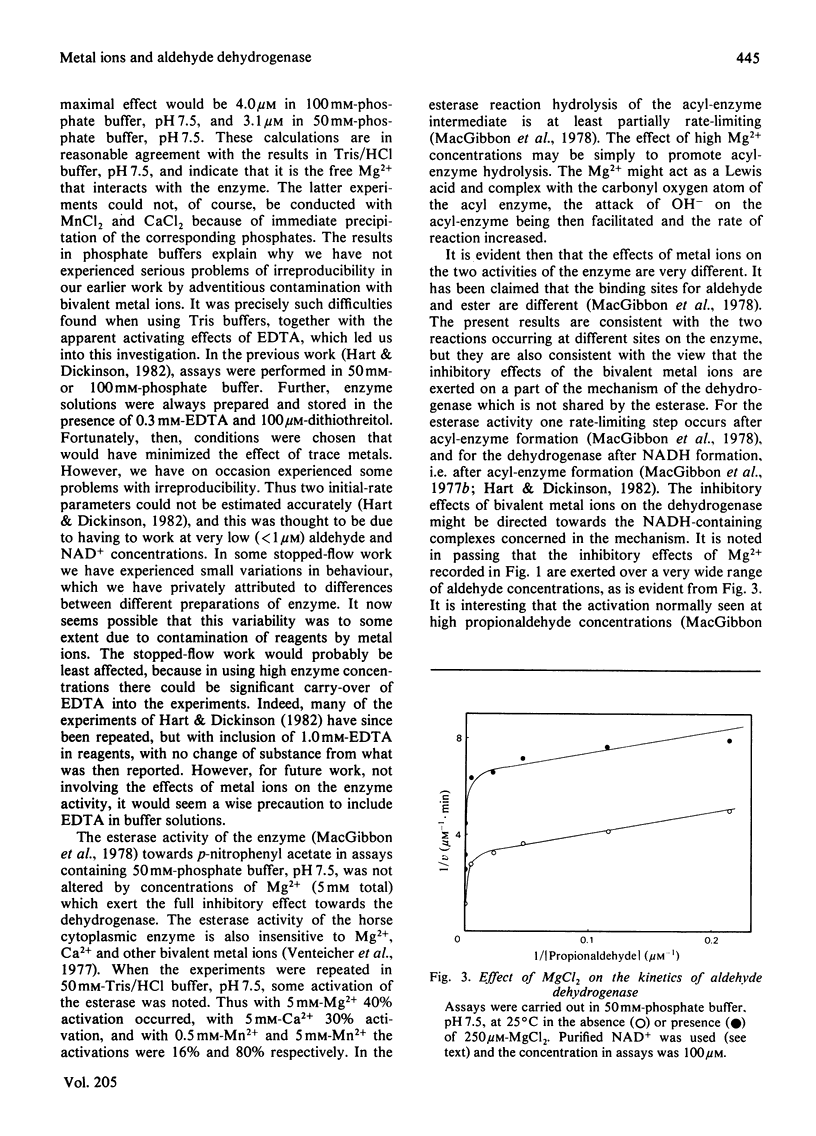
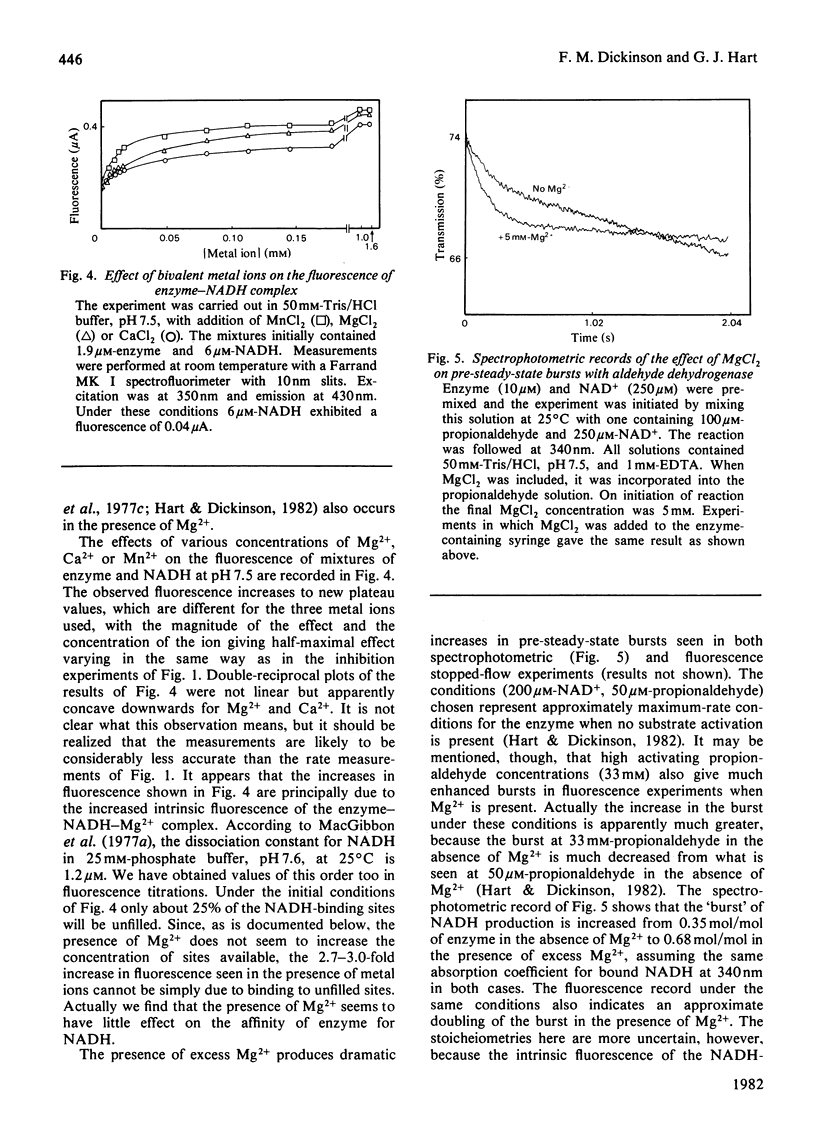
Sheep liver cytoplasmic aldehyde dehydrogenase is strongly inhibited by Mg2+, Ca2+ and Mn2+. The inhibition is only partial, however, with 8-15% of activity remaining at high concentrations of these agents. In 50 mM-Tris/Hcl, pH 7.5, the concentrations giving half-maximal effect were: Mg2+, 6.5 micrometers; Ca2+, 15.2 micrometers; Mn2+, 1.5 micrometer. The esterase activity of the enzyme is not affected by such low metal ion concentrations, but appears to be activated by high concentrations. Fluorescence-titration and stopped-flow experiments provide evidence for interaction of Mg2+ with NADH complexes of the enzyme. As no evidence for the presence of increased concentrations of functioning active centres was obtained in the presence of Mg2+, it is concluded that effects of Mg2+ (and presumably Ca2+ and Mn2+ also) are brought about by trapping increased concentrations of NADH in a Mg2+-containing complex. This complex must liberate products more slowly than any of the complexes involved in the non-inhibited mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crompton M., Capano M., Carafoli E. Respiration-dependent efflux of magnesium ions from heart mitochondria. Biochem J. 1976 Mar 15;154(3):735–742. doi: 10.1042/bj1540735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Dickinson F. M., Hart G. J., Kitson T. M. The use of pH-gradient ion-exchange chromatography to separate sheep liver cytoplasmic aldehyde dehydrogenase from mitochondrial enzyme contamination, and observations on the interaction between the pure cytoplasmic enzyme and disulfiram. Biochem J. 1981 Dec 1;199(3):573–579. doi: 10.1042/bj1990573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Kinetic properties of highly purified preparations of sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1982 Jun 1;203(3):617–627. doi: 10.1042/bj2030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Some properties of aldehyde dehydrogenase from sheep liver mitochondria. Biochem J. 1977 May 1;163(2):261–267. doi: 10.1042/bj1630261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins W. T. Anomalous "saturation kinetics" occurring in the reaction of L-alpha-aminoadipate with pig heart aspartate aminotransferase. Arch Biochem Biophys. 1980 Nov;205(1):57–66. doi: 10.1016/0003-9861(80)90083-1. [DOI] [PubMed] [Google Scholar]
- MacGibbon A. K., Blackwell L. F., Buckley P. D. Pre-steady-state kinetic studies on cytoplasmic sheep liver aldehyde dehydrogenase. Biochem J. 1977 Nov 1;167(2):469–477. doi: 10.1042/bj1670469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Buckley P. D., Blackwell L. F. Evidence for two-step binding of reduced nicotinamide-adenine dinucleotide to aldehyde dehydrogenase. Biochem J. 1977 Sep 1;165(3):455–462. doi: 10.1042/bj1650455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Haylock S. J., Buckley P. D., Blackwell L. F. Kinetic studies on the esterase activity of cytoplasmic sheep liver aldehyde dehydrogenase. Biochem J. 1978 Jun 1;171(3):533–538. doi: 10.1042/bj1710533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Motion R. L., Crow K. E., Buckley P. D., Blackwell L. F. Purification and properties of sheep-liver aldehyde dehydrogenases. Eur J Biochem. 1979 Jun 1;96(3):585–595. doi: 10.1111/j.1432-1033.1979.tb13073.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Weiner H. Magnesium stimulation of catalytic activity of horse liver aldehyde dehydrogenase. Changes in molecular weight and catalytic sites. J Biol Chem. 1980 Sep 10;255(17):8206–8209. [PubMed] [Google Scholar]


