Abstract
Recanalizing acute ischemic stroke with carotid tandem occlusion (CTO) is technically challenging because distal embolic migration during revascularization can significantly influence outcomes. In this study, we aimed to introduce our coaxial balloon technique using a balloon-guiding catheter (BCG), angioplasty catheter, and aspiration catheter sequentially to prevent thrombus migration to a new vascular territory. We used this technique for six patients with CTO. Technical success with good revascularization of the CTO was achieved in all six patients (100%) without neurological complications, including one and five cases of modified Thrombolysis in Cerebral Infarction 2b and 3, respectively. The median duration of the procedure was 61 (interquartile range, 52–90) min. The mean National Institutes of Health Stroke Scale score at discharge was 3.5 (2–8), with favorable clinical outcomes at 90 days for three of six patients (50%). The coaxial balloon technique for CTO is safe and effective for revascularization in patients with acute ischemic stroke. Thrombectomy before proximal stenting was associated with shorter reperfusion times and better clinical outcomes. Therefore, this approach is recommended for tandem occlusions requiring stent angioplasty.
Keywords: Balloon-assisted tracking technique, cerebral blood flow, neuroradiology, reperfusion, revascularization
Introduction
Atherosclerosis with or near occlusion of the extracranial carotid artery in conjunction with embolism of its distal branches (i.e., the middle cerebral artery) represents a specific subgroup of acute ischemic stroke (AIS), namely the carotid tandem occlusion (CTO).1–4 The prognosis of CTO is poor when treated with intravenous (IV) thrombolysis alone because of the reduced supply of the thrombolytic drug due to occlusion of the supplying vessel and a large thrombus.5,6 This stroke subtype is underrepresented or excluded from randomized controlled trials; however, endovascular thrombectomy (EVT) improves its clinical outcomes, as demonstrated in the subgroup analyses of ESCAPE and MRCLEAN trials.7,8
Proximal occlusion of the carotid artery increases the burden of thrombi within the occluded segment and the risk of distal embolization into the territories of the anterior cerebral artery (ACA) and middle cerebral artery (MCA) during reopening of the vessel. 9 Mechanical thrombectomy for this lesion is complex. The procedure often involves multiple catheters and devices, is prolonged, and has worse clinical outcomes than isolated MCA occlusion 10 ; thus, newer recanalization techniques are needed. A balloon-guiding catheter (BGC) and a distal filter can be combined to minimize the risk of embolic complications. 11 A German multicenter study reported seemingly better clinical outcomes in terms of modified Thrombolysis in Cerebral Infarction (mTICI) 2b and 3 and modified Rankin Scale (mRS) score 0–2 after applying their ReWiSed CARe technique in a one-stage treatment for tandem extracranial and intracranial occlusions. 12 The German procedure dealt with the carotid lesion first before performing mechanical thrombectomy for the cerebral arterial thrombus. In this study, we aimed to introduce our innovative modification of the procedure and use exemplary cases to demonstrate the technical feasibility, safety, and outcomes of the coaxial balloon technique for EVT in patients with AIS due to CTO.
Material and methods
Ethics approval
This study was approved by the Institutional Review Board of Kuang Tien General Hospital (approval number KTGH 11322). The Institutional Review Board also waived the need for consent because of the nature of this study, which focused on interventional neuroradiological techniques.
Study design, setting, and population
We developed an endovascular angioplasty procedure for patients with AIS due to CTO. We evaluated the performance of this coaxial balloon technique by analyzing the neurological outcome measures.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were used to ensure the selection of patients most likely to benefit from the coaxial balloon technique while minimizing the risks associated with the procedure. The inclusion criteria were as follows: patients with AIS due to CTO, including both extracranial and intracranial occlusions; eligibility for endovascular thrombectomy and stent angioplasty using the coaxial balloon technique; age of 18 years and older; National Institutes of Health Stroke Scale (NIHSS) score ranging from 8 to 30; confirmed occlusion of the internal carotid artery (ICA) origin and tandem intracranial occlusion using computed tomography angiography (CTA) and/or magnetic resonance angiography (MRA); Alberta Stroke Program Early Computed Tomography (CT) Score (ASPECTS) of at least two on baseline CT; and onset-to-groin puncture time (OTP) within 6 h, with consideration up to 8 h if favorable perfusion imaging is present, indicated by an infarct core of less than 70 mL and a mismatched ratio greater than 1.8. The exclusion criteria included severe comorbidities that would preclude the patient from undergoing endovascular procedures; known bleeding diathesis or contraindications for anticoagulation and antiplatelet therapy; previous disabling stroke with a mRS score >3 before the current stroke; presence of intracranial hemorrhage or significant mass effect on initial imaging; absence of a suitable vascular access route for the endovascular procedure; OTP exceeding 24 h without favorable perfusion imaging; ASPECTS of zero; and patients who received IV thrombolysis within 3 h of symptom onset and achieved signs of recanalization or significant clinical improvement (NIHSS <8).
Coaxial balloon technique
All procedures were performed under general anesthesia in all included patients. After establishing groin access with a 9F sheath, a BGC (Cello; Medtronic, Minneapolis, MA, USA) was navigated into the distal common carotid artery (CCA) over a 0.035-inch guidewire and angiocatheter (JB2; Cook Medical, Bloomington, IA, USA). After carefully navigating the guidewire and angiocatheter through the steno-occlusive site of the proximal ICA, a 0.014-inch microguidewire with a length of 300 cm (Traxcess; Microvention, Aliso Viejo, CA, USA) was used for exchanging with the 0.035-inch guidewire and advancing further into the distal ICA. A balloon angioplasty catheter, usually 3.5 or 4 mm in diameter and 20 or 30 mm in length (Sterling; Boston Scientific, Marlborough, MA, USA), replaced the angiocatheter and was advanced along with the microguidewire.
Subsequently, the balloon of the BGC in the CCA was inflated for flow arrest, establishing proximal protection and minimizing the possibility of distal clot embolic migration. After balloon angioplasty, an aspiration catheter was advanced along the microguidewire into the ICA, and the M1 segment was placed under continuous aspiration. A stent-retriever thrombectomy was performed instead for more peripheral occlusions, such as the M2 segment or unsuccessful clot aspiration. After confirming complete vessel recanalization, the BCG was deflated. Elective carotid stenting of the proximal ICA was performed if postprocedural dynamic CT showed no intracranial hemorrhage and more than 50% residual stenosis was present.
After carotid stenting, an IV bolus of tirofiban, a glycoprotein IIb/IIIa antagonist, was administered, followed by continuous IV infusion for 24 h. Aspirin (500 mg) and clopidogrel (300 mg) were administered orally when the patients exhibited no active hemorrhage. Aspirin (100 mg/day) and clopidogrel (75 mg/day) were continued for 3 months, followed by lifelong antiplatelet monotherapy with either aspirin or clopidogrel. Aspirin monotherapy (100 mg/day) was administered instead of dual antiplatelet therapy in patients with a high risk of bleeding complications.
Post-procedure care
Following the revascularization procedure, all patients were sent to the neurointensive care unit, and neurologists reevaluated their neurological status.
Efficacy measures
All patients underwent magnetic resonance imaging follow-up 24 h post procedure. Post-procedural brain CT was performed within 3 days or if the NIHSS score increased by 4 points. In our hospital, the routine timing for NIHSS assessments is as follows: at admission, 2 h, 4 h, and 8 h; on days 1, 7, 14, 21, and 28 post treatment; and at discharge, to monitor the patient’s progress and treatment complications. The regular schedule for mRS scoring was performed at admission to assess the pre-admission status and at months 1, 3, and 6 post-treatment to evaluate the patient’s recovery and the overall functional status over time. To enhance efficiency, we provided only the NIHSS and mRS scores at the time of discharge. Neurological assessments were performed over telephone after discharge.
Results
We included six patients in this study; all were male, and their mean age was 68 years (range, 57–84 years). The mean NIHSS score at presentation was 12 (range, 9–20). Successful revascularization with subsequent carotid artery treatment was feasible in all six patients. BGC could function as a post-stenting angioplasty balloon. In these six cases, we did not resort to the use of any angioplasty balloon catheter. The estimated post-dilation residual stenosis rate measured by angiogram was less than 10% in all cases. Overall, successful reperfusion mTICI ≥2b was achieved in all patients, and 5/6 (83%) individuals had complete reperfusion (mTICI = 3). Marked clinical improvement was observed in all patients. The median duration of the procedure was 61 min (interquartile range [IQR], 52–90). The median NIHSS at discharge was 3 (IQR 2.5–4), with favorable clinical outcomes at 90 days in three out of six patients (50%). One patient was discharged with an mRS score of 4, which improved to 3 at 6 months. The clinical presentation and outcomes are summarized in Table 1.
Table 1.
Demographic data, clinical characteristics, treatments, prognostic scoring, and outcomes of the case series.
| Case | Age/Sex | Tandem occlusion sites | Stroke risk factors | IV rtPA | ASPECTS | Initial NIHSS | Discharge NIHSS | OTP (min) | Procedure time (min) | Recanalization mTICI | Carotid stenting | sICH | mRS Discharge/3 months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64/M | R-ICA + car-T | Previous stroke HTN Smoking |
Y | 9 | 9 | 3 | 230 | 61 | 3 | Y | N | 2/0 |
| 2 | 62/M | R-ICA + MCA | Previous stroke HTN Smoking |
N | 7 | 10 | 2 | 377 | 115 | 3 | Y | N | 3/3 |
| 3 | 57/M | R-ICA + MCA | HTN T2DM Dyslipidemia |
N | 10 | 12 | 4 | 420 | 46 | 2b | Y | N | 2/0 |
| 4 | 73/M | R-ICA + MCA | Previous stroke | N | 8 | 19 | 3 | 174 | 64 | 3 | Y | Y | 4/3 |
| 5 | 68/M | L-ICA + MCA | Smoking | N | 2 | 17 | 8 | 260 | 60 | 3 | Y | N | 4/3 |
| 6 | 84/M | L-ICA + distal-ICA | Lung cancer HTN |
N | 10 | 20 | 4 | 360 | 57 | 3 | Y | N | 3/2 |
Notes: Abbreviations: ASPECTS: Alberta Stroke Program Early CT Score; car-T: carotid terminus; HTN: hypertension; ICA: internal carotid artery; IV rtPA: intravenous recombinant tissue plasminogen activator; L: left; M: male; MCA: middle cerebral artery; mo: months; mRS: modified Rankin Scale score at 90 days; NIHSS: National Institutes of Health Stroke Scale; OTP: time from onset of stroke to groin puncture; R: right; sICH: secondary intracerebral hemorrhage; T2DM: type 2 diabetes; TICI: Thrombolysis in Cerebral Infarction.
Footnote: Regarding Case 4, the patient fell and sustained scalp laceration at admission. It was determined that rtPA was better not to be administered. Instead, the patient underwent thrombectomy procedure directly. An intracranial hemorrhage was detected on the MRI conducted 24 h after treatment, likely resulting from a hyperperfusion injury. Intracerebral hemorrhage was not found at presentation.
Representative illustrations
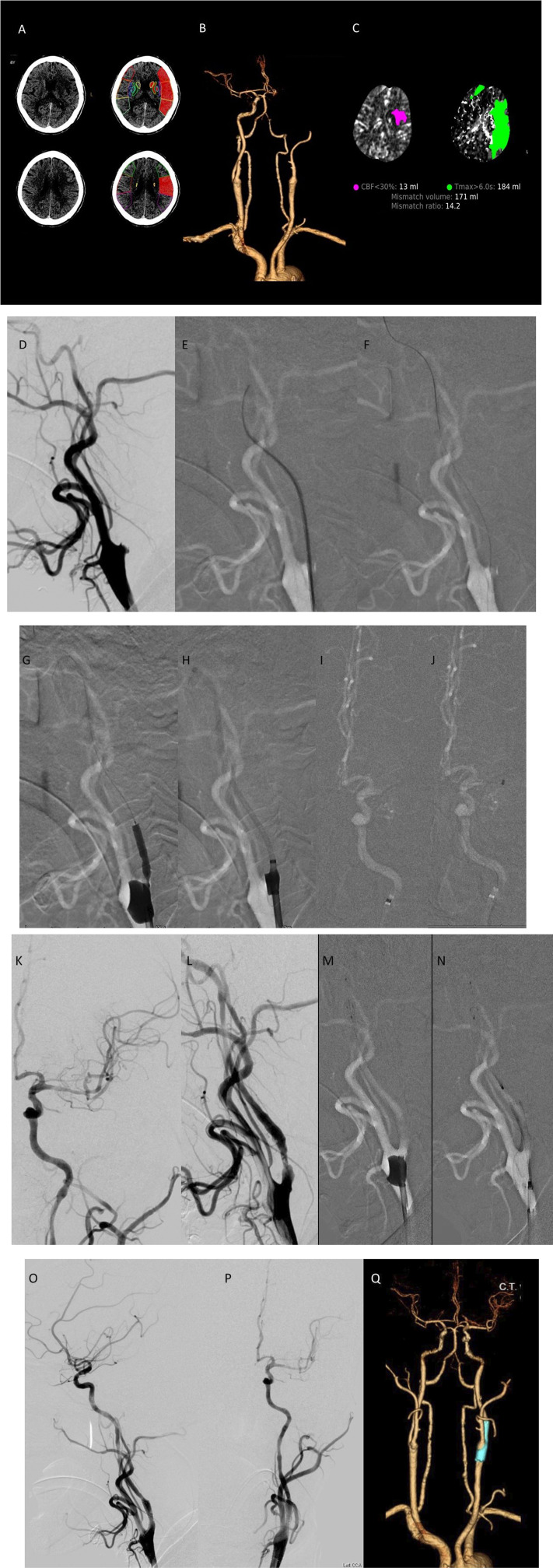
A 68-year-old man (Case 5, Table 1) presented with a sudden onset of right hemiparesis and aphasia. The NIHSS score at admission was 17. Baseline axial CT performed 190 min after symptom onset showed faint hypodensity in the left cerebral hemisphere, with a RAPID-ASPECTS score of 3 (Figure 1(A)). CTA and three-dimensional (3D) reconstruction revealed a left-ICA origin occlusion and tandem intracranial occlusion (Figure 1(B)). CT perfusion (CTP) RAPID maps (IschemaView) showed a perfusion deficit (Tmax >6 s) of 184 mL and an ischemic core (CBF <30%) of 13 mL in the left frontotemporal-parietal lobe (Figure 1(C)).
Figure 1.
Detailed illustrative imaging of Case 5 demonstrating tandem occlusions and successful recanalization using the coaxial balloon technique. (A)–(C) Baseline CT, CTA, and CTP performed 190 min after the symptom onset. (D)–(P) Left carotid angiography showing ICA-origin occlusion and tandem intracranial occlusion and successful recanalization of the vessels after the procedure. (Q) CTA follow-up 1 week after the procedure showing patency of the carotid stent and M1. CT, computed tomography; CTA, computed tomography angiography; CTP, computed tomography perfusion; ICA, internal carotid artery.
Endovascular thrombectomy was performed under general anesthesia. The OTP duration was 260 min. A 9 F sheath was inserted via the right femoral artery, and a 9F BGC (Cello; Medtronic, USA) was placed just proximal to the carotid stump (Figure 1(D)). Left CCA arteriography revealed ICA-origin occlusion (Figure 1(E)). A 0.035-inch angled hydrophilic guidewire (Radifocus; Terumo, Japan) and angiocatheter (JB2; Cook Medical) were cautiously navigated across the occluded segment (Figure 1(E)). After navigating the guidewire and angiocatheter into the distal ICA, a 0.014-inch exchange-length (300-cm) steerable microwire (Transcend; Stryker, Kalamazoo, MI, USA) was used for exchanging with the 0.035-inch guidewire (Figure 1(F)). The JB2 angio-catheter was removed, and a 3.5 mm × 20 mm angioplasty balloon catheter (Sterling; Boston Scientific) was introduced over the microwire and positioned within the area of maximal stenosis. The balloon was inflated to the nominal pressure under fluoroscopy at a rate of 1 atm/15 s and subsequently deflated slowly (Figure 1(G)). The angioplasty balloon catheter was removed after the occlusion segment was opened. The BGC was partially deflated and advanced into the bulb to prevent ICA flow arrest. An aspiration catheter (React 68; Medtronic, Dublin, Ireland) was introduced up to the proximal M1 under continued machinery aspiration (Figures 1(H)–(J)). The left carotid injection revealed a complete reopening of M1 after aspiration (Figure 1(K)), and the aspiration catheter was removed. Left common carotid injection from the BCG demonstrated persistent segmental stenosis at the proximal ICA (Figure 1(L)). After confirming the absence of intracranial hemorrhage using angiography suite cone-beam CT, the BGC was inflated again. A 4-mm embolic protection device (SpiderFX; Medtronic, USA) was deployed in the distal ICA, and a 7 mm × 40 mm self-expanding stent (Protégé; Medtronic, USA) was advanced and deployed over the stenosis (Figures 1(M) and (N)). Before removing the protective device, the BGC was deflated and advanced into the stent for post-dilation (not shown). Left carotid injection after thrombectomy and carotid stenting revealed mTICI 3 reperfusion (Figures 1(O) and (P)). The total duration of the procedure was 60 min. Follow-up CTA 1 week later confirmed stent and MCA patency (Figure 1(Q)). The patient was discharged for rehabilitation on day 9 with an NIHSS score of 8 and mRS score of 3. At the 90-days follow-up, the patient still had an mRS score of 3, with minimal residual aphasia and right-sided weakness.
Discussion
In this study, we introduced our innovative coaxial balloon technique, which represents a significant enhancement over traditional methods for managing AIS with CTO. This technique leverages the combined efficacy of EVT with balloon angioplasty to effectively address both proximal and distal lesions. The challenges associated with CTOs, namely the complexity of lesions and the increased risk of distal embolic events, are met with an approach that ensures minimal embolic spread and optimizes revascularization.
Traditional approaches to AIS with CTO often involve direct stenting and EVT, which can be limited by the need for extensive manipulation within the vascular architecture, possibly leading to higher rates of distal embolization. 13 Among the notable technique advancements, the ReWiSed CARe technique has demonstrated robust results, achieving successful and complete reperfusion in 96% and 44% of patients, respectively, with a median revascularization duration of 63 min 12 A 55% favorable outcome rate was observed at 90 days, showcasing the efficacy and safety of the technique for handling complex atherosclerotic tandem occlusions through simultaneous carotid artery stenting and intracranial thrombectomy. 12 In contrast, our coaxial balloon technique employs a BGC that provides proximal flow arrest during the intervention, thereby reducing the risk of embolic complications. This is crucial, given the proximity of lesions to crucial brain territories supplied by the anterior and middle cerebral arteries. Studies by Gao et al. and Lockau et al. have shown that traditional techniques are effective but do not adequately address the challenge of embolic protection as effectively as the coaxial balloon technique.1,2 The similarities in procedural success and principles of minimizing distal embolization underlying our coaxial balloon technique and the ReWiSed CARe technique underscore a critical advancement in the management of AIS with CTO. Both techniques emphasize a combination of devices and procedural strategies designed to optimize revascularization while protecting against potential complications inherent for these complex interventions and the pathological vascular characteristics of the patients. Table 2 compares the procedural subtleties and outcomes among three different techniques for treating CTOs, demonstrating that the coaxial balloon technique used in the current study achieved 100% successful recanalization with zero thrombus migration, albeit with a higher rate of symptomatic intracerebral hemorrhage compared to the other techniques.
Table 2.
Differences in the subtleties of the procedure compared between the TITANS registry, the ReWiSed CARe, and our coaxial balloon technique.
| Study acronym | Titans14,15 | ReWiSed CARe | Yen et al. (Current study) |
|---|---|---|---|
| Full name | Thrombectomy In TANdem lesion | Retriever Wire Supported Carotid Artery Revascularization | Coaxial balloon technique |
| No. of patients | 395 | 23 | 6 |
| Timepoint of ICA recanalization | One-stage treatment (better outcome) | One-stage treatment | One-stage treatment |
| Head or neck first? | Not specified | Neck first | Head first |
| Type of guide wire | Not specified | Must use the “thin wire” as the guiding wire | No restriction. Any type of stent retriever |
| BGC use | Not specified | Optional | Mandatory |
| Prevention of thrombus migration | Not specified | No special protection. 13% developed distal embolization. | Uses BGC for protection. Zero migration. |
| Modified thrombolysis in cerebral infarction score 2b–3 | 76% | 96% | 100% |
| Modified ranking scale score 0–2 | 52.2% | 55% | 50% |
| Symptomatic ICH | 13.8% | 0% | 16% |
| Mortality | 13.2% | 20% | 0% |
Abbreviations: BGC: balloon guiding catheter; ICA: internal carotid artery; ICH: intracerebral hemorrhage.
A recent systematic review of large multicenter studies demonstrated the efficacy of EVT in achieving successful reperfusion in 88% of cases with tandem occlusions; however, the complete reperfusion was only achieved in 40%. 16 Our method has shown a 100% technical success rate with substantial improvements in NIHSS scores and 50% favorable outcomes at 90 days. Compared with traditional approaches, our technique minimizes distal embolic complications and shortens reperfusion times, demonstrating superior safety and efficacy. The strength of our study lies in its technically feasible and safer procedural approach that effectively addressed both proximal and distal lesions, ensuring minimal embolic spread and optimizing revascularization success. Our results align with the highest standards of current endovascular treatments, offering significant advancements in the management of complex AIS cases with CTOs.
Our findings of a 100% technical success rate in the case series, with substantial improvements in NIHSS scores postprocedure, are consistent with the results of other recent studies, underscoring the efficacy of endovascular techniques for AIS. For instance, the ESCAPE and MRCLEAN trials demonstrated improved outcomes in similar patient cohorts through EVT, although a specific focus on innovative techniques such as ours was lacking.7,17 The coaxial balloon technique has shown promising results related to reperfusion quality and clinical outcomes, which are comparable to or better than those reported in the recent literature.
Our study limitations include a small number of patients enrolled and the procedure has a learning curve and operator-dependent nature. The results of this study are promising; nevertheless, this technique may benefit from further refinement. Advances in catheter technology, imaging, and adjunctive pharmacotherapy have enhanced the precision and efficacy of coaxial balloon techniques. The coaxial balloon technique offers several advantages, including streamlining the procedure, reducing the risk of distal thrombus migration, and achieving high technical success rates; however, it also presents challenges such as the need for a learning curve, potential complications related to balloon-guiding catheter manipulation, and increased initial procedure costs (Table 3). Prospective randomized studies involving larger patient populations are needed to establish definitive evidence of its superiority over traditional methods. Additionally, exploring the integration of this technique with other neuroprotective strategies is a vital area for future research.
Table 3.
The advantages and potential challenges or considerations of the coaxial balloon technique.
| Considerations/variables | Perceived or potential advantages | Potential challenges or other considerations |
|---|---|---|
| One-stage operation | - Streamlines the procedure, reducing total time spent in the interventional suite. - Potentially reduces the overall risk of complications by limiting the number of procedural steps. |
- May be challenging for patients in high risk of intracranial hemorrhage, such as those with a large infarct core about 70 mL |
| Balloon-guiding catheter (BGC) use | - Provides proximal flow arrest, reducing the risk of distal thrombus migration during the procedure. - Enhances control over the intervention site, improving safety and efficacy. - Reduces the procedural time. |
- Requires a learning curve, especially in navigating and positioning the BGC effectively. - The added device cost and the potential for complications related to BGC manipulation. |
| Procedure success rate | - Achieved 100% technical success in the study, indicating high efficacy in appropriate cases. | - The success rate may vary with operator experience and case complexity, particularly in cases with severe stenosis or challenging anatomy. |
| Procedure adaptability | - Versatile technique that can be adapted to various anatomical challenges. | - Requires tailored approaches for different anatomical variations, which may complicate the procedure. |
| Risk mitigation | - BGC use for proximal flow arrest provides enhanced protection against embolic complications. | - Full benefits of risk mitigation may only be realized by experienced operators. |
| Cost-Effectiveness | - Overall cost savings than staged operations. | - The need for specialized equipment like BGC increases initial procedure costs. |
In conclusion, the ability of the coaxial balloon technique to effectively manage thrombus migration is a crucial advantage that an interventional neuroradiology service requires. This capability is even more relevant, given the high-risk nature of CTO, where the proximal component of the occlusion often harbors unstable thrombi that are prone to dislodgement. By incorporating both proximal flow arrest and targeted balloon angioplasty, this technique allows for a controlled environment during the intervention, potentially decreasing the risk of stroke progression and improving patient outcomes. Our coaxial balloon technique for AIS with CTO is robust for managing a challenging subgroup of patients with stroke. It can improve clinical outcomes by mitigating the risks associated with traditional methods and paves the way for further innovations in interventional neuroradiology. Ongoing development and rigorous evaluation of this technique will be essential to define its role in the future landscape of stroke management.
Acknowledgements
We are grateful to the Department of Neurology, Department of Emergency Medicine, and Department of Radiology, all of Kuang Tien General Hospital, Taichung, for providing the necessary support while conducting all procedures using the coaxial balloon technique.
Footnotes
Author contributions: Conceptualization: PSY. Formal Analysis: PSY, VCK. Funding Acquisition: PSY. Investigation: PSY, VCK, YHL, YTW, LYK. Methodology: PSY, VCK. Project Administration: PSY, VCK. Resources: PSY. Supervision: PSY. Validation: PSY, VCK. Visualization: PSY, VCK. Writing – Original Draft Preparation: PSY, VCK. Writing – Review & Editing: PSY, VCK, YHL, YTW, LYK.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement
Ethical considerations
This study was approved by the Institutional Review Board of Kuang Tien General Hospital (approval number KTGH 11322).
Consent to participate
The Institutional Review Board waived the need for consent because of the nature of this study, which focused on interventional neuroradiological techniques.
ORCID iD
Victor C. Kok https://orcid.org/0000-0003-3440-8154
References
- 1.Gao F, Joyce Lo W, Sun X, et al. Combined use of stent angioplasty and mechanical thrombectomy for acute tandem internal carotid and middle cerebral artery occlusion. NeuroRadiol J 2015; 28: 316–321. DOI: 10.1177/1971400915591679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockau H, Liebig T, Henning T, et al. Mechanical thrombectomy in tandem occlusion: procedural considerations and clinical results. Neuroradiology 2015; 57: 589–598. DOI: 10.1007/s00234-014-1465-5. [DOI] [PubMed] [Google Scholar]
- 3.Jadhav AP, Zaidat OO, Liebeskind DS, et al. Emergent management of tandem lesions in acute ischemic stroke. Stroke 2019; 50: 428–433. DOI: 10.1161/STROKEAHA.118.021893. [DOI] [PubMed] [Google Scholar]
- 4.Di Donna A, Muto G, Giordano F, et al. Diagnosis and management of tandem occlusion in acute ischemic stroke. Eur J Radiol Open 2023; 11: 100513. DOI: 10.1016/j.ejro.2023.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozdemir O, Bussière M, Leung A, et al. Intra-arterial thrombolysis of occluded middle cerebral artery by use of collateral pathways in patients with tandem cervical carotid artery/middle cerebral artery occlusion. AJNR Am J Neuroradiol 2008; 29: 1596–1600. DOI: 10.3174/ajnr.A1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anadani M, Marnat G, Consoli A, et al. Endovascular therapy with or without intravenous thrombolysis in acute stroke with tandem occlusion. J Neurointerventional Surg 2022; 14: 314–320. DOI: 10.1136/neurintsurg-2020-017202. [DOI] [PubMed] [Google Scholar]
- 7.Assis Z, Menon BK, Goyal M, et al. Acute ischemic stroke with tandem lesions: technical endovascular management and clinical outcomes from the ESCAPE trial. J Neurointerventional Surg 2018; 10: 429–433. DOI: 10.1136/neurintsurg-2017-013316. [DOI] [PubMed] [Google Scholar]
- 8.Compagne KCJ, Goldhoorn RB, Uyttenboogaart M, et al. Acute endovascular treatment of patients with ischemic stroke from intracranial large vessel occlusion and extracranial carotid dissection. Front Neurol 2019; 10: 102. DOI: 10.3389/fneur.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YC, Rivera J, Peter K. Tandem stenosis to induce atherosclerotic plaque instability in the mouse. Methods Mol Biol 2015; 1339: 333–338. DOI: 10.1007/978-1-4939-2929-0_23. [DOI] [PubMed] [Google Scholar]
- 10.Nolan NM, Regenhardt RW, Koch MJ, et al. Treatment approaches and outcomes for acute anterior circulation stroke patients with tandem lesions. J Stroke Cerebrovasc Dis 2021; 30: 105478. DOI: 10.1016/j.jstrokecerebrovasdis.2020.105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy C, Heger J, Balogh G, et al. Endovascular recanalization of tandem internal carotid occlusions using the balloon-assisted tracking technique. Clin Neuroradiol 2022; 32: 375–384. DOI: 10.1007/s00062-021-01078-2. [DOI] [PubMed] [Google Scholar]
- 12.Maus V, Behme D, Maurer C, et al. The ReWiSed CARe technique : simultaneous treatment of atherosclerotic tandem occlusions in acute ischemic stroke. Clin Neuroradiol 2020; 30: 489–494. DOI: 10.1007/s00062-019-00795-z. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Lee DG, Kwon SU, et al. Relay-balloon technique for recanalization of acute symptomatic proximal internal carotid artery occlusion with short balloon-tipped guiding catheter landing zone. J Neurointerventional Surg 2018; 10: 39–43. DOI: 10.1136/neurintsurg-2016-012900. [DOI] [PubMed] [Google Scholar]
- 14.Gory B, Haussen DC, Piotin M, et al. Impact of intravenous thrombolysis and emergent carotid stenting on reperfusion and clinical outcomes in patients with acute stroke with tandem lesion treated with thrombectomy: a collaborative pooled analysis. Eur J Neurol 2018; 25: 1115–1120. DOI: 10.1111/ene.13633. [DOI] [PubMed] [Google Scholar]
- 15.Papanagiotou P, Haussen DC, Turjman F, et al. Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC Cardiovasc Interv 2018; 11: 1290–1299. DOI: 10.1016/j.jcin.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Grigoryan M, Haussen DC, Hassan AE, et al. Endovascular treatment of acute ischemic stroke due to tandem occlusions: large multicenter series and systematic review. Cerebrovasc Dis 2016; 41: 306–312. DOI: 10.1159/000444069. [DOI] [PubMed] [Google Scholar]
- 17.Berkhemer OA, Borst J, Kappelhof M, et al. Extracranial carotid disease and effect of intra-arterial treatment in patients with proximal anterior circulation stroke in MR CLEAN. Ann Intern Med 2017; 166: 867–875. DOI: 10.7326/M16-1536. [DOI] [PubMed] [Google Scholar]