Abstract
Contactin-associated protein (CNTNAP1) gene mutations have been reported in cases of congenital hypomyelinating neuropathy (CHN), a rare hereditary neuropathy. We present a case of a term male infant born at 39 weeks 4 days with respiratory distress, impaired swallow function, and hypotonia. Neurological workup for structural, autoimmune, neuromuscular, and metabolic etiologies was negative and whole exome sequencing revealed a novel mutation in the CNTNAP1 gene, consistent with a diagnosis of CHN3. While CHN3 cases with mutations in the same domain have required long-term respiratory support, our patient, now 2 years old, has not required respiratory support since his initial birth hospitalization. Neurologically, he now has central hypotonia with hypertonia in the bilateral extremities and global developmental delay. This case adds to a growing number of identified pathological CNTNAP1 mutations and their heterogenous clinical phenotypes and highlights a rare neurological etiology for respiratory distress in newborns.
Keywords: Contactin-associated protein (CNTNAP1), congenital hypomyelinating neuropathy (CHN), neonatology, neurology
Introduction
Congenital hypomyelinating neuropathy (CHN) is a rare hereditary neuropathy that presents heterogeneously at birth but is often characterized by respiratory distress, hypotonia, and cranial nerve abnormalities with decreased nerve conduction velocities and hypomyelination. 1 There is genetic heterogeneity within CHN, with CHN3 (OMIM #618186) being associated with homozygous or compound heterozygous mutations in the contactin-associated protein (CNTNAP1) gene. At least 39 pediatric cases of CNTNAP1-associated disease have been identified. We report a case of a term male infant who presented at birth with respiratory distress, copious oral secretions, hypotonia, and poor swallow function. Whole exome sequencing identified a novel mutation in the CNTNAP1 gene, consistent with a diagnosis of CHN3. In contrast to the majority of CHN3 cases, the patient’s initial respiratory requirements improved, and he was successfully weaned to room air. This case highlights how identification of novel mutations contributes to further understanding of genotype-phenotype correlations in rare diseases and a neurological etiology for neonatal respiratory distress.
Case
The patient is a small gestational age-term male born at 39 weeks and 4 days via repeat c-section to a 34-year-old G3P2102 mother of Afghanistan descent. The mother received routine prenatal care, which was notable for gestational diabetes and prenatal ultrasounds showing intrauterine growth restriction with normal umbilical doppler, small abdominal measurements, and polyhydramnios. At birth, the infant weighed 2555 g. APGAR scores were 8 and 9. The infant was vigorous however had respiratory distress that was supported with continuous airway positive pressure (CPAP) and was subsequently admitted to a level 3 neonatal intensive care unit (NICU). By day 4 of life, he was noted on exam to have copious clear secretions requiring frequent oral suctioning, mild hypotonia, and an absent gag reflex. He had frequent bradycardia and oxygen desaturation episodes, and the inability to tolerate oral feeds.
Pediatric Neurology and Pediatric Otolaryngology were consulted. A bedside flexible laryngoscopy showed swollen and erythematous arytenoids with deficits in symmetrical vocal cord abduction and an absent gag reflex. Initial neurological exam noted a weak gag and cough, and mild truncal hypotonia. Head ultrasound and brain and spine MRI were normal. A chest x-ray was unremarkable for thoracic structural lesions that could cause compression. Evaluation for autoimmune and neuromuscular etiologies showed normal thyroid studies and myasthenic syndromes and a normal creatine kinase (CK) level of 130 U/L. A 24-h video electroencephalogram (EEG) was obtained due to intermittent staring episodes and noted no seizures. Hearing screen was also normal.
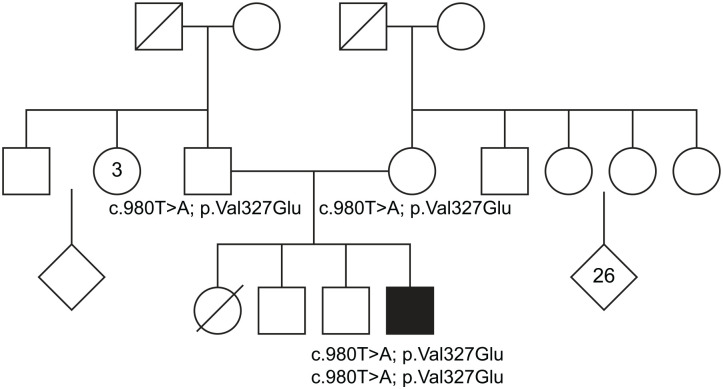
Genetics was consulted. Evaluation included a 3-generation pedigree, which noted 2 healthy brothers born at term and 1 sister born prematurely at 32 weeks who died on the first day of life. Per the parent’s account, the sister’s cause of death was thought to be due to prematurity and respiratory distress. The parents are of Afghanistan descent with a history of remote consanguinity as 6th-generation cousins (Figure 1). Metabolic and genetic testing included Prader Willi chromosomal microarray, metabolic syndromes, and California newborn screen were normal. Whole exome sequencing of the patient and parents identified a novel homozygous, autosomal recessive missense mutation (c.980T>A; p.Val327Glu) in the CNTNAP1 gene that was maternally and paternally inherited. Given the homozygous inheritance of this mutation in the CNTNAP1 gene along with the patient’s clinical presentation, this is most consistent with a diagnosis of congenital hypomyelinating neuropathy-3.
Figure 1.
Family pedigree with identified homozygous CNTNAP1 mutation in parents and patient. CNTNAP1: contactin-associated protein.
The infant continued to have significant secretions and an inability to tolerate oral feeds, requiring gastrostomy-tube placement. He was eventually weaned from CPAP and did not require respiratory support by 2 months of age. Repeat MRI at 3 weeks of age demonstrated normal brain growth and myelination. The patient was discharged from the NICU at 2 months of age and is now approximately 2 years old. A swallow study at 7 months of age demonstrated no evidence of aspiration or laryngeal penetration and the patient continues to be partially fed by mouth with gastrostomy-tube supplementation. The patient continues to have low truncal tone, now with appendicular hypertonia on baclofen, as well as global developmental delay.
Discussion
In this report, we present a case of CHN3 that presented prenatally with polyhydramnios, then respiratory distress, orobulbar dysfunction, and central hypotonia in the neonatal period. Through whole exome sequencing, we identified a novel mutation in the CNTNAP1 gene that has not been previously identified in the literature. This mutation (c.980T>A; p.Val327Glu) was homozygously inherited, and likely the cause of death in the patient’s deceased sister. CNTNAP1 gene mutations (OMIM #602346) are associated with both CHN3 and lethal congenital contracture syndrome 7 (LCCS7), which are thought to be part of a phenotypic spectrum caused by CNTNAP1 mutations. To date, at least 39 patients with CNTNAP1-associated mutations have been identified.2–4 Unlike other types of CHN that only affect peripheral nerves, CNTNAP1-related CHN3 affects the myelination of both central and peripheral nerves. 2 Previous reports of CNTNAP1 mutations report high mortality in early infancy. However, recent reports, including this case, have identified a broader spectrum of clinical outcomes. 2 Frequently reported features include polyhydramnios, respiratory distress, hypotonia, and swallowing/orobulbar dysfunction, consistent with our patients’ clinical presentation.3,4 Other features include developmental delay, hypomyelination on nerve biopsy, and brain MRI abnormalities such as cerebral atrophy and hypomyelination.1,2,4 Interestingly, our patient’s two MRIs did not show evidence of hypomyelination. However, it may be that brain MRI performed at an older age will be abnormal.
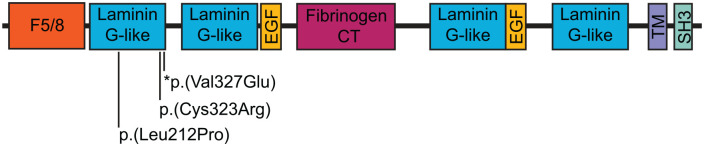
CNTNAP1 encodes CASPR (contactin-associated protein), which forms a key part of the paranodal junctions in both central and peripheral myelinated axons.2,5 Organization of the axon into distinct domains (the node of Ranvier and flanking paranode, juxtaparanode, and internode domains), is necessary for efficient transmission of action potentials in the nervous system. 6 When CASPR is disrupted, the paranodal domain fails to form, and nerve conduction is impaired.6,7 CASPR has also been found to be expressed in radial glial cells and to play a role in cerebral cortex development.8,9 In our patient, the location of the identified mutation (homozygous p.Val327Glu) is in the Laminin G 1 domain of the CASPR protein at p.175-335 (NP_003623.1) (Figure 2). Two other missense mutations in this domain have been previously reported (p.Cys323Arg, p.Leu212Pro) across 3 families (5 patients).1,10,11 Age of death ranged from at birth to 8 years or older.1,10,11 In cases that survived past birth, preterm birth, polyhydramnios, respiratory distress, and altered sensorineural hearing were present. Interestingly, our patient did not exhibit hearing loss (passed the hearing screen) and while respiratory distress was present, our patient required only CPAP at maximal support and was eventually weaned to room air. In prior cases, all non-deceased patients eventually required tracheostomy, suggesting that even mutations within the same domain may lead to significantly different clinical prognoses. Recent advances in genetic testing and the development of translational mouse models of specific CNTNAP1 mutations may provide new insights into the structure and function of CASPR and the expected clinical presentation of specific mutations. 7
Figure 2.
Schematic of CASPR protein and functional domains. Mutations identified in the Laminin G 1 domain are labeled. * indicates the novel mutation identified in patient. F5/8: coagulation factor 5/8 C-terminal domain; EGF: EGF-like domain; TM: transmembrane region; SH3: SH3 binding.
Conclusion
In summary, this report identifies a novel mutation in the CNTNAP1 gene that is pathogenic for CHN3. This adds to a growing number of identified CNTNAP1 mutations and provides further clinical data on the spectrum of presentations at birth, particularly in a case where long-term respiratory support with tracheostomy has not been required and patient survival has been greater than 1 year of age. We recommend the consideration of neurological and genetic workup in neonates with respiratory distress with hypotonia and cranial nerve dysfunction.
Acknowledgments
We would like to thank the patient and their family.
Footnotes
Author contributions: P.D.R. and P.L. conceived of the project. H.W., P.L. wrote and edited the manuscript. D.C., M.D.C., P.D.R., and P.L. reviewed the manuscript and participated in the care of this patient.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient’s parent for their anonymized information to be published in this article.
ORCID iD: Helen Wang  https://orcid.org/0000-0002-2345-3892
https://orcid.org/0000-0002-2345-3892
References
- 1. Low K, Stals K, Caswell R, et al. Phenotype of CNTNAP1: a study of patients demonstrating a specific severe congenital hypomyelinating neuropathy with survival beyond infancy. Eur J Hum Genet 2018; 26: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lesmana H, Vawter Lee M, Hosseini SA, et al. CNTNAP1-related congenital hypomyelinating neuropathy. Pediatr Neurol 2019; 93: 43–49. [DOI] [PubMed] [Google Scholar]
- 3. Sabbagh S, Antoun S, Mégarbané A. CNTNAP1 mutations and their clinical presentations: new case report and systematic review. Case Rep Med 2020; 2020: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garel P, Lesca G, Ville D, et al. CNTNAP1-encephalopathy: six novel patients surviving the neonatal period. Eur J Paediatr Neurol 2022; 37: 98–104. [DOI] [PubMed] [Google Scholar]
- 5. Einheber S, Zanazzi G, Ching W, et al. The axonal membrane protein Caspr, a homologue of Neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol 1997; 139: 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhat MA, Rios JC, Lu Y, et al. Axon-glia interactions and the domain organization of myelinated axons requires Neurexin IV/Caspr/Paranodin. Neuron 2001; 30: 369–383. [DOI] [PubMed] [Google Scholar]
- 7. Chang C, Sell LB, Shi Q, et al. Mouse models of human CNTNAP1-associated congenital hypomyelinating neuropathy and genetic restoration of murine neurological deficits. Cell Rep 2023; 42: 113274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Z-Q, Li D, Huang Y, et al. Caspr controls the temporal specification of neural progenitor cells through notch signaling in the developing mouse cerebral cortex. Cereb Cortex 2017; 27: 1369–1385. [DOI] [PubMed] [Google Scholar]
- 9. Li W, Yang L, Tang C, et al. Mutations of CNTNAP1 led to defects in neuronal development. JCI Insight 2020; 5: e135697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conant A, Curiel J, Pizzino A, et al. Absence of axoglial paranodal junctions in a child with CNTNAP1 mutations, hypomyelination, and arthrogryposis. J Child Neurol 2018; 33: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nizon M, Cogne B, Vallat J-M, et al. Two novel variants in CNTNAP1 in two siblings presenting with congenital hypotonia and hypomyelinating neuropathy. Eur J Hum Genet 2017; 25: 150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]