Abstract
The Total Thrombus-formation Analysis System (T-TAS) is an automated device using coated microchips to assess thrombus formation under flow conditions. Its value to monitor coagulation function in patients under antiplatelet therapy awaits further clarification. This study evaluated T-TAS to detect response to dual antiplatelet therapy (DAPT) in patients with peripheral artery disease (PAD).
T-TAS using the platelet-chip (PL-chip) and atheroma-chip (AR-chip) was performed in 60 patients with PAD on the day after lower extremity revascularization. Results were compared with light transmission aggregometry (LTA) and multiple electrode aggregometry (MEA, ADP- and ASPI-test). To determine T-TAS reference ranges, 30 healthy blood donors were enrolled.
The area under the curve of the PL-chip (AUC-PL) was outside the reference range in 91.2% and AUC-AR in 21.1% of the PAD patients. Low responders in MEA ASPI, MEA ADP or both tests and low responders in LTA induced by ADP had a significantly higher AUC-PL compared to responders (204 vs 70, p = .016 and 140 vs 32, p < .001), respectively. Median AUC-PL in low responders in LTA and MEA, LTA or MEA and in responders in LTA and MEA was 301, 104 and 32 (p = .001), respectively. Our results suggest that the PL-chip can continuously assess the level of response to DAPT and might be helpful to monitor PAD patients.
Keywords: antiplatelet monitoring, clopidogrel, dual antiplatelet therapy, peripheral artery disease, T-TAS
Introduction
Dual antiplatelet therapy (DAPT) with acetylsalicylic acid (ASA) and clopidogrel is standard of care in patients with peripheral artery disease (PAD) for at least one month after percutaneous transluminal angioplasty (PTA) and stent implantation to prevent vascular restenosis. 1 However, there is a large inter-individual variation of the in-vivo effects of these drugs. 2 There are several assays available to evaluate single pathways of platelet activation, but all have various limitations. Laboratory tests such as light transmission aggregometry (LTA), impedance aggregometry (multiple electrode aggregometry, MEA), VerifyNow and vasodilator-stimulated phosphoprotein-phosphorylation (VASP) are performed under unphysiological conditions, and the correlation between those tests remains modest.3–5
The Total Thrombus-formation System (T-TAS) is a standardized whole blood flow chamber system for the measurement of the in-vitro thrombus formation in ready-to-use pre-coated chips with microcapillaries and flow channels. The system includes a platelet chip (PL-chip), using 25 microcapillaries coated with type I collagen and shear rates of 1500/s, and an atheroma chip (AR-chip), which contains a single channel coated with collagen and tissue thromboplastin using 600/s shear rates. Blood flow is measured over time and aggregation of platelets leads to occlusion of the microcapillaries. While the PL-chip rather represents the function of primary hemostasis under arterial shear rates, the AR-chip further focuses processes of secondary hemostasis.6–8
There are several T-TAS studies in coronary artery disease patients (CAD) suggesting that the area under the curve of the PL-chip (AUC-PL) is a useful parameters to assess antiplatelet therapy in these patients 9 and that AUC-PL and the area under the curve of the AR-chip (AUC-AR) may predict bleeding events.10–12 However, studies examining T-TAS in PAD patients are currently lacking. In contrast to CAD patients, where the use of clopidogrel has been largely replaced by ticagrelor, clopidogrel is still the standard of care for PAD patients. 1 Therefore, the aim of this study was to investigate the role of the in-vitro thrombus formation in the T-TAS-system for the detection of low response to DAPT with clopidogrel and ASA in PAD patients and to compare this method with LTA and MEA.
Methods
Study Design and Participants
PAD patients who underwent revascularization of the lower extremity or pelvic vessels at the Department of Angiology, University Hospital Leipzig were prospectively enrolled. All patients were on long-term antiplatelet therapy with 100 mg ASA per day and received a loading dose of 300 mg clopidogrel at the day of intervention, followed by a daily dose of 75 mg clopidogrel. Exclusion criteria were revascularization of the carotid artery or upper extremity, thrombolysis, indication for therapeutic anticoagulation, treatment with antiplatelet drugs other than ASA or clopidogrel, treatment with non-steroidal anti-inflammatory drugs (NSAID) or metamizole, treatment with selective serotonin uptake inhibitors, intake of direct oral anticoagulants 24 h, a therapeutic dose of low molecular weight heparin (LMWH) 24 h or a prophylactic dose of LMWH 12 h before blood sampling. In addition, pregnant and lactating women and subjects < 18 years of age were excluded.
On the day after intervention, blood was drawn to perform T-TAS, LTA and MEA, assuming that inhibition levels close to steady state were reached within a few hours after a loading dose of 300 mg clopidogrel. 13 The glomerular filtration rate (GFR) and lipoprotein (a) were measured as part of the routine blood collection on the same day. Additional data on gender, age, comorbidities, performed intervention and medication were collected from medical records.
The study protocol was approved by the ethics committee of the University of Leipzig (reference 250/20-ek) and conducted in accordance with the Declaration of Helsinki. Written informed consent was given by all patients.
Blood Sampling and Measurements
A total of 17.8 mL blood was drawn from the cubital vein using a 21 Gauge needle and divided into 3 mL BAPA-tubes for T-TAS PL-chip, 14 1.8 mL vacutainers with 3.2% sodium-citrate for the AR-chip (Becton Dickinson, USA), 1.6 mL hirudin-tubes for MEA, and 3 times 3.8 mL of 3.8% sodium-citrate containing tubes (both Sarsted, Germany) for LTA. All samples were kept at room temperature. LTA was measured within two hours from blood sampling. T-TAS and impedance aggregometry were measured after a 30 min rest and within 2 h from blood sampling according to the manufacturer's instructions. Blood was centrifuged at 1800xg for 20 min to produce platelet poor plasma (PPP) and at 170 g for 10 min to produce platelet rich plasma (PRP). Left over plasma was stored at −80 °C until measurement.
T-TAS (Fujimori Kogyo Co. Ltd, ZACROS, Japan) was performed according to the manufacturer's instruction. For the T-TAS measurements, blood was pipetted into the Reservoir Set attached to the chips flow path. In the PL Chip, BAPA anticoagulated blood is pumped through collagen coated microcapillaries leading to a 1500/s wall shear rate which causes activation of platelets and platelet thrombus formation. For the AR-Chip measurement, citrate anticoagulated blood is mixed with 20 µL of the CaCTI reagent (CaCl2 plus corn-derived trypsin inhibitor [CTI], a FXIIa inhibitor) directly prior to the insertion into the Reservoir Set. Microcapillaries in the AR-Chip are coated with collagen and tissue thromboplastin to induce fibrin-rich thrombus formation. Blood is pumped through the capillaries of the AR-Chip inducing a wall shear rate of 600/s. The process in both chips is controlled by a pressure sensor that measures the increase in pressure during thrombus formation. The area under the flow pressure curve (AUC) was measured for ten minutes after the start of the assay for the PL-chip and 30 min for the AR-chip or until the occlusion pressure was reached. In addition, occlusion start time (OST, the time until the flow pressure reaches 10 kPa due to partial occlusion of the capillary) and occlusion time (OT, the time until the flow pressure reaches 60 kPa due to complete occlusion of the capillary) were measured. The technical structure of the T-TAS system and a flow diagram of a T-TAS measurement are given in the Supplementary Figures 1 and 2.
LTA was assessed using the Platelet Aggregation Profiler (PAP-8E, möLab, Germany). Platelet aggregation was induced by adenosine diphosphate (ADP) at the final concentrations 2 µM, 5 µM, 20 µM, and arachidonic acid (AA) at the final concentrations 1 mM, 1.5 mM, 2 mM. Parameters of LTA were monitored for 15 min. In addition, ristocetin was used as positive control (final concentration 1.2 mg/mL, all reagents from möLab, Germany). Read-out parameters were: maximum aggregation (MA) and the final aggregation (FA) for each parameter.
Impedance aggregometry was measured by MEA (Multiplate Analyzer, Roche Diagnostics, Switzerland) with the ADP- and ASPI-test according to the manufacturer's instructions.
Thrombin generation (TGA) was performed using the Calibrated Automated Thrombogram (Diagnostica Stago, France) with commercially available test kits according to the manufacturer's instructions on a Fluoroskan Ascent (Thermo Labsystems, Finland) at 360/460 nm wavelength activated with 5 pM tissue factor.
Frozen leftover samples were used to determine the following hemostatic parameters: von Willebrand factor (VWF) antigen and VWF activity, factor VIII and factor XIII, D-dimer and prothrombin fragments on a BCS XP analyzer (all Siemens Healthineers, Germany).
Clinical Outcome
Outcome data were collected twelve months after the intervention via phone interview and medical records to determine the result of the revascularization. The outcome search included data on vascular (re)-intervention, re-hospitalization, and bleeding events. Bleeding events were classified according to the International Society on Thrombosis and Haemostasis definitions.15,16
A flow chart of the study is given in the Supplementary Figure 3.
Reference Cohort
Whole blood from 30 healthy adult blood donors from the Institute for Transfusion Medicine, University Hospital Leipzig was collected to determine reference values for T-TAS. From each donor, 3 mL whole blood were taken for the PL chip and 1.8 mL for the AR chip. For T-TAS measurements, samples were prepared and stored in the same way as samples from PAD patients. Exclusion criteria were known bleeding disorders, a positive bleeding history in the standardized bleeding propensity questionnaire of the University Hospital Leipzig (Supplementary Table 1), as well as intake of anticoagulants, antiplatelets, NSAID, metamizole or oral contraceptives. The healthy blood donors were not screened for CAD or PAD, but those with known CAD or PAD requiring antiplatelet therapy were excluded.
Statistical Analysis
Continuous variables are expressed as median and interquartile range (IQR) and were compared with Student's t-test for normally distributed data or Mann-Whitney U-test otherwise. Categorial parameter were analyzed with chi-square test or Fisher exact test. A p value <.05 was considered as statistically significant. The ankle-brachial index of each leg was calculated according to current recommendations as the ratio of the higher systolic pressure in the ipsilateral dorsalis pedis and posterior tibial arteries divided by the higher of the left and right brachial artery systolic pressures.1,17 Reference ranges for T-TAS were defined as mean and 2.5%–97.5% percentiles of the healthy population. Target ranges for MEA were defined according to the manufacturer (ASPI-test 0-39 U, ADP-test 0–46 U). For LTA, a final aggregation ≥20% was considered as outside target range for ADP and a maximum aggregation ≥20% for AA, respectively. 18 For defining a low response in LTA, we chose 20 µM ADP and 2 mM AA, because these concentrations induce the strongest activation of platelets. Correlations between T-TAS, LTA, MEA and other coagulation parameters were assessed by Spearman's rho. For correlations, the best fit model was selected, represented by either a linear or non-linear curve. Positive percent agreement (PPA), negative percent agreement (NPA) and overall rates of agreement (ORA) for the PL-chip were calculated by receiver operating characteristic (ROC) analysis using MEA or LTA as the reference, respectively. Cut-offs were determined by using Youden's index. We defined a test result outside the target range in either ASPI, AA- or ADP-test as a positive result in the ROC-analysis. ROC-analysis was performed for the PL-chip only, because the AUC-AR was not able to differentiate between healthy patients and patients with DAPT.
Statistical analysis was performed with IBM SPSS Statistics, version 26.0 (SPSS Inc., USA), Microsoft Office Excel and GraphPad Prism 9.1 (GraphPad Software, USA).
Results
Patient's Characteristics
Between August 2020 and April 2021, 60 consecutive white Caucasian PAD patients with a median age of 65 (IQR 60-71) years were included. Most patients were classified as Fontaine IIb (63%) and the majority (53%) received PTA plus stent implantation for revascularization. Most common risk factors and comorbidities were smoking, dyslipidemia and diabetes mellitus. Elevated Lp(a) levels (>75 nmol/L) were detected in 15 out of 45 patients with available data (33%). Baseline characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of the Study Population.
| PAD patients | |||
|---|---|---|---|
| n | 60 | ||
| Age (years), median (IQR) | 65 (60-71) | ||
| Male sex, n (%) | 54 (90) | ||
| Fontaine classification, n (%) | I | 2 (3.3) | |
| IIa | 6 (10.0) | ||
| IIb | 38 (63.3) | ||
| III | 3 (5.0) | ||
| IV | 11 (18.3) | ||
| ABI, n (%) | intervened side | non-intervened side | |
| >1.4 | 4 (6.7) | 6 (10.0) | |
| >0.9 − ≤ 1.4 | 3 (5.0) | 17 (28.3) | |
| 0.5 − ≤ 0.9 | 28 (46.7) | 16 (26.7) | |
| <0.5 | 22 (36.7) | 17 (28.7) | |
| Not available a | 3 (5.0) | 4 (6.7) | |
| Intervention, n (%) | PTA only | 5 (8.3) | |
| Stent | 32 (53.3) | ||
| DEB | 6 (10.0) | ||
| Complex b | 17 (28.3) | ||
| Prior interventions | 39 (65.0) | ||
| Intervened Vessel, n (%) | Iliac | 23 (38.3) | |
| Femoral | 13 (21.7) | ||
| Popliteal | 4 (6.7) | ||
| Lower leg | 6 (10.0) | ||
| Multiple | 14 (23.3) | ||
| Risk factors and comorbidities, n (%) | Smoking c | 52 (86.6) | |
| Hypertension | 53 (88.3) | ||
| Type II diabetes mellitus | 24 (40.0) | ||
| Dyslipidemia | 48 (80.0) | ||
| CAD | 13 (21.7) | ||
| Lp(a) > 75 nmol/L d | 15 (33.3) | ||
| GFR (mL/min/1,73 m2), mean (SD) | 75.5 ± 19.6 | ||
| Platelet count (103/μL), mean (SD) | 334 ± 112 | ||
The ABI was not measured in three patients on both legs and was not measurable in one patient on the non-intervened side due to a leg amputation.
Complex intervention was defined as the use of several methods for revascularization.
Currently smoking or smoking in the past.
For Lp(a), n = 45, reference range for Lp(a) < 75 nmol/l
Abbreviations: ABI, ankle brachial index; CAD, coronary artery disease; DEB, drug eluting balloon; GFR, glomerular filtration rate; Lp(a), Lipoprotein (a); PTA, percutaneous transluminal angioplasty.
Median age of healthy controls was 38 years (IQR: 28-56) and 50% were female. PAD patients were older than the healthy subjects (p < .001), and fewer patients were female (p < .001).
Reference Ranges
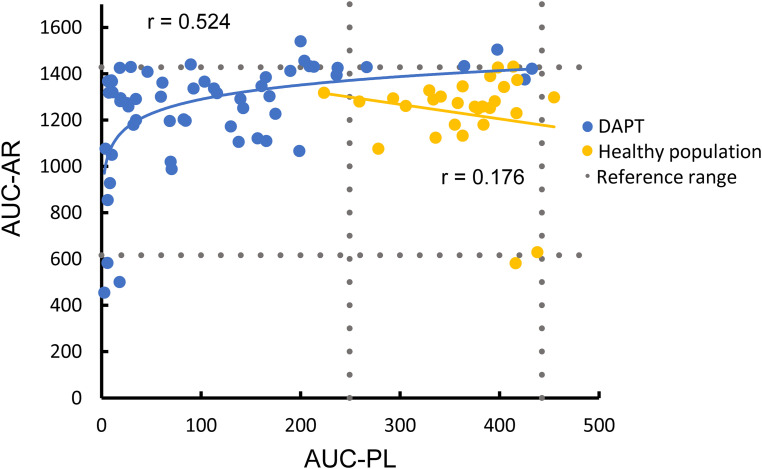
Results for the AUC-PL and AUC-AR of healthy blood donors and PAD patients are shown in Figure 1. Reference ranges for the AUC-PL and the AUC-AR in healthy blood donors were 363 (249-443) and 1224 (616-1428), respectively. Reference ranges for OST and OT are given in the Supplementary Table 1.
Figure 1.
T-TAS parameters in patients with dual antiplatelet therapy and healthy blood donors.
Reference ranges for T-TAS were defined as 2.5%–97.5% percentiles of the healthy population.
Abbreviations: AUC-PL, area under the curve for the PL-chip; AUC-AR, area under the curve for the AR-chip; DAPT, dual antiplatelet therapy.
Comparison of PAD Patients with Healthy Subjects
PAD patients had a median AUC-PL of 76.6 (IQR 19.1-168.1) and a median AUC-AR of 1300 (IQR 1121-1394). Median AUC-PL in PAD patients with DAPT was significantly lower than in healthy subjects (p < .001) but AUC-AR was not different. AUC-PL in PAD patients was outside the reference range in 91.2% and the AUC-AR in 21.1% of the patients. OT and OST in PAD patients are given in the Supplementary Table 2.
Correlations Between T-TAS, LTA and MEA
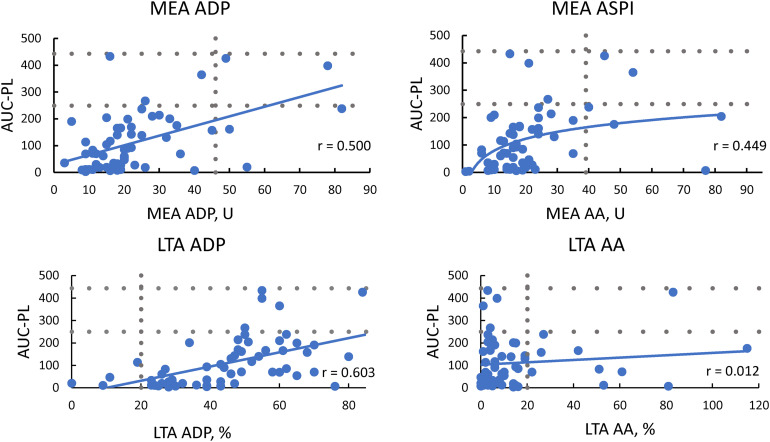
T-TAS and MEA
There was a weak to moderate positive correlation between MEA ADP and the AUC-PL (r = 0.500, p < .001) and the AUC-AR (r = 0.394, p = .002). MEA ASPI correlated better with the AUC-PL (r = 0.449, p < .001) than with the AUC-AR (r = 0.342, p = .008).
T-TAS and LTA
The strongest correlation between LTA and T-TAS was found between the AUC-PL and MA of ADP 20 μmol/L (r = 0.603), while this was moderate with ADP 5 μmol/L (r = 0.459, p < .001). MA of AA was not correlated with the AUC-PL but the AUC-AR had a moderate positive correlation with AA 1 mmol/L (r = 0.437, p = .001) and AA 1.5 mmol/L (r = 0.358, p = .005).
LTA and MEA
There was a weak correlation between MEA ADP and MA of ADP 20 μmol/L in LTA (r = 0.278, p = .031), while there was no significant correlation between the LTA AA and the MEA ASPI. Correlations between T-TAS, MEA and LTA are shown in Figure 2 and the Supplementary Table 3.
Figure 2.
Correlation between T-TAS, LTA and MEA.
Reference and target ranges are shown as dotted lines. For LTA, results are shown for final aggregation after induction with 2 mM AA and 20 μM ADP.
Abbreviations: AA, arachidonic acid; ADP, adenosine diphosphate; ASPI-test, acetylsalicylic acid-test; AUC-PL, area under the curve of the PL-chip; LTA, light transmission aggregometry; MEA, multiple electrode aggregometry; U, units.
AUC-PL and Degree of Inhibition
Nine plasma samples were out of target range in the MEA, four of those were out of target range in the ASPI-test, three in the ADP-test and two were outside target range in both tests. After stimulation with 20 µM ADP, 20 samples were outside target range in the LTA. Another three samples were out of target range after induction with 2 mM AA and eight samples were outside target range in both LTA tests.
Responders and non-responders defined by MEA or LTA did not differ in patient characteristics such as age, gender, stage of PAD, presence of coronary artery disease, arterial hypertension or diabetes.
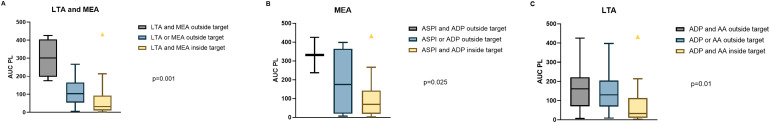
Median AUC-PL was 32 (IQR 11-93) in 27 patients with all LTA and MEA tests within the target range. Median AUC-PL was 104 (IQR 54-165) in 27 patients with at least one LTA or MEA test outside the target range and 301 (IQR 197-405) in 6 patients with at least one LTA and at least one MEA test outside the target range, p = .001 (Figure 3a).
Figure 3.
Area under the curve of the PL-chip (AUC-PL) in dependence of the degree of platelet inhibition.
A: AUC-PL in patients with a) all LTA and MEA tests within target range, b) LTA or MEA tests outside target range, c) both tests outside target range, p = .001.
B: AUC-PL in patients with a) MEA ASPI and ADP test within the target range, b) ASPI or ADP outside target range, c) ASPI and ADP outside target range, p = .025.
C: AUC-PL in patients with a) LTA ADP and AA test within target range, b) ADP or AA test outside target range, c) ADP and AA test outside target range, p = .01.
Abbreviations: AA, arachidonic acid; ADP, adenosine diphosphate; ASPI-test, acetylsalicylic acid-test; LTA, light transmission aggregometry; MEA, multiple electrode aggregometry.
Patients who were low responders in MEA ASPI (n = 4), ADP (n = 3) or both tests (n = 2) had a significantly higher median AUC-PL compared to responders (204 [IQR 90-381] vs 70 [IQR 19-153], p = .016). Median AUC-PL was 175 (IQR 19-365) in seven patients with at least one MEA test (ASPI or ADP) outside the target range, and 331 (IQR 114-301) in two patients with both MEA tests (ASPI and ADP) outside the target range, p = .025 (Figure 3b).
In LTA, patients with a final aggregation ≥20% after stimulation with 20 µM ADP (n = 28) had a higher median AUC-PL than patients who were inside the target range (148 [IQR 70-208] vs 31 [IQR 11-92] p < .001). Overall, 31 patients who had an aggregation <20% after induction with ADP and AA had a median AUC-PL of 32 (IQR 11-113) compared to 130 (IQR 69-204) in 21 patients with at least one ADP or AA test outside the target range and 161 (IQR 70-222) in eight patients with both ADP and AA tests outside the target range, p = .01. (Figure 3c). MA after stimulation with ristocetin was 83% (median, IQR 79-88) and MA was <60% in only four cases.
Table 2 and the Supplementary Table 4 show the number of patients inside and outside the target ranges in MEA and LTA tests and compares the AUC-PL between both groups.
Table 2.
Number of Aggregation Tests Outside the Target Range and Comparison of the Area Under the Curve (AUC) of the PL Chip.
| Test | Outside target range, n (%) | AUC-PL inside target, median (IQR) |
AUC-PL outside target, median (IQR) |
p | |
|---|---|---|---|---|---|
| MEA | ASPI-test a | 4 (7.8) | 69 (19-142) | 189 (49-324) | .197 |
| ADP-test a | 3 (5.5) | 69 (19-142) | 19, 161, 398* | .324 | |
| ADP and ASPI-test | 2 (3.3) | 69 (18-142) | 237, 425* | .008 | |
| LTA | ADP 20 µM a | 20 (40.0) | 31 (10-98) | 139 (73-208) | .01 |
| AA 2 mM a | 2 (9.0) | 31 (10-98) | 11, 83* | .968 | |
| AA and ADP | 8 (13.3) | 32 (11-113) | 161 (70-222) | .440 | |
Absolute values.
Abbreviations: AA, arachidonic acid; ADP, adenosine diphosphate; ASPI-test, acetylsalicylic acid-test; AUC-PL, area under the curve of the PL-chip; IQR, interquartile range; LTA, light transmission aggregometry; MEA, multiple electronic aggregometry.
Including cases of single low response only.
Agreement Between the Three Methods
Considering MEA as reference, the AUC of the ROC-curve for the AUC-PL was 0.754 (CI: 0.539-0.969), Supplementary Figure 4. As a cut-off value, an AUC-PL of 159 showed a PPA of 77.8% and a NPA of 78.4%.
Considering LTA as reference, the AUC of the resulting ROC-curve for AUC-PL was 0.723 (CI: 0.59-0.856), Supplementary Figure 5. An AUC-PL of 65 as cut-off showed a PPA of 82.8% and a NPA of 67.7%.
Plasma samples were grouped according to the AUC-PL of 159 derived from the MEA ROC-curve and 65 calculated from the LTA ROC-curve and the target ranges for LTA and MEA, Table 3. PPA, NPA and ORA for the prediction of low response in MEA and LTA were calculated for the AUC-PL of 159 and 65, Table 4.
Table 3.
Number of Plasma Samples Inside and Outside Target Range for MEA and LTA Depending on AUC-PL cut-Offs Determined by ROC Analysis, Including Single and Double low Responders.
| AUC-PL | Outside target range, n | Inside target range, n | Total, n | |
|---|---|---|---|---|
| MEA ASPI and ADP |
>159 | 7 | 11 | 18 |
| <159 | 2 | 40 | 42 | |
| LTA AA and ADP |
>65 | 23 | 11 | 34 |
| <65 | 5 | 21 | 26 |
Abbreviations: AA, arachidonic acid; ADP, adenosine diphosphate; ASPI-test, acetylsalicylic acid-test; AUC-PL, area under the curve of the PL-chip; LTA, light transmission aggregometry; MEA, multiple electrode aggregometry.
Table 4.
Positive Percent Agreement (PPA), Negative Percent Agreement (NPA) and Overall Rates of Agreement (ORA) for the AUC-PL with a cut-off >159 for MEA and >65 for LTA to Predict low Response in MEA or LTA.
| PPA (%) | NPA (%) | ORA (%) | |
|---|---|---|---|
| Single MEA a | 71.4 | 78.4 | 77.6 |
| Double MEA b | n.a. | 78.4 | 75.5 |
| Single and double MEA c | 77.8 | 78.4 | 78.3 |
| Single LTA a | 81.0 | 67.7 | 73.1 |
| Double LTA b | 87.5 | 67.7 | 71.8 |
| Single and double LTA c | 82.8 | 67.7 | 75.0 |
Abbreviations: AA, arachidonic acid; ADP, adenosine diphosphate; ASPI-test, acetylsalicylic acid-test; LTA; light transmission aggregometry; MEA, multiple electrode aggregometry; n.a. not applicable, due to one value = 0.
“Single MEA” or “Single LTA” includes low responder in one of the MEA or LTA tests.
“Double MEA” or “Double LTA” includes low responders in both MEA or LTA tests.
Including single and double low response.
A total of 22 patients were identified as responders in both LTA and MEA tests and had an AUC-PL < 65. One patient was low responding in LTA ADP and MEA ADP but responded in LTA AA and MEA ASPI and had an AUC-PL of 398. Two patients were low responders in both LTA and MEA tests and had an AUC-PL of 237 and 425. In all other patients (n = 35), the results were inconsistent for at least one test system.
Thrombin Generation Assay and Additional Hemostatic Parameters
Most patients (82%) had elevated FVIII levels (>150%) with a median of 183 (IQR 160-197) %. Median VWF activity was 247 (IQR 198-317) %, median VWF antigen was 230 (IQR 200-263) %. Patients with VWF levels >150% did not show significantly higher AUC-PL or AUC-AR than patients with normal VWF concentration.
Median D-dimer was 0.97 (IQR 0.65-1.51) mg/L and median prothrombin fragments 322 (IQR 220-433) pmol/L. D-Dimer levels were elevated in 93% and prothrombin fragments in 75% of the patients. Patients with elevated D-dimers or prothrombin fragments did not have higher AUC-PL or AUC-AR levels. Median TGA values were 2.67 (IQR 2.0-3.0) min for LT, 2136 (IQR 1878-2315) nM*min for ETP, 371 (IQR 341-401) nM for peak thrombin and 5.4 (IQR 4.7-6.3) min for TTP. Variables of TGA did not correlate with parameters of both T-TAS chips.
There was no correlation between the concentration of Lp(a) and the AUC-PL or AUC-AR. The platelet count showed a moderate correlation with the AUC-PL (r = 0.348, p = .006) and a strong correlation with the AUC-AR (r = 0.503, p < .001).
The correlation between hemostatic parameters and thrombin generation with T-TAS are displayed in the Supplementary Table 5.
Clinical Outcome
One-year follow up data were available for 42 participants. For 33 patients, data were collected by telephone call and medical records; for the remaining 9 patients, only medical records were available. Eighteen were lost to follow up. After 4-6 weeks on DAPT, 31 patients received a daily dose of 100 mg ASA, while 6 patients were switched to a combination of 100 mg ASA and 2.5 mg rivaroxaban twice daily. Another 5 patients were continued with phenprocoumon (n = 1), ASA and ticagrelor (n = 1), ASA and rivaroxaban 20 mg daily (n = 1) or clopidogrel only (n = 2).
Twenty-four patients were re-hospitalized within one year, none of them due to thrombotic occlusion of the intervened vessels. Eight patients were readmitted for a planned intervention in another vessel, whereas 12 patients were readmitted for stenotic arterial occlusion and underwent an unplanned intervention attributable to a stenosis in another (n = 8), a new stenosis in the same vessel (n = 2), and a new stenosis in the same and another vessel (n = 2). Of the eight patients who underwent surgery in a different vessel, seven had a newly diagnosed stenosis, while one was a progression of a known stenosis. Median AUC-PL in patients with planned compared to those with unplanned re-interventions was comparable (69 [IQR: 32-141] vs 64 [IQR: 23-166], p = .671). Other reasons for re-hospitalization were: amputation of the leg due to sepsis of unknown origin (n = 1), hernia-surgery (n = 1), thyroidectomy (n = 1), and polytrauma after car accident (n = 1).
One year bleeding data were available for 33 patients, of whom eleven reported clinically relevant non-major bleeding (hematoma tendency, n = 9 and epistaxis, n = 2). These patients received ASA and clopidogrel (n = 8), clopidogrel and 20 mg rivaroxaban (n = 1), ASA and twice daily 2.5 mg rivaroxaban (n = 1) and clopidogrel only (n = 1). The remaining 22 patients had no bleeding events. Median AUC-PL was not different between both groups (77 [IQR: 27-168] vs 93 [IQR: 19-204], p = .778).
In addition, there was no difference in AUC-AR or in any of the MEA or LTA test in patients with bleeding or unplanned re-interventions compared to those without such events.
Discussion
This study was the first to investigate the effect of DAPT on T-TAS in patients with PAD and to compare it with the currently used MEA and LTA and with plasma samples from healthy controls. Regarding the two used chips in T-TAS the PL-chip in contrast to the AR-chip showed effect of DAPT. Values obtained for the PL-chip correspond well to those previously described in 31 healthy volunteers. 19 Patients with low response to antiplatelet therapy detected with MEA and LTA were also detected with T-TAS in a dose-dependent manner. This may imply that a single flow chamber-based assay may be more representative for the physiological flow conditions than two separate platelet aggregation assays to detect low response to ASA and clopidogrel, as used in MEA and LTA. All available whole blood platelet assays show significant differences in the distribution of the drug effect in terms of linearity and coefficient of variation 20 but the clinical impact remains undefined.
Current cardiology guidelines conclude that aspirin should be given at low doses and platelet function testing to adjust dosing is not recommended. 21 In contrast, low platelet inhibition by clopidogrel is associated with stent thrombosis. While the use of more potent P2Y12-inhibitors ticagrelor or prasugrel may overcome this disadvantage, those drugs are recommended only in acute coronary syndrome. After the promising results for cardiac patients the EUCLID-trial was conducted to compare ticagrelor with clopidogrel in PAD patients. The trial showed comparable results between clopidogrel and ticagrelor in terms of cardiac events and limb events, at the cost of a higher bleeding rate in patients taking ticagrelor, resulting in a higher rate of drug-discontinuation. 22
Current guidelines recommend clopidogrel as the first line therapy in PAD patients because a benefit regarding cardiovascular events compared with ASA monotherapy was shown.1,23,24 In case of recurrent re-occlusions further testing with LTA is recommended to rule out non-response. 25
In a recent meta-analysis, high on treatment platelet reactivity (HTPR) was found in 29% of all PAD patients treated with clopidogrel and was associated with a higher risk of major adverse limb events. 26 Several studies have investigated HTPR in PAD patients receiving ASA and / or clopidogrel. HTPR detected with LTA was found in about one third of all patients treated with clopidogrel but HTPR to ASA was found in 11%–45%. 27
A recent study investigated 300 patients undergoing coronary artery stenting and found that HTPR detected with MEA and VASP was associated with an enhanced risk of major adverse cardiovascular events. Patients with HTPR to three tests (MEA, VASP and the platelet function analyzer P2Y-assay) had the highest risk of vascular events. 28 We showed that the AUC-PL was higher in patients having HTPR in LTA and MEA compared to those with HTPR in only one test or in patients without HTPR, suggesting that T-TAS may predict cardiovascular outcomes when a larger cohort is studied.
We separately set MEA or LTA as the reference for the ROC-analysis and found an AUC-PL of 159 or 65 as the best discriminator for low response to DAPT for MEA and LTA, respectively. We tested both cut-offs of the AUC-PL for the prediction of low response in MEA and LTA. When we set the cut-off for the AUC-PL to 159, PPA was 78% and NPA was about 78% for MEA including single and double low response. LTA measurements have shown an even lower NPA with a comparable PPA when setting the cut-off to >65 which was mainly caused by the comparably high number of low responders in LTA ADP. That is mainly caused by the low standardization of LTA leading to a high variability of the results. Therefore, our data support that the use of LTA for the determination of low response especially for P2Y12-receptor antagonists should be avoided and replaced by more standardized methods.
The AUC-PL was able to predict periprocedural bleeding after PCI under DAPT, while the AUC-AR did not correlate with acute bleeding events. 10 In contrast, AUC-AR but not the AUC-PL showed a small but significant effect on bleeding events in 561 patients one year after PCI. 11 On multivariable analysis only the AUC-AR and the combination of antiplatelets and anticoagulants were found to be predictive for bleeding events. A comparable effect of the AUC-AR was found in patients with stable CAD. 8 Our data failed to show a predictive value of either AUC-AR or AUC-PL for bleeding events. This may be due to the comparably low number of patients who have been treated with a combined therapy of antiplatelets and anticoagulants and the low number of patients with follow up data. Although AUC-PL indicates the antiplatelet effect of DAPT, our data showed no effect on re-intervention or re-occlusion at one year. This was mainly because most re-interventions were either planned at discharge or caused by re-stenosis due to intima hyperplasia rather than thrombotic occlusion of the vessel.
Our study has got several limitations. We only included a small population from a single center and subjects were all of white ethnicity. Age and sex distribution of blood donors and PAD patients differ significantly, which must be considered when applying reference ranges to patients that might be older than the reference group. Patients were tested only once at the day after the intervention, because steady-state conditions of clopidogrel are reached within hours after the loading dose 13 and because PAD patients are usually discharged early after the intervention. However, we cannot exclude that the results of T-TAS may be interfered by the severe endothelial damage caused by the intervention and that the results may be different if they were assessed later after the intervention.
Patients underwent different types of interventions that may have affected platelet activation, but making our data more transferable to a larger cohort of patients. Future studies should include more patients and could be restricted to one revascularization technique only to improve validity. Furthermore, data on clinical outcome were available only from 70% and one-year bleeding data only from 55% of the patients. In addition, patients continued DAPT for a different period of time depending on their individual risk, type of intervention, and comorbidities, which further limits the comparability of clinical outcome in terms of patency and bleeding rates assessed one year after the intervention, so that no reliable conclusion on clinical outcome can be drawn from our study.
Conclusions
Our data indicate that the AUC-PL of T-TAS may assess the level of response to DAPT in PAD patients after revascularization. Further investigation with a larger study population is needed to validate T-TAS cut-offs for clinical outcome parameters in PAD patients and to examine this tool for clinical application.
Supplemental Material
Supplemental material, sj-pdf-1-cat-10.1177_10760296241301412 for Total Thrombus-Formation System in Patients with Peripheral Artery Disease by Christian Pfrepper, Careen Franke, Michael Metze, Maria Weise, Annelie Siegemund, Roland Siegemund, Martin Federbusch, Reinhard Henschler, Sirak Petros and Manuela Konert in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Data Availability Statement: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christian Pfrepper received speakers honoraria from Fujimori Kogyo Co (Zacros). Apart from that, the authors have no relevant conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fujimori Kogyo Co (Zacros),
ORCID iDs: Christian Pfrepper https://orcid.org/0000-0002-0485-7402
Martin Federbusch https://orcid.org/0000-0002-1126-5763
Sirak Petros https://orcid.org/0000-0002-2345-756X
Statements and Declarations: The study protocol was approved by the ethics committee (Ref: 250/20-ek) of the University of Leipzig and conducted in accordance with the Declaration of Helsinki. Written informed consent was given by all patients.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Aboyans V, Ricco J-B, Bartelink M-LEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European stroke organization (ESO)The task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J. 2018;39(9):763-816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 2.Guirgis M, Thompson P, Jansen S. Review of aspirin and clopidogrel resistance in peripheral arterial disease. J Vasc Surg. 2017;66(5):1576-1586. doi: 10.1016/j.jvs.2017.07.065 [DOI] [PubMed] [Google Scholar]
- 3.Stratmann J, Karmal L, Zwinge B, Miesbach W. Platelet aggregation testing on a routine coagulation analyzer: A method comparison study. Clin Appl Thromb Hemost. 2019;25. doi: 10.1177/1076029619885184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36(2):195-198. doi: 10.1007/s10555-017-9677-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaglia MA, Torguson R, Pakala R, et al. Correlation between light transmission aggregometry, VerifyNow P2Y12, and VASP-P platelet reactivity assays following percutaneous coronary intervention. J Interv Cardiol. 2011;24(6):529-534. doi: 10.1111/j.1540-8183.2011.00670.x [DOI] [PubMed] [Google Scholar]
- 6.Hosokawa K, Ohnishi T, Kondo T, et al. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9(10):2029-2037. doi: 10.1111/j.1538-7836.2011.04464.x [DOI] [PubMed] [Google Scholar]
- 7.Hosokawa K, Ohnishi T, Fukasawa M, et al. A microchip flow-chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc Res. 2012;83(2):154-161. doi: 10.1016/j.mvr.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa S, Tsukahara K, Kikuchi S, et al. Impact of total antithrombotic effect on bleeding complications in patients receiving multiple antithrombotic agents. Circ J. 2019;83(6):1309-1316. doi: 10.1253/circj.CJ-18-1236 [DOI] [PubMed] [Google Scholar]
- 9.Arima Y, Kaikita K, Ishii M, et al. Assessment of platelet-derived thrombogenicity with the total thrombus-formation analysis system in coronary artery disease patients receiving antiplatelet therapy. J Thromb Haemost. 2016;14(4):850-859. doi: 10.1111/jth.13256 [DOI] [PubMed] [Google Scholar]
- 10.Oimatsu Y, Kaikita K, Ishii M, et al. Total thrombus-formation analysis system predicts periprocedural bleeding events in patients with coronary artery disease undergoing percutaneous coronary intervention. J Am Heart Assoc. 2017;6(4):1-13. doi: 10.1161/JAHA.116.005263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuse T, Kaikita K, Ishii M, et al. Total thrombus-formation analysis system can predict 1-year bleeding events in patients with coronary artery disease. J Atheroscler Thromb. 2020;27(3):215-225. doi: 10.5551/jat.49700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M, Kaikita K, Sueta D, et al. Total thrombus-formation analysis system (T-TAS) can predict periprocedural bleeding events in patients undergoing catheter ablation for atrial fibrillation. J Am Heart Assoc. 2016;5(1):1-12. doi: 10.1161/JAHA.115.002744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savcic M, Hauert J, Bachmann F, Wyld PJ, Geudelin B, Cariou R. Clopidogrel loading dose regimens: Kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999;25(Suppl 2):15-19. [PubMed] [Google Scholar]
- 14.Hellstern P, Stürzebecher U, Wuchold B, et al. Preservation of in vitro function of platelets stored in the presence of a synthetic dual inhibitor of factor Xa and thrombin. J Thromb Haemost. 2007;5(10):2119-2126. doi: 10.1111/j.1538-7836.2007.02716.x [DOI] [PubMed] [Google Scholar]
- 15.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 16.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126. doi: 10.1111/jth.13140 [DOI] [PubMed] [Google Scholar]
- 17.Gornik HL, Aronow HD, Goodney PP, et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the management of lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2024;149(24):e1313-e1410. doi: 10.1161/CIR.0000000000001251 [DOI] [PubMed] [Google Scholar]
- 18.Khan H, Zamzam A, Gallant RC, et al. Aspirin nonsensitivity in patients with vascular disease: Assessment by light transmission aggregometry (aspirin nonsensitivity in vascular patients). Res Pract Thromb Haemost. 2021;5(8):e12618. doi: 10.1002/rth2.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Moriki T, Igari A, et al. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res. 2013;132(2):263-270. doi: 10.1016/j.thromres.2013.05.026 [DOI] [PubMed] [Google Scholar]
- 20.Dias JD, Pottgiesser T, Hartmann J, Duerschmied D, Bode C, Achneck HE. Comparison of three common whole blood platelet function tests for in vitro P2Y12 induced platelet inhibition. J Thromb Thrombolysis. 2020;50(1):135-143. doi: 10.1007/s11239-019-01971-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aradi D, Storey RF, Komócsi A, et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur Heart J. 2014;35(4):209-215. doi: 10.1093/eurheartj/eht375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiatt WR, Fowkes FGR, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32-40. doi: 10.1056/NEJMoa1611688 [DOI] [PubMed] [Google Scholar]
- 23.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE steering committee. Lancet. 1996;348(9038):1329-1339. doi: 10.1016/S0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 24.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135(12):e686-e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgi MA, Di Girolamo G, González CD. Nonresponders to clopidogrel: Pharmacokinetics and interactions involved. Expert Opin Pharmacother. 2010;11(14):2391-2403. doi: 10.1517/14656566.2010.498820 [DOI] [PubMed] [Google Scholar]
- 26.Zlatanovic P, Wong KHF, Kakkos SK, Twine CP. A systematic review and meta-analysis on the impact of high on-treatment platelet reactivity on clinical outcomes for patients taking ADP receptor inhibitors following lower limb arterial endovascular intervention. Eur J Vasc Endovasc Surg. 2022;63(1):91-101. doi: 10.1016/j.ejvs.2021.09.026 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Chou JW, Huang W-T, Derry K, Humber D. Platelet reactivity testing in peripheral artery disease. Am J Health Syst Pharm. 2022;79(16):1312-1322. doi: 10.1093/ajhp/zxac095 [DOI] [PubMed] [Google Scholar]
- 28.Simonte G, Guglielmini G, Falcinelli E, et al. High-on-treatment platelet reactivity predicts adverse outcome after carotid artery stenting: A prospective study. Thromb Res. 2022;222:117-123. doi: 10.1016/j.thromres.2022.12.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cat-10.1177_10760296241301412 for Total Thrombus-Formation System in Patients with Peripheral Artery Disease by Christian Pfrepper, Careen Franke, Michael Metze, Maria Weise, Annelie Siegemund, Roland Siegemund, Martin Federbusch, Reinhard Henschler, Sirak Petros and Manuela Konert in Clinical and Applied Thrombosis/Hemostasis